Abstract
In the process of organogenesis, different cell types form organized tissues and tissues are integrated into an organ. Most organs form in the developmental stage, but new organs can also form in physiological states or following injuries during adulthood. Feathers are a good model to study post-natal organogenesis because they regenerate episodically under physiological conditions and in response to injuries such as plucking. Epidermal stem cells in the collar can respond to activation signals. Dermal papilla located at the follicle base controls the regenerative process. Adhesion molecules (e.g., NCAM, tenascin), morphogens (e.g., Wnt3a, sprouty, FGF10), and differentiation markers (e.g., keratins) are expressed dynamically in initiation, growth and resting phases of the feather cycle. Epidermal cells are shaped into different feather morphologies based on the molecular micro-environment at the moment of morphogenesis. Chicken feather variants provide a rich resource for us to identify genetic determinants involved in feather regeneration and morphogenesis. An example of using genome-wide SNP analysis to identify alpha keratin 75 as the mutation in frizzled chickens is demonstrated. Due to its accessibility to experimental manipulation and observation, results of regeneration can be analyzed in a comprehensive way. The layout of time dimension along the distal (formed earlier) - proximal (formed later) feather axis makes the morphological analyses easier. Therefore feather regeneration can be a unique model for understanding organogenesis: from activation of stems cell under various physiological conditions to serving as the Rosetta stone for deciphering the language of morphogenesis.
Keywords: feather, regeneration, stem cell, morphogenesis, dermal papilla, branching
Feathers exhibit amazing regenerative behavior both for physiological needs and in response to trauma. Structurally, feathers are the most sophisticated ectodermal organ whose shapes and color patterns show tremendous variation both among body regions of the same bird as well as between different species (Hill and McGraw 2006, Lucas and Stettenheim 1972, Stettenheim 2000, Yu et al. 2004). The functions of feathers include flight, temperature maintenance, ornamentation, and communication and feathers are also important in speciation of birds. Feathers serve as a model to investigate developmental morphogenesis since these abilities are preserved in cultured explants as well as in dissociated cells cultured in vitro which offer easy accessibility to molecular manipulation (Jiang et al. 1999, Widelitz et al. 1999). Recent technological progress for molecular perturbation in regenerating feathers in vivo have made adult feathers a good model in which to investigate stem cell regulation and molecular contributions that shape regenerating organs. Thanks to advances in avian genetic tools and the variety of chicken mutants preserved during domestication and selective breeding (Andersson and Georges 2004, Coquerelle 2000, Reeder 2006, Rubin et al. 2010), feathers are also an emerging model used to investigate congenital ectodermal disorders (Mou et al. 2011, Ng et al. 2012).
Cyclic regenerative ability of feather follicles and feather stem cells
Follicle structure
Feathers can naturally molt and regenerate (Lucas and Stettenheim 1972). In chickens, a precocious bird, feather follicles have already formed when the chick hatches. They form downy feathers. Shortly after birth, the first molting is initiated and the downy feathers are replaced by contour feathers in the first post-natal (juvenal) plumage and successive plumages. As the birds age, down feathers are replace with juvenile feather and eventually adult feather forms. However, these different feather forms are from the same follicle. Sexually dimorphic feathers can appear in different genders. The physiological molting and regeneration gives birds a chance to reshape and recolor their plumage for physiological needs (Chuong et al., 2012).
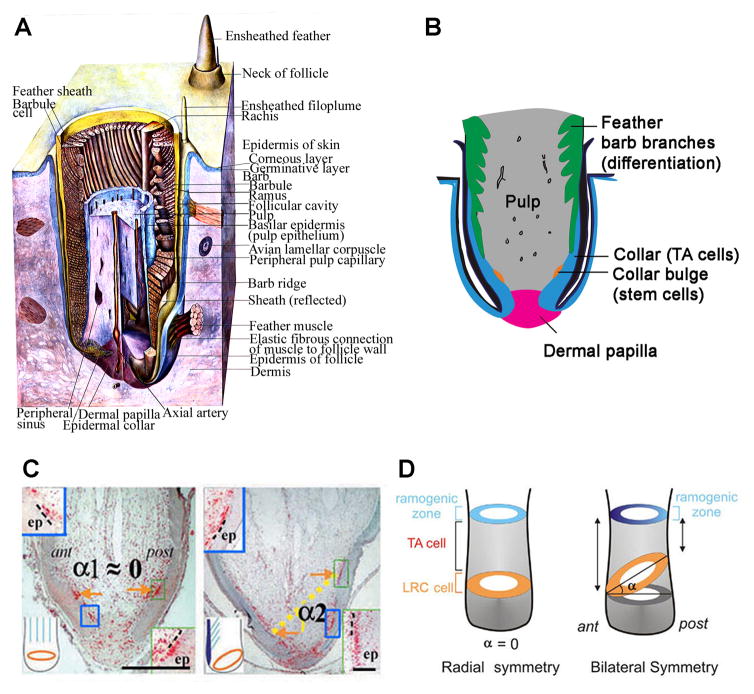
The structure of feather follicles varies during the feather cycles. Feather cycles can be largely divided into two phases: growing phase and resting phase (Lucas and Stettenheim 1972). In the transition from resting phase to growing phase, there are phases of molting and initiation (Yue et al. 2005). The two dimensional feathers are generated from a cylindrical feather follicle which consists of two major components: the epithelium and the mesenchyme (Fig. 1, A and B) (Lillie FR. and Juhn 1938, Yu et al. 2002). The mesenchymal cylinder includes the dermal papilla and the pulp (Lillie F.R. 1940, Lucas and Stettenheim 1972, Yu et al. 2004). The epithelial part includes the epithelium enwrapping the mesenchyme and the feather wall epithelium that is connected with the interfollicular epidermis. The dermal papilla is a permanent structure while the pulp cyclically grows in growing phase and regresses as feathers enter a resting phase. There are no molecular makers available that definitively differentiate dermal papilla from pulp cells. These two structures are conventionally differentiated by their morphology in histology: the dermal papilla is a structure at the follicular base with a compact extracellular matrix while pulp cells above are embedded in a background of loose extracellular matrix with high vascularity. It is believed that pulp cells are derived from the dermal papilla and at least some dermal papilla cells proliferate and give rise to pulp cells in early growing phase (Lillie F.R. 1940, Lucas and Stettenheim 1972). When feathers regrow, the expansion of the mesenchyme with the reestablishment of the vascular channels enlarges the feather germ and provides nutrition and structure support for the growing epithelium (Lillie F.R. 1940, Lucas and Stettenheim 1972).
Fig 1. Structures of feather follicle.
(A) Three dimensional structure of a growing feather (Adopted from Lucas 1973). (B) Schematic drawing of the structure of a growing feather follicle. (C) Label-retaining cells (red) in downy (left) and flight feathers (right) are arranged as a horizontal ring in downy feathers, whereas they are configured as a tilted ring in flight feathers. (Adopted from Yue et al. 2005) (D) Model for the stem cell niche topology in radially symmetric feathers (left) and bilaterally symmetric feathers (right). A ring of stem cells lies parallel to the surface of the skin in radially symmetric feathers but is canted at an angle in bilaterally symmetric feathers. LRC: label-retaining cell; TA: transit amplifying. (Adopted from Yue et al. 2005).
Epidermal stem cells
In parallel, the feather epithelium wrapping around the mesenchymal cylinder also enlarges as the mesenchyme expands in the growing phase. The feather germ epithelium is divided into several unique parts that are associated with distinct behavior in feather growth, including papillaryectoderm, collar bulge, ramogenic zone and barbs (Fig. 1, A and B). The papilla ectoderm is tightly connected with the dermal papilla and this structure is preserved both in the growing and resting phases. When a feather is forcefully removed by plucking, papillary ectoderm is usually preserved and remains connected with the dermal papilla (Lucas and Stettenheim 1972). Cells proliferate quickly and differentiate at the ramogenic zone(Yue et al. 2005). The collar bulge is where keratinocyte stem cells reside in growing phase and the keratinocyte stem cells are arranged as a ring surrounding the feather germ (Fig. 1, D and E)(Yue et al. 2005). By use of BrdU labeling/tracing and in vivo fluorescent labeling, we showed that feather keratinocyte stem cells are slow-cycling cells that give rise to transit amplifying progeny who gradually move vertically upwards and differentiate toward the barbs (Yue et al. 2005, 2006). When feathers enter the resting phase, the collar bulge structure disappears due to shrinkage of collar epithelium (Lucas and Stettenheim 1972, Yue et al. 2005). At this time, the feather keratinocyte stem cells descend to the papillary ectoderm, and become quiescent (Yue et al. 2005). The cells will become active again in the next growing phase.
Dermal papilla as the controlling center for feather regeneration
Origin of dermal papilla cells
What controls the regeneration of feathers? The development of feather follicles results from delicate molecular cross talk between the epithelium and mesenchyme (Chuong 1998). Dermal papilla is a group of specialized dermal fibroblasts that have been demonstrated to be folliculogenic (Lillie FR. and Wang 1944, Lillie F.R. and Wang 1940, Wang 1943). The cell origin of dermal papilla cells has not been characterized until very recently. One question regarding this issue is whether DP cells are derived from a predetermined cell source before feather development or originate from local mesenchymal cells during feather morphogenesis. By reconstitution of embryonic epidermis with dissociated dermal cells at different embryonic developmental stages, our work suggests that before feather bud formation, each dermal cell is equal in its plasticity to differentiate either into a dermal papilla or interbud fibroblast (Jiang et al. 1999). Even in an early stage of feather bud formation when dermal condensates appear, the mesenchymal cells in the developing feather bud or interbud dermis are still plastic and can grow into either new dermal condensates or interbud dermal fibroblasts after they are dissociated and reconstituted with embryonic epidermis (Chuong et al. 1996, Jiang et al. 1999). Our result suggests that DP cell fate is not predetermined before feather morphogenesis and that embryonic dermal mesenchymal cells are true mesenchymal stem cells that can adopt either a bud or interbud fate through interactions with the developing epidermis. In this process, embryonic dermal mesenchymal cells first form dermal condensates which are further specified to dermal papilla fate during later morphogenesis.
Inductive property of dermal papilla
The inductive property of postnatal DP cells was first revealed in early work from Frank Lillie and Hsi Wang on chicken feathers about 7 decades ago (Lillie FR. and Wang 1941, 1944, Wang 1943). It was shown that when the dermal papilla is removed, feather regeneration is prohibited. To further test the inductive property of dermal papilla, the donor dermal papilla was carefully microdissected to remove the attached epithelium and transplanted to a recipient follicle where the dermal papilla and the papillary ectoderm were removed (Fig. 1C). It was revealed that the transplanted dermal papilla induced feather regeneration in the recipient follicle. In addition to the inductivity of post-natal dermal papilla, classical work also demonstrated that only the basal half of the feather follicle epidermis could respond while the interfollicular epidermis fail to regenerate new feather follicles. Results from this delicate microdissection and transplantation study suggest that that the feather dermal papilla can induce the epithelium to regenerate feathers.
Dermal papilla and feather morphology
The shapes of feathers can vary in the same body regions or among different body regions. As the birds age, the shapes of feathers regenerating from the same feather follicles can vary according to physiological needs (Lucas and Stettenheim 1972, Yu et al. 2004). For example, the downy feathers of the embryonic feathers are replaced by contour feathers after birth. The hackle, saddle and sickle feathers even show sexually dimorphic morphologies in adult birds of different genders. In the same body region, feathers often show a gradation of changes in the length, size, shapes and even color patterns in the adjacent follicles. In the adult birds, when feathers are plucked, they regenerate with similar, if not identical, size and morphology. This result raises important questions: how is the feather morphology controlled and where is the information of the feather morphology stored?
These questions were answered using transplantation experiments involving swapping varied components of feather germs between follicles of different feather tracts that display different feather morphologies (Cohen and Espinasse 1961, Lillie FR. and Wang 1941, Wang 1943). When the dermal papilla and the attached papillary ectoderm are transplanted to another follicle whose dermal papilla and the papillary ectoderm are previously removed, the regenerated feather morphology resembles that of the donor follicle. In addition, if the donor dermal papilla is rotated horizontally, the orientation of the regenerated feather vane changes accordingly. Moreover, when half of the dermal papilla is removed from one side of the vane, the feather can regenerate only the other half of the vane. Hence, in addition to the inductivity, the symmetry and orientation of the induced new feather is determined by the donor dermal papilla. The information of feather morphology is stored locally within the feather germ, rather than in the extrafollicular skin.
The classical work is also significant in that dermal papillae of different follicles are not equivalent. In addition, cells in each dermal papilla are not equal, either. There should be topological variation in each dermal papilla or among different dermal papillae that set up and preserve the structural information of regenerating feather structure. Recent work in rodents suggests that these principles may also hold in mammalian follicles (Cohen 1961, Driskell et al. 2009, Jahoda et al. 1984, Oliver 1967). It is unknown how the sexually dimorphic changes of feathers is controlled, but it is possible that the local dermal papilla and the papillary ectoderm may respond to systemic hormones to change the shapes and even color patterns of regenerating feathers. Since sexual dimorphism is not obvious in murine hair, feathers are a good model to investigate how systemic factors reshape the regenerating ectodermal organs.
Molecular expression in regenerating feather follicles
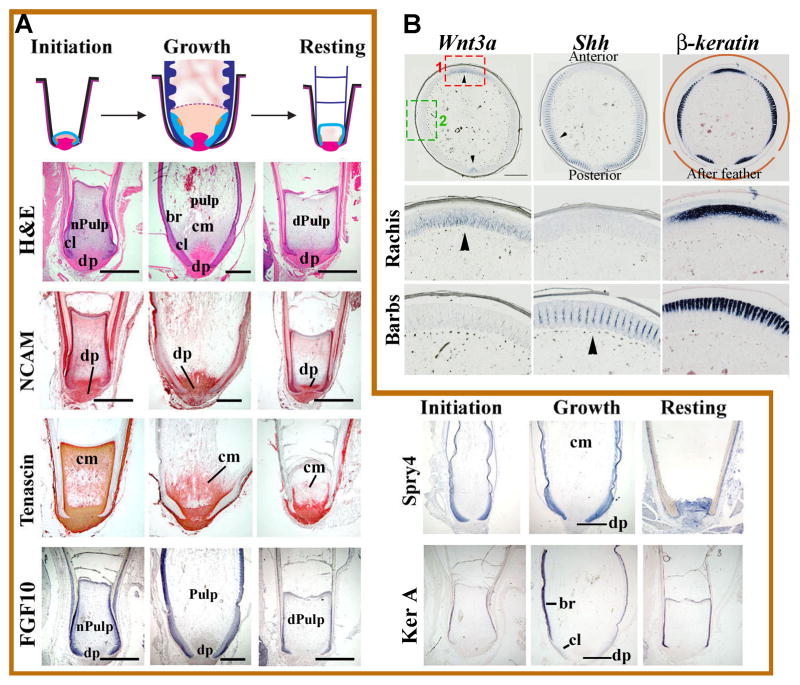
Feathers are lost and regenerate throughout the lifetime of a bird. Young chicks grow radially symmetric downy feathers which help to trap warmth. Once the birds mature, these feathers are replaced by bilaterally symmetric or bilaterally asymmetric feathers. Different regions of birds produce different types of feathers. This offers developmental biologists an opportunity to study cellular and molecular aspects of the feather cycle. Feathers begin to form in the initiation phase of the feather cycle (Fig. 2A). First, there is a re-epithelialization of the dermal papilla by activated papillary ectoderm. At this stage, the epithelial collar is small and in close proximity to the dermal papilla. Normal pulp lies distal to the dermal papilla. In the growing phase, the epithelial collar extends distally and barb ridges begin to form (Fig. 2A). Blood vessels appear within the pulp to bring nutrients to the growing feather. In the resting phase, keratinized pulp caps form as the pulp disintegrates (Fig. 2A).
Figure 2. Molecular expression in regenerating feather follicles.
(A) Schematic diagram of the feather cycle consisting of Initiation, Growth and Resting phases (top panel). The lower panels show longitudinal sections of feathers at each stage stained for H&E, NCAM, Tenascin, FGF10, Spry4 and Ker A. Positive staining is red for immunostaining (NCAM, Tenascin) and blue for in situ hybridization (FGF10, Spry4 and Ker A). br, barb ridge; cl, collar; cm, collar mesenchyme; dPulp, degenerating pulp; dp, dermal papilla; nPulp, newly formed pulp. Bar= 1000 μm. (B) Dynamics of molecular expression patterns in semiplume feathers in cross sections. In situ hybridization shows RNA expression pattern of Wnt3a, Shh and β-keratin in the cross sections of a semiplume feather. Wnt3a is expressed in the rachises of the feather and the after-feather (arrowheads). Shh is present in the marginal plate of barbs (arrowhead). β-keratin shows up in the differentiated region of the barbs and rachises. The 2nd and third rows are magnified graphs from the boxed regions in the top left panel to highlight rachis and barb regions. Bar= 500 μm.
Longitudinal sections of the follicle show dynamic tissue remodeling and molecular expression in different cycle stages
To understand what molecular signals regulate the transition between Initiation, Growth and Resting phases, we and others have characterized molecular expression during these phases by in situ hybridization in longitudinal sections (Fig. 2A). As an example of these studies, 2 cell adhesion molecules, neural cell adhesion molecule (NCAM) and tenascin C, are present in the dermal papilla at all phases of the feather cycle. Tenascin C is also present in the mesenchyme adjacent to the collar epithelium. Researchers also investigated the expression of growth factors and their inhibitors (ie., FGF10 and Spry4). High levels of FGF10 are expressed in the epithelium of feathers during Initiation and Growth, but the expression decreases during Resting phase. Spry 4 is expressed in the feather epithelium during Initiation and Growth phases and then is reduced in the dermal papilla during Resting phase. Finally the expression of the differentiation markers such as Keratin A (Ker A) were explored. Ker A is only expressed in the differentiating branching epithelium during Growth phase (Yue et al., In press).
Cross sections of the follicle show bilateral asymmetry based on rachidial ridge and progressive formation of barb ridges
For adult birds, each cycle of feather regeneration yields a new feather with a branching pattern almost identical to the one replaced. The robust nature of this process makes it a reliable platform for investigating molecular basis of pattern formation. Semiplume feathers on the chicken trunk have an afterfeather attached to each main feather. The rachides of the main feather and the afterfeather are opposite to each other (Fig. 2B, arrowheads). Both express high level of Wnt3a (Fig. 2B). Functional perturbation experiments confirms the importance of Wnt3a in rachis formation (Yue et al. 2006). Efforts have also been made to explain the periodical formation of barbs. A reaction-diffusion model based on Turing’s theory has been proposed (Harris et al. 2005). Shh is considered as a potential activator based on its expression in marginal plates (Fig. 2B). After periodic pattern formation of barb ridges, feather epithelium differentiate in a peripheral to central order (Fig. 2B, β-keratin serves as a differentiation marker).
Modulating feather forms by perturbing molecular pathways
The classical microdissection and transplantation studies provided clues to the key cellular components in feather regeneration and feather morphology and also showed that the structural complexity of regenerating feathers is a readout of information stored in the local dermal papilla and the attached feather ectoderm. However, how is such message relayed and how does it instruct the epithelium to build the complex feather structures? Over the past decade, our lab has provided the molecular basis for the morphogenesis of regenerating feather shapes. Here are some examples of molecular pathways which have been reported.
BMP signalling determines the balance of rachis and barbs
We showed that an activator/inhibitor pair of BMP4 and noggin determined whether the keratinocytes grow into rachis or barbs (Yu et al. 2002). BMP4 promotes rachis formation and the barb fusion, while noggin enhances rachis and barb branching. Hence, when BMP4 is high and noggin is low, rachis forms. In the zones where barbs form, BMP4 is low and noggin is high. By perturbing the ratio of BMP4 and noggin, we are able to convert barbs into rachis. It is intriguing to explore how such a molecular gradient is established by the dermal papilla and the attached papillary ectoderm.
Wnt3a signaling determines bilateral and radial symmetry
The shapes of feathers are critical to their functions. The radially symmetric downy feathers are important for heat insulation while the formation of the bilaterally symmetric vanes is important for ornamentation and flight. We showed that feather keratinocyte stem cells are multipotent cells located in the collar bulge epithelium (Fig. 1, B, D and E)(Yue et al. 2005). How do birds manage their keratinocyte stem cells to create such morphological variation? We found that the keratinocyte stem cell ring is tilted in bilaterally symmetric feathers, low in the anterior side and high in the posterior side, while this ring remains horizontal in the radially symmetric feathers (Fig. 1, D and E). The tilted ring creates an anterior-posterior maturation gradient that might help to form the rachis and bilaterally symmetric vanes. In search of molecular control of such events, we found that there is a molecular gradient of Wnt3a in the bilaterally symmetric feather germ (Yue et al. 2006), high in the anterior side and low in the posterior side. Perturbing this gradient converts bilaterally symmetric feathers into radially symmetric feathers. Since the orientation of the feather vane is determined by the orientation of the dermal papilla, further work will help to clarify how this molecular gradient is set up in the bilaterally symmetric feathers by the dermal papilla.
Sprouty signalling determines branching and proximal/distal morphology
The ectopic expression of Spry4, a negative regulator of receptor tyrosine kinases, converts proximal regions of the feather follicle to more distal fates (Fig. 3, C and D). In other words, the region of the collar epithelium abnormally produces barbs. Ectopic barbs can even be seen within the follicle sheath (equivalent to the hair follicle outer root sheath). The resultant feathers have an expanded vane with a greatly diminished calamus (the unbranched, bottom section of a feather). Feathers overexpressing Spry4 have an expanded pulp and reduced or missing dermal papilla. Since epithelial – dermal papilla interactions are essential for feather initiation of a new feather cycle, these feathers often fail to regenerate. Ectopic expression of FGF10 has the opposite effect (Fig. 3E). The dermal papilla is increased in size and the collar epithelium and adjacent mesenchyme are expanded. Branching morphogenesis and keratin differentiation are inhibited (Yue et al., In Press).
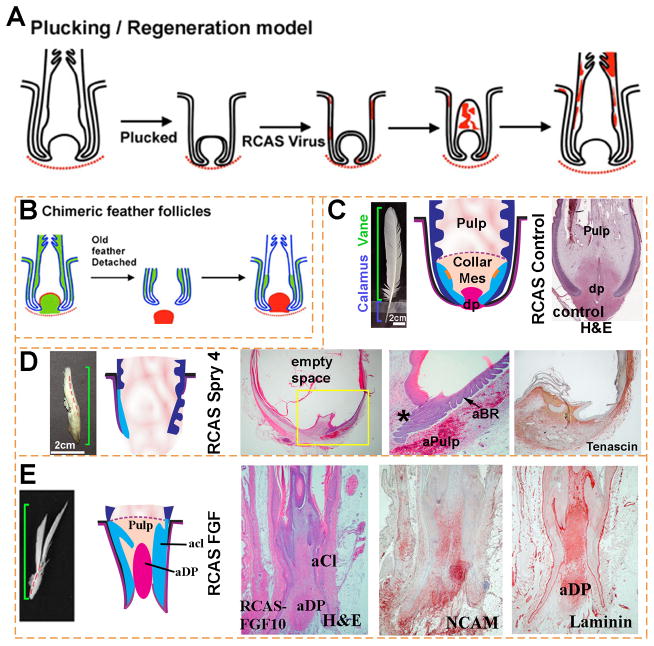
Figure 3. FGF/Sprouty determines the proximal-distal feather morphology and the size of the dermal papilla.
(A, B) Schematic depiction of methods used for studying feather regeneration and morphogenesis. (A). Feathers are plucked to induce the initiation of a new feather. Virus carrying exogenous genes such as β–galactosidase is employed to transduce cells of the newly formed feather. Red color indicates β–galactosidase staining. (B) Classical tissue recombination studies involving microdissection and transplantation of specific components of the follicle. The dermal papilla can be microdissected from the donor follicle and transplanted to the recipient follicle where the dermal papilla has been removed. (C–E) Examples using RCAS sprouty to study the roles of signaling genes on feather morphogenesis. (C) Control feather transduced with RCAS Lac Z. (D) Regenerating feathers transduced with RCAS Spry 4 show miniaturization of the DP, which also becomes Tenascin C negative (compared with Fig. 2A). The perturbed follicle also shows an expanded pulp which is represented by empty space in the section. The follicles also show numerous ectopic branches forming within the follicle and also on the follicle sheath outside of the follicle. (E) Transduction with RCAS FGF10 induces proximal feather structures with a thickened keratinocyte collar and a diffuse dermal papilla which is positive for NCAM and laminin. aCl, abnormal collar; aBr, abnormal barb ridge; aDp, abnormal dermal papilla; aPulp, abnormal pulp.
We next examine how the ectopic expression of Spry4 and FGF10 affects the expression of genes known to play important roles during feather morphogenesis. In Spry4 overexpressing feathers, markers of the dermal papilla, including laminin, tenascin C and NCAM, are greatly diminished (Fig. 3D). The expression domains of NCAM and laminin are extended distally in feathers transduced with RCAS FGF10 (Fig. 3E). These findings are consistent with our observation that formation of the dermal papilla is dependent on FGF signaling and that the dermal papilla is reduced or missing in feathers expressing exogenous Spry4. The results imply that signals along the Sprouty/FGF pathway can regulate epidermal stem cell activation at different feather cycle stages to produce different shapes along the proximal-distal feather axis and likely contributes to feather morphological diversity.
Genetic determinants of feather forms
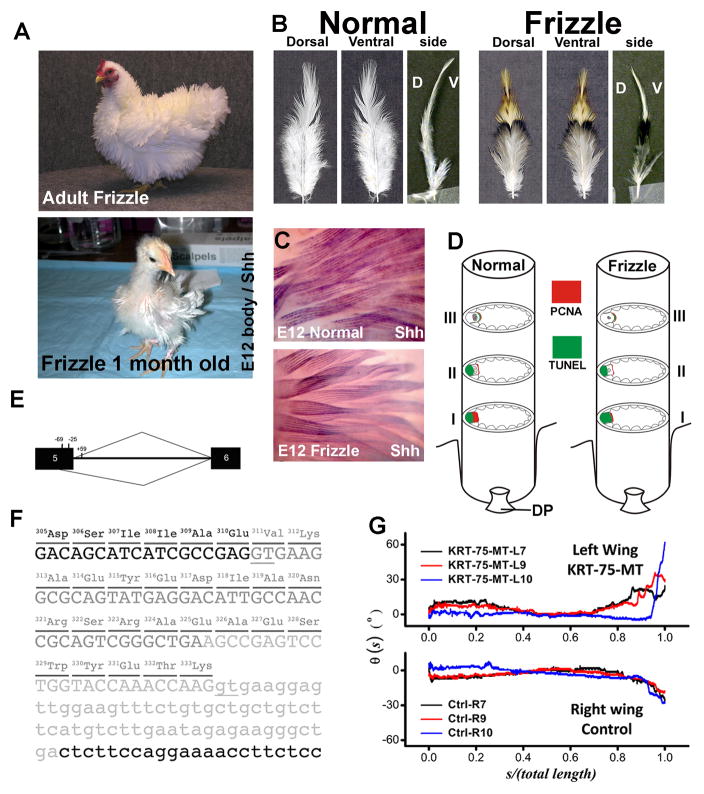
Domestication and selective breeding have fastened phenotypic diversification in chickens, and the plentiful distinct breeds available provide an excellent opportunity to explore the genetic basis underlying the variation in morphology, physiology, and behavior. Frizzle feathers have been described in domesticated chickens (Coquerelle 2000). The contour feathers of the frizzle chicken curl outwards and upwards (Fig. 4A, B), and feathers do not normally rest flat against the body due to the altered rachidial structure. The frizzle phenotype does not become obvious until the first-generation downy feathers are replaced by the pennaceous second-generation feathers (Fig. 4A, lower panel). The frizzle phenotype shows incomplete dominant inheritance, but the cellular, genetic and molecular basis for this trait was previously unknown. When we compare the Shh whole mount in situ hybridization between frizzle and normal feathers at embryonic day 12, we found the frizzle feathers showed the same expression of SHH as controls (Fig. 4C), which suggests that embryonic frizzle feather branching occurred normally, even though the tip of frizzle feathers were randomly twisted.
Figure 4. Frizzle feather phenotype was caused by KRT75 mutation.
(A) Adult and 1-month-old frizzle chickens. Adult frizzle chicken feathers curve away from the body. The second-generation feathers in a one-month old chick start to show a clear frizzle phenotype. (B) Comparison of body feathers from normal white leghorn and frizzle chickens in dorsal view, ventral view and side view. D, dorsal; V, ventral. (C) Shh wholemount in situ hybridization in embryonic day 12 normal and frizzle feather buds. (D) Diagram summary of PCNA and TUNEL staining at different levels of the rachis. (E) Diagram of the chicken KRT75 gene and the cryptic splice site activated by the deletion mutation that covers positions 224 of exon 5 to +59 of intron 5. Black boxes represent exon sequences; intron 5 is designated by a line. The caret designating use of the cryptic site (position 269) is shown below, and the caret designating use of the authentic site is shown above the diagram of the pre-mRNA. (F) Partial sequence of KRT75 gene. The 84-bp deletion in genomic DNA is shown in light gray letters. The additional deletion in exon 5 created by a cryptic splice site is shown in dark gray letters. The deletion in genomic DNA and use of the cryptic splice site together result in a deletion of 23-amino acids (position 311–333) in the K75 protein. Parts of exon 5 and intron 5 are shown in capital and small letters, respectively. The underlines show the authentic and cryptic mRNA splicing sites. (G) Effects of viral misexpression, as shown by qualitative changes in the rachis curvature. Without the gene mutation, the curvature from the contralateral feathers of a normal chicken were expected to exhibit mirror symmetry, which is obviously abrogated in this case.
To understand the cellular basis of the frizzle phenotype, we examine rachidial morphogenesis at different time points of regeneration after feather plucking. Horizontal sections at different levels of the regenerating feathers, from mature to immature, were prepared for examination of histology, cell proliferation and cell death (Fig. 4D). We find the cell proliferation zone at an developing level (level I) of the frizzle rachis is narrower compared to that of a normal rachis, contributing to a smaller medulla in the frizzle rachis (Fig. 4D).
In order to dissect the genetic basis responsible for this phenotype, we conducted a genome-wide linkage analysis scan and indentify the causative mutation in a-keratin (KRT75)(Ng et al. 2012). We found a deletion in KRT75 covering the junction of exon 5 and intron 5 (Fig. 4E). This deletion shows complete segregation with the frizzle phenotype in all the offspring within the F1 generation of the experimental crosses. Sequence analysis of the coding sequence of KRT75 cDNA shows that the loss of the authentic splice site at the exon5/intron 5 junction activates a “cryptic” splice site in exon 5 (Fig. 4E), resulting in a 69-bp in-frame deletion within the coding region (Fig. 4F).
We constructed a KRT75 mutant form (KRT75-MT) to test its function in generating this phenotype. Its involvement was confirmed by retrovirus-mediated misexpression of KRT75-MT. Forced expression of the mutated gene in normal chickens produce a twisted rachis similar to the morphology of a frizzle feather (Fig. 4G). Forced expression of the mutated gene in normal chickens produce a twisted rachis similar to the morphology of a frizzle feather. Unlike the gentle inward bending of the wild type feathers, the end of the K75 mutant-transduced feather twisted abruptly away from the body. Those feathers exhibit anomalous bending and kinky structures that mimic feathers from the frizzle mutant (Ng et al., 2012).
Proximal – distal axis of the feather as a chronological record of changing micro-environment for feather stem cells
As feathers regenerate, there is a chronological order of feather growth: the distal end forms earlier and the proximal end forms later (Figure 5; Lucas and Stettenheim, 1972). The structure and shape of feathers dynamically change from the distal end to the proximal end. The distal pennaceous vane changes in contour. Toward the proximal end (which forms later), the barbs become plumulaceous (downy). The rachis gradually increases in width, and eventually turns into the calamus (Fig. 5).
Figure 5. Time dimension and proximal-distal feather axis during regeneration.
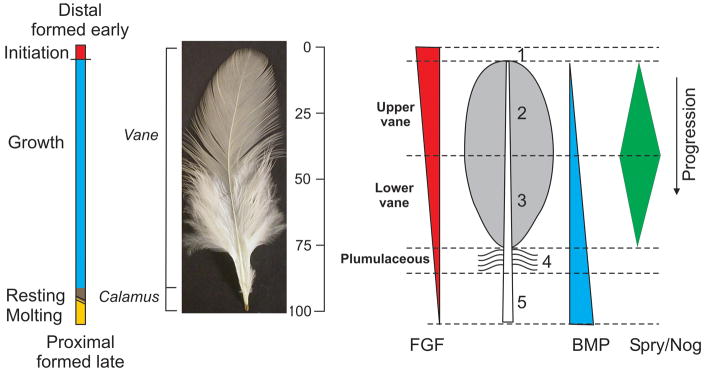
Each feather displays its formation processes. The distal part is formed first and proximal part later. The 0–100 vertical bar is used to indicate that the signaling microenvironment in the feather follicle varies in these different arbitrary time units for feather formation. In the schematic feather on the right, numbers indicate different chronological stages during feather formation. 1) Initiation occurs in what will become the distal tip of the feather. Growth phase can be split into the 2) upper and 3) lower vane. In the typical feathers, upper and lower vane are separated by the line across the widest region of the vane. 4) This is followed by the plumulaceous region in which barbs are not attached to their neighboring barbs and remain fluffy. 5) The calamus is formed in the resting phase at the proximal end of the feather. For the molecular involvement, we know the combination of different ratios of FGF, BMP, sprouty and noggin will modulate feather stem cells into different feather forms at different time points. The quantitative amounts of these signaling molecules, however, are based on idealized speculation.
Feather stem cells are located in the proximal follicle. Collar keratinocytes in the proximal follicle proliferate. The proliferation, apoptosis, arrangement and differentiation of their progeny contribute to the the diversity of feather forms. Structural alterations along the distal-proximal axis leave a physical record of the dynamic changes in the integrated regulatory signals issued at each time point that modulate the behavior of keratinocyte stem cells and their progeny. Analyzing the regulatory signals at each time point can yield a “snap shot” of the regulatory inputs. Serial presentation of such “snap shots” along the distal-proximal axis of feather growth can provide a “time-lapse video” of the dynamic changes of molecular events.
Connecting with adjacent tissues
A feather follicle does not exist independently. It has to be integrated to adjacent tissues.
During development, feather germs first form by epidermal and dermal tissue interactions. The early feather follicle starts to attract muscle, blood vessels, adipose tissues and nerves to come to the feather follicle. This allows the specific dermal muscle network to be connected (Lucas and Stettenheim 1972). Blood vessels originally travel parallel to the skin surface. Now they send out periodically spaced sprouts toward the base of each feather follicle. Eventually blood vessels enter the pulp vial the dermal papilla. Nerve fibers originally are randomly distributed in the dermis. Now the peripheral neurites go around the follicle and eventually enter the follicle via the dermal papilla into the pulp. With these connections, nutrition can be provided to the growing feather follicles. Feather follicles also can be moved with proper muscle control and connected with proper neurites. There is also an adipose layer surrounding the feather follicle, providing cushion and other functions.
During regeneration, muscles remain connected to the follicle sheath which does not degenerate. However, nerve and blood vessels that enter the pulp have to undergo degeneration. They will also have to regenerate, together with the regenerating feathers, to re-establish their function.
Thus feather work as an ideal model for its regeneration and the connection with surrounding tissues, so it is well integrated to the host.
Acknowledgments
This work was supported by US NIH grants AR42177, AR47364, and AR 060306 (to C.M.C.); Taiwan National Health Research Institutes grant NHRI PS9803 (to S.J.L.); National Taiwan University grant 101R7602D4 (S.J.L.); National Taiwan University Hospital grant 101S-1857 (to S.J.L.); Taiwan National Science Council grants NSC99-2320-B-002-004-MY3 and NSC101-2325-B-002-081 (to S.J.L.).
References
- Andersson L, Georges M. Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet. 2004;5:202–212. doi: 10.1038/nrg1294. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Widelitz RB, Ting-Berreth S, Jiang TX. Early events during avian skin appendage regeneration: dependence on epithelial-mesenchymal interaction and order of molecular reappearance. J Invest Dermatol. 1996;107:639–646. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- Cohen J. The transplantation of individual rat and guineapig whisker papillae. J Embryol Exp Morphol. 1961;9:117–127. [PubMed] [Google Scholar]
- Cohen J, Espinasse PG. On the normal and abnormal development of the feather. J Embryol Exp Morphol. 1961;9:223–251. [PubMed] [Google Scholar]
- Coquerelle G. Les poules: Diversité génétique visible. Paris: L’Institut National de la Recherche Agronomique; 2000. [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci U S A. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE, McGraw KJ. Bird Coloration. Cambridge, Massachusetts: Harvard University Press; 2006. [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- Lillie F, Juhn M. Physiology of development of the feather. II. General principles of development with special reference to the after-feather. Physiol Zool. 1938;11:15. [Google Scholar]
- Lillie F, Wang H. Physiology of development of the feather. V. Experimental morphogenesis. Physiol Zool. 1941:33. [Google Scholar]
- Lillie F, Wang H. Physiology of development of the feather. VII. An experimental study of induction. Physiol Zool. 1944;17:30. [Google Scholar]
- Lillie FR. Physiology of development of the feather. III. Growth of the mesodermal constituents and blood circulation in the pulp. Physiol Zool. 1940;13:33. [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. V. Experimental morphogenesis. Physiol Zool. 1940;14:33. [Google Scholar]
- Lucas AM, Stettenheim PR. Agriculture Handbook 362: Agricultural Research Service. Washington DC: US Dept. Agrculture; 1972. Avian Anatomy: Integument. [Google Scholar]
- Mou C, et al. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLoS Biol. 2011;9:e1001028. doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CS, et al. The Chicken Frizzle Feather Is Due to an alpha-Keratin (KRT75) Mutation That Causes a Defective Rachis. PLoS Genet. 2012;8:e1002748. doi: 10.1371/journal.pgen.1002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver RF. The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J Embryol Exp Morphol. 1967;18:43–51. [PubMed] [Google Scholar]
- Reeder B. An Introduction to Color Forms of the Domectic Fowl. Bloomington, Indiana: AuthourHouse; 2006. [Google Scholar]
- Rubin CJ, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 2010;464:587–591. doi: 10.1038/nature08832. [DOI] [PubMed] [Google Scholar]
- Stettenheim PR. The Integumentary Morphology of Modern Birds—An Overview. Amer Zool. 2000;40:17. [Google Scholar]
- Wang H. The morphogenetic functions of the epidermal and dermal components of the papilla in feather regeneration. Physiol Zool. 1943;16:26. [Google Scholar]
- Widelitz RB, Jiang TX, Chen CW, Stott NS, Jung HS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development. 1999;126:2577–2587. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The biology of feather follicles. Int J Dev Biol. 2004;48:181–191. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci U S A. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Wu P, Widelitz R, Chuong CM. Sprouty/FGF signaling regulates the proximal-distal morphology and the size of dermal papillae. Dev Biol. doi: 10.1016/j.ydbio.2012.09.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]