Abstract
Rationale
Δ9-tetrahydrocannabinol (Δ9-THC) modifies dopamine efflux. However, the extent to which cannabinoid and dopamine drugs modify each other’s behavioral effects has not been fully established.
Objectives
This study examined dopamine releasers and/or transport inhibitors alone and in combination with cannabinoids in two drug discrimination assays.
Methods
Experimentally and pharmacologically experienced rhesus monkeys (n=5) discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle while responding under a fixed ratio 5 schedule of stimulus-shock termination. A separate group (n=6) of monkeys responded under the same schedule, received daily Δ9-THC (1 mg/kg/12 h s.c.), and discriminated the cannabinoid antagonist rimonabant (1 mg/kg i.v.), i.e. cannabinoid withdrawal, from vehicle. A sign of withdrawal sign (head shaking) was examined in monkeys receiving Δ9-THC daily.
Results
Rimonabant antagonized the Δ9-THC discriminative stimulus and a dose of Δ9-THC greater than the daily treatment attenuated the rimonabant discriminative stimulus. In monkeys discriminating Δ9-THC, the dopamine transporter ligands cocaine, amphetamine, bupropion, RTI 113, and RTI 177 produced a maximum of 2% responding on the drug lever and blocked the discriminative stimulus effects of Δ9-THC. In Δ9-THC treated monkeys discriminating rimonabant, the dopamine transporter ligands partially substituted for and increased the potency of rimonabant to produce discriminative stimulus effects. The dopamine antagonist haloperidol enhanced the Δ9-THC discriminative stimulus without significantly modifying the rimonabant discriminative stimulus. Imipramine and desipramine, which have low affinity for dopamine transporters, were less effective in modifying either the Δ9-THC or rimonabant discriminations. The dopamine transporter ligands and haloperidol attenuated head shaking, whereas imipramine and desipramine did not.
Conclusions
Dopamine release and/or inhibition of dopamine transport blocks detection of Δ9-THC and is potentially the mechanism by which some therapeutics (e.g. bupropion) reduce the subjective effects of marijuana and enhance the subjective effects of marijuana withdrawal.
Keywords: cannabis, delta-tetrahydrocannabinol, dependence, dopamine, drug discrimination, marijuana, monkey, rimonabant, withdrawal
INTRODUCTION
The cannabinoid agonist Δ9-tetrahydrocannabinol (Δ9-THC) can increase dopamine synthesis, turnover, and efflux (Holtzman et al. 1969; Poddar and Dewey 1980; Chen et al. 1990; Tanda et al. 1997; Cheer et al. 2004; Solinas et al. 2006). Δ9-THC also increases dopamine cell firing (Gessa et al., 1998) and chronic Δ9-THC treatment results in tolerance to this effect (Wu and French 2000; Moranta et al. 2009). Δ9-THC withdrawal, induced by abrupt discontinuation of chronic Δ9-THC treatment or administration of rimonabant, results in decreased dopamine efflux and neurotransmission (Diana et al. 1998; Tanda et al. 1999). Collectively, these studies show that dopamine neurotransmission varies as a function of the acute and chronic effects of Δ9-THC.
The involvement of dopamine in, as well as the potential for dopaminergic ligands to modify, the in vivo effects of cannabinoids have not been fully established. When cannabinoids and dopaminergic ligands share effects, their combined effects are generally additive. Marijuana, cocaine, and amphetamine shared some physiological and behavioral effects in humans and, when combined, marijuana had additive effects with cocaine and amphetamine (Zalcman et al. 1973; Evans et al. 1976; Foltin et al. 1987; 1993). Additivity in rats was reported for the effects of Δ9-THC and amphetamine on ingestive behavior and body weight (Hattendorf et al. 1977), for the effects of dopamine receptor agonists and a cannabinoid antagonist (rimonabant) on motor activity (Compton et al. 1996; Giuffrida et al. 1999; Masserano et al. 1999), and for the cataleptic effects of a cannabinoid agonist and dopamine antagonist (Anderson et al. 1996). These results suggest that cannabinoid and dopaminergic ligands do not interact under most conditions. However, Δ9-THC and amphetamine have opposing effects on locomotor activity and body temperature and their combined effects result in functional antagonism (Hattendorf et al. 1977; Lew and Richardson 1981; Gorriti et al. 1999).
In the current study, a pharmacologically selective measure of the in vivo effects of Δ9-THC (i.e. drug discrimination; Balster and Prescott 1992) was used to examine the combined effects of dopamine releasers/uptake inhibitors and cannabinoids. Even though Δ9-THC increases dopamine efflux, dopamine is not sufficient to mimic the discriminative stimulus effects of Δ9-THC inasmuch as cocaine does not produce Δ9- THC like effects (Järbe 1984; McMahon 2006) although dopamine releasers and uptake inhibitors were 4 reported to increase the potency of Δ9-THC to produce discriminative stimulus effects (Solinas et al. 2010). In monkeys discriminating Δ9-THC (0.1 mg/kg i.v.), Δ9-THC was combined with non-selective monoamine transporter ligands (cocaine, amphetamine, and bupropion), dopamine-transporter selective ligands (RTI 113 and RTI 177; Kotian et al. 1995), a dopamine antagonist (haloperidol), and monoamine transporter ligands with relatively low affinity for dopamine transporters (imipramine and desipramine; Koe 1976). After having demonstrated that dopamine transporter ligands attenuated the discriminative stimulus effects of Δ9-THC, the generality of these findings was examined in another discrimination assay sensitive to cannabinoid antagonism and withdrawal, i.e. the discriminative stimulus effects of rimonabant (1 mg/kg i.v.) in monkeys receiving chronic Δ9-THC (1 mg/kg/12 h s.c.). The effects of the test compounds to substitute for or to modify the rimonabant discriminative stimulus effects were compared to effects on a Δ9-THC withdrawal sign, i.e., rimonabant-induced head shaking.
MATERIALS AND METHODS
Subjects
Five adult rhesus monkeys (Macaca mulatta; two female and three male) discriminated Δ9- THC from vehicle; six other rhesus monkeys (four female and two male) discriminated rimonabant while receiving Δ9-THC (1 mg/kg/ 12 h s.c.) treatment. Monkeys were housed separately on a 14-h light/10-h dark schedule, were maintained at 95% free-feeding weight (range 5.6–10.1 kg) with a diet consisting of fresh fruit, peanuts, and primate chow (High Protein Monkey Diet, Harlan Teklad, Madison, WI). Water was provided in the home cage. Monkeys received non-cannabinoids and cannabinoids in previous studies (McMahon 2010); Stewart and McMahon 2010). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Surgery
A catheter (heparin coated polyurethane, od = 1.68 mm, id = 1.02 mm, Instech Solomon, Plymouth Meeting, PA) was inserted 5 cm into a femoral or subclavian vein while monkeys were anesthetized with ketamine (10 mg/kg i.m.) and isoflurane (1.5–3.0% inhaled via facemask). Suture silk (coated vicryl, 5 Ethicon Inc., Somerville, New Jersey) anchored the catheter to the vessel and was used to ligate the section of the vessel adjacent to the catheter insertion. The opposite end of the catheter was attached to a vascular access port (Mida-cbas-c50, Instech Solomon) located s.c. at the mid-scapular region of the back.
Apparatus
Experiments were conducted in ventilated, sound-attenuating operant conditioning chambers containing two levers and red lights. Monkeys were seated in chairs (Model R001, Primate Products, Miami, FL). Feet were placed in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator. Experimental events were controlled and recorded by an interface (MedAssociates, St. Albans, VT), a computer, and Med-PC software.
Drug discrimination procedures
Five monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle consisting of 1 part absolute ethanol, 1 part Emulphor-620, and 18 parts saline. Six monkeys received 1 mg/kg/12 h of Δ9-THC (at 0600) and 1800 h) and discriminated rimonabant (1 mg/kg i.v.) from the same vehicle at 1200 h. Both groups responded under a fixed ratio 5 (FR5) schedule of stimulus-shock termination. The experimental sessions were divided into multiple, consecutive cycles. The beginning of each cycle was a 5-min timeout; responses during the timeout resulted in no programmed consequence. The timeout was followed by a 5-min schedule of stimulus-shock termination, which was signaled by illumination of two red lights (one positioned above each lever). Five consecutive responses on the correct lever extinguished the red lights, prevented delivery of an electric stimulus, and initiated a 30-s timeout. Otherwise, an electric stimulus was delivered every 40 s (Δ9-THC discrimination) or 10 s (rimonabant discrimination). Responding on the incorrect lever reset the response requirement on the correct lever. Determination of correct levers varied among monkeys (i.e. left lever associated with drug; right lever associated with vehicle) and remained the same for that monkey for the duration of the study.
During training sessions, the training drug (Δ9-THC or rimonabant) or vehicle was administered in the first min of a cycle; vehicle or sham (dull pressure applied to the skin overlying the vascular access port) was administered in two additional cycles for a total of three cycles. Three drug-training cycles were preceded by 0–3 vehicle-training cycles. Some training sessions included administration of vehicle or sham in the first min of 6 3–6 cycles. Completion of the FR on the correct lever was required for a reinforcer during each training cycle. Monkeys had previously satisfied the criteria for testing, i.e. at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever before completion of the FR on the correct lever for all cycles for 5 consecutive or 6 of 7 training sessions. Tests were conducted after performance for consecutive training sessions, including both vehicle and drug training sessions, satisfied the test criteria.
During test sessions, five consecutive responses on either lever postponed the shock schedule. Control dose-effect curves for Δ9-THC or rimonabant were determined by administering vehicle in the first cycle followed by cumulative intravenous doses increasing by 0.5 log unit in subsequent cycles. Test drugs were administered at the beginning of the first cycle followed by vehicle at the beginning of each of five cycles or by cumulative doses of the training drug (Δ9-THC or rimonabant) in subsequent cycles. Rimonabant (1 mg/kg i.v.) was studied prior to cumulative doses of Δ9-THC; moreover, Δ9-THC (3.2 mg/kg i.v.) was studied prior to cumulative doses of rimonabant. Test compounds studied prior to the training drugs included cocaine (0.1–3.2 mg/kg s.c.), amphetamine (0.1–1 mg/kg s.c.), bupropion (1–10 mg/kg s.c.), RTI 113 (0.32 and 1 mg/kg s.c.), RTI 177 (1 and 3.2 mg/kg s.c.), desipramine (3.2–17.8 mg/kg s.c.), imipramine (3.2 and 10 mg/kg s.c.), and haloperidol (0.0032–0.1 mg/kg s.c.). Experimental sessions with haloperidol consisted of 20-min cycles (i.e. a 15-min timeout followed by a 5-min period of responding for stimulus shock-termination). RTI 177 was administered 1 h before the first cycle and all other test compounds were administered at the beginning of the first cycle.
Head shaking
Head shaking was measured in Δ9-THC treated monkeys while monkeys were seated in chairs inside the operant conditioning chambers. Head shaking and discriminative stimulus effects were measured on different days in the same monkeys and the order of drug treatment was non-systematic. The experimenters were blind to treatment. Head shaking was defined as rapid, horizontal, side-to-side oscillation of the head for a minimum of 1 s. Individual bouts of head shaking were separated by at least 1 s. When vehicle, a test compound, or rimonabant were studied alone, head shaking was measured for 40 min immediately after administration. When a test compound was studied in combination with rimonabant, the test 7 compound was administered 40 min before a dose of rimonabant; head shaking was measured for 40 min after each drug. The exception was RTI 177, which was administered 1 h and 40 min before rimonabant; measurement of head shaking began 1 h after RTI 177.
Drugs
Rimonabant and Δ9-THC (100 mg/ml in absolute ethanol; The Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD) were dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia Inc., Cranbury, NJ), and 18 parts physiologic saline and were administered i.v. Haloperidol (Sigma Chemical Co., Saint Louis, MO) was dissolved in the same vehicle and administered s.c. Cocaine hydrochloride and D-amphetamine sulfate (The Research Technology Branch of the National Institute on Drug Abuse), bupropion hydrochloride (AK Scientific, Union City, CA), 3β-(4-chlorophenyl)tropane-2β- carboxylic acid phenyl ester hydrochloride (RTI 113, synthesized by F.I.C.), 3β-(4-chlorophenyl) tropane-2β-(3-phenylisoxazol-5-yl) hydrochloride (RTI 177, synthesized by F.I.C.), desipramine (Sigma Chemical), and imipramine (Sigma Chemical) were dissolved in saline and administered s.c. Drugs were administered in a volume of 0.1–1 ml/kg; doses were expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
Data analyses
A dose-effect curve for a particular drug or drug combination was determined in at least four monkeys and each monkey served as its own control (i.e. observations were within-subjects). Discrimination data were expressed as a percentage of responses on the drug lever out of total responses on both the drug and vehicle levers. Rate of responding on both levers (i.e. drug and vehicle) was calculated as responses per s excluding responses during timeouts. Rate of responding during a test was expressed as the percentage of the control response rate for individual animals. Control response rate was defined as the average response rate for all cycles during the five previous vehicle training sessions excluding sessions during which the test criteria were not satisfied. Head shaking was expressed as frequency during a 40-min observation period. Discrimination, rate, and head shaking data were averaged among subjects (± S.E.M.) and plotted as a function of dose.
For drug discrimination and response rate data, control dose-effect curves (i.e. the training drugs alone) were determined before and after every second test with a test compound in combination with the training drug and all control dose-effect determinations were averaged for an individual subject for further analysis. For head shaking, the control dose-effect curve was determined twice, before and after studies with the various test compounds. The order of testing with various test compounds was non-systematic.
Individual dose-effect data were analyzed with linear regression (GraphPad Prism version 5.0 for Windows; San Diego, CA). The slopes of dose-effect functions for a training drug alone and in combination with various doses of a test compound were compared with an F-ratio test. If the slopes were not significantly different, then parallel line analyses with the common, best-fitting slope was used to calculate ED50 values in monkeys discriminating Δ9-THC (Tallarida 2000). In monkeys discriminating rimonabant, some of the test drugs produced greater than 50%, but less than 75%, responding on the rimonabant lever; therefore, ED75 values were calculated for rimonabant. The ED50 or ED75 values were considered significantly different from each other when the 95% confidence limits of the potency ratio did not include 1. When the training drug did not produce greater than 50% effect in the presence of a test compound, a significant difference from control was evidenced by slopes or intercepts that were significantly different from each other, as determined by an Fratio test (p<0.05). When at least three doses were studied, a test compound was considered to have produced a significant effect when the slope of the dose-effect function was significantly different from 0. If two or fewer doses were studied, then repeated measures ANOVA or a paired t-test was used to examine significance relative to control.
RESULTS
Rimonabant and Δ9-THC: mutual antagonism of discriminative stimulus effects
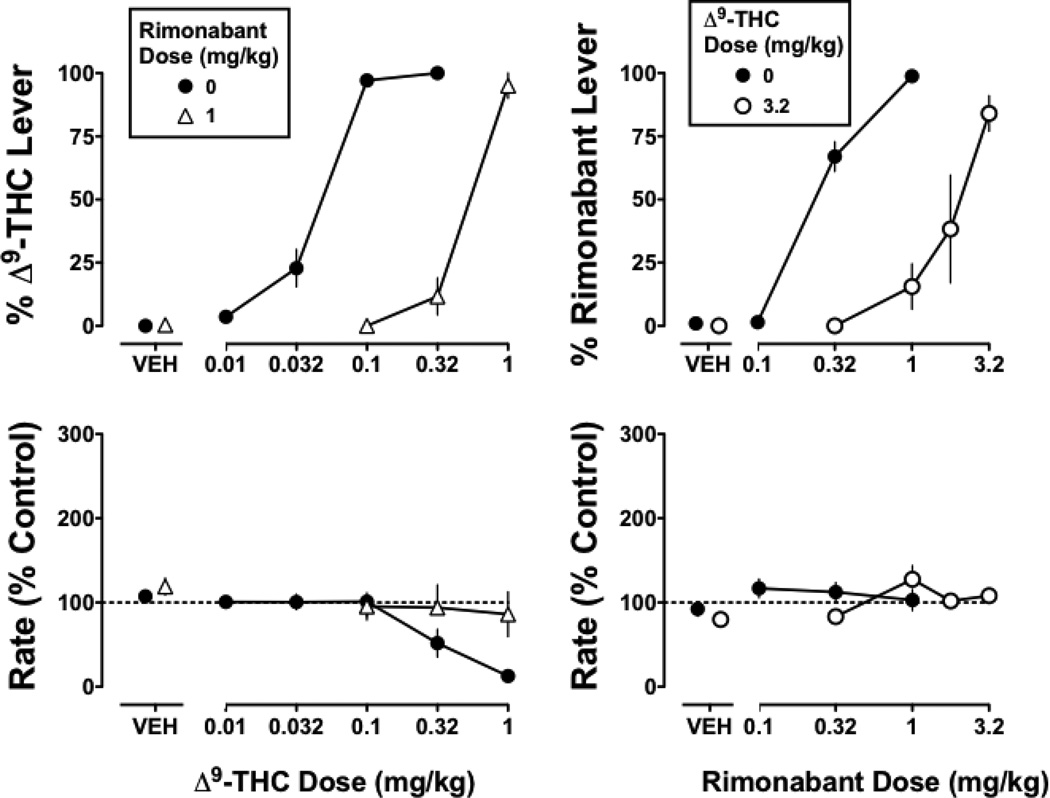
In monkeys discriminating Δ9-THC from vehicle, the training drug dose-dependently increased responding on the Δ9-THC lever (Figure 1 top left, circles). Mean (± S.E.M.) responding on the Δ9-THC lever was 4 ± 3% at a dose of 0.01 mg/kg and was increased to 97 ± 1% at the training dose (0.1 mg/kg). The ED50 value of Δ9-THC to produce 9 discriminative stimulus effects was 0.042 mg/kg (Table 1). Following vehicle, mean (± S.E.M.) responding on the Δ9-THC lever was 0%. The absolute rate of responding in the five monkeys was 0.71, 1.49, 1.51, 2.18, and 2.63 responses per s. Up to the training dose, Δ9-THC did not modify rate of responding (Figure 1 bottom left), whereas larger doses (0.32 and 1 mg/kg) decreased response rate to 52% and 13% of control, respectively. The ED50 value (95% confidence limits) of Δ9-THC to decrease response rate was 0.29 (0.17 – 0.61) mg/kg.
Figure 1. Antagonism of the Δ9-THC (0.1 mg/kg i.v) discriminative stimulus by rimonabant (left) and antagonism of the rimonabant (1 mg/kg i.v.) discriminative stimulus in Δ9-THC (1 mg/kg/12 h s.c.) treated moneys by an additional dose (3.2 mg/kg i.v.) of Δ9-THC (right).
Abscissae: vehicle (VEH) and dose of Δ9-THC (left) or rimonabant (right) in mg/kg body weight. Ordinates: Percentage of responding on the Δ9-THC lever (top left) or rimonabant lever (top right) and response rate expressed as a percentage of control (VEH training days) rate [Rate (% Control)] (bottom). Symbols represent mean (± S.E.M.) values from each of 4 monkeys.
Table 1.
F-ratios and p-values comparing the slope of the control dose response curve (i.e. Δ9-THC alone) to that for Δ9-THC determined in the presence of a dose of test compound, as well as ED50 values, potency ratios, and corresponding 95% confidence limits (95% CL), in monkeys discriminating Δ9-THC (0.1 mg/kg i.v.)
| Test drug | Slope: Δ9-THC alone compared with Δ9- THC + test compound |
ED50 value in mg/kg |
Potency ratio (95% CL)† |
|---|---|---|---|
| Δ9-THC alone | 0.042 (0.031–0.060) | ||
| + Rimonabant (1 mg/kg) | Not different | 0.45 (0.34–0.65) | 11 (8.1–16)* |
| + Cocaine (1 mg/kg) | Not different | 0.10 (0.054–0.30) | 2.5 (1.4–4.4)* |
| + Cocaine (3.2 mg/kg) | F1,19=20; p<0.001 | 0.32*** | NA |
| + Amphetamine (0.32 mg/kg) | Not different | 0.14 (0.083–0.32) | 3.3 (1.4–4.4)* |
| + Amphetamine (1 mg/kg) | F1,22=24; p<0.001 | 0.32*** | NA |
| + Bupropion (3.2 mg/kg) | Not different | 0.070 (0.049–0.10) | 1.7 (0.9–2.4) |
| + Bupropion (10 mg/kg) | F1,18=4.0; p<0.05 | 0.32*** | NA |
| + RTI 113 (1 mg/kg) | F1,22=14; p<0.01 | 0.32*** | NA |
| + RTI 177 (3.2 mg/kg) | F1,25=17; p<0.001 | 0.32*** | NA |
| + Haloperidol (0.0032 mg/kg) | Not different | 0.046 (0.031–0.063) | 1.1 (0.73–1.6) |
| + Haloperidol (0.01 mg/kg) | Not different | 0.021 (0.015–0.030) | 0.52 (0.35–0.78)** |
| + Imipramine (3.2 mg/kg) | Not different | 0.081 (0.047–0.16) | 1.9 (0.9–3.3) |
| + Imipramine (10 mg/kg) | Not different | 0.088 (0.054–0.19) | 2.1 (1.1–3.2)* |
| + Desipramine (17.8 mg/kg) | Not different | 0.066 (0.046–0.094) | 1.6 (1.0–2.5) |
Potency ratios and 95% confidence limits are the ED50 value of Δ9-THC determined in the presence of the test drug divided by the control ED50 value of Δ9-THC NA, not applicable, i.e. slopes were significantly different
Significant decrease in the potency of Δ9-THC
Significant increase in the potency of Δ9-THC
The ED50 value is the largest dose that did not decrease response rate to less than 20% control
Rimonabant (1 mg/kg) produced 0% responses on the Δ9-THC lever and antagonized the discriminative stimulus effects of Δ9-THC (Figure 1 top left, triangles). In the presence of rimonabant (1 mg/kg), the training dose (0.1 mg/kg) of Δ9-THC produced 0% responses on the Δ9-THC lever. A one-log unit increase in dose (1 mg/kg) was required for Δ9-THC to produce the same level of responding on the drug lever as that produced by the training dose. The slopes of the dose-effect curves for Δ9-THC alone and in combination with 1 mg/kg of rimonabant were not significantly different from each other. In the presence of rimonabant, the ED50 value of Δ9-THC (0.45 mg/kg) was significantly (11-fold) different from the control ED50 value (Table 1). Rimonabant (1 mg/kg) also antagonized the effects of Δ9-THC to decrease response rate (Figure 1, bottom left), as evidenced by the dose-effect curve for Δ9-THC in combination with rimonabant having a slope that was not significantly different from 0 and significantly different from control.
In Δ9-THC treated monkeys discriminating rimonabant, doses of 0.32 and the training dose of 1 mg/kg of rimonabant increased mean (± S.E.M.) responding on the drug-lever to 67 ± 6% and 98 ± 1%, respectively (Figure 1 top right, closed circles). The ED75 value of rimonabant to produce discriminative stimulus effects was 0.52 mg/kg (Table 2). Following vehicle, mean (± S.E.M.) responding on the rimonabant lever was 1%. Δ9-THC (3.2 mg/kg) produced 0% responses on the rimonabant lever and attenuated the discriminative stimulus effects of rimonabant (Figure 1 top right, open circles), as evidenced by a significant 6.9-fold increase in the ED75 value of rimonabant (Table 2). The absolute rate of responding in the six monkeys was 1.52, 2.01, 2.55, 2.63, 2.93, and 2.93 responses per s. Response rate was not significantly modified by rimonabant and Δ9-THC, alone or in combination (Figure 1 bottom right).
Table 2.
F-ratios and p-values comparing the slope of the control dose response curve (i.e. rimonabant alone) to that for rimonabant determined in the presence of a dose of test compound, as well as ED75 values, potency ratios, and corresponding 95% confidence limits (95% CL), in Δ9-THC treated monkeys discriminating rimonabant (1 mg/kg i.v.)
| Test drug | Slope: rimonabant alone compared with rimonabant + test compound |
ED75 value (95% CL) in mg/kg |
Potency ratio (95% CL)† |
|---|---|---|---|
| Rimonabant alone | 0.52 (0.43–0.64) | ||
| + Δ9-THC (3.2 mg/kg) | Not different | 3.6 (1.7–7.7) | 6.9 (4.3–11)* |
| + Cocaine (1 mg/kg) | F1,20=51; p<0.001 | NA | NA |
| + Cocaine (3.2 mg/kg) | Not different | 0.13 (0.051–0.32) | 0.24 (0.13–0.46)** |
| + Amphetamine (0.32 mg/kg) | F1,17=15; p<0.01 | NA | NA |
| + Amphetamine (1 mg/kg) | F1,17=7.5; p<0.05 | NA | NA |
| + Bupropion (3.2 mg/kg) | Not different | 0.20 (0.11–0.37) | 0.38 (0.22–0.64)** |
| + Bupropion (10 mg/kg) | F1,18=8.7; p<0.01 | NA | NA |
| + RTI 113 (0.32 mg/kg) | Not different | 0.48 (0.32–0.73) | 0.92 (0.64–1.3) |
| + RTI 113 (1 mg/kg) | F1,17=7.9; p<0.05 | NA | NA |
| + RTI 177 (1 mg/kg) | F1,19=5.8; p<0.05 | NA | NA |
| + RTI 177 (3.2 mg/kg) | F1,16=20; p<0.001 | NA | NA |
| + Haloperidol (0.01 mg/kg) | Not different | 0.44 (0.28–0.69) | 0.83 (0.58–1.2) |
| + Haloperidol (0.032 mg/kg) | Not different | 0.73 (0.34–1.6) | 1.4 (0.85–2.3) |
| + Imipramine (10 mg/kg) | Not different | 0.27 (0.14–0.52) | 0.51 (0.30–0.86)** |
| + Desipramine (17.8 mg/kg) | Not different | 0.42 (0.11–1.5) | 0.79 (0.37–1.7) |
Potency ratios and 95% confidence limits are the ED75 value of rimonabant determined in the presence of the test drug divided by the control ED75 value of rimonabant NA, not applicable, i.e. slopes were significantly different
Significant decrease in the potency of rimonabant
Significant increase in the potency of rimonabant
Dopamine transporter ligands: effects on drug discrimination, response rate, and rimonabant-induced head shaking
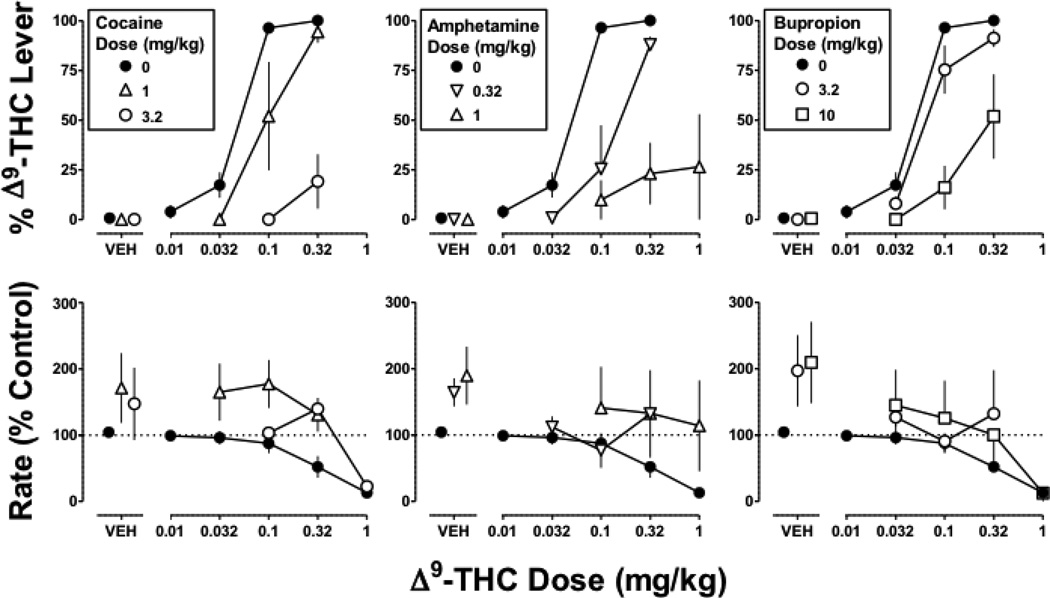
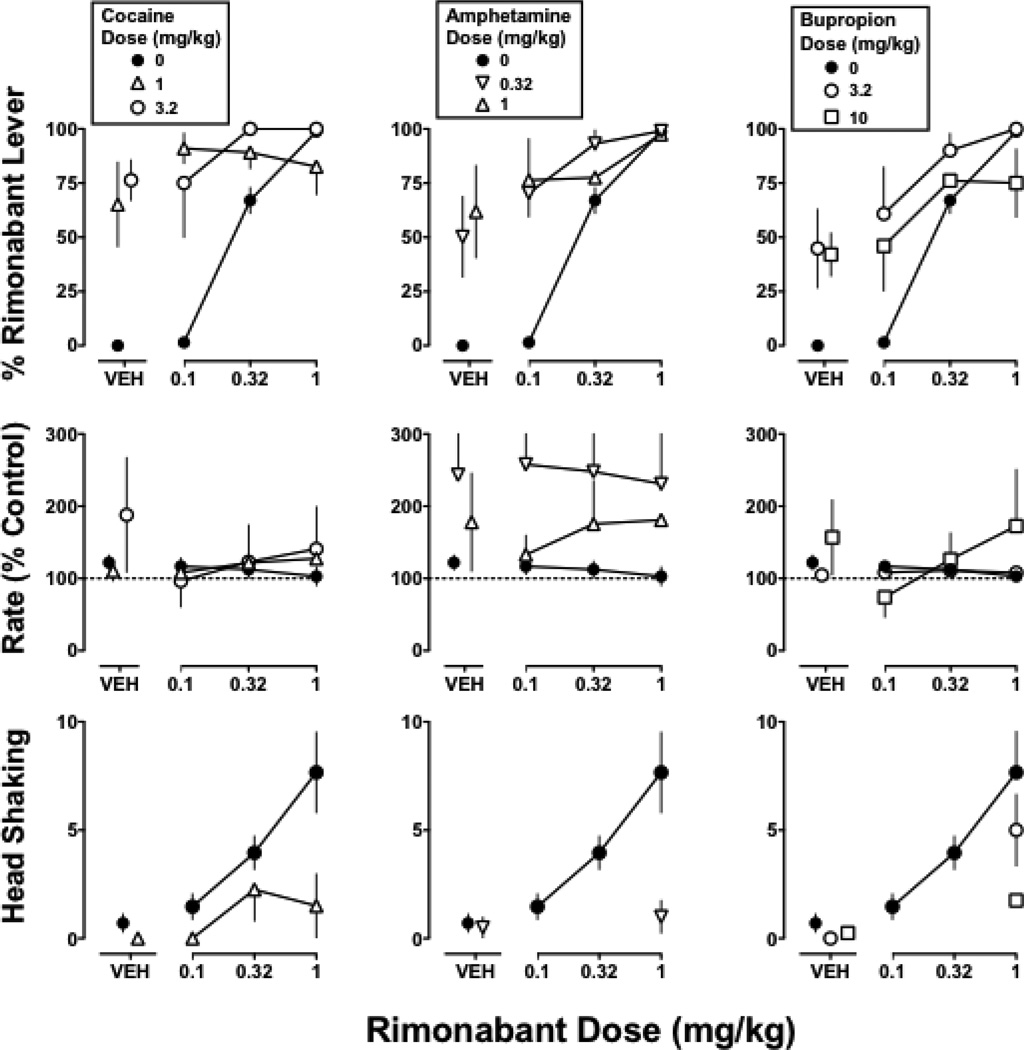
In monkeys discriminating Δ9-THC from vehicle, responding on the Δ9-THC lever was no more than 2% after cocaine, amphetamine, bupropion, RTI 113, or RTI 177 (Figures 2 and 3 top, VEH). In contrast, cocaine, amphetamine, bupropion, RTI 133, and RTI 177 markedly antagonized the discriminative stimulus effects of Δ9-THC. The slopes of the Δ9-THC dose-effect curves determined alone and in combination with 1 mg/kg of cocaine did not significantly differ; cocaine (1 mg/kg) significantly increased the ED50 value of Δ9-THC to 0.1 mg/kg or 2.5-fold (Table 1). A larger dose (3.2 mg/kg) of cocaine produced even greater antagonism of the Δ9-THC discriminative stimulus, as evidenced by a significant difference in slope and maximum response on the Δ9-THC lever (19%) up to the largest dose of Δ9-THC that did not disrupt responding (Figure 2 top left). Amphetamine (0.32 mg/kg) produced a significant 3.3-fold rightward shift of the Δ9-THC discrimination dose-effect curve (Figure 2 top middle; Table 1). Still greater antagonism was obtained with a larger dose (1 mg/kg) of amphetamine, which resulted in a slope for the Δ9-THC dose-effect curve that was significantly different from control (i.e. Δ9-THC alone) but not significantly different from 0. Bupropion (10 mg/kg) produced significant antagonism (Figure 2 top right), as evidenced by a significant difference in slope versus Δ9-THC alone (p<0.05); a smaller dose (3.2 mg/kg) of bupropion was ineffective.
Figure 2. Discriminative stimulus and rate effects of Δ9-THC (0.1 mg/kg i.v.) alone and in combination with cocaine (left), amphetamine (middle), or bupropion (right).
Abscissae: vehicle (VEH) or dose of Δ9-THC in mg/kg body weight. Ordinates: Percentage of responding on the Δ9-THC lever (top) and response rate expressed as a percentage of control (VEH training days) rate [Rate (% Control)] (bottom). The control data (closed circles) are re-plotted from Figure 1 left and are the same in the respective rows of panels. Symbols represent mean (± S.E.M.) values from each of 4 monkeys, except for the test compounds in combination with the largest dose (1 mg/kg) of Δ9-THC, which represent a mean of 2–4 monkeys.
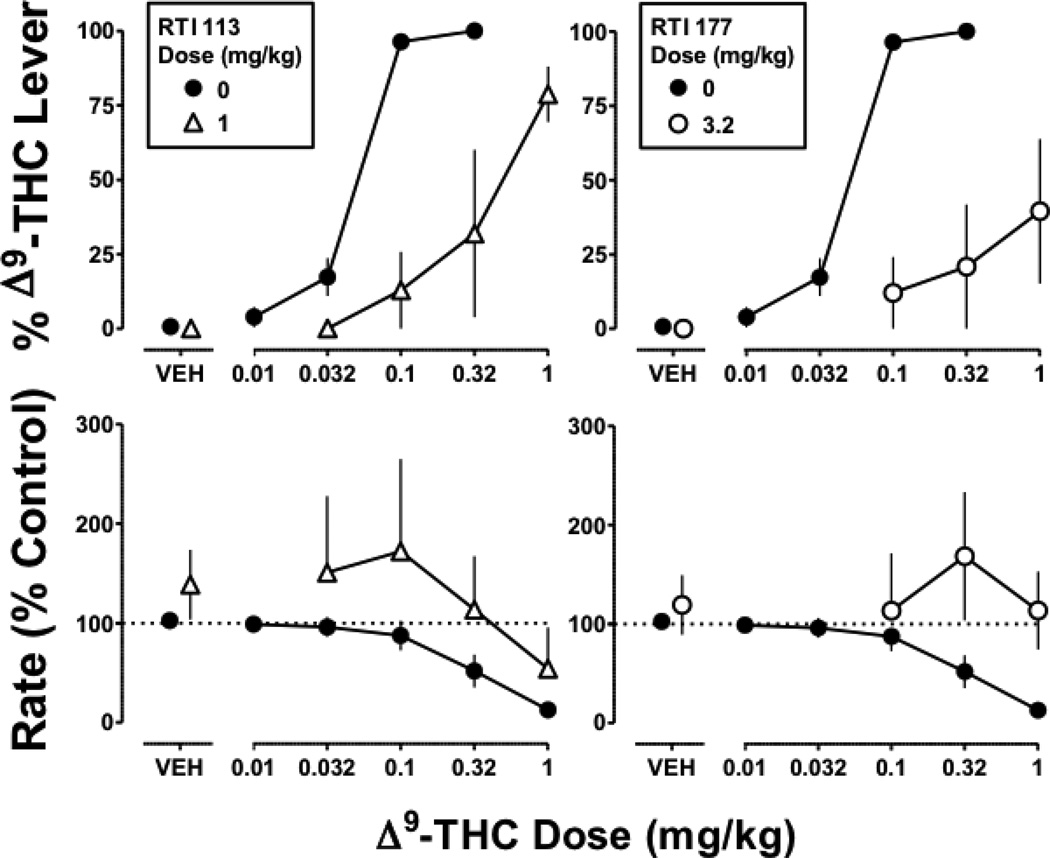
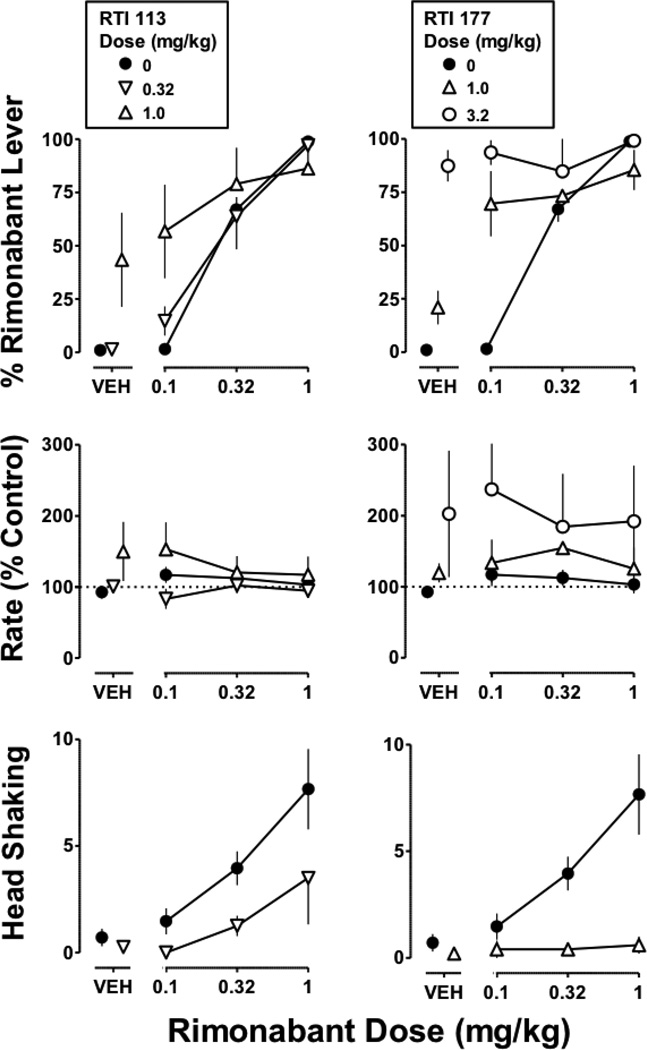
Figure 3. Discriminative stimulus and rate effects of Δ9-THC (0.1 mg/kg i.v.) alone and in combination with RTI 113 (left) or RTI 177 (right).
Symbols represent mean (± S.E.M.) values from each of 4 monkeys, except for the test compounds in combination with the largest dose (1 mg/kg) of Δ9-THC, which represent a mean of 2–3 monkeys. Details as in Figure 2 legend
Cocaine, amphetamine, and bupropion did not significantly modify rate of responding. However, there was a tendency for response rate to be increased (Figure 2 bottom, VEH) and one dose of each compound significantly antagonized the rate-decreasing effects of Δ9-THC, i.e. 1 mg/kg of cocaine (F2,30=9.3; p<0.001), 1 mg/kg of amphetamine (F2,36=3.5; p<0.05), and 3.2 mg/kg of bupropion (F2,31=4.7; p<0.05).
The dopamine transporter-selective ligands RTI 113 (1 mg/kg) and RTI 177 (3.2 mg/kg) significantly antagonized the discriminative stimulus effects of Δ9-THC (Figure 3 top), as evidenced by a significant difference in slope for the Δ9-THC dose-effect curve determined in the presence of a test compound versus control (p<0.05). Maximum responding on the Δ9-THC lever was 69% for Δ9-THC (1 mg/kg) in combination with RTI 113 and 39% for Δ9-THC (1 mg/kg) in combination with RTI 177. When administered alone, neither RTI 113 nor RTI 177 significantly altered response rate. However, both compounds significantly antagonized the rate-decreasing effects of Δ9-THC, as evidenced by a significant difference in intercept for RTI 113 11 (F1,36=4.7; p<0.05) and RTI 177 (F1,36=7.0; p<0.05) relative to control (Figure 3 bottom).
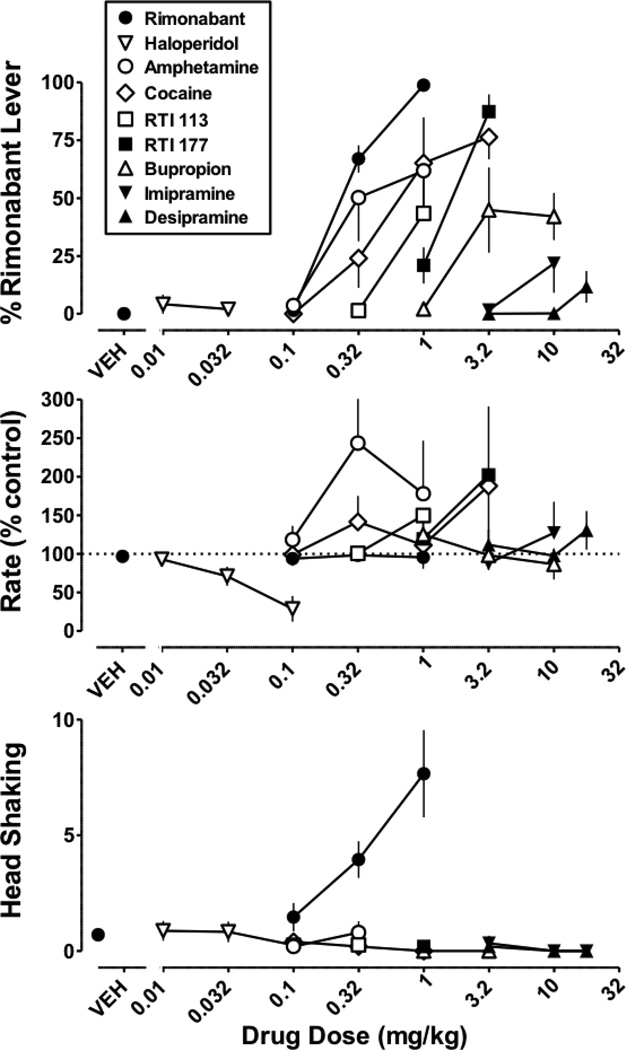
In Δ9-THC treated monkeys discriminating rimonabant, each dopamine transporter ligand dosedependently increased responding on the drug lever (Figure 4 top). Maximum responding on the rimonabant lever was 76% after cocaine (3.2 mg/kg), 62% after amphetamine (1 mg/kg), 45% after bupropion (3.2 mg/kg), 43% after RTI 113, and 87% after RTI 177. Each dopamine transporter ligand also had a tendency to increase rate of responding (Figure 4 middle). Rimonabant dose-dependently increased the frequency of head shaking to 4.0 and 7.6 counts at doses of 0.32 and 1 mg/kg, respectively. In contrast, cocaine, amphetamine, bupropion, RTI 113, and RTI 177 did not produce head shaking in Δ9-THC treated monkeys (Figure 4 bottom). Haloperidol, imipramine, and desipramine produced relatively little responding on the rimonabant lever and did not produce head shaking.
Figure 4. Effects of test compounds on drug discrimination (top), response rate (middle), and head shaking (bottom) in Δ9-THC (1 mg/kg/12 h s.c.) treated monkeys discriminating rimonabant (1mg/kg i.v.).
Abscissae: vehicle or dose of test compound in mg/kg body weight. Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (top), mean (± S.E.M.) response rate expressed as a percentage of control (VEH training days) rate [Rate (% Control)] (middle), and head shaking frequency in 40 min (bottom). The control data (closed circles) in the top and middle panels are re-plotted from Figure 1. Symbols represent mean (± S.E.M.) values from each of 4–5 monkeys.
Cocaine, amphetamine, bupropion, RTI 113, and RTI 177, at doses that produced rimonabant-lever responding, shifted the dose-effect curve for rimonabant upward and in some cases leftward (Figures 5 and 6 top). In a majority of cases, i.e. 1 mg/kg of cocaine, both doses (0.32 and 1 mg/kg) of amphetamine, 10 mg/kg of bupropion, 1 mg/kg of RTI 113, and both doses (1 and 3.2 mg/kg) of RTI 177, the slope of the control rimonabant dose-effect curve was significantly different from that determined in the presence of the test compound (Table 2). In these cases, responding on the rimonabant lever at the smallest dose (0.1 mg/kg) of rimonabant alone was similar to or somewhat greater than rimonabant-lever responding produced by the test compound alone. For example, responding on the rimonabant lever was 57% and 94% for 0.1 mg/kg of rimonabant in combination with RTI 113 and RTI 177, respectively, which was similar to rimonabant-lever responding obtained with each test compound alone (43% and 87%, respectively). In three cases, i.e. 3.2 mg/kg of cocaine, 3.2 mg/kg of bupropion, and 0.32 mg/kg RTI 113, the slopes of the rimonabant dose-effect curves determined in the presence and absence of the test compound were not significantly different, thereby providing for calculation of ED75 values for rimonabant. Cocaine (3.2 mg/kg) and bupropion (3.2 mg/kg) significantly decreased the ED75 value of rimonabant 4.0-and 2.6-fold, respectively, whereas RTI 113 (0.32 mg/kg) did not significantly alter the rimonabant ED75 value (Table 2). The combination of rimonabant with cocaine, 12 amphetamine, bupropion, RTI 113, or RTI 177 did not significantly modify response rate (Figures 5 and 6 middle).
Figure 5. Discriminative stimulus effects of rimonabant (1 mg/kg i.v.) in monkeys receiving chronic Δ9-THC (1 mg/kg/12 h s.c.): tests with cocaine (left), amphetamine (middle), or bupropion (right).
Abscissae: vehicle or dose of rimonabant in mg/kg body weight. Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (top), mean (± S.E.M.) response rate expressed as a percentage of control (VEH training days) rate [Rate (% Control)] (middle), and head shaking frequency in 40 min (bottom). The control data (closed circles) are re-plotted from Figure 4 and are the same in the respective rows of panels. Symbols represent mean (± S.E.M.) values from each of 4 monkeys.
Figure 6. Discriminative stimulus effects of rimonabant (1 mg/kg i.v.) in Δ9-THC (1 mg/kg/12 h s.c.) treated moneys: tests with RTI 113 (left) and RTI 177 (right).
Symbols represent mean (± S.E.M.) values from each of 4 monkeys. Details as in Figure 5 legend
In contrast to discriminative stimulus effects, cocaine, amphetamine, bupropion, RTI 113, and RTI 177 attenuated rimonabant-induced head shaking in Δ9-THC treated monkeys (Figures 5 and 6 bottom). This was evidenced by a significant rightward shift of the rimonabant dose-response curve in the presence of cocaine (1 mg/kg) and RTI 113 (0.32 mg/kg), a significant downward shift of the rimonabant-dose-response curve in the presence of RTI 177 (1 mg/kg), and significant antagonism of the training dose (1 mg/kg) of rimonabant by amphetamine (0.32 mg/kg) and bupropion (10 mg/kg).
Haloperidol: enhancement of Δ9-THC and antagonism of rimonabant-induced head shaking
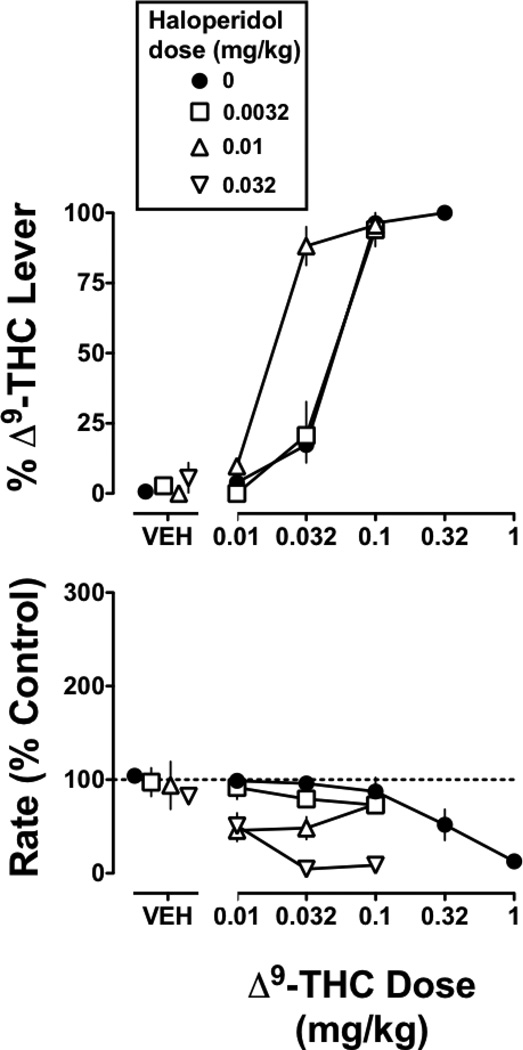
Haloperidol, when studied up to a dose of 0.032 mg/kg, produced a maximum of 6% drug-lever responding in monkeys discriminating Δ9-THC. When combined with Δ9-THC, a dose of 0.01 mg/kg of haloperidol produced a significant 2-fold decrease in the ED50 value of Δ9-THC (Figure 7 top; Table 1). A smaller dose (0.0032 mg/kg) of haloperidol did not significantly modify the ED50 value of Δ9-THC, whereas a larger dose (0.032) of haloperidol significantly decreased responding when combined with Δ9-THC (Figure 7 bottom). In Δ9-THC treated monkeys discriminating rimonabant, haloperidol (0.01 and 0.032 mg/kg) produced a maximum of 4% responding on the rimonabant lever; a larger dose (0.1 mg/kg) decreased response rate to 29% of control. When combined with rimonabant, haloperidol (0.01 and 0.032) did not significantly modify the ED75 value of rimonabant to produce discriminative stimulus effects (Table 2). In contrast, haloperidol (0.032 and 0.1 mg/kg) antagonized rimonabant-induced head shaking in Δ9-THC treated monkeys, as evidenced by a rightward and downward shift in the rimonabant dose-response curve (data not shown).
Figure 7. Effects of haloperidol in monkeys discriminating Δ9-THC (0.1 mg/kg i.v.).
Abscissae: vehicle (VEH) and dose of Δ9-THC in mg/kg body weight. Ordinates: Percentage of responding on the Δ9-THC lever (top) and response rate expressed as a percentage of control (VEH training days) rate [Rate (% Control)] (bottom). Symbols represent mean (± S.E.M.) values from each of 4 monkeys. The control data are re-plotted from Figure 1 left.
Imipramine and desipramine: drug discrimination and rimonabant-induced head shaking
When administered alone in monkeys discriminating Δ9-THC, imipramine and desipramine produced a maximum of 1% responding on the Δ9-THC lever. Imipramine antagonized the discriminative stimulus effects of Δ9-THC, but the magnitude of antagonism was less than that obtained with high-affinity dopamine transporter ligands (Table 1). A dose of 10 mg/kg of imipramine produced a modest (2.1-fold) though significant antagonism of the 13 Δ9-THC discriminative stimulus, whereas a smaller dose (3.2 mg/kg) of imipramine was ineffective (Table 1). Imipramine alone did not significantly alter response rate. However, the relatively large dose (10 mg/kg) of imipramine produced a significant, 2.9-fold leftward shift in the Δ9-THC dose-effect curve for producing ratedecreasing effects. Desipramine did not significantly modify the Δ9-THC dose-effect curve for discriminative stimulus or rate-decreasing effects (Table 1).
In Δ9-THC treated monkeys discriminating rimonabant, imipramine (10 mg/kg) alone produced 22% responding on the rimonabant lever and, when combined with rimonabant, produced a significant 2-fold decrease in the ED75 value of rimonabant (Table 2). Desipramine produced 12% responding on the rimonabant lever and did not significantly modify the ED75 value of rimonabant. Neither imipramine (10 mg/kg) nor desipramine (17.8 mg/kg) significantly modified response rate or head shaking, either alone or when combined with rimonabant (data not shown). A larger dose (17.8 mg/kg) of imipramine produced seizures and doses of desipramine larger than 17.8 mg/kg were not studied to avoid adverse effects.
DISCUSSION
Dopamine transporter ligands blocked the discriminative stimulus effects of Δ9-THC in rhesus monkeys, whereas the dopamine receptor antagonist haloperidol enhanced the effects of Δ9-THC. Moreover, the dopamine transporter ligands partially substituted for and increased the potency of rimonabant to produce discriminative stimulus effects in Δ9-THC treated monkeys (i.e. withdrawal). However, haloperidol did not significantly modify the rimonabant discriminative stimulus and norepinephrine and serotonin uptake inhibitors were much less effective in modifying the discriminative stimulus effects of either training drug (i.e. Δ9-THC or rimonabant). The dopamine transporter ligands and haloperidol attenuated a Δ9-THC withdrawal sign, i.e. rimonabant-induced head shaking, whereas norepinephrine and serotonin uptake inhibitors were without effect. These results suggest that increased extracellular dopamine is associated not only with an inability of animals to detect Δ9-THC, but also mimics a component of the Δ9-THC withdrawal syndrome.
According to behavioral and binding data reported elsewhere, the effects of the uptake inhibitors 14 described in the current study appear to be due to increased synaptic dopamine. Rank order potency for attenuating the discriminative stimulus effects of Δ9-THC was amphetamine = RTI 113 > cocaine = RTI 177 > bupropion. The drugs have similar rank order potency in substituting for the discriminative stimulus effects of cocaine or amphetamine in rhesus monkeys (Kamien and Woolverton 1989; Kleven et al. 1990; Kimmel et al. 2008); both discriminations are strongly linked to increased synaptic dopamine. RTI 113 is highly selective for dopamine transporters (i.e. 1490- and 1180-fold more selective for dopamine relative to norepinephrine and serotonin transporters, respectively), as is RTI 177 (i.e. the corresponding values are 394- and 1890-fold, respectively; Kotian et al. 1995). In contrast, imipramine and desipramine have low binding affinity at dopamine transporters (Koe 1976) and were much less effective in modifying the discriminative stimulus effects of Δ9-THC.
Increased synaptic dopamine is one of the primary mechanisms associated with the reinforcing effects of many abused drugs and is suggested to mediate, at least in part, the effects of Δ9-THC. Therefore, a further increase in synaptic dopamine, such as that produced by a dopamine releaser and/or uptake inhibitor, might be expected to enhance the effects of Δ9-THC. Consistent with this notion, amphetamine and cocaine increased the potency of Δ9-THC to produce discriminative stimulus effects in rats responding for food (Solinas et al. 2010). The opposite results obtained in the current study could be due to a variety of factors including procedural differences across studies as well as Δ9-THC treatment history. Procedural differences include species (monkey versus rodent) and reinforcer (food versus stimulus-shock termination). Moreover, Δ9-THC treatment history could determine the magnitude or the direction of the effect of Δ9-THC on dopamine signaling. Chronic cannabinoid agonism resulted in tolerance to its effects on dopamine cell firing (Wu and French 2000; Moranta et al. 2009). To the extent that 2 mg/kg/day of Δ9-THC (i.e. the treatment used in the rimonabant discrimination assay) was previously demonstrated to result in tolerance to some behavioral effects (McMahon 2010), monkeys discriminating rimonabant might also have been tolerant to effects on dopamine signaling. There are some examples of Δ9-THC decreasing dopamine neurotransmission, including dopamine receptor binding (Marcellino et al. 2008) and mesocortical dopamine cell firing (Pistis et al. 2001; Cheer et al. 2003). Although 15 the current results are consistent with such decreases in dopamine signaling, it is possible the current results are unrelated to any effects of Δ9-THC on dopamine neurotransmission.
Whereas antagonism of Δ9-THC by rimonabant is simple, competitive, and reversible (i.e. mediated by a common receptor type), antagonism by dopamine transporter ligands is potentially functional or due to drugs exerting opposite effects at two different receptor types, i.e., cannabinoid receptors and dopamine transporters. For discriminative stimulus effects, in general, and the discriminative stimulus effects of Δ9-THC, in particular, a reduction in effect might not be due to pharmacologic antagonism, but rather to another mechanism referred to as perceptual masking. One example of perceptual masking involves auditory stimuli (i.e. pure tones) or a pure tone blocking detection of a second pure tone (Wegel and Lane 1924). When applied to the discriminative stimulus effects of a drug, perceptual masking occurs when a test drug produces a stimulus that is distinct from and more robust than the stimulus to which the animals have been trained (i.e. training drug), thereby blocking detection of the training drug. Amphetamine is an example of a drug that produces a stimulus distinct from morphine and that attenuates the discriminative stimulus effects of morphine (Gauvin and Young 1989). In this case, the test drug does not oppose the effect of the training. Even though the current results might reflect perceptual masking, functional antagonism can occur between cannabinoid agonists and dopamine transporter ligands for effects on operant responding (current results), locomotor activity, and body temperature (Hattendorf et al. 1977; Lew and Richardson 1981; Gorriti et al. 1999). In addition to pharmacologic mechanisms, perceptual masking could be another mechanism responsible for attenuation of the effects of Δ9-THC by indirect-acting dopamine agonists.
As previously reported in monkeys dependent on Δ9-THC, supplemental administration of Δ9-THC attenuated not only the discriminative stimulus effects of rimonabant, but also rimonabant-induced head shaking. In contrast, the dopamine releasers and/or uptake inhibitors partially substituted for and increased the potency of rimonabant to produce discriminative stimulus effects. The effects of the dopamine transporter ligands are consistent with attenuation of the discriminative stimulus effects of Δ9-THC; that is, the dopamine transporter ligands produced rimonabant-like effects in both drug discrimination assays. To the extent that the 16 rimonabant discrimination in Δ9-THC treated monkeys reflects a cannabinoid withdrawal syndrome, dopamine releasers and uptake inhibitors appear to produce a withdrawal-like syndrome in Δ9-THC dependent animals. However, the dopamine transporter ligands produced the opposite effect on (i.e. attenuated) rimonabant-induced head shaking. The qualitatively different effects on drug discrimination and head shaking might reflect distinct neuropharmacologic mechanisms responsible for the two types of cannabinoid withdrawal sign. An alternative and more parsimonious explanation is that other behavioral effects (e.g. hyperactivity) of the dopamine transporter ligands were incompatible and interfered with head shaking. That the dopamine antagonist and cataleptic haloperidol also attenuated rimonabant-induced head shaking is compatible with this interpretation. The failure of imipramine and desipramine to modify rimonabant-induced head shaking suggests that increases in synaptic norepinephrine and serotonin do not modify cannabinoid withdrawal, at least after acute administration.
One of the drugs studied here (bupropion) has been demonstrated to attenuate the effects of cannabis in humans. Bupropion significantly attenuated marijuana-induced increases in the ratings “I feel high”, “I feel friendly”, “I feel social”, and “I can’t concentrate” (Haney et al. 2001). In the same study, bupropion enhanced marijuana withdrawal. However, qualitatively different results have been reported for cocaine and amphetamine, with marijuana producing additive subjective and cardiovascular effects with amphetamine (Evans et al. 1976) or cocaine (Foltin et al. 1987; 1993). In another study, haloperidol enhanced some of the effects of Δ9-THC on performance in humans (D’Souza et al. 2008), consistent with the current results. Taken together, the current and previous studies suggest indicate that there is marked potential for dopamine transporter ligands and dopamine antagonists to interact with marijuana. The nature of the interaction (i.e. antagonism or not) between cannabinoid and dopamine ligands could depend on dose and/or pharmacologic history of the experimental subjects.
In summary, these results suggest that CB1 receptor agonism and increased synaptic dopamine produce qualitatively different effects. Dopamine release and/or uptake inhibition appears to attenuate the discriminative stimulus effects of Δ9-THC as well as the effects of Δ9-THC to decrease operant responding. 17 Perceptual masking of Δ9-THC appears to be responsible for attenuation of discriminative stimulus effects, whereas effects on response rate are consistent with functional antagonism. These results underscore the potential for some therapeutics (bupropion) and/or abused drugs (cocaine and amphetamine) to modify the subjective effects of cannabis.
Acknowledgments
This research was supported by grants from the U.S. Public Health Service, National Institutes of Health, National Institute on Drug Abuse [R01-DA19222 and R01-DA26781].
REFERENCES
- Anderson JJ, Kask AM, Chase TN. Effects of cannabinoid receptor stimulation and blockade on catalepsy produced by dopamine receptor antagonists. Eur J Pharmacol. 1996;295:163–168. doi: 10.1016/0014-2999(95)00661-3. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Mason R, Marsden CA. Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology. 2003;44:633–641. doi: 10.1016/s0028-3908(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MA, Martz R, Rodda BE, Lemberger L, Forney RB. Effects of marihuana-dextroamphetamine combination. Clin Pharmacol Ther. 1976;20:350–358. doi: 10.1002/cpt1976203350. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology. 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gorriti MA, Rodríguez de Fonseca F, Navarro M, Palomo T. Chronic (−)-delta9-tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. Eur J Pharmacol. 1999;365:133–142. doi: 10.1016/s0014-2999(98)00851-6. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Hattendorf C, Hattendorf M, Coper H, Fernandes M. Interaction between Δ9-tetrahydrocannabinol and damphetamine. Psychopharmacology. 1977;54:177–182. doi: 10.1007/BF00426776. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Lovell RA, Jaffe JH, Freedman DX. 1-Δ9-tetrahydrocannabinol: neurochemical and behavioral effects in the mouse. Science. 1969;163:1464–1467. doi: 10.1126/science.163.3874.1464. [DOI] [PubMed] [Google Scholar]
- Järbe TUC. Discriminative stimulus properties of cocaine. Effects of apomorphine, haloperidol, procaine and other drugs. Neuropharmacology. 1984;23:899–907. doi: 10.1016/0028-3908(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. A pharmacological analysis of the discriminative stimulus properties of damphetamine in rhesus monkeys. J Pharmacol Exp Ther. 1989;248:938–946. [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- Kotian P, Abraham P, Lewin AH, Mascarella SW, Boja JW, Kuhar MJ, Carroll FI. Synthesis and ligand binding study of 3 beta-(4'-substituted phenyl)-2 beta-(heterocyclic)tropanes. J Med Chem. 1995;38:3451–3453. doi: 10.1021/jm00018a004. [DOI] [PubMed] [Google Scholar]
- Lew EO, Richardson JS. Neurochemical and behavioural correlates of the interaction between amphetamine and Δ9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 1981;8:93–101. doi: 10.1016/0376-8716(81)90104-6. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Müller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Masserano JM, Karoum F, Wyatt RJ. SR 141716A, a CB1 cannabinoid receptor antagonist, potentiates the locomotor stimulant effects of amphetamine and apomorphine. Behav Pharmacol. 1999;10:429–432. doi: 10.1097/00008877-199907000-00010. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol. 2010;162:1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranta D, Esteban S, García-Sevilla JA. Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:61–72. doi: 10.1007/s00210-008-0337-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Pistis M, Porcu G, Melis M, Diana M, Gessa GL. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci. 2001;14:96–102. doi: 10.1046/j.0953-816x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- Poddar MK, Dewey WL. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J Pharmacol Exp Ther. 1980;214:63–67. [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration aone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Wertheim CE, Goldberg SR. Dopaminergic augmentation of Δ9-tetrahydrocannabinol (THC) discrimination: possible involvement of D2-induced formation of anandamide. Psychopharmacology. 2010;209:191–202. doi: 10.1007/s00213-010-1789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. Rimonabant-induced Δ9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334:347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2000. pp. 44–50. [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Loddo P, Di Chiara G. Dependence of mesolimbic dopamine transmission on delta9-tetrahydrocannabinol. Eur J Pharmacol. 1999;376:23–26. doi: 10.1016/s0014-2999(99)00384-2. [DOI] [PubMed] [Google Scholar]
- Wegel RL, Lane CE. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Physiol Rev. 1924;23:266–285. [Google Scholar]
- Wu X, French ED. Effects of chronic Δ9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Liskow B, Cadoret R, Goodwin D. Marijuana and amphetamine: the question of interaction. Am J Psychiatry. 1973;130:707–708. doi: 10.1176/ajp.130.6.707. [DOI] [PubMed] [Google Scholar]