Abstract
Small GTPases play critical roles in diverse biological events including regulating both the cytoskeletal and adhesive properties of cells. The importance of small GTPases to these events stems from their ability to be turned on and off, respectively, by specific GEFs and GAPs. In neurons, for example, regulation of small GTPase activity by extracellular guidance cues controls axonal and dendritic process shape, extension and navigation. Here, we discuss recent findings that indicate a specific regulator of small GTPase signaling, the Plexin transmembrane GAP, is differentially controlled by specific extracellular cues to guide growing axons. In particular, Plexins are receptors for one of the largest families of axon guidance cues, Semaphorins and negatively regulate cell morphology and motility by serving as GAPs for Ras/Rap family GTPases. Recent observations reveal that Plexin’s GAP activity is controlled by the cAMP-dependent protein kinase (PKA), which phosphorylates Plexin and generates a binding site for the phospho-serine/threonine binding protein 14-3-3ε. This PKA-mediated Plexin-14-3-3ε interaction prevents Plexin from associating with its GTPase substrate, and thus antagonizes Semaphorin signaling. We now further examine these interactions and how they provide a new logic by which axon guidance signaling pathways over-ride one another to steer growing axons. We also further explore how Plexin interacting proteins, including Ras, PKA and 14-3-3 may interact with the Plexin GAP domain. Our observations also further indicate that 14-3-3 proteins may have conserved roles in the regulation of GTPase activity.
Keywords: motor axon guidance, semaphorin, Plexin receptor signaling, GAP, protein kinase A, 14-3-3, Drosophila
Cells and their elongating processes require the concerted function of cytoskeletal and adhesive complexes in order to change their shape, move and correctly navigate.1 A simplified view of this movement is that initial cellular protrusions such as filopodia and lamellipodia develop by actin nucleation/polymerization.2-4 These protrusions are stabilized by attaching to the extracellular matrix (ECM) or adjacent cells, which are connected to the actin cytoskeleton and provide “feet” for migration.4-6 These steps cycle in the course of motility.
Guiding Axons
Our interests are to understand the cellular, molecular and biochemical mechanisms underlying cellular shape change, motility and guidance. In particular, we are very interested in how the cellular extensions/processes of cells, including, the axonal processes of neurons, perform these functions. At early stages of nervous system development, the leading edge of a growing axon, the growth cone, begins its elongation toward its final target, which is often located a long distance away. The growth cone is a highly motile structure comprised of spike-like filopodia and web-like lamellipodia.3,4 Filopodia are supported in part by bundled, parallel-organized filamentous actin, while lamellipodia are comprised of a mesh-like network of actin. In addition to these actin structures, microtubules strengthen the long axon shaft and also stabilize growth cones. Likewise, axonal growth and guidance also depends on the ability of the growth cone to adhere to a substrate, which may take the form of the ECM, other cell types and even other axons.4,7 Environmental cues direct the organization of these actin, microtubule and adhesive components by triggering the activation of specific receptors present on the membranous surface of growth cones. These receptors then activate intracellular signaling cascades that specify a change—either growth/elongation-promoting or inhibitory/retraction-inducing effects—in cytoskeletal dynamics and adhesion.8 Thus, by expressing different sets of guidance receptors, or by modulating cellular signaling states, each growth cone differentially regulates cytoskeletal dynamics and adhesion and so extends further, steers in another direction, or stops growing.7,8 While much has been learned of the cellular aspects of axon guidance, our understanding of the molecular and biochemical mechanisms enabling precise axon guidance is still far from complete.
Small GTPases Are Key Regulators of Cytoskeletal and Adhesive Dynamics
In neurons, as in other cells, small GTP binding proteins (small GTPases) have emerged as key players in multiple cell biological processes including the regulation of cell shape, movement and navigation.9 Among the five Ras superfamily GTPases, Ras, Rho, Rab, Arf and Ran, Rho family proteins have been best characterized as regulators of the actin cytoskeletal structures that allow for changes in cell morphology and motility (reviewed in refs. 9 and 10). For example, classic microinjection studies using fibroblast cells revealed that RhoA induces actin stress fibers and focal adhesion. Likewise, loading of Rac with GTP results in the formation of lamellipodia, as does Cdc42 for the formation of filopodia. Small GTPases have GTP hydrolysis activity, thereby working as molecular switches that are functional in the GTP bound form and turned-off in the GDP bound form. GTP bound GTPases preferentially associate with downstream effectors such as Rho kinase (ROCK), p21-activated kinase (PAK), phosphatidylinositol-4-phosphate 5-kinase (PI4P5K) and N-WASP (neuronal Wiskott-Aldrich Syndrome protein) and activate these proteins to regulate cytoskeletal dynamics by, among other means, recruiting them to the cell membrane and/or by inducing conformational changes.9,10
In addition to the regulation of cytoskeletal dynamics, Ras superfamily GTPases also regulate cell-cell and cell-substrate adhesion by associating with among other proteins, Cadherins and Integrins.4,11 Cadherins cluster together through Ca2+- dependent homophilic dimerization, which results in cell-cell adhesion. Likewise, Integrins are receptors for extracellular matrix (ECM) proteins including Laminin, Fibronectin and Collagen.5 Integrins consist of α and β subunits, which form heterodimers, bind to their ligands and enable substrate adherence. Binding to ECM components activates Integrins, which initiate downstream signaling through diverse proteins such as focal adhesion kinase (FAK) to regulate cell-cell adhesion and reorganize the actin cytoskeleton.5 Integrins also connect with the actin cytoskeleton through the formation of protein complexes involving talin, paxillin and vinculin.5,6 Small GTPases in the Ras family including R-Ras and Rap1 have also emerged as key regulators of Integrin-mediated cell adhesion.11 Although the molecular mechanisms of how R-Ras and Rap1 activate Integrin-mediated adhesion is not completely understood, the activation of Ras family GTPases increases Integrin-mediated cell adhesion by inducing clustering of Integrins and conformational changes in the extracellular domains of Integrins, which consequently enhances affinity toward the ECM.11 Likewise, R-Ras has also been linked to enhancing Integrin activity by regulating endocytosis of Integrins through increasing membrane dynamics.12 Furthermore, Rap1 activates its effectors, RIAM and RAPL, which directly associate with Integrin complex and enhance Integrin activity.11
Semaphorins: Key Regulators of GTPase-Mediated Axon Guidance
More than half-a-century of intense study has now resulted in the identification of a number of axon guidance cues and their receptors.7 The Semaphorin (Sema) family of secreted and membrane-associated proteins has emerged as one of the largest families of these axon guidance cues, and also function in similar events in multiple other cell types.7,13 Plexin family transmembrane proteins serve as receptors for Semas, activating critical downstream signaling in the growth cone.14 Interestingly, Semas combine with their Plexin receptors to push-away (repel) axons, and thus serve to guide axons by preventing their extension into inappropriate areas.13 In particular, to avoid Sema repellents, the growth cone shifts its orientation by reorganizing the actin cytoskeleton and decreasing its substrate adhesion on the side of the growth cone contacting Semas. Thus, Sema/Plexin guidance is thought to work by regulating both cytoskeletal and adhesive interactions through its downstream signaling proteins.
Significant advances have been made by a number of groups over the past 15 years into the identification of the intracellular molecules that mediate the repulsive responses of Semas/Plexins. As summarized in recent reviews,13,14 these studies have revealed that prominent components in Sema/Plexin axon guidance signaling are small GTPases. In particular, Plexins have been found to interact with a number of different small GTPases, and some of these small GTPases appear to work in Sema/Plexin signaling by serving to activate different effector proteins.9,14-16 Plexin binding may also sequester these small GTPases from interacting with their downstream effectors.13,14 In particular, Plexins have a RhoGTPase binding domain (RBD) and different classes of Plexins have been found to interact with members of Rho GTPase family. For example, as reviewed in references 14−16, some Plexins associate with Rnd GTPases, which regulate Plexin activity. Other small GTPases like RhoD, compete with Rnd family GTPases for binding to Plexin. Plexins also bind to Rac and RhoA and this binding has been postulated to sequester Rac from its downstream effector PAK and enhance the effects of RhoA. Rac1-Plexin interactions have also been found to promote surface expression of Plexin.
An increasing amount of literature indicates that Plexins not only directly bind to several different small GTPases, but they also regulate GTPase activity by functioning as GTPase activating proteins (GAPs) (Reviewed in refs. 14, 16 and 17). In particular, the intrinsic rate of GTP hydrolysis by GTPases is typically slow and GAPs function to accelerate this chemical step and thereby control the signal duration of GTPases. The role of GAPs is therefore in contrast to guanine nucleotide exchange factors (GEFs), which stimulate the release of bound GDP and allow for replacement with GTP, which is abundantly present in the cytoplasm. Therefore, GEFs turn-on and GAPs turn-off GTPase function. The cytoplasmic region of Plexins exhibit sequence similarity to GAPs for Ras and Rap family GTPases, and an overview of a number of recent studies suggests that different Plexins regulate the activity of several Ras and Rap family members.14,16,17 Likewise, crystal structure of the Plexin cytoplasmic region reveals that the Plexin GAP structure is comparable to that of canonical RasGAPs.18,19 While much remains to be learned of this Plexin GAP activity and the differences in GAP specificity/activity between different Plexin family members, these results are exciting in light of the role of small GTPases in cytoskeletal regulation and adhesive interactions. For example, both Ras and Rap family small GTPases are known to activate Integrin-mediated cell adhesion,11,14,17 and results indicate that Plexins decrease the level of active GTP-bound forms of these GTPases (Reviewed in ref. 14). These effects result in decreased Integrin adhesion (de-adhesion) and contribute to the repulsive guidance effects seen with Sema/Plexin signaling. This Plexin RasGAP activity has also been found to underlie Sema/Plexin repulsive signaling by regulating other intracellular proteins including phosphatidylinositol-3 kinase (PI3K) and phosphatase and tensin homolog (PTEN) (Reviewed in ref. 14).
These exciting observations on the Plexin GAP domain have led us and others to question how the Plexin GAP domain is activated and inactivated. The Plexin GAP homology domain is unusual in that it is divided by Plexin’s Rho GTPase binding domain (RBD).14 In at least some contexts, direct binding of a GTPase to the RBD has been found to be important for the stimulation of the Plexin GAP activity (Reviewed in refs. 14 and 16). It is also thought that activation of the Plexin GAP requires binding of Plexin to its Sema ligand and/or perhaps even a combination of Sema binding to the extracellular region and Rho family GTPase binding to the RBD in the cytoplasmic region.14 Although a complete understanding on how Semas and Rho family GTPases contribute to the activation of the Plexin GAP awaits further study, Sema binding likely induces clustering of Plexins and GAP activity,18,20,21 while GTPase binding also induces a conformational change in Plexin and increases the amount of Plexin at the cellular membrane.14 These studies suggest that the Plexin GAP functions as a coincidence detector whose activity depends on both extracellular stimulus by a Sema and intracellular status, which is regulated by other guidance cues and the cellular capacity to respond to those cues.
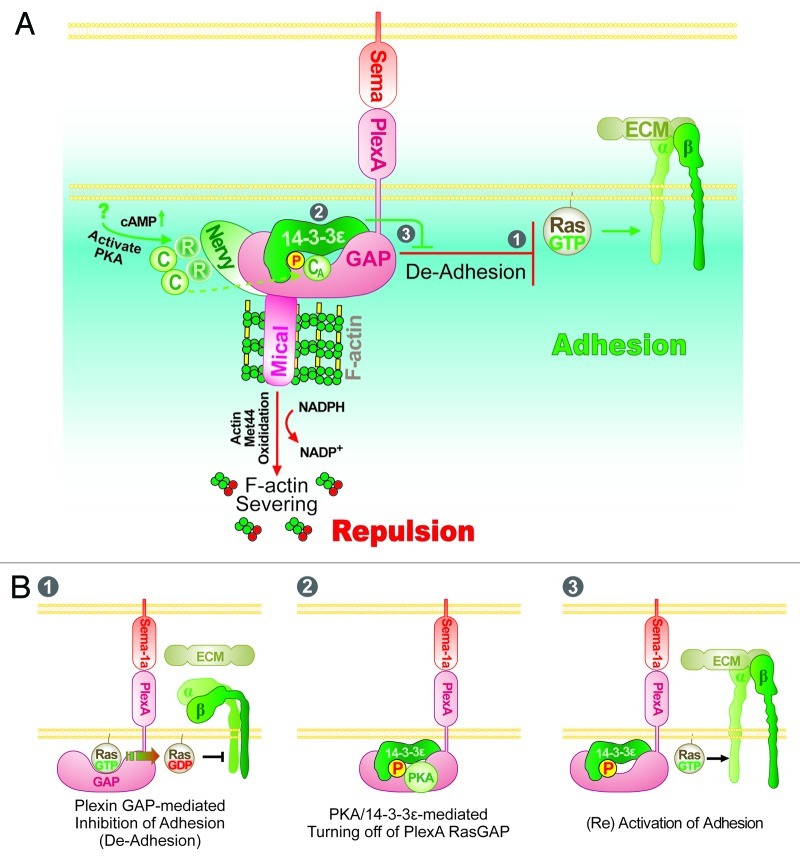
Regulating Sema/Plexin-Mediated Small GTPase Signaling to Guide Axons
To better understand the mechanisms of Sema signaling and its component parts, we along with other groups have been using the simple model system of Drosophila to find and characterize these molecules. Using these strategies, a number of new molecules have been found that have furthered the understanding of Sema-mediated signaling. For example, we have recently characterized a new family of enzymes, the MICALs, which interact with cytoplasmic region of Plexin A (PlexA) (Fig. 1A).13 MICALs are multi-domain oxidoreductase (Redox) enzymes whose activity is required for the propagation of PlexA- mediated repulsion.13,22 Our recent results also reveal that Mical directly binds and disassembles F-actin by oxidation of the Met-44 residue of actin, a residue that is within the Subdomain 2 portion of actin that is critical for actin monomer-monomer contact (Fig. 1A).23,24 Hence, Mical directly links Sema repulsive activity to actin reorganization. Moreover, PlexA has also been found to physically interact with Nervy/MTG family proteins, which among other roles have been found to function as protein kinase A (A kinase) anchoring proteins (AKAPs)25,26 and link protein kinase A (PKA) to the Plexin A receptor (Fig. 1A).27,28 Interestingly, in contrast to Mical, multiple genetic interaction experiments indicate that Nervy and PKA antagonize Sema/PlexA-mediated repulsive axon guidance.27,29 These cAMP-dependent protein kinase (PKA)-mediated antagonistic effects on PlexA-dependent repulsion is reminiscent of observations revealing that increasing cAMP can silence the repulsive effects of specific Semas on cultured Xenopus and chick growth cones (reviewed in refs. 8,29). Thus, this conserved role of cAMP signaling raises interesting questions about the substrates of PKA and how PKA inactivates Sema/Plexin repulsive signaling.
Figure 1. Working model of the mechanisms underlying Drosophila Sema-1a/PlexA-mediated repulsive axon guidance. (A) Drosophila Sema-1a exerts repulsive guidance effects by activating PlexA, which then induces repulsion. In particular, the cytoplasmic region of PlexA interacts with Mical, a novel F-actin disassembly factor that directly oxidizes the Met44 residue of actin to disassemble F-actin and limit actin polymerization. Plexin family proteins also have a GAP domain within their cytoplasmic region, which inactivates members of the Ras/Rap family of small GTPases to inhibit Integrin-mediated cell adhesion (De-Adhesion). Our recent results indicate that this Sema/Plexin “De-adhesion” can be turned-off by phosphorylation of the GAP domain of PlexA by protein kinase A (PKA), which allows binding of 14-3-3ε to PlexA and disrupts the interaction of the PlexA GAP domain with its Ras substrate. These interactions effectively allow Integrin-mediated adhesion to be turned back on (see also B). Several other molecules including Off-track, Gyc76C and Plexin B have also been implicated in this signaling pathway but their mechanistic role in this pathway is not yet clear.51 The circled numbers (1, 2 and 3) refer to the panels in (B) which provide more detail. (B) Panel 1: Plexin RasGAP facilitates GTPase activity of its substrate Ras/Rap family GTPases. Subsequently, GDP-bound inactive Ras is unable to activate Integrin-mediated cell adhesion. Panel 2: PKA phosphorylates a serine residue in the Plexin RasGAP domain and generates a binding site for 14-3-3ε. Panel 3: The interaction between 14-3-3ε and Plexin inhibits Plexin GAP activity by preventing it from associating with its Ras substrate. GTP-bound active Ras is able to activate Integrin-mediated adhesion.
Recently, while further investigating proteins that might direct Sema/Plexin signaling, we were particularly intrigued by a new Plexin binding protein, 14-3-3ε, that had been identified in a yeast two-hybrid screen.29 Members of the 14-3-3 family of proteins are well-known for their interactions with phosphorylated serine/threonine residues in target proteins and play important roles in cellular signaling.30,31 Interestingly, our analysis of the interaction between PlexA and 14–3-3ε revealed that a single serine residue situated within the Plexin RasGAP domain was critical for the PlexA-14-3-3ε interaction.29 Thus, we wondered if this interaction between PlexA and 14-3-3ε could regulate Plexin RasGAP-mediated axon guidance.
14-3-3 proteins have long been known to be highly expressed in the nervous system (discussed in ref. 29) but their in vivo roles in axon guidance have only been poorly defined. Using the Drosophila nervous system as a model, we found that 14-3-3ε is highly expressed within developing neurons and their axonal processes and can be co-immunoprecipitated with neuronal PlexA in vivo.29 Turning to genetic assays in Drosophila, we found that loss of 14-3-3ε generated axon guidance defects, as did raising the neuronal levels of 14-3-3ε 29. These results revealed that 14-3-3ε is both necessary for correct axon guidance and sufficient to alter the guidance of axons. Interestingly, further analysis revealed that the guidance defects that resulted from manipulations of 14-3-3ε levels were opposite in effect to similar manipulations of Sema-1a or PlexA.29 This potential antagonistic relationship between 14-3-3ε and Sema/PlexA was confirmed using enhancer-suppressor genetic interaction assays and revealed, for example, removing 14-3-3ε increased the severity of Sema/PlexA-mediated axonal repulsion.29 These results were similar to the effects that had previously been observed with PKA and Nervy,27 and revealed that 14-3-3ε, despite directly interacting with PlexA, functioned to antagonize Sema/PlexA repulsive guidance signaling.
To better understand the mechanisms underlying this antagonistic relationship between 14–3-3ε and Sema/PlexA signaling, we looked further at the site of interaction between 14–3-3ε and PlexA. In particular, we wondered if the single serine residue situated within the GAP domain of PlexA that was required for the interaction with 14-3-3ε might be phosphorylated. Indeed, this PlexA serine residue exhibited the consensus hallmarks of a PKA phosphorylation site.29 Likewise, using in vitro kinase assays, a phospho-specific antibody (phospho-Serine 1794), and in vivo analyses of PKA mutants, we found that the single serine residue within PlexA that is critical for its interaction with 14-3-3ε is selectively phosphorylated by PKA.29 Furthermore, we found that this phosphorylation is critical for the interaction between PlexA and 14-3-3ε 29. Moreover, mutating this single serine residue to prevent PKA phosphorylation and subsequent 14-3-3ε binding, generated a hyperactive PlexA in vivo. Thus, these results indicated that PKA-mediated phosphorylation of PlexA and 14-3-3ε binding antagonize Sema/PlexA-mediated repulsive axon guidance.29
In light of the position of this phosphorylated serine residue within Plexin’s GAP domain, we wondered if PKA-mediated phosphorylation and subsequent 14-3-3ε binding to this serine residue might provide a mechanism to limit PlexA’s ability to associate with its small GTPase substrate. Using in vitro binding assays and purified 14-3-3ε and Ras family GTPases, we found that the association between PlexA and its Ras substrate was significantly reduced in the presence of 14-3-3ε.29 Moreover, this disruptive ability of 14-3-3ε was dependent on the single 14-3-3ε-interacting serine residue in the GAP domain of PlexA.29 Likewise, the GAP activity of PlexA toward its Ras family GTPase substrate was also reduced in the presence of 14-3-3ε (but not by phosphorylation alone).29 Thus, our results are in agreement with a hypothesis that the specific interaction that occurs between 14-3-3ε and PlexA inhibits Plexin GAP function.
To further test this “GAP inhibition” hypothesis we turned to genetic assays and reasoned that if 14–3-3ε functions to occlude PlexA’s access to its small GTPase substrate then the axon guidance defects that result from loss of 14–3-3ε might be rescued by increasing the levels of active/GTP-bound Ras (i.e., in this situation where the levels of 14–3-3ε are decreased, the amount of bound Ras/Plexin GAP activity would be predicted to be higher and result in more inactive/GDP-bound Ras). Indeed, we found that raising the levels of a specific GTP-bound Ras family GTPase (using a constitutively active Ras) rescues the axon guidance defects present in 14–3-3ε mutants.29 Furthermore, increasing the activity of this Ras family GTPase reverses the effects of hyperactive Sema/PlexA signaling that occur with a loss of 14-3-3ε 29. Moreover, we reasoned that since activation of Ras/Rap family GTPases is known to increase cellular adhesion through Integrins, the effects we see in 14-3-3ε mutants might result from loss of Integrin-mediated adhesions. Indeed, increasing the levels of specific Integrins also rescues the axon guidance defects present in 14–3-3ε mutants and reverses the hyperactive Sema/PlexA repulsive axon guidance signaling that occur in the absence of 14-3-3ε 29. Thus, our recent results indicate a cAMP-PKA-14-3-3ε-dependent signaling pathway that functions to tightly control/turn-off Sema/Plexin/RasGAP-mediated axon repulsion and restore Integrin-mediated adhesion (Fig. 1A and B).
PKA-Mediated Phosphorylation/14-3-3ε Binding as a Molecular Switch to Turn-Off Sema/Plexin GAP-Mediated Signaling
These recent results identify the novel Plexin-interacting protein 14-3-3ε as a site of convergence for cAMP and GTPase signaling. Interestingly, this interaction between PlexA and 14-3-3ε functions to turn off the GAP activity of the Plexin receptor (and this consensus 14-3-3 binding motif is also conserved in mammalian Plexins that signal for Semas within classes 3, 4, 5, 6 and 7).29 Controlled inactivation of receptor signaling is critical for normal physiological function and prevents the prolonged activation of a receptor that may result in pathologies including cancer and degenerative diseases. Likewise, growing axons require coordinated activation and inactivation of multiple guidance receptors to precisely wire the nervous system.7 For instance, persistent repulsive guidance signaling would alter the ability of a growth cone to extend toward its target in vivo. Thus, the Plexin RasGAP-mediated repulsive response needs to be tightly regulated. Guidance receptors are inactivated by various mechanisms including receptor endocytosis,8,32,33 ubiquitin-mediated degradation,34 decreases in surface levels,35-37 and changes in receptor compositions.8,38,39 Our study indicates that cAMP/PKA-mediated posttranslational modification in conjunction with binding to an adaptor protein can also inactivate receptor function in axon guidance. This relatively rapid second messenger/phosphorylation-mediated inhibition of Sema/Plexin signaling also suggests that Sema/Plexin-dependent repulsive responses may only be activated for a short period of time in vivo or even quickly silenced depending on the context.
Conserved Roles for 14-3-3 Proteins in the Regulation of GTPase/GAP activity
The mechanism of 14-3-3ε in the inhibition of the Plexin RasGAP, although very specific in nature, is in line with previous roles of 14-3-3 proteins in regulating the function of enzymes in a phospho-specific manner.30,31 In particular, the function of 14-3-3 as a regulator in multiple cellular processes can be summarized by its mechanisms of action, none of which are mutually exclusive (Reviewed in30,31). For example, 14-3-3 can serve as a scaffolding protein by binding to two target proteins through its homo/hetero dimerization. Likewise, by binding to two sites in the same target protein, 14-3-3 can induce conformational changes and modulate enzymatic activity. Furthermore, 14-3-3 binding can protect phosphorylated residues from phosphatase-mediated dephosphorylation. 14-3-3 binding can also sequester specific binding sites from both association with other proteins and further post-translational modifications such as ubiquitination. 14-3-3 binding has also been found to regulate surface expression of target proteins. The specific effect of 14-3-3 proteins appears to depend on the particular 14-3-3 protein mediating the interaction, the function of the specific target proteins and the spatiotemporal context of its involvement.30 For example, we find no evidence that 14-3-3ε changes the surface expression level of Plexin on axons.29 Instead, our results reveal a competition between 14-3-3ε and Ras for binding to PlexA. Likewise, the proximity between the 14-3-3ε binding Serine residue on Plexin and the conserved Arginine residue that is necessary for Plexin GAP activity14 indicate that 14-3-3ε binding sequesters the active site of the Plexin GAP domain. In this regard, it is interesting that the RasGAP NF1, and a regulator of G protein signaling, RGS7, both employ a similar mechanism to regulate their GAP activity.40,41 In particular, the Ser-424 residue in RGS7 is located near the contacting residues for the interaction with its substrate Gαi subunit and has been found to bind to 14-3-3τ, which silences RGS GAP activity.40
Further Investigations into the Regulation of the Plexin GAP Activity
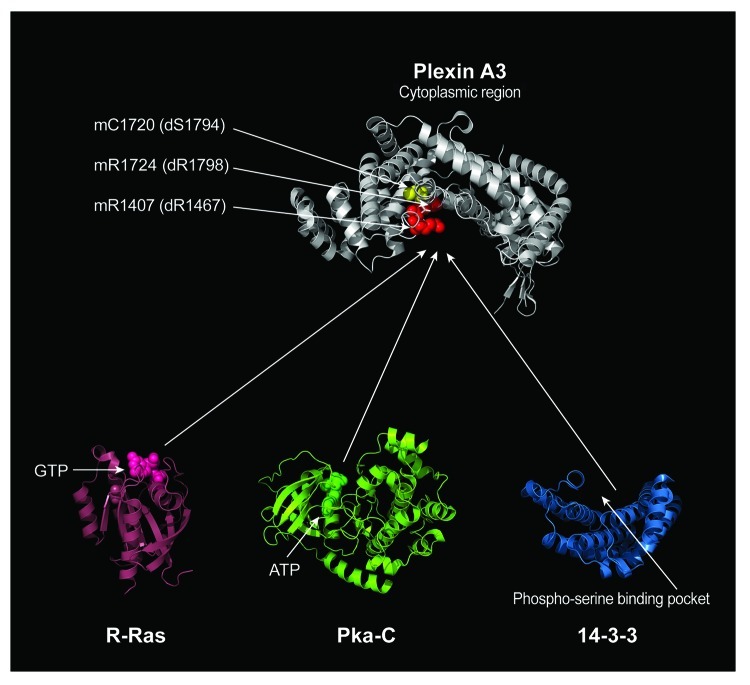
The cytoplasmic region of A class Plexins has been found to interact with several different proteins. In Drosophila, for example, evidence indicates that Mical, Nervy, Ras2 and 14-3-3ε directly interact with PlexA.22,27,29 In addition, PKA interacts with and phosphorylates the PlexA cytoplasmic region.27-29 Among these interacting proteins, Ras2, 14-3-3ε and PKA share the same interacting region within the Plexin GAP domain. As mentioned above, activation of the Ras/Rap GAP activity of Plexin is thought to occur in vivo by a conformational change brought on by Sema binding, Rho GTPase binding, and/or some other modification (Fig. 2; reviewed in refs. 14, 16 and 17). Likewise, the Plexin structures determined thus far suggest that the Plexin GAP domain also needs to undergo a conformational change in order to be phosphorylated at the Serine 1794 residue (the site we find to be critical for 14-3-3ε binding) (Fig. 2). Thus, it is reasonable to predict that Sema binding, Rho GTPase binding, and/or some additional post-translational modifications in the cytoplasmic region of PlexA induces the conformational change that also allows the catalytic subunit of PKA access to its Serine 1794 substrate residue within the Plexin GAP domain. Interestingly, structural examination of Ras, 14-3-3ε and the catalytic subunit of PKA reveals that they are similar in size but are different in shape (Fig. 2). This analysis suggests that each of them must access the Plexin GAP domain in a unique manner. For example, 14-3-3ε is longer and narrower than the ball-shaped Ras family GTPase. Therefore, while the ball-shaped Ras may fit into the GAP domain directly, the saddle-shaped 14-3-3ε may access the GAP domain from the side (Fig. 2). Future studies should be aimed at further exploring the conformational changes that occur when Plexin is activated and how these different proteins access the PlexA GAP domain.
Figure 2. Structural Comparison of Proteins Interacting with the Plexin GAP domain. Our results indicate that Ras family GTPases, 14-3-3ε, and a catalytic subunit of PKA (Pka-C) share a binding site within the GAP domain of Plexin A. The conserved arginine residues that are critical for Plexin RasGAP activity are labeled in red. The residue corresponding to a 14-3-3ε binding site is labeled in yellow, which is veiled by the arginine residues. The amino acid residues for these arginine and serine in both mouse Plexin A3 (m) and Drosophila PlexA (d) are indicated by arrows. The structural model of the Plexin A3 cytoplasmic region (some portions of the cytoplasmic region were not resolved in the crystal structure) is oriented with the concave surface of RasGAP domain facing downward. Active sites for proteins that interact with the Plexin RasGAP domain are indicated such as GTP for R-Ras and ATP for Pka-C. The phospho-serine-binding pocket of 14-3-3 is also indicated (14-3-3 is thought to often function as a dimer and bind two different phosphorylated residues. For clarity, a monomer of 14-3-3 is depicted). Protein data bank identification numbers: 3IG3 for mouse Plexin A3, 2FN4 for human R-Ras, 2F7X for cow catalytic subunit of PKA and 2BR9 for human 14-3-3ε.
Integration of Different Axon Guidance Signals
These results also uncover a critical axon guidance integration point subject to control by different guidance signaling pathways. For example, silencing Plexin RasGAP activity by a phosphorylation-mediated interaction with 14-3-3ε suggests that distinct molecular pathways antagonize the repulsive response of Sema/Plexin signaling by increasing the level of cAMP. Thus, what are these antagonizing pathways that increase cAMP levels? One candidate is Netrin, which is a phylogenetically conserved attractive guidance cue,7 that seems to be expressed in the right place (muscles) to perform this function in developing Drosophila embryos.42,43 At least some Netrin-dependent axon guidance has been linked to increases in cAMP/PKA signaling,44-47 although additional work is needed to fully define its involvement. cAMP is produced by adenylate cyclase (AC) and among the members of the AC family in vertebrates, at least AC1 and AC8 are known to be activated by calcium/calmodulin as well as heterotrimeric Gαs proteins.14,48 Intracellular calcium signaling is an important mediator of growth cone guidance49 and Sema activity is also regulated by calcium signaling.14,50 Thus, extracellular signals that lead to calcium-stimulated AC activity should also be further investigated. In addition, one possibility is that Semas may not only activate the Plexin GAP but also inactivate Plexin as a feedback inhibition through calcium signaling50—such that Semas also control the duration of repulsive signaling in vivo. Further studies will focus on which antagonizing factors decrease Sema/Plexin repulsive signaling.
Acknowledgments
We thank M. Buck for comments on the manuscript and members of the Terman lab and T. Yang’s thesis committee (J. Bibb, C. Cowan, E. Ross and X. Zhang) for comments and feedback on this work. Supported by the NIH (NIMH; MH085923) to J.R.T.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/22765
References
- 1.Bray D. Cell Movements: From Molecules to Motility. Garland Science, 2000. [Google Scholar]
- 2.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73:1068–81. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesner S, Legate KR, Fässler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–99. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3:3. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 11.Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–76. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- 12.Conklin MW, Ada-Nguema A, Parsons M, Riching KM, Keely PJ. R-Ras regulates beta1-integrin trafficking via effects on membrane ruffling and endocytosis. BMC Cell Biol. 2010;11:14. doi: 10.1186/1471-2121-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung RJ, Terman JR. Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly. Cytoskeleton (Hoboken) 2011;68:415–33. doi: 10.1002/cm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Püschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- 16.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–71. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos JL, Pannekoek WJ. Semaphorin signaling meets rap. Sci Signal. 2012;5:pe6. doi: 10.1126/scisignal.2002913. [DOI] [PubMed] [Google Scholar]
- 18.He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci USA. 2009;106:15610–5. doi: 10.1073/pnas.0906923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Y, Hota PK, Penachioni JY, Hamaneh MB, Kim S, Alviani RS, et al. Structure and function of the intracellular region of the plexin-b1 transmembrane receptor. J Biol Chem. 2009;284:35962–72. doi: 10.1074/jbc.M109.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell CH, Aricescu AR, Jones EY, Siebold C. A dual binding mode for RhoGTPases in plexin signalling. PLoS Biol. 2011;9:e1001134. doi: 10.1371/journal.pbio.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/S0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 23.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334:1710–3. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, et al. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–7. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuyama T, Sueoka E, Sugio Y, Otsuka T, Niho Y, Akagi K, et al. MTG8 proto-oncoprotein interacts with the regulatory subunit of type II cyclic AMP-dependent protein kinase in lymphocytes. Oncogene. 2001;20:6225–32. doi: 10.1038/sj.onc.1204794. [DOI] [PubMed] [Google Scholar]
- 26.Schillace RV, Andrews SF, Liberty GA, Davey MP, Carr DW. Identification and characterization of myeloid translocation gene 16b as a novel a kinase anchoring protein in T lymphocytes. J Immunol. 2002;168:1590–9. doi: 10.4049/jimmunol.168.4.1590. [DOI] [PubMed] [Google Scholar]
- 27.Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–7. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- 28.Fiedler SE, Schillace RV, Daniels CJ, Andrews SF, Carr DW. Myeloid translocation gene 16b is a dual A-kinase anchoring protein that interacts selectively with plexins in a phospho-regulated manner. FEBS Lett. 2010;584:873–7. doi: 10.1016/j.febslet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Yang T, Terman JR. 14-3-3ε couples protein kinase A to semaphorin signaling and silences plexin RasGAP-mediated axonal repulsion. Neuron. 2012;74:108–21. doi: 10.1016/j.neuron.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aitken A. Post-translational modification of 14-3-3 isoforms and regulation of cellular function. Semin Cell Dev Biol. 2011;22:673–80. doi: 10.1016/j.semcdb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Tzivion G, Shen YH, Zhu J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene. 2001;20:6331–8. doi: 10.1038/sj.onc.1204777. [DOI] [PubMed] [Google Scholar]
- 32.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–17. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Williamson WR, Yang T, Terman JR, Hiesinger PR. Guidance receptor degradation is required for neuronal connectivity in the Drosophila nervous system. PLoS Biol. 2010;8:e1000553. doi: 10.1371/journal.pbio.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim TH, Lee HK, Seo IA, Bae HR, Suh DJ, Wu J, et al. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J Neurochem. 2005;95:1–8. doi: 10.1111/j.1471-4159.2005.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–15. doi: 10.1016/S0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 36.Nawabi H, Briançon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–27. doi: 10.1016/S0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 38.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–38. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 39.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–41. doi: 10.1016/S0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 40.Benzing T, Yaffe MB, Arnould T, Sellin L, Schermer B, Schilling B, et al. 14-3-3 interacts with regulator of G protein signaling proteins and modulates their activity. J Biol Chem. 2000;275:28167–72. doi: 10.1074/jbc.M002905200. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, Yunoue S, Tokuo H, Ozawa T, Zhang D, Patrakitkomjorn S, et al. PKA phosphorylation and 14-3-3 interaction regulate the function of neurofibromatosis type I tumor suppressor, neurofibromin. FEBS Lett. 2004;557:275–82. doi: 10.1016/S0014-5793(03)01507-2. [DOI] [PubMed] [Google Scholar]
- 42.Winberg ML, Mitchell KJ, Goodman CS. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 1998;93:581–91. doi: 10.1016/S0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–15. doi: 10.1016/S0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 44.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–35. doi: 10.1016/S0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 45.Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chédotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–50. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 46.Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–64. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicol X, Hong KP, Spitzer NC. Spatial and temporal second messenger codes for growth cone turning. Proc Natl Acad Sci USA. 2011;108:13776–81. doi: 10.1073/pnas.1100247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76:935–41. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–25. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 50.Nishiyama M, Togashi K, von Schimmelmann MJ, Lim CS, Maeda S, Yamashita N, et al. Semaphorin 3A induces CaV2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol. 2011;13:676–85. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]