Abstract
Organ weight is one of the most sensitive drug toxicity indicators, and its changes often precede morphological changes. So far, no background data about organ weight and its coefficient in SD rats at different weeks of age have been reported in China. The aim of this study was to summarize and analyze the change trends of organ weight and organ weight coefficients in SD rats at different weeks of age. The absolute of the weights of the brain, spleen, heart, lungs, liver, kidneys, adrenal glands and testes were increased in male SD rats from 13 to 78 weeks, and the weights of the brain, heart, lungs, liver, kidneys and especially the testes were decreased from 78 to 104 weeks. On the other hand, the absolute weight of the adrenal glands showed an increasing trend from 13 to 104 weeks. The absolute weight of the brain, spleen, heart, lungs, liver, kidneys, adrenal glands and ovaries showed an increasing trend from 13 to 104 weeks. A significant increase was observed in adrenal gland and ovary weights, whereas no obvious change trends were observed for the other organ weights mentioned above. It was surprising that the absolute of weight of the adrenal glands and organ-to-brain and organ-to-body weight ratios in female rats were significantly higher than those in males from 13 to 104 weeks. This study was the first to establish background data for organ weights in SD rats at different weeks of age and their reference ranges in line with the experimental animal status in China and to summarize their summarized their changes trend.
Keywords: Sprague-Dawley rat, organ weight, reference range, organ coefficient
Introduction
Organ weight can be the most sensitive indicator of an effect of drug toxicity, as significant differences in organ weight between treated and control animals may occur in the absence of any morphological changes. Evaluation of organ weight changes in the presence of body weight differences has resulted in the use of organ-to-body weight and organ-to-brain weight to assess treatment effects in toxicology studies1. Understanding the relationship between absolute organ weight, body weight, and brain weight will improve organ weight interpretation. Background data reflects the organ weight of control animals, and it is significantly important for the evaluation of drug safety. During the process of nonclinical drug safety evaluation, the toxicity of some drugs may only result in animal organ weight or organ coefficient changes. Therefore, organ background data is particularly important because they not only can be used to determine whether treatment group animal organ weights are in the range of background data or not but also provide an important reference to provide to pathologists for gross anatomy and microscopic examinations2. In many institutes including our laboratory in China, CD(SD) (Sprague-Dawley) rats are used predominantly as experimental models in toxicity studies, and they have been used as traditional animal models for assessing carcinogenic potential of various kinds of compounds3. However, relatively few background data for organs weight from SD rats at different weeks of age are available. Under these circumstances, we face an urgent task to collect background data on SD rats in China. Therefore, the present study was performed to obtain organ weight background data for SD rats at different weeks of age demonstrate the features of their change trends as an aid for interpretation of results.
Materials and Methods
Animals
CD(SD) SPF rats (3147 male and 3132 female) were obtained from shanghai Laboratory Animal Center (Shanghai, China) at 5 weeks of age for use as the control group. The animals were quarantined and acclimatized for 1 week and used at 6 weeks of age. They were housed individually in stainless steel wire mesh cages in an animal room that was maintained at 22 ± 2°C and 50 ± 20% relative humidity with air ventilation 10 to 15 times per hour and a 12-hour light-dark cycle. Pelleted diet (radiation sterilized CR-LPF, Yuhong District, Shenyang City, China) and drinking water (tap water, via automatic water supply system) were provided ad libitum. The experiment was conducted in compliance with the laws or guidelines relating to animal welfare in China including Standards Relating to the Care and Management of Experimental Animals and Guidelines for Animal Experimentation.
Organ weights
According to the protocol, the animals were completely necropsied at 13, 26, 52, 78 and 104 weeks, which refers to the weeks of the experiment rather than weeks of age. Generally, anatomical examinations are performed at 13 weeks in subchronic experiments, while 26, 52, 78 and 104 weeks are commonly used for chronic toxicity/carcinogenic tests. At the time of necropsy at weeks 13, 26, 52, 78 and 104, blood samples were collected from the abdominal aorta under diethyl ether anesthesia into blood collection tubes. Prior to blood sample collection, the animals were deprived of food overnight. After collection of blood samples, the animals were euthanized by exsanguination and subjected to complete necropsy. Then, the weights of the following organs were measured: brain, heart, adrenals, kidneys, liver, testes, ovaries, lungs and spleen. All organ weight data were directly input into the “organs weighing 1.0” software.
Data analysis
We selected organ weight data including those of the brain, heart, adrenals, kidneys, liver, testes, ovaries, lungs and spleen from the control group animals at 13, 26, 52, 78 and 104 weeks of age. Based on the wet weight (absolute weight) and body weight at necropsy, the organ-to-body weight or organ-to-brain weight ratio (relative weight) was calculated. Inappropriate organs were excluded in accordance with the recommendations of Grubbs in the Statistical interpretation of data-Detection and treatment of outliers in the normal sample (GB/T488882-2008). The level of outliers excluded was 1%.
Results
The reference range of organ weights / organ coefficients in rats at different ages
The results are shown in Table 1 and Table 2.
Table 1. Absolute Organ Weight Data for Nontreated SD Rats.
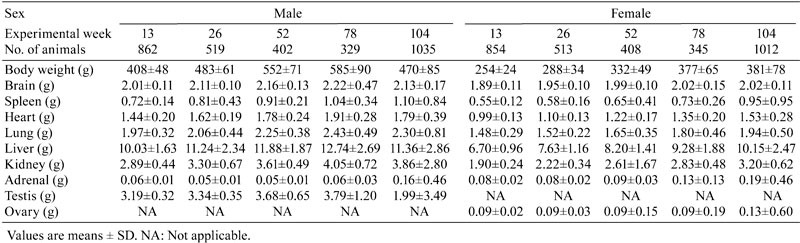
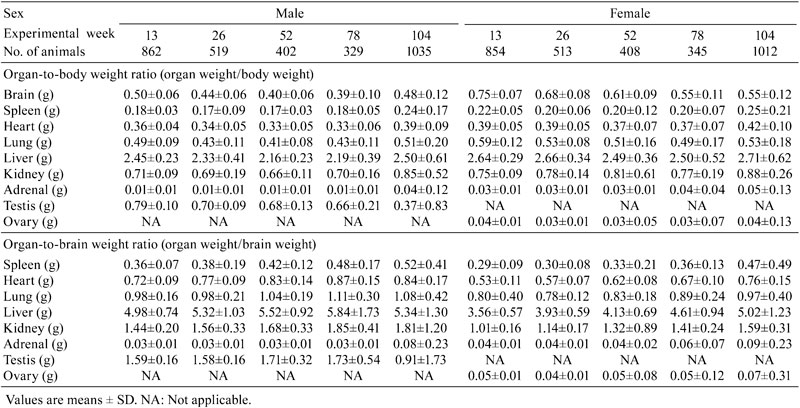
Table 2. Relative Organ Weight Data for Nontreated SD Rats.
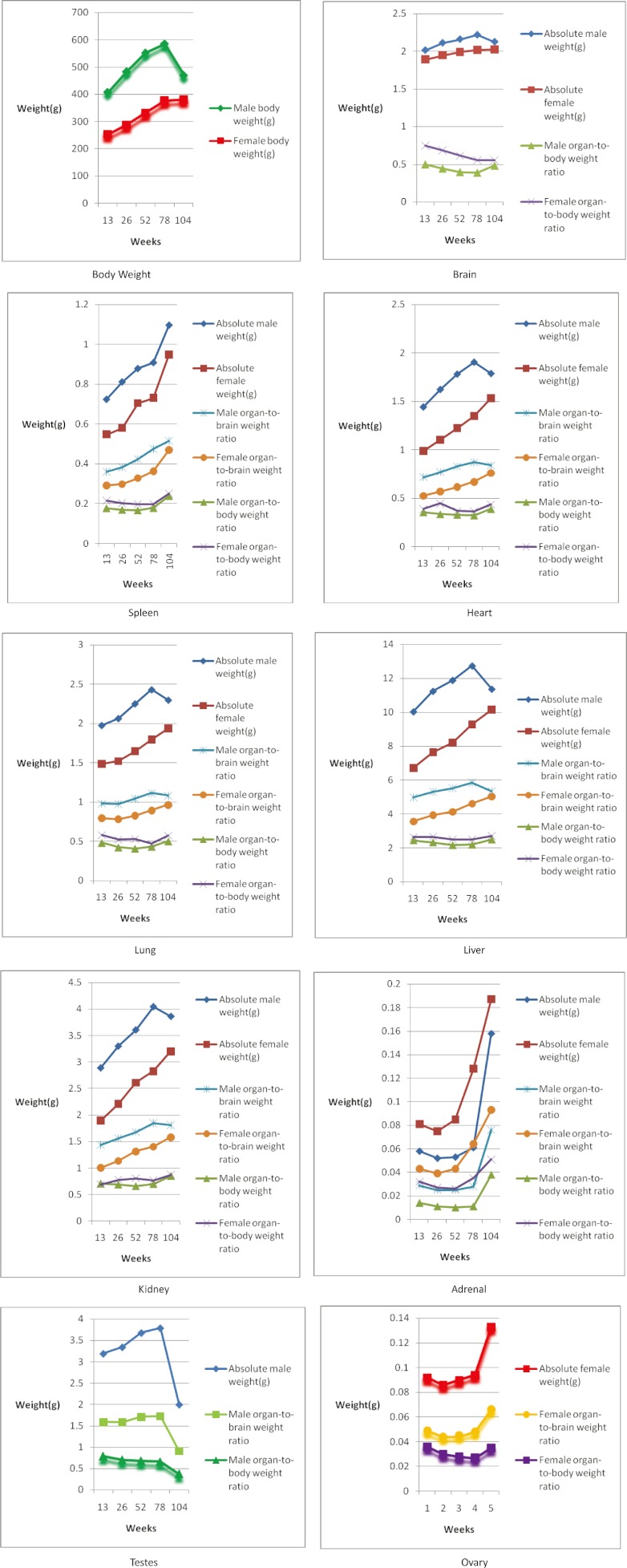
Change trends of organ weights and organ coefficients in male rats at different ages
The absolute weights of the brain, spleen, heart, lungs, liver, kidneys, adrenal glands and testes were increased in male SD rats at weeks 13 to 78, and the weights of the brain, heart, lungs, liver, kidneys and especially the testes were decreased at weeks 78 to 104. On the other hand, the absolute weights of the adrenal glands and spleen showed an increasing trends from weeks 78 to 104. A similar trend was observed in the organ-to-brain weight ratio, while the opposite trend was seen for the organ-to-body weight ratio during the same time period (Fig.1).
Fig. 1.
Changes trend of organ weights and organ weights coefficients in SD rats at different ages.
Change trends of organ weights and organ coefficients in female rats at different ages
The absolute weight of the brain, spleen, heart, lungs, liver, kidneys, adrenal glands and ovaries showed an increasing trend from weeks 13 to 104. A significant increase was observed in adrenal gland and ovary weights, whereas the change trends of the other organ weights mentioned above were not obvious. As for the organ-to-brain weight ratio, the change trends were similar to those of the absolute weights (Fig.1).
Comparison of organ weight and organ coefficients in SD rats at different ages
The absolute weights of the brain, spleen, heart, lungs, liver and kidneys of male rats were higher than those of the female rats, and a similar trend was shown for the organ-to-brain weight ratio, whereas the organ-to-body weight ratios of these organs mentioned above in female rats were slightly higher than those in the male rats. It was surprising that the absolute weight of the adrenal glands and organ-to-brain and organ-to-body weight ratios in female rats were significantly higher than those in males from weeks 13 to 104 (Fig.1).
Discussion
Organ weight is one of the most sensitive drug toxicity indicators, and it changes often precede morphological changes. A number of factors have been reported that may influence animal organ weights including strain of animal, age, sex and environmental and experimental conditions4. The establishment of organ weight reference values at each testing facility for laboratory animals used in toxicological studies has become a standard practice. So far, no background data about organ weights and their coefficients in SD rats at different weeks of age have been reported in China. Therefore, this study aimed to summarize and analyze the change trends of organ weights and organ weight coefficients in SD rats at different weeks of age. The present study demonstrated that the absolute weights and organ-to-brain weight ratios of the brain, spleen, heart, lungs, liver and kidneys in male rats were higher than those in female rats, whereas an opposite result was seen for the organ-to-body weight ratio, which might be associated with animal sex, because the body weights of the male rats were usually higher than those of the rats as the rats got older. Generally, the body weight of the male rat was approximately two times that of the female rat.
The differences in body weight were considered a potential source of bias in the analysis of the organ weight data. When considering organ weight data, differences in body weight must be taken into account using an appropriate analysis. The brain weight was low variability compared with other organs mentioned above and is not considered to be influenced by nutritional factors5. The organ-to-brain weight ratio was partially successful in reducing the variability among the organ weights. Some consider evaluation of the organ-to-brain weight ratio helpful when terminal body weights are affected by the test item or to normalize organ weight data when there is large interanimal variability6. The STP recommends collection of brain weights so that organ-to-brain weight ratios may be calculated if needed. Bailey SA et al.7 thought that, based on analysis of control animal data, liver and thyroid gland weights were best compared using organ-to-body weight ratios and that adrenal gland and ovary weights are best compared using organ-to-brain weight ratios. The present study found that the absolute weights of some organs including the heart, liver, kidney and lungs, showed significant gender differences, in other words, the absolute weights of male rats were higher than those of female rats. However, the absolute organ weight and organ-to-brain weight and organ-to-body weight ratios of the adrenal glands in female rats were higher than those in male rats, which increased with age. During the process of autopsy, the adrenal glands of the female rats were found to be larger than those of the male rats which were often surrounded by more adipose tissue. It is reported that the absolute organ weight and organ-to-body weight ratio of the adrenal glands in F344 female rats were significantly higher than those in male rats from weeks 4 to 104, while a similar trend was observed from weeks 13 to 104 in B6C3F1 mice8, and this was consistent with our results. The change in adrenal gland weight might suggest the appearance of hypertrophy, hyperplasia, tumor or test item effects9. Some studies have reported that the adrenal cortex was thicker in female F344 rats and B6C3F1 mice compared with males, in particular, the reticular zone thickness increased significantly in the area that was mainly responsible for the secretion of androgen and a small amount of estrogen10. However, some reports have also shown that the absolute weight of the adrenal gland in male hamsters was higher than that in females. Microscopically, the reticular zone thickness of the male hamster was about three times that of the female hamster11. The results suggested that the adrenal gland weight variation might be a gender difference in species or strains, which may be related to the level of a hormone secreted by the adrenal zona reticularis. The present study found that the absolute organ weight of the spleen of both sexes increased from weeks 78 to 104. As we all know increased spleen weight is associated histologically with severe congestion in red pulp and enhanced extramedullary hematopoiesis, as well as depletion of lymphoid follicles in white pulp12. The splenic white pulp volume gradually becomes small with red pulp volume expansion from weeks 78 to 104. More important, the incidence of tumors such as malignant lymphoma, and large granular lymphocyte leukemia in the spleen increased from weeks 78 to 104 in the present study (data not shown).
In addition, the weights of the brain, heart, lung, liver, kidneys and especially the testis in male rats showed a decreasing trend from weeks 78 to 104. The SD rat life span is about 2 to 3 years, and 104 weeks is considered to be old age. The decrease in testes weight was associated with organ dysfunction and atrophy, because the incidence of testis atrophy was about 67 percent at 104 weeks in our facility. However, the ovarian weight was significantly increased from weeks 78 to 104, microscopically, there was a significant reduction in ovarian follicles, stromal cell proliferation and ovarian cyst formation. It is necessary to study further the underlying causes of the organ weight changes in combination with pathological background data.
This study was the first to establish organ weight background data and their reference ranges in SD rats at different weeks of age in line with the experimental animal status in China and to summarize their change trends.
Acknowledgments
The authors thank Ying Wang and Donghua Yang for their technical support and Jie Wang for assistance with the experiments and valuable discussion.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, and Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 35: 742–750 2007. [DOI] [PubMed] [Google Scholar]
- 2.Okamura T, Suzuki S, Ogawa T, Kobayashi J, Kusuoka O, Hatayama K, Mochizuki M, Hoshiya T, Okazaki S, and Tamura K. Background data for general toxicology parameters in RccHanTM:WIST rats at 8, 10, 19 and 32 weeks of age. J Toxicol Pathol. 24: 195–205 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Liu J, Zhou B, Xu R, Tao L, Ji M, Zhu L, Jiang J, Shen J, Gui X, Gu L, Bai W, Sun W, and Cheng J. Acute and sub-chronic toxicity studies of Danshen injection in Sprague-Dawley rats. J Ethnopharmacol. 141: 96–103 2012. [DOI] [PubMed] [Google Scholar]
- 4.Gur E, and Waner T. The variability of organ weight background data in rats. Laboratory Animals. 27: 65–72 1993. [DOI] [PubMed] [Google Scholar]
- 5.Long GG, Symanowski JT, and Roback K. Precision in data acquisition and reporting of organ weights in rats and mice. Toxicol Pathol. 26: 316–318 1998. [DOI] [PubMed] [Google Scholar]
- 6.Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, and Schafer K. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol. 35: 751–755 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bailey SA, Zidell RH, and Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 32: 448–466 2004. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, and Utagawa A. Safety trials and experimental animal technology. Sugiyama printing, Japan. 1995 [Google Scholar]
- 9.Lloyd RV. Adrenal cortical tumors, pheochromocytomas and paragangliomas. Mod Pathol. 24: 858–865 2011. [DOI] [PubMed] [Google Scholar]
- 10.Devečerski VD. The reticular zone of the rat adrenal gland 60 days after exposure to a chemical fertilizer. Rev Environ Health. 11: 79–83 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gattermann R, Fritzsche P, Weinandy R, and Neumann K. Comparative studies of body mass, body measurements and organ weights of wild-derived and laboratory golden hamsters (Mesocricetus auratus). Lab Anim. 36: 445–454 2002. [DOI] [PubMed] [Google Scholar]
- 12.Domingues A, Grassi TF, Spinardi-Barbisan AL, and Barbisan LF. Developmental exposure to diuron causes splenotoxicity in male Sprague-Dawley rat pups. J Environ Sci Health B. 47: 420–426 2012. [DOI] [PubMed] [Google Scholar]