Abstract
In rats, it is sometimes difficult to distinguish malignant reticuloses from astrocytomas in routine histopathological assessment. In the present study, four spontaneous brain neoplasms developing in the cerebrum of one Wistar Hannover rat and three Sprague-Dawley rats were immunohistochemically examined using microglia and macrophage markers. Histopathologically, these neoplasms were localized mainly in the cerebral cortex, hypothalamus or piriform lobe, and the portions showing solid growth did not show characteristic cellular arrangement but had an indistinct boundary with the surrounding brain parenchyma. Neoplastic cells had oval or pleomorphic small nuclei with abundant eosinophilic cytoplasm. Two cases showed neoplastic cell infiltration into the meninges and perivascular spaces. Silver staining showed lack of reticulin fiber production in the stroma of the neoplasms. Immunohistochemically, the neoplastic cells were strongly positive for Iba-1 and sporadically positive for CD68 in all four cases. On the basis of these results, all the neoplasms examined here could be distinguished from astrocytomas and diagnosed as malignant reticuloses. Thus, immunohistochemical demonstration of microglia/macrophage characters, such as using Iba-1, is considered to be helpful for differential diagnosis of malignant reticuloses from astrocytomas among spontaneously occurring primary brain neoplasms in rats.
Keywords: malignant reticulosis, astrocytoma, rat, immunohistochemistry, Iba-1, differential diagnosis
The incidence of spontaneous neoplasms in the central nervous system (CNS) is higher in rats (>1%) than in mice (>0.001%)1. The most common primary CNS neoplasms in rats are glial and meningeal neoplasms, and the common glial neoplasms in rats are astrocytomas, oligodendrogliomas and mixed gliomas1,2,3,4. In contrast, the incidence of malignant reticulosis derived from lymphoreticular cells5 is low in rats1,2,4,6.
Microscopically, astrocytomas in rats usually show diffuse proliferation of fusiform to round neoplastic cells displaying spindle to round nuclei and a moderate amount of eosinophilic cytoplasm with indistinct cell borders. Pseudopalisading of neoplastic cells around foci of hemorrhage or necrosis may occasionally occur. Astrocytomas occurs in the brain parenchyma, and the neoplastic cells frequently show perivascular and meningeal invasion1,2,4,5,6,7. Reticulin fiber formation may sometimes be encountered in astrocytomas8. With regard to malignant reticuloses in rats, the neoplasm is characterized by perivascular and subarachnoid infiltration of neoplastic cells having pale nuclei, with morphological characteristics resembling lymphocytic, plasmacytic, monocytic, reticular, or microglial cells2,5,9. Reticulin fibers are usually abundant in malignant reticulosis8,9.
In humans, immunohistochemical demonstration of glial fibrillary acidic protein (GFAP) is useful for confirming the diagnosis of astrocytomas, which are the most frequent subtype of adult glial neoplasms next to glioblastomas10. However, spontaneous astrocytomas in rats have been uniformly negative for GFAP1,4,6,8,11. Therefore, it is definitely difficult to confirm diagnosis of astrocytomas by means of GFAP immunohistochemistry. Because of the similarity in morphological features between malignant reticuloses and astrocytomas lacking oligodendrocytic cellular components, it is now important to establish cellular markers for malignant reticuloses. The present case report immunohistochemically examined spontaneous brain neoplasms primarily diagnosed as glial neoplasms using microglia and macrophage markers in rats.
Four spontaneous brain neoplasms were examined, one from a non-treated male Wistar Hannover (RccHanTM:WIST, Japan Laboratory Animals, Inc., Hanno, Japan) rat and three from male Sprague-Dawley (Crl:CD [SD], Charles River Laboratories Japan, Inc., Atsugi, Japan) rats administered orally 0.5% methyl cellulose. The animals were treated in accordance with the Guide for Animal Experimentation. None of the animals showed any neurological symptoms during clinical observation, and there were no abnormal findings at necropsy. The Wistar Hannover rat (Case 1) was euthanized by exsanguination under anesthesia and necropsied when the animal was 84 weeks old. One of the SD rats (Case 2), which became moribund at the age of 84 weeks, was euthanized by exsanguination under anesthesia. The two other SD rats (Cases 3 and 4) were found dead and necropsied at the age of 90 and 99 weeks, respectively. After necropsy, removed brains were fixed in 10% neutral buffered formalin, cut coronally for embedding in paraffin, sectioned and stained with hematoxylin and eosin (H&E) and by Watanabe’s silver impregnation method. For immunohistochemical examination, sections were incubated either with antibody against the ionized calcium binding adaptor molecule 1 (Iba-1, rabbit polyclonal, 1:200, Wako Pure Chemical Industries, Ltd., Osaka, Japan), CD68 (mouse monoclonal, ED1, 1:500, AbD Serotic, Oxford, UK), glial fibrillary acidic protein (GFAP, rabbit polyclonal, 1:500, Dako Japan Inc., Kyoto, Japan), Olig2 (rabbit polyclonal, 1:100, Immuno-Biological Laboratories Co., Ltd., Fujioka, Gunma, Japan), vimentin (mouse monoclonal, V9, 1:100, Dako Japan Inc.), neurofilament (mouse monoclonal, 2F11, 1:200, Dako Japan Inc.), CD8 (mouse monoclonal, OX-8, 1:100, AbD Serotic), and Ki-67 (mouse monoclonal, MIB-5, 1:50, Dako Japan Inc.). All these antibodies react or cross-react with the respective rat recognition proteins. Prepared tissue sections were incubated with the antibodies in a refrigerator overnight (Olig2) or at room temperature for 30 minutes (all others), and immunodetection was performed by the peroxidase-labeled polymer method using an EnVision+ kit (Dako Japan Inc.) or the labeled streptavidin-biotin method with an LSAB2 kit (Dako Japan Inc.) with 3,3’-diaminobenzidine/H2O2 as the chromogen. For assessment of the Ki-67-positive cell rates, we selected regions including large amounts of Ki-67-positive cells and calculated the percentage of Ki-67-positive cells among 500 neoplastic cells.
Histopathologically, the neoplasms were located in the piriform lobe and invaded into the meninges (Case 1), the hypothalamus and invaded into the cerebral cortex (Case 2), the piriform lobe (Case 3) and the hypothalamus (Case 4), respectively. There was no characteristic cellular arrangement in these neoplasms. The boundaries of the neoplasms and the surrounding normal brain parenchyma were not clear. The neoplastic cells were characterized by diffuse proliferation, abundant eosinophilic cytoplasm with indistinct cell borders and oval to round or pleomorphic small nuclei. Mitotic figures and apoptosis were scarcely observed in these neoplastic cells. Two cases (Case 1 and 2) showed neoplastic cell infiltration into the meninges or perivascular spaces. Staining with Watanabe’s silver impregnation method revealed no reticulin fibers in the stroma of any of these neoplasms.
Immunohistochemically, all four neoplasms were positive for Iba-1 and CD68 (Table 1, Figs. 1–4). In particular, almost all of the neoplastic cells were strongly positive for Iba-1, which is expressed by cells of the microglia/macrophage lineage and is specifically found in microglia but not in neurons, astrocytes or oligodendrocytes in the brain of rats12. In the case of CD68, positive neoplastic cells were sparsely observed. All cases were negative for GFAP, Olig2, vimentin and neurofilament. The Ki-67-positive cell rates were 4.8% (Case 1), 9.0% (Case 2), 12.6% (Case 3) and 0.4% (Case 4), respectively. On the basis of these results, we diagnosed all four neoplasms as malignant reticulosis. In the one SD rat case (Case 2) positive for CD8, the CD8-positive cells were diffusely localized in the neoplastic mass, and their cellular morphology was the same as the other neoplastic cells; therefore, we considered them to be neoplastic components. Neoplastic cells of the microglia/macrophage lineage may express the CD8 antigen; it has been reported that infiltration of CD8-positive microglias/macrophages was found in the lesions of traumatic brain injury13,14. This finding also supports the origin of this neoplasm to be microglias/macrophages.
Table 1. Immunohistochemical Staining Results of Four Spontaneous Brain Neoplasms in Rats.
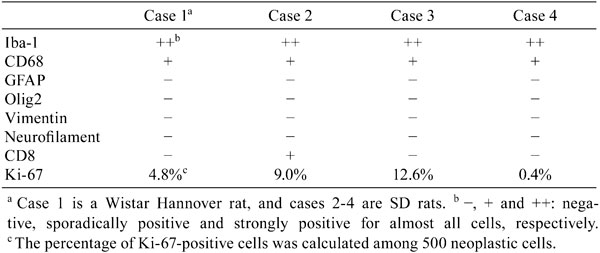
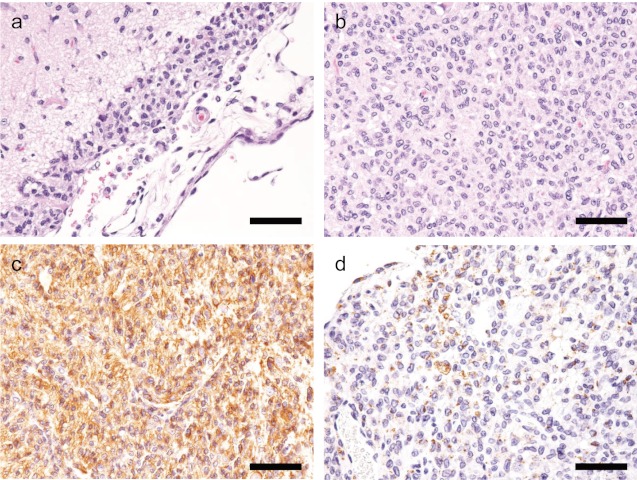
Fig 1.
H&E and immunohistochemical staining of Case 1 (Wistar Hannover rat).a: Neoplastic cells infiltrate into the meninges. H&E stain. Bar=50 μm. b: Neoplastic cells proliferate in the piriform lobe. H&E stain. Bar=50 μm. c: Neoplastic cells are strongly positive for Iba-1. Bar=50 μm. d: Neoplastic cells are sporadically positive for CD68. Bar=50μm.
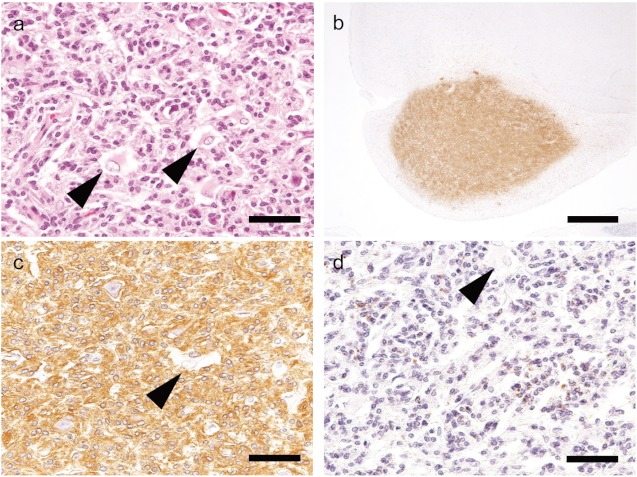
Fig 4.
H&E and immunohistochemical staining of Case 4 (SD rat).a: Neoplastic cells proliferate in the hypothalamus. H&E stain. Bar=50 μm. b: Iba-1 shows strong immunoreactivity in the region of neoplastic cell infiltration. Bar=1 mm. c: Neoplastic cells are strongly positive for Iba-1. Bar=50 μm. d: Neoplastic cells aresporadically positive for CD68. Bar=50 μm.
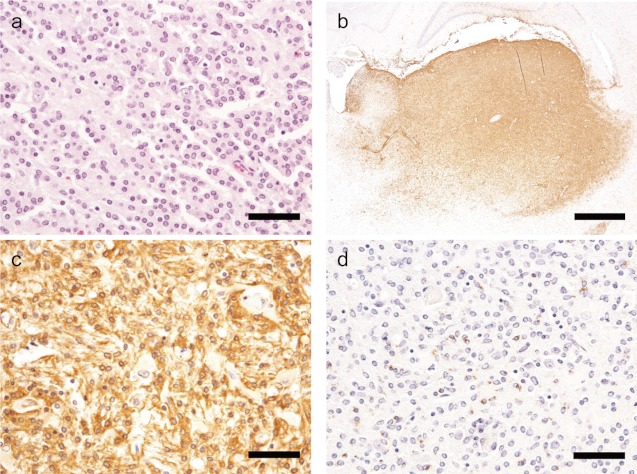
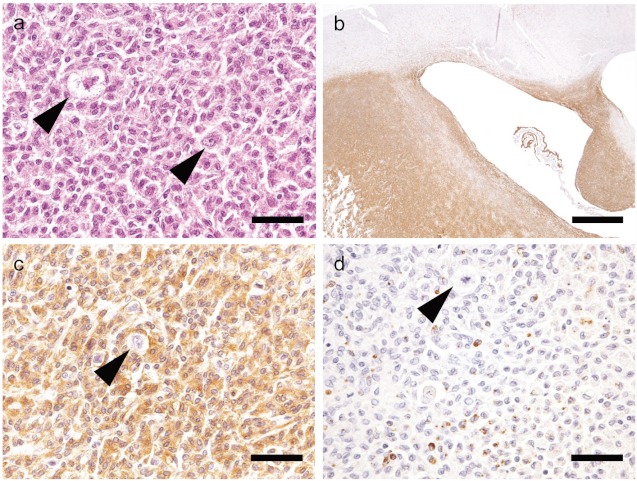
In all cases, small numbers of cells with moderately eosinophilic cytoplasm and large nuclei accumulated at the margin of the neoplasms. These cells were considered to be reactive astrocytes, because they were negative for Iba-1 and CD68 but positive for GFAP and vimentin. In addition, we observed a few large cells with eosinophilic granules in the cytoplasm with large nuclei in two cases of SD rats (Cases 2 and 3, Figs. 2 and 3), and none of the antibodies reacted with these cells. Appearance of granular cells has been reported in a case of malignant astrocytoma in an SD rat, but their origin was unidentified15. In the present study, we also observed this type of granular cells, but we also could not identify their cellular origin using an immunohistochemical approach.
Fig 2.
H&E and immunohistochemical staining of Case 2 (SD rat).a: Neoplastic cells proliferate in the cerebral cortex, and large granular cells are observed (arrow). H&E stain. Bar=50 μm. b: Iba-1 shows strong immunoreactivity in the region of neoplastic cell infiltration. Bar=1 mm. c: Neoplastic cells are strongly positive for Iba-1, but the large granular cells exhibit negative immunoreactivity (arrow). Bar=50 μm. d: Neoplastic cells aresparsely positive for CD68, but the large granular cellsexhibitnegative immunoreactivity (arrow). Bar=50 μm.
Fig 3.
H&E and immunohistochemical staining of Case 3 (SD rat).a: Neoplastic cells proliferate in the piriform lobe, and reactive astrocytes are observed (arrow). H&E stain. Bar=50 μm. b: Iba-1 shows strong immunoreactivityin the region of neoplastic cell infiltration. Bar=1 mm. c: Neoplastic cells are strongly positive for Iba-1, but reactive astrocytes are negative. Bar=50 μm. d: Neoplastic cells are sporadically positive for CD68, but the reactive astrocytes exhibit negative immunoreactivity. Bar=50 μm.
It has been reported that spontaneous astrocytoma-like brain neoplasms in the rat brain are stained positive for microglia/macrophage markers such as CD684,8, RM-48, RCA-14,16 and Iba-117. Brain glial neoplasms positive for these markers in rats might have a microglia/macrophage origin, although the radial glia lineage has also been suggested as a possible site of origin of the tumor8,17. However, radial glias have been proven to be stem cells for both neuronal and glial cell lineages18,19.
Glial neoplasms are rarely observed in rats in breeding condition younger than one year old, except in cases where the neoplasms have been induced by chemicals2. They are occasionally found in 2-year carcinogenicity studies in which neoplasms are routinely diagnosed solely with H&E-stained sections, and the immunohistochemical approach is seldom applied. However, in some cases, differentiation between the diagnoses of astrocytoma and malignant reticulosis is difficult in H&E-stained sections. Therefore, immunohistochemical staining using microglia/macrophage markers, such as Iba-1, may be helpful for the differential diagnosis of malignant reticuloses from astrocytomas in spontaneous brain neoplasms in rats.
Acknowledgments
The authors would like to thank Ms. Chieko Azuma for skillful technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Krinke GJ, Kaufmann W, Mahrous AT, and Schaetti P. Morphologic characterization of spontaneous nervous system tumors in mice and rats. Toxicol Pathol. 28: 178–192 2000. [DOI] [PubMed] [Google Scholar]
- 2.Zwicker GM, Eyster RC, Sells DM, and Gass JH. Spontaneous brain and spinal cord/nerve neoplasms in aged Sprague-Dawley rats. Toxicol Pathol. 20: 576–584 1992. [DOI] [PubMed] [Google Scholar]
- 3.Janisch W. Tumours of the central nervous system. In: Pathology of Tumours in Laboratory Animals Volume 1 - Tumours of the Rat, 2nd ed., VS Turusov, and U Mohr (eds). IARC, Lyon. 677-698. 1990 [PubMed] [Google Scholar]
- 4.Weber K, Garman RH, Germann PG, Hardisty JF, Krinke G, Millar P, and Pardo ID. Classification of neural tumors in laboratory rodents, emphasizing the rat. Toxicol Pathol. 39: 129–151 2011. [DOI] [PubMed] [Google Scholar]
- 5.Greaves P. Nervous System and Special Sense Organs. In: Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, Third Edition. Elsevier Inc., Oxford, UK. 861-933. 2007 [Google Scholar]
- 6.Walker VE, and Swenberg JA. Gliomas, Rat. In: Monographs on Pathology of Laboratory Animals. Nervous System. TC Jones, U Mohr, and RD Hunt (eds). Springer-Verlag, Berlin. 134-143. 1988 [Google Scholar]
- 7.Kaufmann W, Bolon B, Bradley A, Butt M, Czasch S, Garman RH, George C, Groters S, Krinke G, Little P, McKay J, Narama I, Rao D, Shibutani M, and Sills R. Proliferative and nonproliferative lesions of the rat and mouse central and peripheral nervous system. Toxicol Pathol. 40: 87S–157S 2012. [DOI] [PubMed] [Google Scholar]
- 8.Nagatani M, Ando R, Yamakawa S, Saito T, and Tamura K. Histological and immunohistochemical studies on spontaneous rat astrocytomas and malignant reticulosis. Toxicol Pathol. 37: 599–605 2009. [DOI] [PubMed] [Google Scholar]
- 9.Garman RH. Malignant Reticulosis, Rat. In: Monographs on Pathology of Laboratory Animals. Nervous System. TC Jones, U Mohr, and RD Hunt (eds). Springer-Verlag, Berlin. 117-123. 1988 [Google Scholar]
- 10.Larjavaara S, Mantyla R, Salminen T, Haapasalo H, Raitanen J, Jaaskelainen J, and Auvinen A. Incidence of gliomas by anatomic location. Neuro Oncol. 9: 319–325 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solleveld H, Gorgacz E, and Koestner A. Central Nervous System Neoplasms in the Rat. In: Guides for Toxologic Pathology. STP/ARP/AFIP, Washington, DC. 1991 [Google Scholar]
- 12.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, and Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research. 57: 1–9 1998. [DOI] [PubMed] [Google Scholar]
- 13.Popovich PG, Rooijen NV, Hickey WF, Preidis G, and McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Experimental Neurology. 182: 275–287 2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Artelt M, Burnet M, Trautmann K, and Schluesener HJ. Early infiltration of CD8+ macrophages/microglia to lesions of rat traumatic brain injury. Neuroscience. 141: 637–644 2006. [DOI] [PubMed] [Google Scholar]
- 15.Pruimboom-Brees IM, Brees DJ, Shen AC, and Ibebunjo C. Malignant astrocytoma with binucleated granular cells in a Sprague-Dawley rat. Vet Pathol. 41: 287–290 2004. [DOI] [PubMed] [Google Scholar]
- 16.Krinke GJ, and Germer M. Binding of lectin Ricinus communis agglutinin-1 (RCA-1) to rat brain tumors. Vet Pathol. 30: 300–303 1993. [DOI] [PubMed] [Google Scholar]
- 17.Kolenda-Roberts H, and Hardisty F. Immunohistochemical staining of spontaneous and chemically-induced brain tumors in the rat. Society of Toxicologic Pathology (STP), Annual Meeting. Abstract. 2011 [DOI] [PubMed]
- 18.Götz M, Hartfuss E, and Malatesta P. Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Research Bulletin. 57: 777–788 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gubert F, Zaverucha-do-Valle C, Pimentel-Coelho PM, Mendez-Otero R, and Santiago MF. Radial glia-like cells persist in the adult rat brain. Brain Research. 1258: 43–52 2009. [DOI] [PubMed] [Google Scholar]