Abstract
Uterine deciduomas were found in two female virgin rats, a 15-week-old Lewis rat and a 7-week-old Sprague-Dawley rat. The firm white nodules were located at the base of unilateral uterine horns and were approximately 6 mm and 4 mm in diameter. Histopathologically, the nodules were composed of three areas, each with a distinct type of proliferating cells: large epithelioid decidual cells with round nuclei, prominent nucleoli and abundant eosinophilic cytoplasm (antimesometrial region); compact spindle-shaped cells with oval nuclei and vacuolar cytoplasm (transitional region); and pleomorphic and spiny cells with round to oval nuclei and compact eosinophilic cytoplasm (mesometrial region). These cells proliferated in sheet-like arrangements and transformed into the other types of cells located in surrounding regions. Immunohistochemically, proliferating cells in all regions were strongly positive for proliferating cell nuclear antigen. The proliferating cells were positive for vimentin, and large decidual cells were positive for common acute lymphoblastic leukemia antigen 10, a marker of uterine interstitial cells. Large decidual cells were positive for α-smooth muscle actin and desmin, suggesting differentiation into muscular cells. Progesterone receptor was expressed in all cell types; however, estrogen receptor α was not expressed in the antimesometrial region. These extremely rare tumor-like nodules represent nonneoplastic lesions referred as decidual reactions of endometrial interstitial cells, and their biological behavior is that of a space-occupying benign tumor in young rats. Our cases might provide information as a historical control in toxicity and pharmacological studies in rats.
Keywords: deciduoma, decidual reaction, immunohistochemistry, rat, uterus
Spontaneous primary tumors and tumor-like lesions of the rodent uterus are extremely rare in young rodents. Hyperplastic lesions of decidual stromal cells, so-called decidual reactions/decidual tumors, have been found in the uterus or subserosal abdominal locations in rats1,2, mice3,4, guinea pigs5, rabbits6, dogs7, monkeys8, and humans9,10. These lesions are characterized by focally extensive areas of hyperplastic and hypertrophied eosinophilic, round, oval to somewhat elongated cells, which are thought to be endometrial stromal cells. Decidual stromal cells are derived from the fibroblast-like cells within the endometrium, which maintain their progesterone receptors in the presence of progesterone11. This proliferating lesion is often referred to as deciduoma and is thought to be a proliferative response of stromal endometrial cells that is histologically similar to decidual implantation sites2. This change is infrequently seen in young adult rats used in subacute toxicity studies, and the incidence of decidual reactions/deciduoma in young female F344 rats examined in National Toxicity Program studies is 1.3% (2 of 159 rats)12. Only 5 spontaneous cases of decidual reactions/deciduoma were identified in approximately 12000 Sprague-Dawley (SD) rats at 3- to 8-months of age13. In B6C3F1 mice used in National Toxicity Program studies, this change was seen in only one mouse3. Few studies on the immunohistochemical characterization of spontaneously occurring deciduoma have been reported13. We encountered two uterine deciduomas in a young Lewis rat and a SD rat and examined their histopathological and immunohistochemical features in the present study.
The experimental protocol and all animal procedures were approved by the Animal Care and Use Committee of Kansai Medical University and were in accordance with the university’s guidelines for animal experimentation. Case 1 was a 15-week-old female LEW/CrlCrlj (Lewis) rat, and Case 2 was a 7-week-old female Crl:CD (SD) rat. The 2 rats were purchased from Charles River Laboratories Japan (Kanagawa, Japan) and used in different mechanistic studies of chemical-induced toxicity and carcinogenesis. The rats were housed in plastic cages with paper-chip bedding (Paper Clean, SLC, Hamamatsu, Japan) in an air-conditioned room at 22 ± 2°C and 60 ± 10% relative humidity with a 12-h light/dark cycle and fed a commercial diet (CMF 30 Gy; Oriental Yeast, Chiba, Japan) and tap water ad libitum.
The rats were anesthetized with isoflurane (Forane®; Abbott Japan, Tokyo, Japan), sacrificed by exsanguinations via abdominal aortic transection, and subjected to complete necropsies. One small mammary carcinoma was seen in Case 1, but none were seen in Case 2. No abnormalities were observed except in the mammary glands macroscopically. The uterus and other reproductive organs were fixed overnight in 10% neutral buffered formalin. Samples of uterine nodules, the other uterine horns, vaginas, and ovaries were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). Sequential sections of nodules were stained with periodic acid Schiff (PAS). Immunohistochemistry was performed with antibodies to proliferating cell nuclear antigen (PCNA) to detect the proliferative activity14, pancytokeratin (CK) as an epithelial cell marker, vimentin as a mesenchymal cell marker15, the common acute lymphoblastic leukemia antigen 10 (CD10) as a uterine interstitial cell marker16, α-smooth muscle actin (SMA) and desmin as muscular cell markers15, S-100 as a Schwann cell and adipocyte marker17, and estrogen receptor α (ER) and progesterone receptor (PgR) to detect steroid hormone receptors18. Detailed staining protocols for all antibodies are listed in Table 1. Briefly, sections were deparaffinized, hydrated, and blocked for endogenous peroxidase. Heat-induced epitope retrieval was performed for all antibodies except SMA, desmin, and S-100. The antigen-antibody complexes were identified by using a streptavidin-biotin (LSAB) staining kit (Dako) according to the manufacturer’s instructions. The reaction products were visualized with 3-3’-diaminobenzidine tetrahydrochloride. The intensity of immune staining was scored as negative (–), weak (+), moderate (++), or strong (+++). Histopathological examination including immunohistochemical scoring was conducted by two toxicologic pathologists certified by the Japanese Society of Toxicologic Pathology and/or the International Academy of Toxicologic Pathology (A. T. and K.Y.), according to the previously defined histopathological terminology and diagnostic criteria (goRENI, http://www.goreni.org/; Leininger and Jokinen, 1990).
Table 1. Primary Antibodies and Reaction Conditions for Immunohistochemistry.
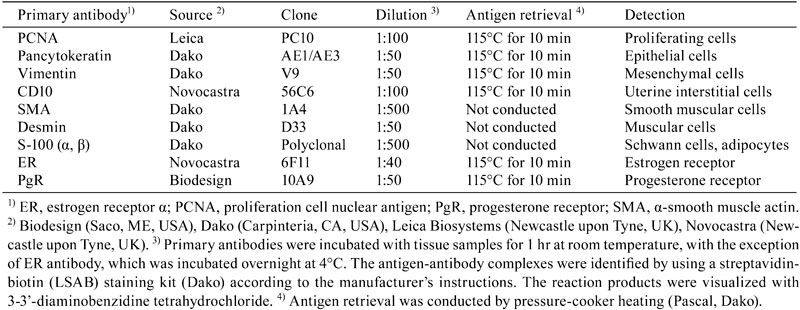
A solitary uterine nodule was detected in only one rat of approximately 100 animals used in each study. The white and firm solitary nodules were found in both rats at the base of unilateral uterine horns and were approximately 6 mm and 4 mm in diameter. Histopathologically, these nodules had collapsed the uterine lumens with erosion of the overlying endometrial surface and hemorrhage (Fig. 1a, 1b). The nodules contained 3 areas of different proliferating cell types. Large epithelioid giant cells with round nuclei containing coarsely vesicular chromatin in large oval nuclei with one to four variably shaped nucleoli and abundant eosinophilic and coarsely granular cytoplasm proliferated in the antimesometrial side of the uterus (decidual cells) (Fig. 2a). Occasionally, multinucleate or binucleate cells were seen in this region. A small number of large cells possessed abundant PAS-positive granules in this region (data not shown). The mesometrial region contained pleomorphic and spiny cells with round to oval nuclei and abundant coarse clear cytoplasmic vacuoles (Fig. 2c). Small spindle-shaped cells with oval nuclei and finely vacuolar cytoplasm were located between both regions (transitional region) (Fig. 2b). These cells proliferated in sheet-like arrangements and transformed into the other cell types located in surrounding regions. The cells of the nodules were supported by a fine fibrovascular stroma with small congested blood vessel profiles (Fig. 2c). On the antimesometrial side, there was an expansile, partially encapsulated nodular mass composed of three poorly defined layers: the capsule, the basal zone with residual endometrial glands, and the myometrium (Fig. 2d). No abnormal changes were detected histopathologically in the other uterine horns, vaginas, and bilateral ovaries of these rats (data not shown).
Fig. 1.
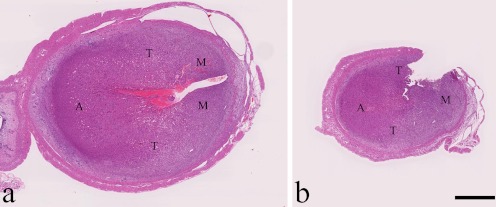
Solitary nodules in the uterus of Case 1, a 15-week-old Lewis rat, (a) and Case 2, a 7-week-old SD rat (b). Note the collapsed uterine lumens. The antimesometrial region (A), transitional region (T), and mesometrial region (M) are shown. HE stain, bar = 1 mm.
Fig. 2.
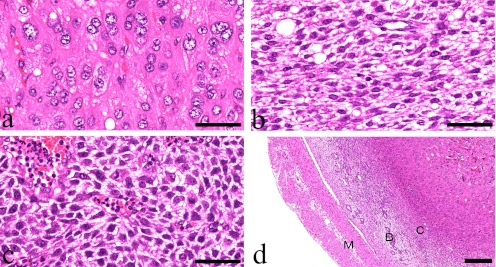
Histopathological findings. (a) Large epithelial-like decidual cells can be seen in the antimesometrial region. Case 1, HE stain, bar = 50 μm. (b) Spindle cells with vacuolar cytoplasm and prominent intercellular spaces can be seen in the transitional region. Case 1, HE stain, bar = 50 μm. (c) Starry-formed and spiny cells with vacuolar cytoplasm cam be seen in the mesometrial region. Case 2, HE stain, bar = 50 μm. (d) The outside of this nodule is composed of the myometrium (M), basal zone (B), and capsule (C). A residual endometrial gland can be seen in the basal zone. Case 1, HE stain, bar = 200 μm.
The results of the immunohistochemical analysis are summarized in Table 2. The nuclei of most decidual cells in both rats were strongly positive for PCNA in all three regions (Fig. 3a), showing highly proliferative activity of the nodules. The tumor cells in all regions were also strongly positive for vimentin, and the cytoplasm of large decidual cells was weakly positive for CD10, showing that the tumor cells originated from uterine interstitial cells (Fig. 3b). The cytoplasm of these cells was also moderately or strongly positive for SMA and desmin, probably suggesting differentiation into muscular cells (Fig. 3c, 3d). ER was expressed weakly to strongly in the nuclei of compact spindle-shaped cells and starry-formed cells in the transitional and mesometrial regions (Fig. 3e); however, no signals were seen in the antimesometrial region. In contrast, PgR was expressed moderately to strongly in the nuclei of all types of decidual cells in the three regions (Fig. 3f). No proliferative cells were positive for S-100 in any regions. The cells comprising the capsule and myometrium were weakly to moderately positive for vimentin, SMA, desmin, ER, and PgR. Endometrial glands in the basal zone were positive for CK and ER, which was similar to the normal endometrial gland (data not shown).
Table 2. Immunohistochemical Expression of Antigens in the Nodules.
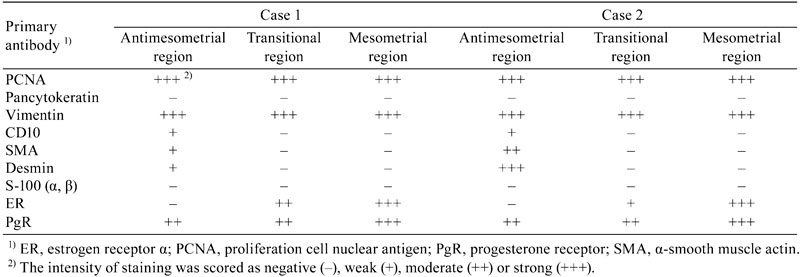
Fig. 3.
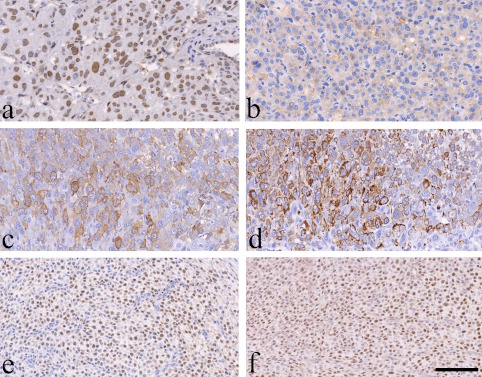
Immunohistochemical characteristics. PCNA signals can be seen in most nuclei of the decidual cells in the antimesometrial region of Case 2 (a). CD10 is expressed weakly in the cytoplasm of the decidual cells in the antimesometrial region of Case 1 (b). The cytoplasm of decidual cells is moderately to strongly positive for SMA (c) and desmin (d) in the antimesometrial region of Case 2. The nuclei of most proliferative cells are diffusely positive for ER (e) and PgR (f) in the mesometrial region of Case 1. Bar = 100 μm.
In two virgin rats, single and discrete round nodules were located in the unilateral uterine horns. They had a high level of structural organization due to the proliferation of large epithelioid cells with large round nuclei, prominent nucleoli and abundant eosinophilic cytoplasm, which are the characteristics typical of decidual cells. Histopathological findings revealed hyperplasia of decidual stromal cells, referred to as decidual reactions/deciduomas, in the rodent uterus2,19. Spontaneously occurring deciduoma is infrequently seen in young adult rats. Deciduomas can be induced in rats by mechanical irritation or trauma; electrical stimulation; intrauterine instillation of sesame oil, Hank’s balanced salt solution, histamine, air, or prostaglandins; and the administration of progestin13,20. In rats, the life history of an artificially induced deciduoma may be divided into three stages: (1) proliferation of stromal cells and their differentiation into decidual cells during days 4 to 8 of pseudopregnancy; (2) maintenance of the deciduoma, a stage in which there is no further increase in decidual weight, during days 9 to 11 of pseudopregnancy; and (3) regression of the decidual tissue during days 12-16 of pseudopregnancy21. The life cycle of spontaneously occurring deciduomas is not different from the induced lesions13. Our two cases are considered to be spontaneously occurring lesions because the rats had no history of mechanical irritation or trauma to the uterus or systemic hormonal effects and the lesions were detected in only one of approximately one hundred rats used in each study.
Immunohistochemically, the expression of vimentin and desmin increases in rat decidual cells in both in vitro and in vivo models, as these proteins are valid markers of stromal cell differentiation22,23. During the implantation stage of the normal rat pregnancy, the localization of vimentin is widespread in decidual cells, while desmin is present densely in decidual cells of the antimesometrial region where implantation occurs24. In our cases, the proliferative decidual cells showed strong and diffuse expression of vimentin; however, desmin was expressed in the decidual cells in the antimesometrial region. Similar to the expression of desmin, SMA was expressed in the decidual cells of the antimesometrial region, suggesting myogenic or myofibroblastic differentiation25. In our deciduomas, large decidual cells at the antimesometrial region also weakly expressed CD10, a marker for uterine interstitial cells16,26. Although normal endometrial stromal cells are positive for CD10 in humans16, these signals were not detected in the normal endometrial stromal cells in rats.
ER and PgR are expressed in the myometrium of the rat during the decidualization process and in spontaneously occurring deciduomas21. Progesterone and small amounts of estrogen are required for the sensitization of the uterus before a decidualizing stimulus. Continuous presence of progesterone is essential and adequate for proliferation of the decidual cells and for the growth and maintenance of the deciduoma21. In our cases, PgR and ER were expressed strongly in the mesometrial region; however, ER was not expressed in the antimesometrial region. According to a previous report27, the involvement of both receptors may suggest that as the decidual cells become differentiated, they become enriched in PgR and impoverished in ER.
Although Lewis rats and SD rats are frequently used in pharmacological studies and toxicity studies, respectively, historical control data for uterine decidual lesions in young rats of both strains have not been well reported. In our cases, proliferating decidual cells expressed PCNA, vimentin, CD10, SMA, and desmin. PgR was expressed in all cell types, but ER was not expressed in the antimesometrial region. Although additional immunohistochemical analyses are needed to understand the biology of spontaneously occurring deciduomas, our two cases might provide valuable information as a historical control for Lewis and SD rats.
Acknowledgments
This study was supported in part by a Grant-in Aid for Scientific Research C from the Japan Society for the Promotion of Science (22591954). We thank Ms. T. Akamatsu for her technical assistance and Dr. T. Sasaki, Maruho Co. Ltd., for her excellent scientific advice. All authors read and approved the final manuscript. We declare that we have no competing financial interests.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Bryant CM. Wednesday Slide Conference 2009-2010 Conference 7. The Armed Forces Institute of Pathology, Department of Veterinary Pathology. 1–6. 2009
- 2.Leininger JR, and Jokinen MP. Oviduct, uterus and vagina. In: Pathology of the Fischer Rat: Reference and Atlas. Boorman GA, Eustis SL, Elwell MR, Montgomery Jr. CA, and MacKenzie WF (eds.). Academic Press, London. 450-452. 1990 [Google Scholar]
- 3.Davis BJ, Dixon D, and Herbert RA. Decidual reaction. In: Pathology of the Mouse. Ovary, Oviduct, Uterus, Cervix, and Vagina. Maronpot RR, Boorman GA, and Gaul BW (eds). Cache River Press, Vienna. 436. 1999 [Google Scholar]
- 4.Karbe E. “Mesenchymal tumor” or “decidual-like reaction”? Toxicol Pathol. 27: 354–362 1999. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JA, and Garris DR. Deciduoma formation in response to uterine trauma in the guinea pig. Biol Reprod. 19: 1135–1141 1978. [DOI] [PubMed] [Google Scholar]
- 6.Cooper TK, Adelsohn D, and Gilbertson SR. Spontaneous deciduosarcoma in a domestic rabbit (Oryctolagus cuniculus). Vet Pathol. 43: 377–380 2006. [DOI] [PubMed] [Google Scholar]
- 7.Koguchi A, Nomura K, Fujiwara T, Kawai Y, and Okaniwa A. Maternal placenta-like endometrial hyperplasia in a beagle dog (Canine deciduoma). Exp Anim. 44: 251–253 1995. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh D, Bell SC, and Sengupta J. Immunohistological localization of insulin-like growth factor binding protein-1 in primary implantation sites and trauma-induced deciduomal tissues of the rhesus monkey. Placenta. 25: 197–207 2004. [DOI] [PubMed] [Google Scholar]
- 9.Carter D, True L, and Otis CN. Serous membranes. In: Histopathology for Pathologists. Mills SE (ed). Lippincott Williams & Wilkins, Philadelphia. 559, 2007 [Google Scholar]
- 10.Hendrickson MR, Atkins KA, and Kempson RL. Uterus and fallopian tubes. In: Histopathology for Pathologists. Mills SE (ed). Lippincott Williams & Wilkins, Philadelphia. 559. 2007 [Google Scholar]
- 11.Dunn CL, Kelly RW, and Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 7: 151–161 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dixon D, Heider K, and Elwell MR. Incidence of nonneoplastic lesions in historical control male and female Fischer-344 rats from 90-day toxicity studies. Toxicol Pathol. 23: 338–348 1995. [DOI] [PubMed] [Google Scholar]
- 13.Elcock LH, Stuart BP, Mueller RE, and Hoss HE. Deciduoma, uterus, rat. In: Monographs on Pathology of Laboratory Animals: Genital System. Jones TC, Mohr U, and Hunt RD (eds). Springer-Verlag, Berlin. 140-145. 1987 [Google Scholar]
- 14.Sasaki T, Yoshizawa K, Kinoshita Y, Miki H, Kimura A, Yuri T, Uehara N, and Tsubura A. Spontaneous occurring intracranial lipomatous hamartoma in a young BALB/c mouse and a literature review. J Toxicol Pathol. 25: 179–182 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizawa K, Oishi Y, Makino N, Suzuki J, Matsumoto M, Fukuhara Y, Fujihira S, and Fujii T. Congenital mesoblastic nephroma in a young beagle dog. J Toxicol Pathol. 9: 101–105 1996. [Google Scholar]
- 16.Toki T, Shimizu M, Takagi Y, Ashida T, and Konishi I. CD10 is a marker for normal and neoplastic endometrial stromal cells. Int J Gynecol Pathol. 21: 41–47 2002. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa K, Oishi Y, Makino N, Suzuki J, Matsumoto M, Yamauchi K, Fujihira S, and Fujii T. Malignant schwannoma of the intracranial trigeminal nerve in a 19-week-old female Sprague-Dawley rat. J Toxicol Pathol. 9: 107–112 1996. [Google Scholar]
- 18.Yuri T, Lai YC, Kanematsu S, Kuwata M, Yoshizawa K, and Tsubura A. Effects of short-term estrogen treatment on the progression of N-methyl-N-nitrosourea-induced premalignant mammary lesions in female Lewis rats. Med Mol Morphol. 44: 125–130 2011. [DOI] [PubMed] [Google Scholar]
- 19.goRENI. Global Open Registry Nomenclature Information System. http://www.goreni.org/
- 20.Finn CA, and Keen PM. The induction of deciduomata in the rat. J Embryol Exp Morph. 11: 673–682 1963. [PubMed] [Google Scholar]
- 21.Peleg S, Bauminger S, and Lindner HR. Oestrogen and progestin receptors in deciduoma of the rat. J Steroid Biochem. 10: 139–145 1979. [DOI] [PubMed] [Google Scholar]
- 22.Glasser SR, and Julian J. Intermediate filament protein as a marker of uterine stromal cell decasualization. Biol Reprod. 35: 463–474 1986. [DOI] [PubMed] [Google Scholar]
- 23.Glasser SR, Lampelo S, Munir MI, and Julian J. Expression of desmin, laminin and fibronectin during in situ differentiation (decidualization) of rat uterine stromal cells. Differentiation. 35: 132–142 1987. [DOI] [PubMed] [Google Scholar]
- 24.Korgun ET, Cayli S, Asar M, and Demir R. Distribution of laminin, vimentin and desmin in the rat uterus during initial stages of implantation. J Mol Histol. 38: 253–260 2007. [DOI] [PubMed] [Google Scholar]
- 25.Oliver C, Montes MJ, Galindo JA, Ruiz C, and Olivares EG. Human decidual stromal cells express α-smooth muscle actin and show ultrastructural similarities with myofibroblasts. Hum Reprod. 14: 1599–1605 1999. [DOI] [PubMed] [Google Scholar]
- 26.Klemmt PAB, Carver JG, Kennedy SH, Koninckx PR, and Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 85: 564–572 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martel D, Monier MN, Psychoyos A, and DeFeo VJ. Estrogen and progesterone receptors in the endometrium, myometrium, and metrial gland of the rat during the decidualization process. Endocrinology. 114: 1627–1634 1984. [DOI] [PubMed] [Google Scholar]


