Abstract
Abstract: Background data during the gestation period were obtained from 128 Wistar Hannover GALAS rats and 26 Crl:CD(SD) pregnant rats in the control groups of our previous toxicity studies. The body weights of dams in the Wistar Hannover GALAS rats were significantly lower throughout the gestation period than those in the Crl:CD(SD) rats. In contrast, the time-dependent change in the body weight gain (%) of dams showed very similar trends in both strains. The mean number of live embryos/fetuses in the Wistar Hannover GALAS rats was 12.0, and was lower than that (14.5) in the Crl:CD(SD) rats. The placental weights gradually increased with pregnancy progression and reached a plateau on gestation day (GD) 19, although the embryo/fetal weights rapidly increased from GD 17 to GD 21. The embryo/fetal weights in the Wistar Hannover GALAS rats were significantly lower on only GD 21 than those in the Crl:CD(SD) rats. It is considered that this fetal weight difference between the strains develops during the fetal period, but not during the organogenesis period. In contrast, there were no differences in the placental weights between the two strains. Microscopically, the thickness of the labyrinth zone in the Wistar Hannover GALAS rats was thicker throughout the gestation period than that in the Crl:CD(SD) rats.
Keywords: background data, Crl:CD(SD), development, rat, Wistar Hannover GALAS
Reproductive and developmental toxicity studies in rats are necessary for safety evaluation of pharmaceutical drugs, pesticides and food additives. There have been many reports on background data on gestation day (GD) 21 in pregnant rats1,2,3,4,5,6. However, there have been few reports on background data of cesarean section during the gestation period7. Therefore, we put the data concerning fetal and placental development from the pregnant rats in the control groups in our previous 3 fetal toxicity studies8,9,10 and 7 placental toxicity studies11,12,13,14,15,16,17 together in order to obtain background data during the gestation period in Wistar Hannover GALAS rats and Crl:CD(SD) rats. In addition, we compared the developmental parameters between the two strains.
These data were obtained from 128 Wistar Hannover GALAS and 26 Crl:CD(SD) pregnant rats that were the control animals in the above mentioned 10 toxicity studies and a few other toxicity studies. The Wistar Hannover GALAS rats (CLEA Japan, Inc., Japan) and Crl:CD(SD) rats (Charles River Laboratories Japan, Inc., Japan) were purchased at approximately 10-14 weeks of age. A female rat was housed together with a male rat of the same strain and source for mating. The occurrence of copulation was established by daily inspection for a vaginal plug. GD 0 was designated as the day when the presence of vaginal plug was identified. The maternal animals were housed individually in plastic cages on softwood chip bedding in an air-conditioned room (22 ± 2ºC; humidity, 55 ± 10%; light cycle, 12 hr/day). Feed (CRF-1, Oriental Yeast Co., Ltd., Japan) and water were available ad libitum. Maternal body weights were recorded on GDs 0, 6–17, 19, and/or 21. The dams were sampled on GDs 11, 13, 15, 16, 17, 19, and 21 in the Wistar GALAS Hannover rats and on GDs 13, 15, 17, and 21 in the Crl:CD(SD) rats. The dams were euthanized by exsanguination under anesthesia and necropsied. All embryos/fetuses were removed from the placentas. About 1/2 to 1/3 of the placentas were separated between the basal zone and the decidua basalis, and removed from the uterus wall. The embryos/fetuses and removed placentas were weighed, and the embryo/fetal-placental weight ratio was calculated individually. Some placentas in the Crl:CD(SD) rats were measured along the major axis and minor axis, and for thickness. The fetuses on GD 21 were macroscopically examined for external malformations. All placentas were fixed in 10% neutral buffered formalin. Four placentas per dam were obtained randomly from the live embryos/fetuses. The selected placentas were embedded in paraffin, sectioned at a thickness of 4-µm, and stained routinely with hematoxylin and eosin (H&E). The thicknesses of the labyrinth zone, basal zone, decidua basalis and metrial gland close to the central portion were measured in placentas from each dam with the aid of an image analyzer (WinROOF, Mitani Corporation, Japan, or IPAP, Processor for Analytical Pathology, Sumika Technoservice Corporation. Japan).
Means and standard deviations (SD) of the individual litter values were calculated. Comparison between the Wistar Hannover GALAS rats and the Crl:CD(SD) rats was analyzed with the Leven’s test. When variances were homogeneous, the Student’s t-test was performed. The Aspin-Welch t-test was performed when variances were not homogeneous. Comparison of parameters between GD 21 and other sampling points was analyzed with the Bartlett’s test. When variances were homogeneous, the Dunnett’s multiple comparison test was performed. The Steel’s multiple comparison test was performed when variances were not homogeneous. The levels of significance were set at P<0.05 and P<0.01. All these experiments were conducted according to the Guidelines for Animal Experimentation, Japanese Association for Laboratory Animal Science, 1987.
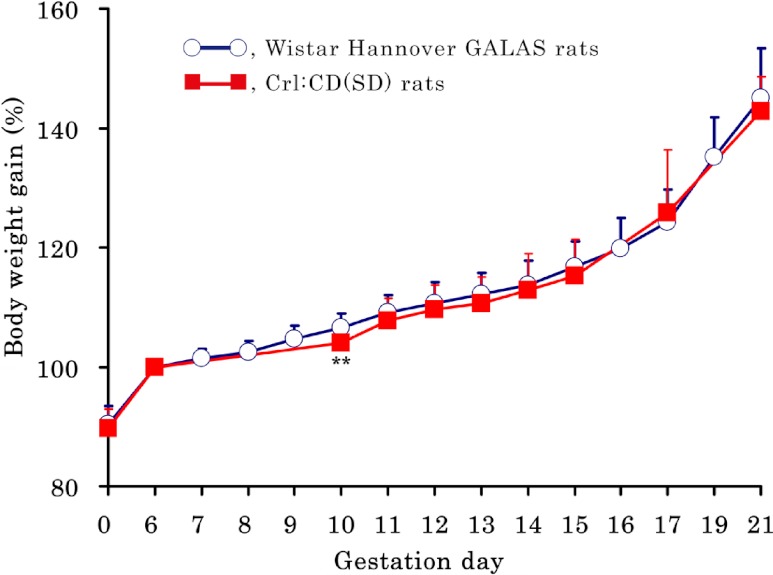
There were no notable clinical signs in any dams during the gestation period. The body weights and body weight gains (%) of dams (based on the body weight on GD 6 as 100%) are shown in Table 1 and Fig. 1, respectively. The body weights of dams in the Wistar Hannover GALAS rats were significantly lower throughout the gestation period than those in the Crl:CD(SD) rats. In contrast, the time-dependent change in the body weight gain (%) of dams showed very similar trends in both strains, although there was a significant transient increase on GD 10 in the Wistar Hannover GALAS rats, as compared with the Crl:CD(SD) rats.
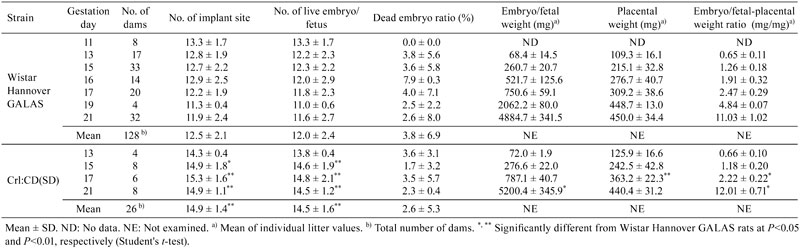
Table 1. Body Weight of Dams During the Gestation Period (g).
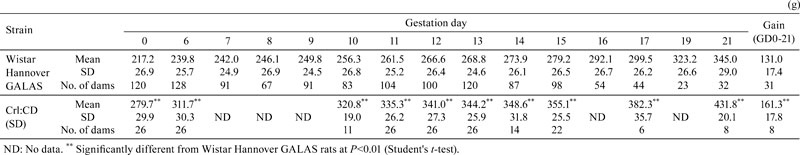
Fig. 1.
Body weight gain (%) of dams during the gestation period. The body weight gain (%) of dams shows very similar trends in both strains, although there is a significant increase on GD 10 in the Wistar Hannover GALAS rats, as compared with the Crl:CD(SD) rats. Each value represents the mean ± SD.** Significantly different from Wistar Hannover GALAS rats at P<0.01 (Student-t test).
The findings at cesarean section are shown in Table 2. There were no external abnormalities in the fetuses of both strains. The mean numbers of implantation sites and live embryos/fetuses during the gestation period were 12.5 and 12.0 in the Wistar Hannover GALAS rats, and 14.9 and 14.5 in the Crl:CD(SD) rats, respectively. Both parameters in the Wistar Hannover GALAS rats were significantly lower than those in the Crl:CD(SD) rats. The mean of the dead embryo/fetus ratio during the gestation period was 3.8% in the Wistar Hannover GALAS rats and 2.6% in the Crl:CD(SD) rats, with no significant difference between the two strains. In addition, there were no significant differences in these 3 parameters between GD 21 and other sampling points in both strains.
Table 2. Observation at Cesarean Section of Dams During the Gestation Period.
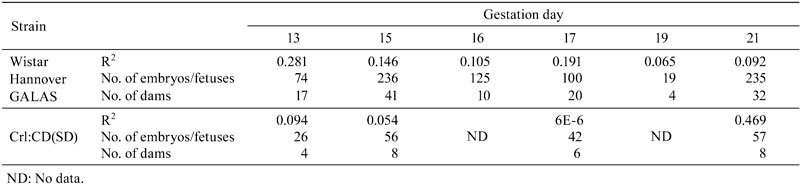
The placental weights gradually increased with pregnancy progression and reached a plateau on GD 19, although the embryo/fetal weights rapidly increased from GD 17 to GD 21. The embryo/fetal weights and embryo/fetal-placental weight ratio in the Wistar Hannover GALAS rats were significantly lower on only GD 21 than those in Crl:CD(SD) rats. In other background data2,3,4,5,6, the fetal weights on GD 21 in the Crl:CD(SD) rats are more than 5 g (5.29-5.56 g in males and 5.06-5.30 g in females), and are also heavier than those in the Wistar Hannover GALAS rats in the present study. In addition, it has been reported that the fetal weights on GD 20 in the Crl:CD(SD)BR rats are more than those in Wistar Hannover (Tac:Glx:WIfBR) rats18. Therefore, we conclude that the embryo/fetal weights in the Wistar Hannover GALAS rats are lower at parturition than those in the Crl:CD(SD) rats. Furthermore, this fetal weight difference between the strains develops during the fetal period, but not during the organogenesis period. In contrast, the placental weights and embryo/fetal-placental weight ratio in the Wistar Hannover GALAS rats were significantly lower and higher on GD 17 than those in the Crl:CD(SD) rats, respectively. However, since these changes were transient, it appeared that there were no differences in the placental weights between the strains. The relationships between the embryo/fetal weights and the placental weights are shown in Table 3. In the present rat study, no clear correlation between them was detected at any sampling points under conditions with no distinction of gender, although it has been reported that a strong relationship is observed between the fetal and placental weights in humans19. This could be attributed to the fact that no dwarf fetus or small placenta was included in the present study and that the embryo/fetal and placental weights were within the range of normal variability.
Table 3. Squared Correlation Coefficients (R2) Between Embryo/Fetal Weights and Placental Weights.
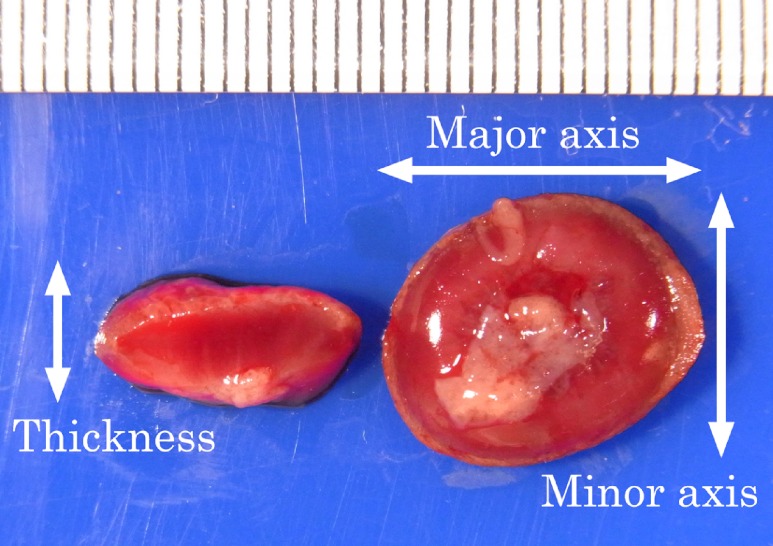
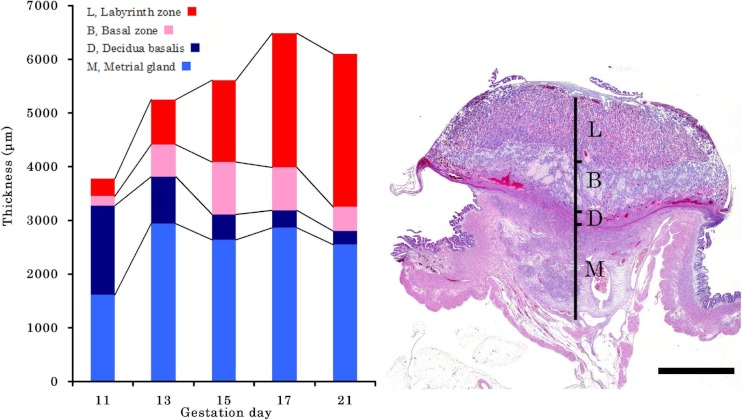
Macroscopically, the diameter and thickness of the placenta in the Crl:CD(SD) rats are shown in Table 4 and Fig. 2. The thickness of the placenta reached a plateau on GD 17, although the diameter gradually increased until GD 21. Histologically, the thickness of each layer of the placenta is shown in Table 5 and Fig. 3. The placenta is composed of the fetal part and maternal part20. The fetal part of the placenta consists of the labyrinth zone and basal zone. The basal zone was fully developed on GD 15, leading to regression gradually before parturition. The labyrinth zone was developed with advancing pregnancy, and formed the majority of the fetal part of the placenta. The maternal part of the placenta consists of the decidua and metrial gland. The decidua basalis underwent regression after GD 11. The metrial gland was fully developed on GD 13 and was maintained until parturition. The metrial gland formed the majority of the maternal part of the placenta. The labyrinth zone in the Wistar Hannover GALAS rats was thicker throughout the gestation period than that in the Crl:CD(SD) rats, which appeared to result from strain differences. However, it appeared that there was no difference in the total volume of the labyrinth zone between the Crl:CD(SD) rats and the Wistar Hannover GALAS rats, because the placental weights, which mainly consisted of the labyrinth zone and basal zone, were almost the same. Therefore, it is considered that the difference in the thickness of the labyrinth zone between them is not a major cause of the strain difference in reproductive and developmental toxicity. In contrast, the basal zone and decidua basalis in the Wistar Hannover GALAS rats were significantly thicker on GD 21 and thinner on GD13, as compared with those in the Crl:CD(SD) rats, respectively. Since these changes in the basal zone and decidua basalis seemed to be transient, it is necessary to examine many more placentas in the Crl:CD(SD) rats.
Table 4. Macroscopic Size of the Placenta During the Gestation Period.
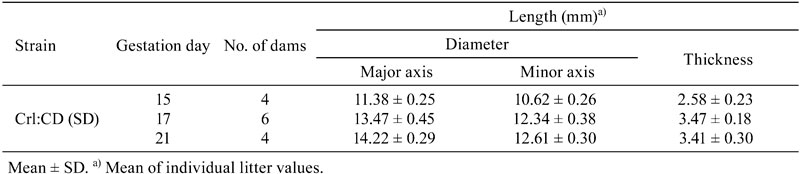
Fig. 2.
Gross appearance of the placenta on GD 21.
Table 5. Thickness of Each Layer of the Placenta During the Gestation Period.
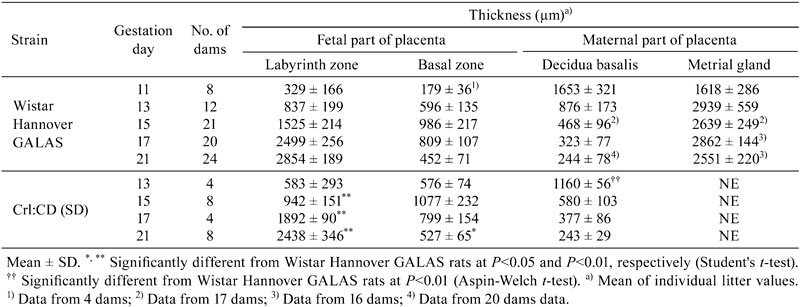
Fig. 3.
Time-dependent change in thickness of each placental layer in Wistar Hannover GALAS rats and microscopic appearance of the placenta on GD 15. H&E stain, Bar=2000 μm. The labyrinth zone developes with advancing pregnancy. The basal zone is fully developed on GD 15, leading to regression gradually before parturition. The decidua basalis undergoes regression after GD 11. The metrial gland is fully developed on GD 13, and is maintained until parturition.
In conclusion, this study indicated that there were some differences in parameters between the Wistar Hannover GALAS rats and the Crl:CD(SD) rats. The data in the present study would contribute to evaluate the results in reproductive and developmental toxicity studies in both rats. Further accumulation of background data during the gestation period should be performed for detailed consideration and better understanding of the mode of action in chemical-induced developmental toxicity.
Acknowledgments
The authors would like to thank Mr. Akihisa Endo (CLEA Japan, Inc.) for valuable advice, and Mr. Kiyoshi Kobayashi, Ms. Kaori Maejima, Ms. Hiromi Asako, Mr. Atsushi Funakoshi, and Mr. Yoshinori Tanaka for their excellent technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
Refrences
- 1.Aoyama H, Kikuta M, Shirasaka N, Hojo H, Takahashi KL, Shimizu N, Harigae M, Taguchi F, and Teramoto S. Historical control data on reproductive abilities and incidences of spontaneous fetal malformations in Wistar Hannover GALAS rats. Congenit Anom (Kyoto). 42: 194–201 2002. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, and Sugimoto S. Accumulation of background Data in Crj: CD (SD) IGS rats on reproductive and developmental toxicity study. In: Biological Reference Data on CD(SD) IGS Rats-1998. CD(SD)IGS Study Group (ed). Yokohama. 164-166. 1998 [Google Scholar]
- 3.Sakimura M, Hisada T, Zhou Y, Ohiwa K, and Horimoto M. Comparison between Crj:CD(SD)IGS and Slc:SD rats in reproductive parameters: Embryo-fetal development. In: Biological Reference Data on CD(SD) IGS Rats-1999. CD(SD)IGS Study Group (ed). Yokohama. 176-181. 1999 [Google Scholar]
- 4.Fujioka M, Yoshioka M, Chihara K, Funabashi H, and Matuoka N. Background control data of reproductive and developmental parameters in Crj:CD(SD)IGS rats. In: Biological Reference Data on CD(SD) IGS Rats-2000. CD(SD)IGS Study Group (ed). Yokohama. 126-130. 2000 [Google Scholar]
- 5.Mako T, Kato M, Fukushima T, Isogai Y, Matsui J, Horimoto M, Hamada Y, and Horii I. Historical control data of skeletal findings in Crj:CD(SD)IGS rat fetuses–Bone and cartilage alterations-. In: Biological Reference Data on CD(SD) IGS Rats-2002/2003. CD(SD)IGS Study Group (ed). Yokohama. 152-155. 2003 [Google Scholar]
- 6.Fujii Y, Furuyama Y, Kohayakawa Y, and Goto K. A comparison of incidence of lumber ribs between Crj:CD(SD)IGS and Crj:CD(SD) rats in developmental studies. In: Biological Reference Data on CD(SD) IGS Rats-2002/2003. CD(SD)IGS Study Group (ed). Yokohama. 168-172. 2003 [Google Scholar]
- 7.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Histological expression of metallothionein in the developing rat placenta. J Toxoicol Pathol. 21: 223–227 2008. [Google Scholar]
- 8.Furukawa S, Usuda K, Abe M, and Ogawa I. Histopathological findings of cleft palate in rat embryos induced by triamcinolone acetonide. J Vet Med Sci. 66: 397–402 2004. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa S, Abe M, Usuda K, and Ogawa I. Indole-3-acetic acid induces microencephaly in rat fetuses. Toxicol Pathol. 32: 659–667 2004. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa S, Usuda K, Abe M, and Ogawa I. Effect of indole-3-acetic acid derivatives on neuroepithelium in rat embryos. J Toxicol Sci. 30: 165–174 2005. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Busulfan-induced apoptosis in rat placenta. Exp Toxicol Pathol. 59: 97–103 2007. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Effect of 6-mercaptopurine on rat placenta. J Vet Med Sci. 70: 551–556 2008. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. Histopathological effect of ketoconazole on rat placenta. J Vet Med Sci. 70: 1179–1184 2008. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa S, Hayashi S, Hayashi S, Usuda K, Abe M, and Ogawa I. The relationship between fetal growth restriction and small placenta in 6-mercaptopurine exposed rat. Exp Toxicol Pathol. 63: 89–95 2011. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. The impairment of metrial gland development in tamoxifen exposed rats. Exp Toxicol Pathol. 64: 121–126 2012. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, and Ogawa I. Effect of cisplatin on rat placenta development. Exp Toxicol Pathol. 65: 211–217 2013. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Kuroda Y, and Ogawa I. Effect of estrogen on rat placental development depending on gestation stage. Exp Toxicol Pathol. (in press). [DOI] [PubMed]
- 18.Liberati TA, Roe BJ, and Feuston MH. An oral (gavage) control embryo-fetal development study in the Wistar Hannover rat. Drug Chem Toxicol. 25: 109–130 2002. [DOI] [PubMed] [Google Scholar]
- 19.Cetin I, and Taricco E. Clinical causes and aspects of placental insufficiency. In: The Placenta and Human Developmental Programming, 1st ed. Burton GJ, Barker DJP, Moffett A, Thornburg K (eds). Cambridge University Press. 114-125. 2011 [Google Scholar]
- 20.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. Toxicological pathology in rat placenta. J Toxicol Pathol. 24: 95–111 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]