Abstract
Arousal has long been known to influence behavior and serves as an underlying component of cognition and consciousness. However, the consequences of hyper-arousal for visual perception remain unclear. The present study evaluates the impact of hyper-arousal on two aspects of visual sensitivity: visual stereoacuity and contrast thresholds. Sixty-eight participants participated in two experiments. Thirty-four participants were randomly divided into two groups in each experiment: Arousal Stimulation or Sham Control. The Arousal Stimulation group underwent a 50-second cold pressor stimulation (immersing the foot in 0–2° C water), a technique known to increase arousal. In contrast, the Sham Control group immersed their foot in room temperature water. Stereoacuity thresholds (Experiment 1) and contrast thresholds (Experiment 2) were measured before and after stimulation. The Arousal Stimulation groups demonstrated significantly lower stereoacuity and contrast thresholds following cold pressor stimulation, whereas the Sham Control groups showed no difference in thresholds. These results provide the first evidence that hyper-arousal from sensory stimulation can lower visual thresholds. Hyper-arousal's ability to decrease visual thresholds has important implications for survival, sports, and everyday life.
Introduction
Conscious sensory perception is dependent upon the coordinated activity of arousal and sensory systems in the brain. [1] Arousal serves as a substrate of consciousness that is a) important for providing a basic aptitude for behavioral response to the environment and b) a means for optimizing behavior. [2]–[5] Just as hypo-arousal can compromise our ability to respond to the environment (e.g., coma), hyper-arousal holds the potential to enhance our performance (e.g., fight or flight). [4]–[11] In most domains of vision science, however, arousal is not incorporated into theoretical models. Arousal fluctuates naturally over the course of the day, and many activities, social situations, foods, and health factors can influence arousal. If arousal does play a role in modulating visual perception, many current models fail to capture a nearly ubiquitous source of variation. Thus, there is a pressing need to investigate the possible consequences of increased arousal for visual perception.
The present study begins an investigation of the influence of hyper-arousal on perceptual judgments of visual information in the environment. Hyper-arousal is any increase in arousal above an organism's normal awake arousal state. [3], [5] Normal arousal, or normo-arousal, is the average level of arousal in an awake, non-brain damaged organism and serves as the baseline state of responsiveness for conscious behavior. [1], [2] Hyper-arousal can range from small increases due to caffeine intake or startle response to abnormally high levels associated with post-traumatic stress disorder or high-voltage electric shock. [5], [12] In particular, the present study investigates whether inducing a state of hyper-arousal through sensory stimulation improves the discriminability of 1) depth relationships signaled by binocular disparity and 2) contrast gratings.
Visual space perception is a particularly crucial perceptual domain, in that it allows us to represent the shape and layout of things in the environment, interact effectively with people and other objects, and plan future spatial behaviors. Furthermore, sensitivity to contrast provides us with information about the boundaries of objects, facilitates object identification, and guides attentional prioritization. Arousal-based improvement in depth and contrast processing would have obvious implications for survival. In situations involving immediate threats in the environment, increased appreciation of depth could prove essential for avoiding obstacles, localizing targets for reaching or grasping actions, or planning routes through the environment. Increased discrimination of contrast could prove critical for threat detection and accurate representation of obstacle boundaries. Even in less dire situations, such improvement in depth and contrast processing would potentially benefit performance in sports, driving, and many other contexts.
A particularly well-studied means of increasing arousal is cold pressor stimulation (CPS). CPS administration of 1–2 min is commonly used in clinical evaluations of autonomic nervous system function and as a cardiovascular response test. [13]–[17] At longer durations (e.g., 4–5 min), it has also been used in studies of pain threshold and tolerance. [18] Stimulation triggers sympathetic activation leading to vasoconstriction. [15] Heart rate and blood pressure are normally elevated within the first minute of CPS and then return to baseline minutes after stimulation ends. [13]–[17] This response is reliable and demonstrates minimal attenuation when re-tested 2 weeks later. [19] Functional magnetic resonance imaging studies also suggest that CPS activates a wide range of cortical and subcortical structures in the brain, including: the lateral and inferior postcentral gyrus; aspects of the inferior, middle, and superior frontal gyri; anterior insula; anterior cingulate gyrus; occipital and temporal cortices; the thalamus; the anterior and posterior hypothalamus; amygdala; hippocampus; cerebellar cortex; and pontine areas. [20]–[23] This wide range neural activation is consistent with the broad pattern of effect expected with change in arousal.
In the present study, we used a 50 second CPS to induce a hyper-arousal response. Previous electrophysiological research demonstrated that immersing the foot for 50 seconds in 0–2° C water results in a state of hyper-arousal lasting for approximately ten minutes. [3], [4] For example, using the P50 evoked-response potential, a marker of ascending reticular activating system (ARAS) output, Woods et al (2011) demonstrated that CPS induces an arousal regulation response in healthy participants. [4] Specifically, the amplitude of the P50 ERP increased or decreased relative to a person's initial state of arousal. That is to say, if a person is already hyper-aroused at the time of stimulation, arousal-related output of the ARAS is downregulated, thus tending to prevent a state of hyper-arousal that might otherwise be detrimental for behavioral performance, and vice versa for a person in a state of hypo-arousal. These results, in addition to other recent findings, suggest that regulation of arousal output is likely mediated by brain systems exerting regulatory control over the ARAS and serves to promote an optimal state for responding to the surrounding environment. [24]–[28]
Recent animal research demonstrates that arousal states modulate the responsiveness of neurons in the early visual system. [29]–[30] These effects occur as early as the lateral geniculate nucleus, before information reaches the visual cortex. Thus, although rarely considered in theories of higher-level visual perception, arousal has strong implications for the vision sciences. Unfortunately, the mechanisms behind arousal-related improvement in response to the environment remain unclear. Previous research demonstrates that transient exogenous attentional cuing to a particular spatial location or object can enhance the visual appearance of contrast at the cued location and lower visual contrast thresholds there. [31]–[33] Hyper-arousal-related improvements may also function through facilitation of attentional mechanisms. Arousal and attention have long been known to share a reciprocal relationship. For example, while states of hypo- and hyper-arousal modulate attentional processes, sustained and focused attentional processes modulate arousal state in tasks requiring sustained performance. [3], [34]–[37] In a similar vein, recent research demonstrates that emotionally arousing visual stimuli (e.g., fearful faces) gain preferential access to awareness, predominate over less arousing stimuli, and selectively impact different aspects of low-level contrast sensitivity. [38]–[40] These data collectively suggest that transient increases in emotional arousal provide an advantage for processing certain arousing visual stimuli in the environment. Recent research by Phelps et al (2006) suggests that effects of fearful faces on low-level vision can be explained, at least in part, by attentional mechanisms. [35]
However, unlike transient emotional arousal or attentional cueing manipulations, CPS-induced hyper-arousal responses are sustained and generalized (i.e., lasting approximately 10 minutes and irrespective of the side of the body stimulated). [3], [4] Significant effects from a sustained and generalized arousal manipulation would be an important discovery on several fronts. As many factors can result in sustained changes in arousal, arousal-related effects on visual thresholds could broadly impact survival, sports performance, and everyday life. Furthermore, significant decreases in visual thresholds using a sustained and generalized manipulation, rather than a transient spatial cue, would provide an important foundation for future investigations into the role of attention in arousal-related effects. Finally, significant effects of arousal on visual thresholds would provide evidence for the importance of including this factor in models of visual perception.
To assess the impact of hyper-arousal on human visual thresholds, we measured stereoacuity and contrast thresholds before and immediately after CPS in the current study. There are three plausible patterns of result in the current study: 1) both stereoacuity and contrast thresholds will decrease, 2) only one of the two thresholds will decrease, or 3) neither of the two thresholds will decrease following a CPS-induced hyper-arousal response. The first pattern of result would suggest that the role of arousal in visual thresholds is not restricted to a single visual domain, but instead spans multiple domains. In contrast, the second pattern would suggest that the role of arousal is more limited in scope. In contrast, the last pattern of result would suggest that there is likely limited benefit in accounting for arousal states in models of visual perception. A fourth, albeit unlikely, possible outcome is that CPS could lead to a decrement in performance. This might happen if CPS were to induce an extreme state of hyper-arousal. [5] However, past work suggests that 50 sec of CPS stimulation does not produce the kind of extreme hyper-arousal associated with performance decrements, but rather an arousal regulation response. [3]–[4]
Experiment 1: Stereoacuity
Experiment 1 investigated whether a CPS induced hyper-arousal response can increase people's ability to discriminate subtle depth relationships signaled by binocular disparity (i.e., lower thresholds).
Materials and Methods
Ethics statement
The study was approved by the George Washington University Institutional Review Board. All participants in the present research (n = 68) gave written informed consent.
Subjects
Participants were 34 college age volunteers who received course credit for participation. Participants were randomly divided into either an Arousal Stimulation Group (n = 17, mean age±SD = 19.3±1.0 years, 12 females) or a Sham Control Group (n = 17, mean age±SD = 19.4±0.8 years, 11 females). All participants were naïve to the purpose of the study and reported normal or corrected to normal vision.
Design
The experiment took place in a well-lit indoor classroom. Participants in the Arousal Stimulation Group underwent CPS-immersing the foot for 50 seconds in 0–2° C water. Rather than performing CPS, participants in the Sham Control Group underwent a “sham” stimulation-immersing the foot in room temperature water (22–24° C) for 50 seconds. [4] Neither group was aware of the opposing group. Participants in a given group only received one form of stimulation (i.e., CPS or Sham) and underwent depth threshold testing before (Baseline) and after (Post-Stimulation) the appropriate stimulation procedure. A between subjects design was chosen to minimize engagement of demand characteristics associated with conscious knowledge of experimental manipulations in experiments and focus results on relative changes associated with different forms of stimulation.
Participants in both groups received the same instructions. The experimenter (AJW) used a neutral affect and followed a scripted conversation with participants in both groups. A scripted conversation was used so that the CPS or Sham stimulation were always referred to consistently between subjects and never discussed using language that referred to arousal, cold water, etc. Participants first underwent practice trials, followed by Baseline test trials. Following Baseline testing, participants underwent the CPS or Sham stimulation for 50 seconds. Immediately following stimulation, participants underwent a final set of Post-Stimulation test trials. Side of stimulation (i.e., left or right foot) was counterbalanced across participants.
Cold pressor and sham apparatus
CPS was prepared in a closable insulated cooler measuring 14 inches by 10 inches. Equal volumes of water and ice were placed in the cooler. A digital aquarium thermometer was attached below the water line to allow monitoring of water temperature. CPS was prepared 15 minutes prior to the participant's arrival and allowed to attain the targeted temperature between 0 and 2 degrees Celsius. Sham stimulation was prepared 1 hour prior to participant arrival using the same cooler but water was added and allowed to sit with the top open until the targeted 22–24 degrees range was attained. Targeted temperatures could be maintained for over one hour once the cooler was closed.
Stereoscopic depth apparatus
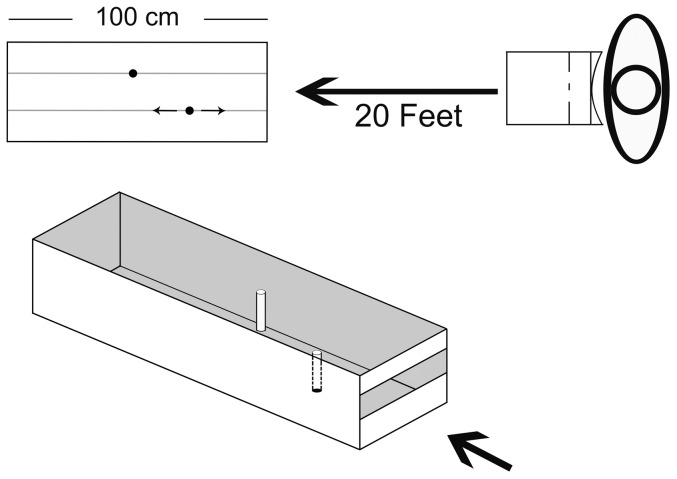
Stereoscopic depth threshold was measured using a two-alternative forced choice technique. The task required participants to indicate which of two rods was closer in depth to their location (i.e., left or right). The target holder was a wooden box mounted on a tripod. The target holder was constructed such that the participant observed two white rods (5 cm×0.7 cm) at various intervals of depth (Figure 1). The rods had a 2.5 cm separation and the left-most rod was stationary. The right-most rod was placed on a hidden slider that allowed it to be adjusted along a range of 100 cm with different test depth intervals ranging from 50 cm (or approximately 140 arcsec of disparity) in front or behind the stationary rod. All surfaces on the target holder were painted matte black to present a consistent texture. The target holder was placed 20 feet from the participant's viewing location to minimize the influence of egocentric distance cues, leaving binocular disparity as the primary stimulus cue to the relative depth between the rods. Participants viewed the target holder through a table-mounted occluder with adjustable chin-rest allowing binocular viewing of the target holder at eye-level.
Figure 1. Depth threshold device.
Participants viewed two white rods through an occluder placed at optical infinity (20 feet). Participants judged which rod (left or right) was closer in depth to their location. The maximum depth interval was 50 cm.
The table-mounted occluder allowed participants to place their face in a molded viewing aperture contoured to fit the forehead and nose firmly in place, with eye-height maintained via the chin-rest. The occluder had a card slot that provided full occlusion of the environment between trials. The card could be removed by the participant at the beginning of each trial to view the target holder. The target holder was built to provide viewing of the two rods at a height of 110 cm with the front “open” surface built to occlude the top and bottom surface of the target rods. Thus, in addition to the disparity between the rods, a potentially useful disparity signal was also present between the occluder and each of the rods. This was equally true in all conditions, however, and thus did not impact our ability to test our primary research question, which hinged on group differences relative to their own baseline performance.
We used a variant of the QUEST adaptive threshold-seeking algorithm written by Denis Pelli for the Psychtoolbox in MATLAB (The Mathworks, Natick, MA). [41]–[43] On each trial, the experimenter entered the accuracy of a participant's response and the program calculated the optimal size of the next depth interval to be presented. The size of the depth interval varied adaptively from trial to trial based on the participant's prior responses. Side of depth (e.g., left rod closer or vice versa) was randomized across trials. Participants underwent a total of 40 trials. The stereoscopic depth threshold was defined as the linear separation between the rods along the line of sight that yielded 82 percent correct performance across the 40 trials.
Procedure
Prior to testing, the chin-rest on the table-mounted occluder was adjusted so that viewing height was 110 cm. Participants received instructions to respond either “Left” or “Right” indicating which of the two rods was closer to their eyes. Participants donned foam earplugs and tight fitting over-ear hearing protectors to remove any auditory cues generated by moving the rod in the depth apparatus between trials (i.e., a slight scraping noise that could provide temporal feedback on relative changes to rod depth between trials). Next, 6 practice trials were conducted. Practice trials were given at depth intervals of±8,±21, and±45 cm of separation in random order (approximately 23.8, 61.3, and 127 arcsec of separation; ± refers to the side of greatest depth, +8 = Left rod 8 cm farther than the right rod, −8 = Left rod 8 cm closer than the right rod). The practice trials were intended to accustom participants with the testing procedure. No error feedback was given.
In both the practice and experimental trials, participants kept their head located in the table-mounted occluder device. At the start of each trial, a card occluding the testing environment was put in place. The experimenters prepared the stimulus by placing the moveable rod at the proper location on the target box. When ready, the experimenter verbally signaled the participant to raise the occlusion card and look through the occluder. Participants verbally indicated which rod appeared closer to their location (i.e., Left or Right). After the response, the experimenter entered the participant's response into the computer and cued the participant to lower the occlusion card for the next trial.
Analyses
Data (depth thresholds) were evaluated performing a 2 (Group: Arousal Stimulation vs. Sham Stimulation) x 2 (Block: Baseline vs. Post-Stimulation) RM-ANOVA. Evidence of a significant Group x Block interaction would suggest at least one of the two groups evidenced a significant effect of Stimulation. Planned comparisons were paired samples t-tests. Numerical differences at baseline between groups were checked for significance using an independent t-test. Depth was analyzed in units of linear separation (cm), but approximate values in arcsec of separation are also provided (assuming an average interpupillary distance, IPD, of 6.3 cm).
Results
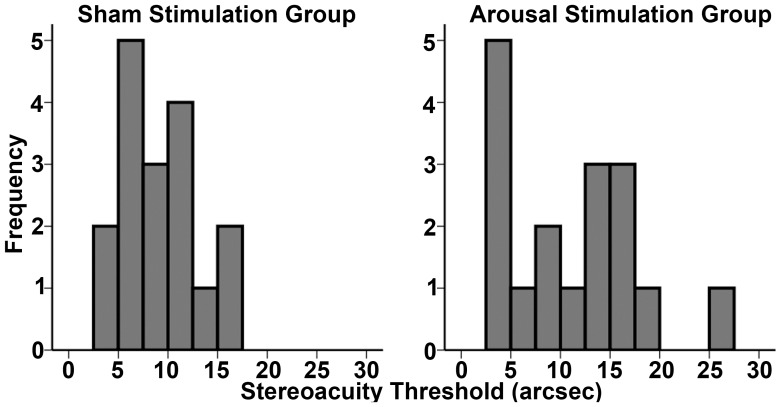
The RM-ANOVA demonstrated a significant Group x Block interaction (F(1,16) = 4.66, Mean Square Error (MSE) = 3.4, p = 0.04, Partial Eta Squared (ηp2) = 0.27). Neither Group (F(1,16) = 0.12, MSE = 0.6, p = 0.73) nor Block (F(1,16) = 3.49, MSE = 3.7, p = 0.08) demonstrated a significant main effect. Planned paired samples t-tests of depth thresholds in the Arousal Stimulation group demonstrated a significant effect of arousal stimulation on stereoacuity thresholds (t = 3.71, DF = 16, p = 0.002, Cohen's d = 1.35; Figure 2) from baseline (mean threshold±standard error (M±SE) = 3.73±0.54 cm; ≈11.17±1.6 arcsec) to post-stimulation (M±SE = 2.81±0.54 cm; ≈8.42±1.3 arcsec). However, Sham stimulation had no effect on depth thresholds from baseline (M±SE = 3.09±0.31 cm; ≈9.27±0.9 arcsec) to post-stimulation (M±SE = 3.07±0.51 cm; ≈9.20±1.5 arcsec; t = 0.05, DF = 16, p = 0.9). There was no significant difference between baseline thresholds in the Arousal versus Sham stimulation groups (independent t-test: t = 1.02; DF = 32; p = .31). Thus, although slightly numerically different at baseline, there was not a significant difference between groups in terms of baseline stereoacuity thresholds (Figure 2). Nevertheless, frequency plots of baseline thresholds for the Arousal and Sham Stimulation Groups demonstrate some differences in the distribution of threshold values at baseline (Figure 3). To determine whether non-significant differences at baseline between groups account for significant effects of Arousal Stimulation, we performed an analysis of covariance (ANCOVA) with signed difference in threshold (post-stimulation - baseline) as the dependent variable with Group and Baseline as independent factors. When controlling for differences in baseline thresholds between groups, there remained a significant effect of Group (F(1,30) = 4.4, MSE = 7.6, p = .04). Thus, numerical differences at baseline between Arousal and Sham Stimulation groups do not explain significant effects of CPS on stereoacuity thresholds.
Figure 2. Stereoacuity thresholds.
Mean stereoacuity threshold from Baseline to Post-Stimulation for the Arousal Group and Sham Control Group. The left y-axis reports thresholds in units of linear separation (cm). The right y-axis reports thresholds in approximate units of angular separation (arcsec). Arcsec was calculated using an assumed average IPD of 6.3 cm. Baseline performance was not significantly different between groups (t = 1.02; DF = 32; p = .31). * = p<.05
Figure 3. Frequency plots for baseline stereoacuity thresholds by group.
Discussion
Hyper-arousal from CPS sensory stimulation increased participants' sensitivity to subtle depth differences. However, sham stimulation had no influence on stereoacuity. These results suggest that sensitivity to at least some subtle visual differences in the environment is facilitated by changes in arousal state. However, it remains unclear whether these effects are specific to stereoacuity or generalize to other aspects of human visual perception. If arousal effects generalize to other aspects of visual perception, this would provide strong evidence for the importance of accounting for this nearly ubiquitous source of variation in models of visual perception.
Experiment 2: Contrast
Experiment 2 investigated whether hyper-arousal effects generalize to another aspect of visual perception: contrast discrimination. As discussed earlier, consistent effects across stereoacuity and contrast would suggest that hyper-arousal has a relatively broad impact on detection of subtle visual differences in the environment. This pattern of result would provide strong evidence supporting future investigations into the role of arousal in other aspects of visual perception. Furthermore, this result would suggest that a sustained and non-spatial manipulation of arousal produces effects on visual thresholds similar to those previously shown in studies using transient attentional cueing to spatial locations. In contrast, lack of hyper-arousal related effects on contrast discrimination would suggest that the role of arousal is more limited in scope and may not broadly impact detection of subtle visual differences in the environment.
Materials and Methods
Subjects
Participants for this study were 34 college age volunteers who received course credit for participation in the study. Participants were divided into either an Arousal Stimulation Group (n = 17, mean age ± SD = 19.3±1.0 years, 12 females) or a Sham Stimulation Control Group (n = 17, mean age±SD = 19.4±0.8 years, 11 females). All participants were naïve to the purpose of the study and reported normal or corrected to normal vision.
Design, cold pressor, and sham apparatus
CPS and Sham stimulation procedures and the pre-post testing design were identical to Experiment 1.
Contrast discrimination apparatus and stimuli
Contrast threshold was measured using a two-alternative forced choice task. The program was modified from an open access program (QuestDemo) written by Denis Pelli for the Psychtoolbox in MATLAB (The Mathworks, Natick, MA) on a Macintosh 2GHz PowerPC G5. [41]–[43] Screen resolution was 1440×900 with graphics acceleration via an ATI Radeon 9600 128MB graphics card. The program used the well-documented QuestMean procedure to determine trial-by-trial levels of contrast discriminability for stimuli. [44]
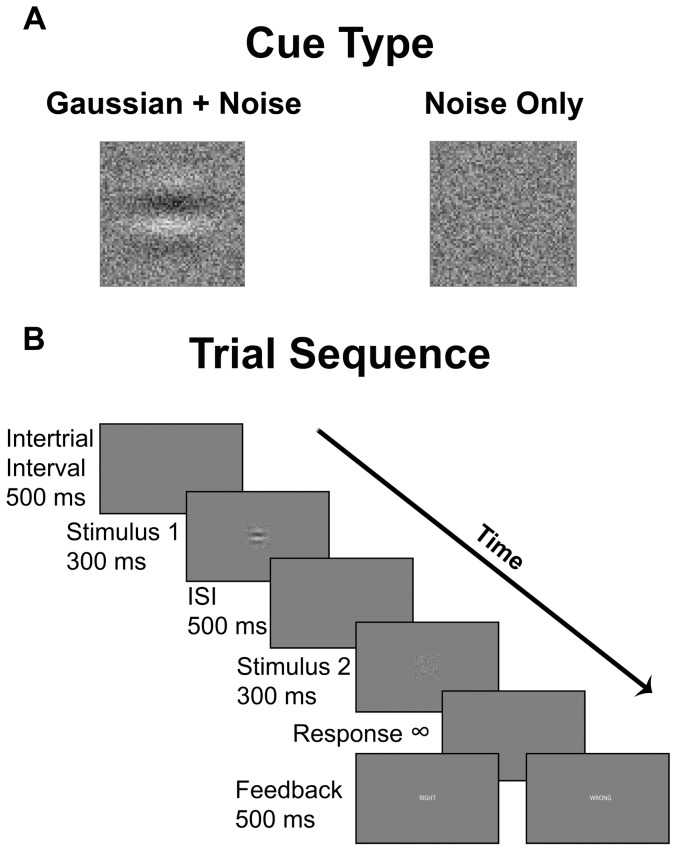
The task required participants to indicate which of two squares contained a Gaussian grating (described to the participants as a ripple in the visual noise – “as if a single rain drop hit calm water”). Participants were presented with a uniform gray background on which two squares (eye to screen distance ≈60 cm; ≈6.7°×6.7° visual angle) filled with visual noise were presented serially at the central location of the screen. One of the two squares contained a Gaussian grating (approximately 3 cycles per degree, horizontally-oriented) inside the visual noise, while the other contained only noise (Figure 4a). The ratio of signal to noise was determined by QuestMean. Specifically, the grating contrast was manipulated while the noise contrast remained constant. The order of presentation of the square containing the Gaussian grating was randomized. Visual stimuli were presented for 300 ms with an inter-stimulus interval (ISI) of 500 ms. After presentation of the visual stimuli, participants were presented with the uniform gray background until they responded. Participants were required to press the left mouse button once or twice after both visual stimuli had appeared and disappeared from the screen, corresponding to the square containing the Gaussian grating (once for the 1st square, twice for the 2nd square). Following their response, participants received error feedback, followed by an intertrial interval of 500 ms before the next pair of stimuli was presented (see Figure 4b). As the trials progressed, the discriminability of stimulus pairs became more difficult (as determined by the QuestMean algorithm).
Figure 4. Contrast threshold task.
A) Illustration of cue types. Gaussian + Noise example represents the highest contrast stimulus presented on the first trial of the test block. Noise only represents the noise stimulus common to all trials. B) Sequence of events in a trial. In the given example, Stimulus 1 represents the Gaussian + Noise example in 1a and Stimulus 2 represents the Noise only example. Feedback was given in white font as right or wrong based on the accuracy of the participant's response. In the given example, participants would click once to correctly identify the 1st stimulus presented as containing the stimulus with more contrast. ISI = inter-stimulus interval.
Contrast discrimination procedure
Participants underwent three blocks of practice trials containing 10 trials each to become accustomed to the procedure. Following practice, participants were administered a block of 80 test trials (Baseline testing). Following Baseline testing, participants underwent the stimulation procedure appropriate to their group. Immediately following stimulation, participants completed another block of 80 test trials (Post-Stimulation testing). Contrast threshold was defined as the value of contrast that yielded 82 percent correct performance across the 80 trials. [42]–[43]
Analyses
To assess the effects of cold pressor stimulation (CPS) on contrast thresholds we performed a 2 (Group: Arousal vs. Sham) x 2 (Block: Baseline vs. Stimulation) repeated measures analysis of variance (RM-ANOVA). If CPS influenced sensitivity to contrast in the Arousal Stimulation group, we would expect to find a significant Group x Block interaction in the RM-ANOVAs.
Results
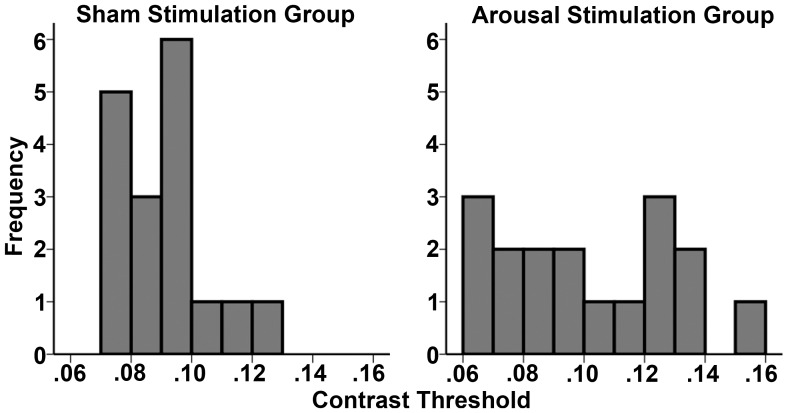
Results for contrast thresholds demonstrated a significant Group x Block interaction (F1,16 = 5.24, p = 0.03, Mean Square Error (MSE) = 0.001, ηp2 = 0.25). Neither Group (F1,16 = 0.06, p = 0.8) nor Block (F1,16 = 3.92, p = 0.07) were significant in the RM-ANOVA model. Planned contrasts utilizing paired-samples t-test between Baseline (mean threshold ± standard error (SE) = 0.102±0.006) and Stimulation (mean threshold ± standard error (M±SE) = 0.086±0.004) for each group demonstrated a significant effect of CPS on contrast thresholds from Baseline to Stimulation for participants in the Arousal group (t = 2.38, DF = 16, p = 0.03, Cohen's d = 0.84; Figure 5. Performance from Baseline (M±SE = 0.092±0.003) to Stimulation (M±SE = 0.093±0.003) in the Sham Stimulation group was not significantly different (t = −0.07, DF = 16, p = 0.9). As is evidenced from the planned contrasts, the marginal significance of Block was a result of the large difference from Baseline to Stimulation in the Arousal group. There was not a significant difference between baseline contrast thresholds in the Arousal versus Sham stimulation groups (independent t-test: t = 1.29; DF = 32; p = .21; Figure 5). Nevertheless, frequency plots of baseline thresholds for the Arousal and Sham Stimulation Groups demonstrate some differences in the distribution of threshold values at baseline (Figure 6). To determine whether non-significant differences at baseline between groups account for significant effects of Arousal Stimulation, we performed an analysis of covariance (ANCOVA) with signed difference in threshold (post-stimulation - baseline) as the dependent variable with Group and Baseline as independent factors. When controlling for differences in baseline thresholds between groups, there remained a significant effect of Group (F(1,30) = 4.6, MSE = .001, p = .03). Thus, slight numerical differences at baseline between Arousal and Sham Stimulation groups do not explain significant effects of CPS on contrast thresholds.
Figure 5. Contrast thresholds.
Mean contrast threshold from Baseline to Post-Stimulation for the Arousal Group and Sham Control Group. Baseline performance was not significantly different between groups (t = 1.29; DF = 32; p = .21). * = p<.05
Figure 6. Frequency plots for baseline contrast thresholds by group.
Discussion
Participants contrast thresholds significantly decreased following CPS. There was no change in participant's contrast thresholds following room temperature sham stimulation. Consistent effects across stereoacuity and contrast thresholds suggest that hyper-arousal has a relatively broad impact on detection of subtle visual differences in the environment. This pattern of result provides strong evidence supporting future investigations into the role of arousal in other aspects of visual perception. Furthermore, these data suggest that a sustained and generalized manipulation of arousal produces similar decreases in thresholds found using transient cued attention to a spatial location and transient manipulations of emotional arousal. As many factors can produce sustained changes in arousal, these findings have broad practical and scientific implications.
General discussion and conclusions
The present research presents the first evidence that hyper-arousal from sensory stimulation can influence aspects of human visual perception. Participants' sensitivity to subtle depth and contrast differences increased after exposure to CPS, but not after sham stimulation. Decreased visual thresholds following a sustained and generalized manipulation of arousal has a number of important implications. First, this suggests that visual perceptual processing of depth and contrast, and perhaps judgments of other visual relationships, might vary systematically depending upon a variety of factors, including time of day, health status, food consumption, drug history, anxiety, sleep history, etc. These effects could play a role in enhancing survival, driving safety, sports performance, and so on.
Second, understanding the scope and origin of hyper-arousal effects following CPS is an important next step in identifying its applicability to real-world tasks. In addition to depth processing, hyper-arousal could influence other aspects of visual perception, other types of sensory perception, or even non-sensory processes. Future research evaluating these possibilities will help to establish the breadth of hyper-arousal's effect and future directions of its application. For example, Woods and colleagues (2012) recently demonstrated that CPS arousal stimulation temporarily ameliorated inattention and sensory magnitude estimation symptoms of unilateral spatial neglect and normalized the P50 ERP biomarker of arousal in a patient with chronic right-hemisphere stroke. [3] Furthermore, in acute clinical settings, physicians use drugs like modafinil to treat arousal-related deficits following brain injury. [10] Better understanding of the perceptual and cognitive effects of hyper-arousal will inform efforts to identify other populations that might benefit from such treatment and the brain mechanisms that underlie clinical improvement.
Our results also motivate the need to understand what psychological and physiological factors are impacted when behavioral performance is influenced by hyper-arousal. When depth thresholds are reduced by hyper-arousal, for example, is this because depth intervals appear slightly larger due to facilitation of attentional processes? The reciprocal relationship between attention and arousal, as described by Carrasco and colleagues as well as the emotional arousal literature [31]–[33], [35], [38]–[40], provide strong empirical and theoretical foundations for investigating attentional mechanisms in hyper-arousal-related effects. Furthermore, significantly decreased visual thresholds by a sustained and generalized manipulation of arousal provide an important starting point for future investigations into the relationship between attention and arousal. Research demonstrating that stereoacuity improves as a function of stimulus contrast also provides a plausible avenue for future investigations into the underlying mechanisms of arousal-related improvement in visual perception. [45] Specifically, this research could suggest that arousal-related improvements in contrast sensitivity serve as the mechanism underlying changes in stereoacuity thresholds in the present study. If true, other aspects of visual perception modulated contrast sensitivity may also benefit from hyper-arousal. Progress in each of these domains will help identify other tasks that stand to benefit most from stimulation. It will also help generate evidence-based predictions for other aspects of perception and cognition potentially influenced by hyper-arousal. As the responsiveness of neural systems is strongly modulated by arousal state [29]–[30], [46]–[48], a better understanding of the behavioral and neural mechanisms of brain arousal systems will have far reaching implications for multiple fields of science.
In summary, arousal serves as an underlying component for human cognition and consciousness. Hypo-arousal can compromise a broad range of behaviors. In contrast, the present research demonstrates that induction of a hyper-arousal response can decrease visual thresholds. Hyper-arousal effects may also extend to many other components of human behavior. As many factors influence our arousal state, understanding its role in human behavior will be an important area of investigation for future research.
Funding Statement
This research was supported in part by the National Institute of Neurological Disorders and Stroke (T32NS007413). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Llinas R (1998) The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1841–1849 doi: 10.1098/rstb.1998.0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones BE (2005) Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier Saunders. pp. 136–153. [Google Scholar]
- 3. Woods AJ, Mennemeier M, Garcia-Rill E, Huitt T, Chelette KC, et al. (2012) Improvement in arousal, visual neglect, and perception of stimulus intensity following cold pressor stimulation. Neurocase 18: 115–122 doi: 10.1080/13554794.2011.568498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods AJ, Philbeck JW, Chelette K, Skinner RD, Garcia-Rill E, et al. (2011) Cold pressor stimulation diminishes P50 amplitude in normal subjects. Acta Neurobiol Exp (Wars) 71: 348–358 Available: doi:http://www.ane.pl/linkout.php?pii=7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yerkes RM, Dodson JD (1908) The relation of strength of stimulus to rapidity of habit formation. J Comp Neurol Psychol 18: 459–482 doi: 10.1002/cne.920180503 [Google Scholar]
- 6. Garcia-Rill E (1997) Disorders of the reticular activating system. Med Hypotheses 49: 379–387 doi: 10.1016/S0306-9877(97)90083-9 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Rill E, Skinner RD, Clothier J, Dornhoffer J, Uc E, et al. (2002) The sleep state-dependent midlatency auditory evoked P50 potential in various disorders. Thalamus Relat Syst 36: 1–11 doi: 10.1016/S1472-9288(02)00032-8 [Google Scholar]
- 8. Skinner RD, Homma Y, Garcia-Rill E (2004) Arousal mechanisms related to posture and locomotion, 2. Ascending modulation. Prog Brain Res 143: 291–298 doi: 10.1016/S0079-6123(03)43028-8 [DOI] [PubMed] [Google Scholar]
- 9. Skinner RD, Miyazato H, Garcia-Rill E (2002) The sleep state-dependent P50 auditory evoked potential in neuropsychiatric diseases. Int Congr Ser 1232: 813–825 doi: 10.1016/S0531-5131(01)00800-7 [Google Scholar]
- 10. Woods AJ, Mennemeier M, Garcia-Rill E, Meythaler J, Mark VW, et al. (2006) Bias in magnitude estimation following left hemisphere injury. Neuropsychologia 44: 1406–1412 doi: 10.1016/j.neuropsychologia.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woods AJ, Mark VW, Pitts A, Mennemeier M (2011) Pervasive cognitive impairment in acute rehabilitation patients “without” brain injury. PM R 3: 426–432 doi: 10.1016/j.pmrj.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner RD, Rasco LM, Fitzgerald J, Karson CN, Matthew M, et al.. (1999) AID-DA4%3E3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 13. Findlay IN, Gillin G, Cunningham AD, Elliott AT, Aitchinson T, et al. (1988) A comparison of isometric exercise, cold pressor stimulation and dynamic exercise in patients with coronary heart disease. Eur Heart J 9: 657–664. [DOI] [PubMed] [Google Scholar]
- 14. McLaren A, Kerr S, Allan L, Steen IN, Ballard C, et al. (2005) Autonomic function Is impaired in elderly stroke survivors. Stroke 36: 1026–1030 doi: 10.1161/01.STR.0000160748.88374.ce [DOI] [PubMed] [Google Scholar]
- 15. Mizushima T, Tajima F, Nakamura T, Yamamoto M, Lee K, et al. (1998) Muscle sympathetic nerve activity during cold pressor test in patients with cerebrovascular accidents. Stroke 29: 607–612 doi: 10.1161/01.STR.29.3.607 [DOI] [PubMed] [Google Scholar]
- 16. Northcote R, Cooke M (1987) How useful are the cold pressor test and sustained isometric handgrip exercise with radionuclide ventriculography in the evaluation of patients with coronary artery disease? Br Heart J 57: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waters D, Szlachcic J, Bonan R, Miller D, Dauwe F, et al. (1983) Comparative sensitivity of exercise, cold pressor and ergonovine testing in provoking attacks of variant angina in patients with active disease. Circulation 67: 310–315. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell LA, Raymond A, MacDonald R, Brodie EE (2004) Temperature and the cold pressor test. J Pain 5: 233–238 doi: 10.1016/j.jpain.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 19. Saab P, Llabre M, Hurwitz B, Schneiderman N, Wohlgenuth W, et al. (1993) The cold pressor test: vascular and myocardial response patterns and their stability. Psychophysiology 30: 366–373 doi: 10.1111/j.1469-8986.1993.tb02058.x [DOI] [PubMed] [Google Scholar]
- 20. Frankenstein UN, Richter W, McIntyre MC, Remy F (2001) Distraction modulates anterior cingulate gyrus activations during the cold pressor test. NeuroImage 14: 827–36 doi: 10.1006/nimg.2001.088 [DOI] [PubMed] [Google Scholar]
- 21. Fulbright RK, Troche CJ, Skudlarski P, Gore JC, Wexler BE (2001) Functional MR imaging of regional brain activation associated with the affective experience of pain. Am J Roentgenol 177: 1205–10 doi: 10.2214/ajr.177.5.1771205 [DOI] [PubMed] [Google Scholar]
- 22. Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, et al. (1998) Regional brain activation in humans during respieratory and blood pressure challenges. Clin Exp Pharmacol Physiol 25: 483–6 doi: 10.1111/j.1440-1681.1998.tb02240.x [DOI] [PubMed] [Google Scholar]
- 23. Woo Ma, Macey PM, Keens PT, Kumar R, Fonarow GC, et al. (2005) Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 11: 437–46 doi: 10.1016/j.cardfail.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 24. Ermutlu MN, Karamursel S, Ugar EH, Senturk L, Gokhan N (2005) Effects of cold stress on early and late stimulus gating. Psychiatry Res 136: 201–9 doi: 10.1016/j.psychres.2003.03.002 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Rill E, Moran K, Garcia J, Findley WM, Walton K, et al. (2008) Magnetic sources of the M50 response are localized to frontal cortex. Clin Neurophysiol 119: 388–398 doi: 10.1016/j.clinph.2007.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson MR, Adler LE (1993) Transient impairment in P50 auditory sensoty gating induced by a cold pressor test. Biol Psychiatry 33: 380–7 doi: 10.1016/0006-3223(93)90328-B [DOI] [PubMed] [Google Scholar]
- 27. Knight RT, Scabini D, Woods DL (1989) Prefrontal cortex gating of auditory transmission in humans. Brain Res 504: 338–42 doi: 10.1016/0006-8993(89)91381-4 [DOI] [PubMed] [Google Scholar]
- 28. Rasco LM, Skinner RD, Garcia-Rill E (2000) Effects of age on sensory gating of sleep state dependent P1/P50 midlatency auditory evoked potentials. Sleep Res Online 3: 97–105. [PubMed] [Google Scholar]
- 29. Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alanso JM, et al. (2006) Thalamic burst mode and inattention in the awake LGNd. Neuron 49: 421–432 doi: 10.1016/j.neuron.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 30. Neill CM, Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 56: 472–479 doi: 10.1016/j.neuron.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carrasco M, Penpeci-Talgar C, Eckstein M (2000) Spatial covert attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Res 40: 10–12 doi: 10.1016/S0042-6989(00)00024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pestilli F, Carrasco M (2005) Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Res 45: 1867–1875 doi: 10.1016/j.visres.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 33. Yeshurun Y, Carrasco M (1998) Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilman KM, Watson RT, Valenstein E (1985) Neglect and related disorders. In K.M. Heilman KM, Valenstein E, editors. Clinical Neuropsychology, 2nd edition. New York: Oxford University Press. pp. 243–293. doi: 10.1002/ana.410100433
- 35. Phelps EA, Ling S, Carrasco M (2006) Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci 17: 292–299 doi: 10.1111/j.1467-9280.2006.01701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14: S76–S84 doi: 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- 37.VaezMousavi M, Barry RJ, Rushby JA, Clarke AR (2007) Arousal and activation effects on physiological and behavioral responding during a continuous performance task. Acta Neurobiol Exp 67: : 461–470. Acta Neurobiologiae Experimentalis website. Available: http://www.ane.pl/linkout.php?vol=67&no=4&fpp=461. Last accessed 2013 Mar 14. [DOI] [PubMed]
- 38. Alpers GW, Gerdes ABM (2007) Here is looking at you: emotional faces predominate in binocular rivalry. Emotion 7: 495–506 doi: 10.1037/1528-3542.7.3.495 [DOI] [PubMed] [Google Scholar]
- 39. Bocanegra BR, Zeelenberg R (2009) Emotion improves and impairs early vision. Psychol Sci 20: 707–713 doi: 10.1111/j.1467-9280.2009.02354.x [DOI] [PubMed] [Google Scholar]
- 40. Yang E, Zald DH, Blake R (2007) Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion 7: 882–886 doi: 10.1037/1528-3542.7.4.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brainard DH (1997) The Psychophysics Toolbox. Spat Vis 10: 433–436 doi: 10.1163/156856897X00357 [PubMed] [Google Scholar]
- 42. Pelli DG (1997) The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis 10: 437–442 doi: 10.1163/156856897X00366 [PubMed] [Google Scholar]
- 43. Watson AB, Pelli DG (1983) Quest: a baysian adaptive psychometric method. Atten Percept Psychophys 33: 113–120 doi: 10.3758/BF03202828 [DOI] [PubMed] [Google Scholar]
- 44. King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A (1994) Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision Res 34: 885–912 doi: 10.1016/0042-6989(94)90039-6 [DOI] [PubMed] [Google Scholar]
- 45. Westheimer G, Pettet MW (1990) Contrast and duration of exposure differentially affect vernier and stereoscopic acuity. Proc R Soc Lond B 241: 42–46 doi: 10.1098/rspb.1990.0063 [DOI] [PubMed] [Google Scholar]
- 46. Jones BE (2004) Activity modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res 145: 157–169 doi: 10.1016/S0079-6123(03)45011-5 [DOI] [PubMed] [Google Scholar]
- 47. Jones BE (2008) Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann NY Acad Sci 1129: 26–34 doi: 10.1196/annals.1417.026 [DOI] [PubMed] [Google Scholar]
- 48. Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, et al. (1998) Neural systems underlying arousal and attention: Implications for Drug Abuse. Ann NY Acad Sci 846: 222–237 doi: 10.1111/j.1749-6632.1998.tb09740.x [PubMed] [Google Scholar]