Abstract
The mammalian heart lacks the capacity to replace the large numbers of cardiomyocytes lost due to cardiac injury. Several different cell-based routes to myocardial regeneration have been explored, including transplantation of cardiac progenitors and cardiomyocytes into injured myocardium. As seen with cell-based therapies in other solid organ systems, inherent limitations, such as host immune response, cell death and long-term graft instability have hampered meaningful cardiac regeneration. An understanding of the cell biology of cardiac progenitors, including their developmental origin, lineage markers, renewal pathways, differentiation triggers, microenvironmental niche, and mechanisms of homing and migration to the site of injury, will enable further refinement of therapeutic strategies to enhance clinically meaningful cardiac repair.
Keywords: Heart disease, Myocardium, Cardiomyocyte, Stem cell, Regenerative medicine
In the United States and developed countries, cardiovascular disease stands as the leading cause of death despite significant advancements in vascular and cardiovascular prophylaxis, diagnosis, and treatment (Heron et al., 2009). A variety of cardiovascular diseases, including coronary artery disease, viral and idiopathic cardiomyopathies, valvular heart disease, and diabetes may all result in cardiomyocyte death (Narula et al., 1996). The surviving cardiomyocytes remodel by compensatory hypertrophy in order to maintain the level of cardiac output (Katz, 1994). This hypertrophic response, however, is maladaptive in the long-term and further promotes cardiomyocyte death by apoptosis leading, ultimately, to heart failure. Because the adult heart lacks significant intrinsic regenerative capability, replacement of lost cardiomyocytes is one of the major goals of cardiac regenerative medicine.
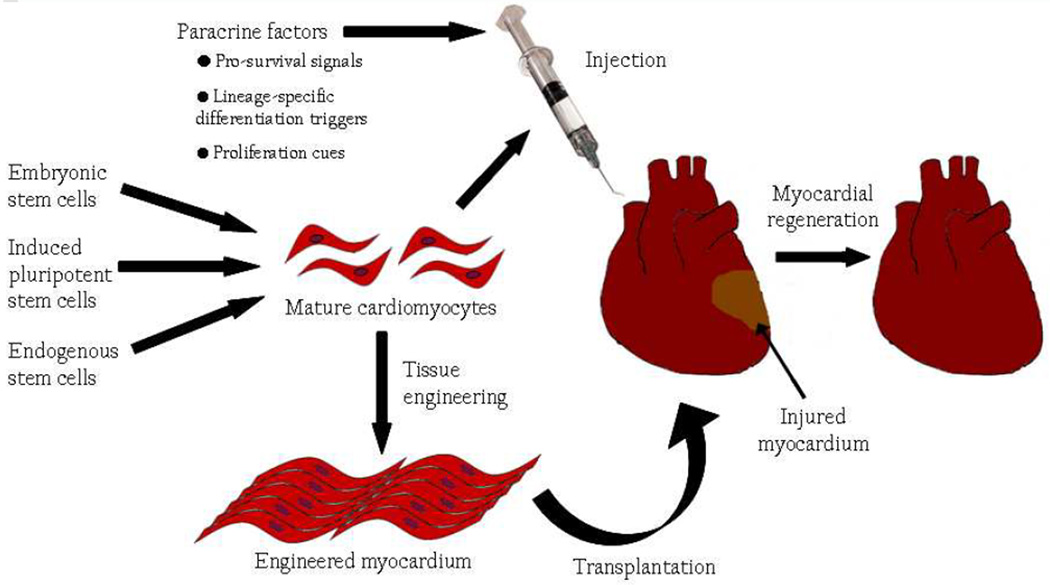
Currently, several routes to cardiomyocyte regeneration are being explored (Figure 1), including, but not limited to: 1) paracrine factors that can mobilize resident cardiac progenitor populations and promote proliferation and differentiation into mature cardiomyocytes; 2) transplantation of cardiac progenitor or stem cells, from a variety of sources including ES cell, iPS cell, or endogenous sources; and 3) transplantation of engineered cardiac tissues. Regardless of the approach used, successful deployment of each of these routes will depend on the availability of a comprehensive understanding of cardiac progenitor cell biology, including knowledge of their developmental origin, defining markers, renewal pathways, lineage-specific differentiation triggers, microenvironmental niche, and mechanisms of homing and migration to specific sites. Knowledge of unique markers for cardiac progenitor cells enables their facile isolation for in vitro expansion, differentiation and subsequent transplantation. The efficiency of this expansion and differentiation is enhanced by understanding the paracrine factors and signals that cooperate to achieve proliferation and cardiomyocyte-specific differentiation. As an alternative to cell transplantation, paracrine factors can also be used to mobilize endogenous cardiac progenitor cells and enhance their contributions to cardiomyocyte renewal. Furthermore, knowledge and manipulation of cardiomyocyte survival cues and migration mechanisms may improve the ability of transplanted cardiac progenitor cells to engraft and repopulate the injured heart.
Figure 1. Routes to myocardial regeneration.
Methods to regenerate functional myocardium currently being explored include, but are not limited to: 1) injection of paracrine factors that enhance cardiomyocyte survival, trigger cardiac lineage-specific differentiation of endogenous cardiac progenitor cells, promote cardiac progenitor and/or cardiomyocyte proliferation; 2) injection of mature cardiomyocytes derived from embryonic, induced pluripotent, or endogenous stem cells; and 3) transplantation of engineered myocardium.
Cardiogenesis and Embryonic Cardiac Progenitors
Myocardial cells are lineage descendents of the developing the mesoderm, which emerges from the primitive streak during gastrulation (Rawles, 1943). From the anterior primitive streak, cardiac precursors migrate under the head folds and divide into two populations, one on either side of the midline. Cells then extend toward the midline, forming the cardiac crescent, where committed cardiovascular cells are first observed. The cardiac crescent then fuses along the midline, forming the linear heart tube, which undergoes rightward looping, the first asymmetric event during organogenesis. Finally, with further hypertrophy of the left and right ventricles and atria, the four heart chambers undergo several phases of remodeling and septation before assuming their fully mature structure.
At the primitive streak stage, cardiac precursors are not yet irreversibly committed to a cardiac lineage and can also contribute to the paraxial mesoderm forming the skeletal muscle in the head and neck (Saga et al., 1999). Markers such as Mesp1 and Mesp2 have been used to identify these earliest cardiac and skeletal precursors (Lindsley et al., 2008; Wu et al., 2008). When mesodermal precursors restrict their fate to cardiovascular and hematopoietic lineages, they begin to express Mesp1 and Flk1 (Wu, 2008). Flk1 is used to denote primitive precursors for cardiovascular cells (Kattman et al., 2006) and has been detected in undifferentiated embryonic stem cells (Kouskoff et al., 2005). Mesp1 and Mesp2 are expressed transiently during the primitive streak stage and their expression is turned off as cardiac precursors migrate away from the primitive streak (Kitajima et al., 2000). Moreover, descendants of Mesp1+ and Mesp2+ cells colonize the entire myocardium (Saga et al., 2000), enabling Mesp1 and Mesp2 to be reliably used as cardiac progenitor markers. Mesp1 is not only a useful marker of cardiac precursors, but plays an important role in cardiac lineage commitment. Inducible Mesp1 overexpression during embryonic stem cell differentiation results in induces myocardial expansion in a Wnt-independent fashion (Lindsley et al., 2008). In zebrafish, ubiquitous Mesp1 overexpression leads to the formation of ectopic cardiac cells that show a beating phenotype (David et al., 2008). Mesp1 drives commitment of mesodermal precursors to the cardiac lineage by promoting the stable expression of cardiomyogenic transcription factors, including Nkx2.5, Gata4, Isl1, and myocardin, in a cell-autonomous manner (Bondue et al., 2008).
At the cardiac crescent stage, cardiac precursors irreversibly commit to cardiovascular lineages and begin to express transcription factors such as Nkx2.5, Gata4 and Isl1 (Moretti et al., 2006; Wu et al., 2006). These cardiac progenitors undergo rapid expansion to provide the necessary cells for the increase in size concomitant with heart tube formation, looping, and chamber formation. The expression of Nkx2.5 is cardiac-selective (Komuro and Izumo, 1993) and has been used as a marker of cardiac progenitor cells during embryonic development. While Isl1 is not strictly cardiac-specific, its expression is often used for the identification of cardiac progenitor cells because it is transiently expressed in cardiac mesoderm but is then turned off during cardiomyocyte maturation (Cai et al., 2003). Cardiogenic Nkx2.5+ and Isl1+ cells are tri-potent and contribute cardiomyocytes, endothelial cells, and vascular smooth muscle cells to the developing heart (Kattman et al., 2006; Moretti et al., 2006; Wu et al., 2006). These multipotent cardiac progenitor cells contribute to the formation of a functional heart by making lineage choice decisions at a single-cell level (Wu et al., 2006).
In addition to myocardial expansion within the heart tube, two heart fields contribute cardiac progenitor cells at the anterior and venous poles of the heart tube. The first heart field is located bilaterally in the anterior splanchnic mesoderm and gives rise to cells that contribute to the left ventricle and both atria (Meilhac et al., 2004). Reliable molecular markers of the first heart field are lacking, though Tbx5 is associated with the first heart field (Takeuchi et al., 2003). The second heart field is pharyngeal mesoderm-derived and is marked by Isl1 expression (Cai et al., 2003). Cardiac progenitors migrate from the second heart field into the heart tube contributing cells to the atria, the right ventricle, and the outflow tract (Cai et al., 2003; Meilhac et al., 2004; Waldo et al., 2001; Zaffran et al., 2004). Lineage tracing experiments demonstrate that the second heart field also contributes cells to the inflow region (Cai et al., 2003).
It has been proposed that the first and second heart fields arise from a common cardiac progenitor. This model is suggested by retrospective clonal analysis in mice showing that two groups of β-gal+ cells (presumably clonally related) are found in the looping stage heart in both right and left ventricles (Meilhac et al., 2004). The first and second heart field precursors are presumably derived from a common progenitor that segregates into distinct heart field precursors early in development, probably at the onset of gastrulation (Huber et al., 2004). Further supporting this idea, recent collaborative work from Chien, Parker, and our lab, using a two-color fluorescent reporting system to isolate first and second heart field progenitors has revealed that the first and second heart field show distinct molecular signatures (Domian et al., 2009). Based on their differential expression patterns, we report that miRNA199a/b and miRNA200a/b may be useful as markers of the first and second heart fields, respectively. While a wealth of evidence exists that support the distinctness of the first and second heart field, identification of their common progenitor has proved quite challenging. Using mouse ES cells as an in vitro model of cardiac development,Kattman et al. (2006) identified a heterogeneous population of Bry+/Flk-1+ cells with cardiomyogenic potential that appeared in two successive waves. The first wave of Bry+/Flk-1+ cells were hemangioblasts and differentiated into hematopoietic and vascular lineages. The second wave of Bry+/Flk-1+ cells gave rise to colonies that could differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells. Some colonies derived from this second wave were Tbx5+ while others were Isl1+, suggesting that this second Bry+/Flk-1+ population may capture precursor cells for the first and second heart fields. It remains to be seen whether a Bry+/Flk-1+ population gives rise to both first and second heart field cells in vivo.
In addition to the first and second heart fields, recent studies support the existence of an epicardium-derived cardiac progenitor cell population. Zhou and colleagues (2008a; 2008b) found that Wilm’s Tumor 1 (WT1) expression marked cells in the developing proepicardium/epicardium that contribute to a minor population of cardiomyocytes during normal heart development. These WT1+ cells overlapped in part with Nkx2.5+ and Isl1+ cardiac progenitors, suggesting that these epicardial progenitors share a developmental program with other, previously described multipotent cardiac progenitors. In addition to WT1, Tbx18 has also been used to identify proepicardial cells that give rise to cardiomyocytes in the ventricular septum and atrial and ventricular walls (Cai et al., 2008). Because Tbx18 is expressed in septal and LV myocardium, the use of Tbx18 to label epicardial progenitor cells has recently been challenged however, because direct myocardial Tbx18 expression is present (Christoffels et al., 2009). Epicardium-derived cardiomyocytes appear to contribute to all four chambers of the heart (Cai et al., 2008; Zhou et al., 2008a; Zhou et al., 2008b). While epicardial progenitors theoretically provide an attractive population of cells to be used in cardiac regeneration or repair, their ability to produce functional cardiomyocytes in an infarcted heart remains to be demonstrated. In zebrafish, the epicardium promotes cardiac regeneration by invading the wound site and creating a complex vascular network (Lepilina et al., 2006). Therefore, it is possible that the epicardium’s greatest contribution to cardiac regeneration lies not in its ability to differentiate directly into new cardiomyocytes, but in its ability to promote endogenous cardiac progenitor cell expansion and neovascularization by paracrine signaling to surrounding cells. Future research on the regenerative role of epicardium-derived cardiac progenitors should include an assessment of both its ability to directly contribute cardiomyocytes and its role in mediating paracrine effects.
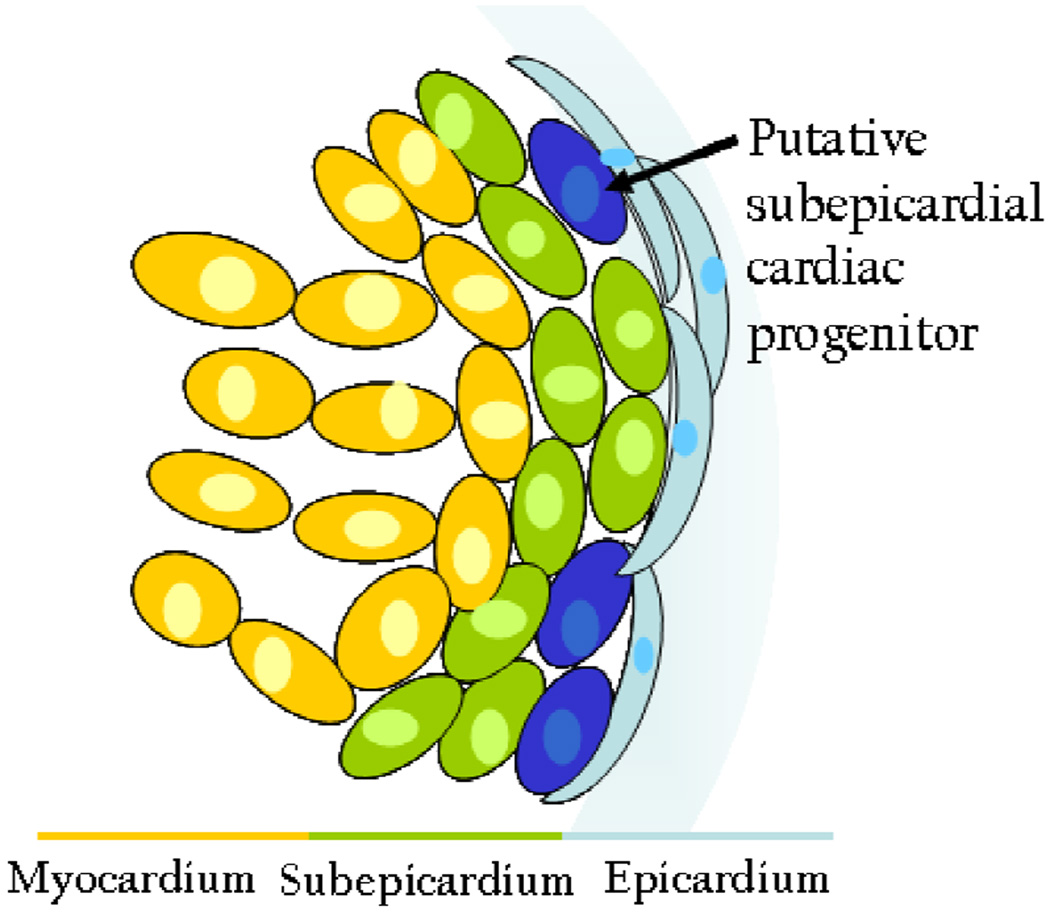
During embryonic development, the epicardium regulates myocardial proliferation and differentiation through FGF signaling (Lavine et al., 2005). Furthermore, work from our lab shows that in mouse, all new cardiomyocytes arise from the subepicardium (S.M. Wu, unpublished data). Our findings are consistent with recent reports that cardiomyocytes derived from the subepicardium constitute the major contribution to zebrafish cardiac regeneration (Kikuchi et al., 2010). Considering the role of the epicardium during both cardiac development and regeneration, we support the notion that the epicardium may serve as a niche for cardiac progenitors (Figure 2).
Figure 2. The epicardium as a niche for cardiac progenitor cells.
During development, the epicardium regulates myocardial proliferation via FGF signaling, and recent studies indicate that cardiomyocytes arise from the subepicardium during cardiac regeneration. Given these roles, the epicardium may serve as a niche for developing cardiac progenitor cells.
Neonatal and Adult Cardiac Progenitors
Though it has long been thought that a resident cardiac progenitor population does not exist in the postnatal heart (Rosenthal, 2003), the discovery of embryonic cardiac progenitors prompted a search for such progenitors in the neonatal and adult heart. The persistence of cardiac progenitors into the adult heart would provide an avenue to direct regeneration of cardiomyocytes that are lost due to cardiac injury. The opportunity to bypass the need for cell transplantation would overcome one of the major challenges in regenerative medicine.
Laugwitz and colleagues (2005) report the identification of neonatal Isl1+ cardiac progenitors in mouse, rat, and human that can develop into fully mature, functional cardiomyocytes. Notably, the conversion of Isl1+ progenitors to mature cardiomyocytes is not the result of fusion of cardiac progenitors with mature cardiomyocytes (Laugwitz et al., 2005). It is unclear, however, whether neonatal and embryonic Isl1+ cardiac progenitors are related and whether Isl1+ cardiac progenitors are lineage precursors to any of the described adult cardiac progenitor populations.
In the adult mouse, three populations of adult cardiac progenitor cells have been identified: Lin−/c-Kit+, Sca-1+, and side population (SP). Self-renewing, clonogenic Lin−/c-Kit+ cells can reportedly give rise to cardiomyocytes, endothelial, and smooth muscle cells in vitro and form functional myocardium in vivo (Beltrami et al., 2003). Based on expression of Sca-1+, a population of cardiac cells were found to have the potential for self-renewal and differentiate into cardiomyocytes in vitro (Matsuura et al., 2003; Oh et al., 2003). Furthermore, transplanted Sca-1+ cardiac progenitor cells can migrate to and engraft the site of injured myocardium by fusing with resident cardiomyocytes (Oh et al., 2003). Stem cell-like side population (SP) cells, identified by their expression of ABCG2 and ability to exclude Hoechst dye, have been isolated from many adult tissues and are known to contribute to diverse lineages (Akasura and Rudnicki, 2002). Cardiac SP cells persist throughout development into the adult heart and are capable of proliferation and differentiation into both cardiac and hematopoietic lineages (Martin et al., 2003).
In humans, atrial and ventricular biopsy cells can develop into “cardiospheres” in culture, which are composed of cells expressing the stem cell marker c-Kit and endothelial progenitor markers. Human cardiosphere cells and human c-Kit+ cells are reportedly capable of long-term self-renewal and multilineage differentiation into cardiomyocytes, endothelial cells, and vascular smooth muscle cells when transplanted into immunodeficient mice (Bearzi et al., 2007; Messina et al., 2004). Furthermore, human c-Kit+ cells were shown to regenerate myocardium in infarcted rodent hearts via a fusion-independent mechanism (Bearzi et al., 2007).
Despite the wealth of published studies on adult cardiac stem/progenitor cells, there is relatively little known about the developmental origin of these cells and their contribution to myocardial homeostasis and/or repair after injury. Using an eGFP-labeled bone marrow-derived cells transplantation system, it was found that a majority of the c-Kit+ cells within the injured adult heart originated from the circulation (Fazel et al., 2006). Since c-Kit, Sca-1, and ABCG2 (SP) are all known markers for hematopoietic cells, it is likely that at least some of these cell populations are bone marrow/circulatory in origin.
Enhancing the Therapeutic Potential of Cardiac Progenitor Cells and Cardiomyocytes
For cardiac progenitor and cardiomyocyte populations to be therapeutically useful, we must be able to produce them in sufficient numbers and direct them to differentiate into fully mature, functional cardiomyocytes. Transplantation of cardiac progenitors into the infarcted heart has shown very limited ability to mediate cardiac regeneration and repair due to massive cell death and inefficient differentiation of progenitors into cardiomyocytes. These limitations have prompted investigations into specific factors that promote cardiac progenitor and cardiomyocyte survival, proliferation, and differentiation.
To improve cell engraftment after transplantation,Laflamme et al. (2007) employed a pro-survival cocktail that broadly inhibits multiple mechanisms of cell death, including anoikis and apoptosis via mitochondrial death pathways, ATP channel activity, Akt signaling, and caspase activity. This pro-survival cocktail improved the rate of successful ES cell-derived myocardial graft formation in infarcted rat hearts without teratoma formation. Treatment of c-Kit+ cardiac progenitor cells with HGF (hepatocyte growth factor) and IGF-1 (insulin-like growth factor 1) activated these cells to proliferate while reducing the levels of cell death due to anoikis following transplantation into damaged myocardium (Tillmanns et al., 2008).
Although its mechanism remains to be clarified, the cytokine HMGB1 (high motility group protein B1) activates adult c-Kit+ stem cells to proliferate and transmigrate following infarction in mouse, leading to myocardial regeneration and improved cardiac performance (Limana et al., 2005). Additionally, signals such as retinoic acid, FGF (fibroblast growth factor), Thymosin β4, and Tbx18 have been reported to trigger epicardial mobilization after cardiac injury suggesting that paracrine, non-cell-autonomous pathways drive angiogenesis (and possible myogenesis) following cardiac injury (Lepilina et al., 2006; Smart et al., 2007). Moreover, it has been suggested that the binding of the α2-chain of laminin and fibronectin to α4-integrins on cardiac stem cells is involved in maintaining cardiac stem cells in an undifferentiated state (Urbanek et al., 2006). Altogether, these data support the notion that promoting ligand/receptor interaction on cardiac progenitor/stem cells may be a reasonable therapeutic strategy.
To address whether a developmental remnant cardiac progenitor cell population truly exists in the postnatal heart and participate, potentially, in myocardial repair after injury, we recently generated a line of inducible Nkx2.5 cardiac enhancer-eGFP-Cre transgenic mice (Liu et al., unpublished data). These mice were bred with ROSA26-LacZ reporter mice to lineage trace postnatal cardiomyogenic precursor cells. By suppressing Cre expression with doxycycline during embryonic development, we found robust contribution of Nkx2.5+ cells in the neonatal heart to cardiomyogenesis and in the myocardium of a post-injury heart (Liu et al., unpublished data). While the extent of Nkx2.5+ cell contribution is modest (~4% of total myocardium), it raises the possibility that these cells in the adult heart may be modulated pharmacologically to enhance cardiomyogenesis post-myocardial infarction.
Enhancing cardiomyocyte division
Although cardiomyocytes were long believed to be terminally differentiated and incapable of cell division, recent studies demonstrate that human cardiomyocytes renew over the adult lifespan, albeit at a very slow rate that declines with age (Bergmann et al., 2009; Hsieh et al., 2007). The mechanism of this renewal and the source of the new cardiomyocytes are unknown, but it is possible that cardiomyocytes may arise de novo from the epicardium (Laugwitz et al., 2005). The dogma that cardiomyocytes are in a terminally differentiated state and do not normally undergo cell division has recently been challenged. In vivo, neuregulin-1, FGF1, and periostin can induce differentiated cardiomyocytes to reenter the cell cycle and undergo cell division, promoting cardiac repair (Bersell et al., 2009; Engel et al., 2005; Kuhn et al., 2007).
Future Perspective
While results from cell-based transplantation to achieve cardiac regeneration have gained some early successes so far, there remain a significant number of limitations to this approach. The first challenge lies in obtaining sufficient quantities of mature cardiomyocytes. Current techniques are unable to drive cardiac progenitor cells to undergo the proliferation and efficient differentiation needed to generate a sufficient supply of cardiomyocytes for transplantation. The use of ES cells is theoretically able to overcome the cell number limitation; however, ES cell-derived cardiomyocytes display an immature phenotype (Mummery et al., 2003). It is unclear if ES cell-derived cardiomyocytes will ever be able to reach a similar degree of maturation after transplantation as adult cardiomyocytes, aside from the ethical concerns regarding the use of human embryonic or fetal cells. Much excitement surrounded the discovery that human somatic cells can be reprogrammed into induced pluripotent stem (iPS) cells by cell fusion or somatic cell nuclear transfer because it offered a means of generating stem cells from adult cells, thereby bypassing the need to for embryo destruction (Takahashi et al., 2007; Yu et al., 2007). However, mouse iPS cell-derived cardiomyocytes show a greater degree of heterogeneity compared with ES cell-derived cardiomyocytes and some iPS cell lines exhibit an impaired ability to mature into well-differentiated, functional cardiomyocytes (S.M. Wu, unpublished data). Very recently, it was reported that iPS cells differ epigenetically from embryonic stem cells (Urbach et al., 2010), which may limit their ability to form mature, functional cardiomyocytes.
Provided that these cell number and maturation concerns can be overcome, immune rejection of the transplanted cells will continue to be of significant concern unless iPS cells are used. The use of pluripotent stem cells such as ES and iPS cells poses a significant risk of teratoma formation unless the transplanted cells are exquisitely pure—a feat which has not been achieved satisfactorily for any pluripotent stem cell lineage thus far. Further studies to identify surface markers that allow for isolation of highly purified cardiac progenitor cells will help overcome this issue.
If the transplanted cells are able to successfully engraft the infarcted myocardium, the graft must be long-lived and able to electrically couple with the host myocardium to achieve cardiac repair. To-date, no long-term functional improvements have been documented following cardiac progenitor or cardiomyocyte transplantation into infarcted myocardium. In most cell-based transplantation studies thus far, the cardiac benefit is short-lived which is likely due to effects from paracrine signaling (Chien, 2006). Therefore, it is crucial that the neocardiomyogenesis achieved for cell-based therapies is durable in the long-term. To do so, a comprehensive understanding of the mechanism by which functional myocardium is generated during embryonic development will be required. Ultimately, this knowledge will guide and refine our strategy for cardiac regeneration in order to address this important unmet medical need.
References
- Akasura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30(11):1339–1345. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Tromifova I, Siggins RW, LeCapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajsutra J, Leri A, Anversa P. Human cardiac stem cells. Proc Nat Acad Sci USA. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajsutra J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3(1):69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Cai C, Liang X, Shi Y, Chu P, Pfaff SL, Chen J, Evans SM. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR. Lost and found: Cardiac stem cell therapy revisited. J Clin Invest. 2006;116(7):1838–1840. doi: 10.1172/JCI29050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MTM, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–E9. doi: 10.1038/nature07916. [DOI] [PubMed] [Google Scholar]
- David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H, Rupp R, Franz WM. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10(3):338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326(5951):426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li R. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: Final Data for 2006. National Vital Statistics Reports. 2009;57(14):1–135. [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13(8):970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Katz AM. Cardiomyopathy of overload: An unnatural growth response in the hypertrophied heart. Ann Intern Med. 1994;121(5):363–371. doi: 10.7326/0003-4819-121-5-199409010-00009. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, MacRae CA, Stainier DYR, Poss KD. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of the cardiac mesoderm. Development. 2000;127:3215–3226. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- Komuro I, Izumo S. Csx: A murine homeobox-containing gene specifically expressed in the developing heart. Proc Nat Acad Sci USA. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Nat Acad Sci USA. 2005;102(37):13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang Y, Lebeche D, Arab S, Keating MT. Periostic induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13(8):962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Laugwitz K, Moretti A, Lam JT, Gruber P, Chen Y, Woodard S, Lin L, Cai C, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans SM, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;460(7251):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kukuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Limana F, Germani A, Zacheo A, Kajsutra J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac c-kit+ cell proliferation and differentiation. Circ Res. 2005;97:73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Murphy TL, Langer EM, Cai M, Mashayekhi M, Wang W, Niwa N, Nerbonne JM, Kyba M, Murphy KM. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell. 2008;3(1):55–68. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2003;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2003;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–598. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronica MVG, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L MS, Martin-Puig S, Sun Y, Evans SM, Laugwitz K, Chien KR. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward-can Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335(16):1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Nat Acad Sci USA. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawles ME. The heart-forming areas of the early chick blastoderm. Physiol Zool. 1943:22–42. [Google Scholar]
- Rosenthal N. Prometheus's vulture and the stem-cell promise. N Engl J Med. 2003;349:267–274. doi: 10.1056/NEJMra020849. [DOI] [PubMed] [Google Scholar]
- Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. [Review] [37 refs] Trends Cardiovasc Med. 2000;10(8):345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki JI, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses KA, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:1–12. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Ohgi M, Koshiba-Takeuchi K, Shiratori H, Sakasi I, Ogura K, Saijoh Y, Ogura T. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development. 2003;130:5953–5964. doi: 10.1242/dev.00797. [DOI] [PubMed] [Google Scholar]
- Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, LeCapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajsutra J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Nat Acad Sci USA. 2008;105(5):1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of Fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajsutra J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Nat Acad Sci USA. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kuminski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wu SM. Mesp1 at the heart of mesoderm lineage specification. Cell Stem Cell. 2008;3(1):1–2. doi: 10.1016/j.stem.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132(4):537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto M, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008a;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT. Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun. 2008b;375(3):450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]