Removal of 9-O-acetyl residues from the cell surface N-acetylneuraminic acid makes ALL cells drug sensitive.
Abstract
The development of resistance to chemotherapy is a major cause of relapse in acute lymphoblastic leukemia (ALL). Though several mechanisms associated with drug resistance have been studied in detail, the role of carbohydrate modification remains unexplored. Here, we investigated the contribution of 9-O-acetylated N-acetylneuraminic acid (Neu5Ac) to survival and drug resistance development in ALL cells. A strong induction of 9-O-acetylated Neu5Ac including 9-O-acetyl GD3 was detected in ALL cells that developed resistance against vincristine or nilotinib, drugs with distinct cytotoxic mechanisms. Removal of 9-O-acetyl residues from Neu5Ac on the cell surface by an O-acetylesterase made ALL cells more vulnerable to such drugs. Moreover, removal of intracellular and cell surface–resident 9-O-acetyl Neu5Ac by lentiviral transduction of the esterase was lethal to ALL cells in vitro even in the presence of stromal protection. Interestingly, expression of the esterase in normal fibroblasts or endothelial cells had no effect on their survival. Transplanted mice induced for expression of the O-acetylesterase in the ALL cells exhibited a reduction of leukemia to minimal cell numbers and significantly increased survival. This demonstrates that Neu5Ac 9-O-acetylation is essential for survival of these cells and suggests that Neu5Ac de-O-acetylation could be used as therapy to eradicate drug-resistant ALL cells.
Pre-B acute lymphoblastic leukemia (ALL) develops through malignant transformation of precursor B lineage cells in the BM. ALLs are stratified into different risk categories based on characteristics such as age and the presence of chromosomal abnormalities. For example, overall cure rates of pediatric patients currently exceed 80% to >90–95% for some patient subsets, whereas those of older patients is only 40% (Pui et al., 2008; Salzer et al., 2010; Gaynon et al., 2010; Moorman, 2012). However, ALLs in all age groups that are characterized by the presence of the Philadelphia (Ph)-chromosome have a worse prognosis, with a rapid development of resistance to drug treatment (Lee et al., 2011; Schrappe et al., 2012). The Ph-chromosome arises as one product of a reciprocal translocation between chromosomes 9 and 22. At the breakpoint, on the Ph-chromosome, a chimeric BCR/ABL oncogene is generated encoding a fusion protein with deregulated tyrosine kinase activity (Groffen and Heisterkamp, 1997; Pasternak et al., 1998; Ren, 2005). We generated a transgenic BCR/ABL mouse model for this type of ALL and are investigating mechanisms through which such leukemias are able to become drug tolerant and resistant (Kaur et al., 2007; Fei et al., 2010a,b; Parameswaran et al., 2010, 2011, 2012; Duy et al., 2011; Park et al., 2011; Feldhahn et al., 2012). However, it is currently unknown whether pre-B ALL cells that develop drug resistance have modified cell surface glycosylation.
Sialic acids (Sia, e.g., N-acetylneuraminic acid [Neu5Ac]) are a family of 9-carbon acidic carbohydrates that are widely distributed in animal tissues, mostly found as α-ketosidically linked capping structures on the glycans associated with glycoconjugates such as glycoproteins and gangliosides (Yu and Ledeen, 1969). The Neu5Ac-associated hydroxyl groups may be further modified with various functional groups including acetyl, lactyl, methyl, sulfate, or phosphate groups. Sialylation, the addition of sialic acid moieties, is an important type of glycosylation that may alter the physical properties of molecules in the plasma membrane and may regulate immune cell function, as well as serve as specific ligands for certain toxins and lectins (Varki and Varki, 2007; Schauer, 2009).
Lectins are proteins that can detect specific carbohydrate structures and are widely used as species-independent detection tools. Interestingly, Achatinin H, a lectin which has a preferential specificity toward terminal 9-O-acetylated sialic acids α2-6-linked to N-acetylgalactosamine, has been proposed to be a useful diagnostic marker for childhood ALL and detect minimal residual disease (Chowdhury and Mandal, 2009). Furthermore, Mukherjee et al. (2008), using thin-layer chromatography on total cell lysates from childhood ALL peripheral blood (PB) mononuclear cells, found that a percentage of the disialoganglioside GD3 is modified by acetylation on the C9 position of the terminal Neu5Ac residue (Ren et al., 1992). Their experiments with a T cell leukemia cell line further suggested a link between 9-O-acetylation of GD3 and T lymphocyte apoptosis because chemical removal of the 9-O-acetyl group by treatment with salicylic acid caused cell death.
In the current study, we have investigated whether O-acetylation of Neu5Ac in glycoproteins/lipids, and in the ganglioside GD3 in particular, plays a role in the development of drug resistance and survival of adult pre-B ALL cells. We also used a lectin, isolated from the Californian crab Cancer antennarius, that binds specifically to Neu5Ac-containing glycoproteins and gangliosides that have been further modified by 4-O or 9-O-acetylation (9-O-Ac; Ravindranath et al., 1985) as a detection tool. Herein we report that induction of 9-O-acetylation on Neu5Ac is associated with drug resistance in ALL cells and also represents an unexpected Achilles heel for such leukemia cells because its removal is incompatible with ALL cell survival, regardless of whether these cells are co-cultured with stromal cells or are located in the very protective BM environment.
RESULTS
Malignant pre-B cells express 9-O-acetylated GD3 (9-O-Ac–GD3)
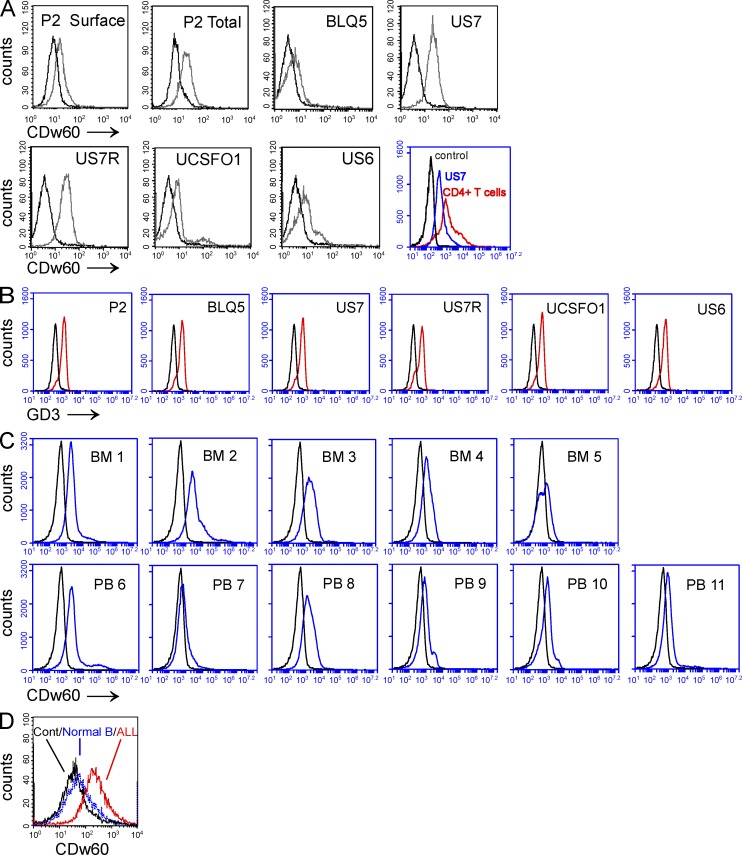
The human pre-B ALL cells used in this study were primary samples from patients that were passaged in NSG mice and then grown ex vivo on OP9 stromal cells. We have used this co-culturing technique to model the environment of leukemic cells in the BM (Fei et al., 2010b; Parameswaran et al., 2010, 2011; Duy et al., 2011; Park et al., 2011). We included both Ph-positive and -negative ALLs in this and subsequent analysis because these distinct subclasses of ALL have a different prognosis and first-line therapy. We first investigated whether these cells express disialoganglioside GD3 and whether it is modified by 9-O-acetylation, using antibodies that specifically detect the CDw60 (also known as CD60b) epitope, representing 9-O-Ac–GD3. Because GD3 is synthesized in the Golgi (Chen and Varki, 2002) and subsequently transported to the plasma membrane, we analyzed both intracellular and surface expression of 9-O-Ac–GD3. As shown in Fig. 1 A, 9-O-Ac–GD3 was clearly expressed on the surface of P2 ALL cells. The right shift in CDw60 detection after permeabilization indicated the presence of 9-O-Ac–GD3 inside the ALL cells as well. We also confirmed CDw60 and GD3 expression in several other human ALL samples (Fig. 1, A and B; and three additional samples not depicted). We used T cells from human PB gated on CD4 as a positive control for CDw60 detection (Fig. 1 A). Because these ALL cells have been passaged in mice and cultured in vitro, we also analyzed CDw60 expression in gated CD19+, CD10+ pre-B cells directly from BM and PB of several ALL patients (Fig. 1 C) to exclude the possibility that CDw60 induction has occurred during the cell culture process. CDw60 expression levels were also compared with those of pre-B cells in normal human BM. As shown in Fig. 1 D, there was little, if any CDw60 in normal CD19+, CD10+ IgM− pre-B cells but clear expression in leukemic pre-B cells from primary human BM samples. This showed that 9-O-acetylation of GD3 is specific for transformed malignant pre-B cells.
Figure 1.
Expression of 9-O-Ac–GD3 in human pre-B ALL cells. (A) Presence of cell surface or total (intracellular + cell surface) 9-O-Ac–GD3 measured using FACS and antibodies against the CDw60 epitope, as exemplified by human relapse Ph-positive ALL P2 and total 9-O-Ac–GD3 expression in different human ALLs including Ph positive at relapse (BLQ5 and UCSFO1), Ph negative ALL (US6), and the same patient at presentation (US7) and relapse (US7R). The experiment was performed two times. Control: CD4-positive T cells gated out from PB of a normal donor and analyzed for 9-O-Ac–GD3 surface expression (red) compared with US7 ALL cells (blue) and isotype control (black). Gray lines indicate 9-O-Ac–GD3 staining. (B) Total GD3 expression in different ALL cells, control (black) and GD3 staining (red). (C) Cell surface expression of 9-O-Ac–GD3 in CD19+, CD10+ pre-B cells from BM and PB from ALL patients, as detected by the anti-CDw60 antibody. (D) CDw60 staining in primary CD19+, CD10+, sIgM− pre-B cells from the BM of a normal donor (blue), ALL patient (red), and isotype control (black). B–D show single experiments.
Absence of GD3 protects leukemia cells from drug-induced apoptosis
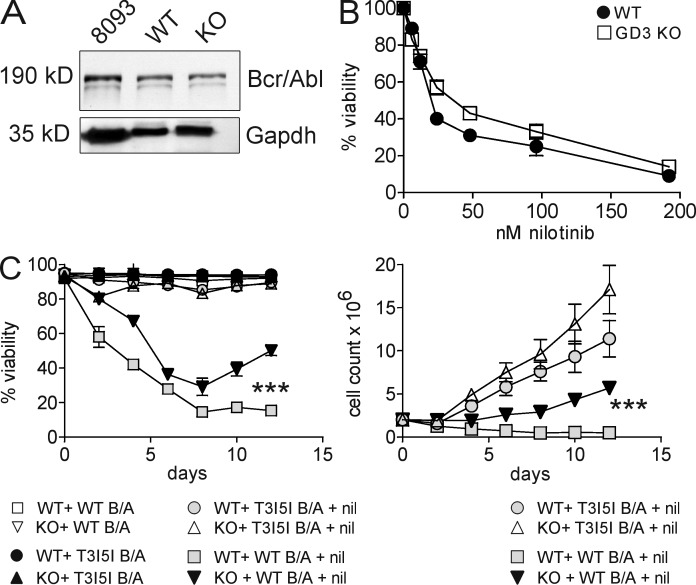
To study the function of GD3 and 9-O-Ac–GD3 expression in pre-B leukemia cells, we generated BCR/ABL-transduced pre-B ALL cells from mice that lack GD3 synthase (St8sia1), the enzyme critical for GD3 synthesis (Chen and Varki, 2002; Fukuda et al., 2005). Equal expression of Bcr/Abl was confirmed by Western blot analysis (Fig. 2 A). We next compared the sensitivity of these cells to nilotinib. This is one of the targeted drugs that is used to treat Ph-positive leukemias and inhibits the tyrosine kinase activity of Bcr/Abl. The IC50nilotinib values of pre-B ALL cells lacking GD3 and of WT were 36 nM and 20 nM, respectively (Fig. 2 B). Interestingly, loss of GD3 also significantly affected the ability of the pre-B ALL cells to recover from long-term nilotinib treatment compared with WT pre-B ALL cells. As shown in Fig. 2 C, whereas WT pre-B ALL cell cultures were eradicated by treatment with 24 nM nilotinib over a 12-d period, lack of GD3 synthase delayed the effect of this drug on ALL cell viability (Fig. 2 C, left) and viable cell counts (Fig. 2 C, right) and allowed pre-B ALL cells to persist and resume proliferation. As a negative control, we also transduced pre-B cells with a BCR/ABL construct that carries the T3151. This mutation in the ATP-binding site of the Abl kinase makes the cells resistant to nilotinib. As expected, pre-B cells transduced with the T315I BCR/ABL mutant did not respond to nilotinib. These data demonstrate that the expression of GD3 in pre-B ALL cells in fact impairs the development of drug resistance.
Figure 2.
Pre-B ALL cells lacking GD3 synthase show reduced drug sensitivity compared with WT pre-B ALL cells. (A) Western blot analysis of transduced pre-B cells from WT (st8sia1+/+) and KO (st8sia1−/−) mice using anti-Bcr antibodies to detect Bcr/Abl P190 protein. 8093 mouse ALL cells from a leukemic BCR/ABL transgenic mouse served as a positive control; Gapdh was a loading control. (B) Dose–response curve to nilotinib showing IC50 of transduced WT and KO pre-B ALL cells after 72 h of incubation. (C) Viability (left) and viable cell numbers (right) of nonmutated or T315I-mutated Bcr/Abl-transduced cells treated with 24 nM nilotinib or DMSO control. ***, P < 0.001 (viability and cell counts) for WT-WT Bcr/Abl + nil compared with KO-WT Bcr/Abl + nil day 12. Error bars show the standard deviation of triplicate samples. Experiments were performed two times.
We also investigated whether GD3 surface expression correlated with drug sensitivity to nilotinib by examining five different human Ph-positive ALLs lacking point mutations in the Abl tyrosine kinase domain but with distinct sensitivity to nilotinib. The untreated cells had different levels of GD3 cell surface expression, but there was no clear correlation of this with nilotinib response (not depicted).
Increasing GD3 levels causes apoptosis in ALL cells
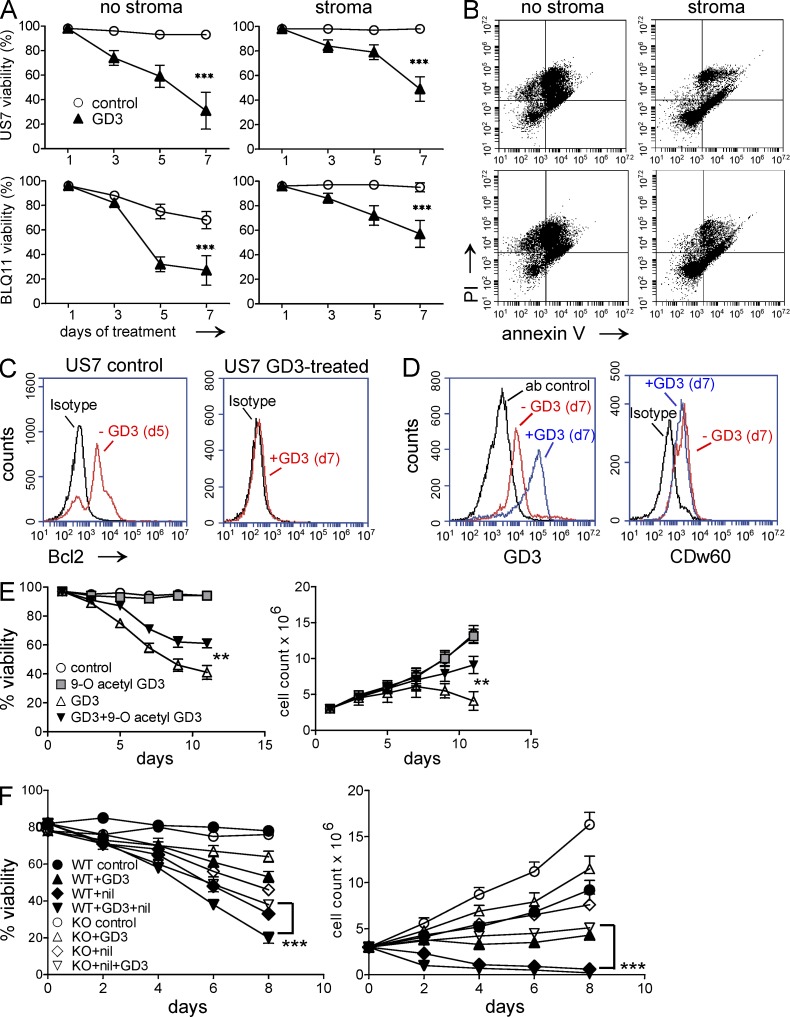
In HEK-293, T cell, melanoma, and glioblastoma cell lines, the 9-O-Ac modification of GD3 was shown to be protective in nature, by neutralizing the proapoptotic effect of GD3 (Birklé et al., 1999, 2000; Malisan et al., 2002; Kniep et al., 2006). Thus 9-O-acetylation of GD3 as found in the ALL cells could be a defense mechanism to escape from apoptosis. If the balance of GD3 with its acetylated form determines cell survival, the disturbance of that balance, by increasing GD3 levels or by de-O-acetylating existing 9-O-Ac–GD3, should lead to cell death. To test this, we incubated the ALLs without OP9 stroma in the presence of exogenously added GD3. The human patient–derived pre-B ALL cells used in this study lost viability in the absence of stromal support, but, as shown in Fig. 3 A (left), cultures treated with GD3 were more severely affected. The effect on diagnosis (US7 and TXL2) and relapsed (BLQ-1, BLQ-11, and US7R) patient samples were similar (not depicted). We then added GD3 to Ph-positive and -negative ALL cells co-cultured with stroma to investigate whether stroma has a protective effect against GD3-mediated ALL cell apoptosis. Although stromal support improved survival of untreated cells, GD3 treatment still caused significant loss of viability (Fig. 3 A, right), which correlated with increased Annexin V/propidium iodide (PI) staining of these cells (Fig. 3 B). Exogenous addition of GD3 had no effect on cell cycle, as observed after 12 and 24 h of incubation of ALL cells with 50 µM GD3 (not depicted). Rippo et al. (2000) reported that enforced expression of Bcl-2 prevents GD3-induced mitochondrial changes, suggesting that targets of GD3-mediated death are controlled by Bcl-2. In concordance with this, we found that the decrease in viability caused by GD3 exposure correlated with a marked decrease in prosurvival Bcl-2 protein levels as determined by FACS (Fig. 3 C).
Figure 3.
Exogenously added GD3 causes apoptosis of human and mouse pre-B ALL cells. (A) Viability of human pre-B ALLs in the absence (left) or presence (right) of irradiated OP9 stroma after treatment with 20 µM GD3. Experiments were performed independently three times. Control versus GD3: ***, P < 0.001 day 7. (B) Annexin V/PI staining of GD3 treated cells from A at day 7. (C) Bcl-2 expression before and after 7 d of exposure to 20 µM GD3 in US7 cells in the presence of stroma. The experiment was independently replicated once. (D) Expression of total GD3 and 9-O-acetylated GD3 on day 7 of 20-µM GD3 treatment in the presence of stroma. Samples are as indicated. The experiment was performed four times. (E) Viability (left) and viable cell numbers (right) of US7 cells treated with 50 µM GD3, 100 µM 9-O-Ac–GD3, or a combination of both. GD3 and 9-O-Ac–GD3 were added every alternate day. Untreated cells served as control. **, P < 0.01 (viability and cell counts day 12) for GD3- compared with GD3 + 9-O-Ac–GD3–treated cells. (F) Viability (left) and viable cell numbers (right) of BCR/ABL-transduced WT and KO cells not treated or treated with 50 µM GD3, 12 nM nilotinib, or both. Experiments in E and F were performed twice. Error bars show the standard deviation of triplicate samples. ***, P < 0.001 (viability and cell count d8) for WT + GD3 + nil compared with KO + GD3 + nil.
It was reported that in Chinese hamster ovary (CHO) and skin fibroblast cell lines, newly synthesized or exogenously added GD3 is converted to 9-O-Ac–GD3 inside the cell and thus protects from GD3-mediated apoptosis (Chen et al., 2006). We therefore investigated whether pre-B ALL cells can incorporate exogenous GD3 and modify it to the 9-O-acetylated form. As shown in Fig. 3 D, there was a clear increase in GD3 detected by FACS on the cells treated with GD3, compared with untreated cells. Unexpectedly, levels of 9-O-Ac–modified GD3 were not increased (Fig. 3 D, right).
We next examined whether exogenously added 9-O-Ac–GD3 can reverse the proapoptotic effect induced by GD3. Interestingly, as shown in Fig. 3 E, addition of 9-O-Ac–GD3 significantly increased both viability and viable cell counts of GD3-treated cells. St8sia1−/− pre-B ALL cells proliferated more rapidly and showed less sensitivity toward nilotinib or GD3 monotreatment than pre-B st8sia1+/+ ALL cells (Fig. 3 F). The combination treatment using nilotinib and GD3 further decreased viability and cell numbers of both st8sia1−/− and st8sia1+/+ pre-B ALL cells. These data show that GD3 is cytotoxic to ALL cells and indicate that the balance between GD3 and 9-O-acetylated GD3 determines ALL survival.
ALL cells express 9-O–Neu5Ac-containing glycoconjugates different from GD3
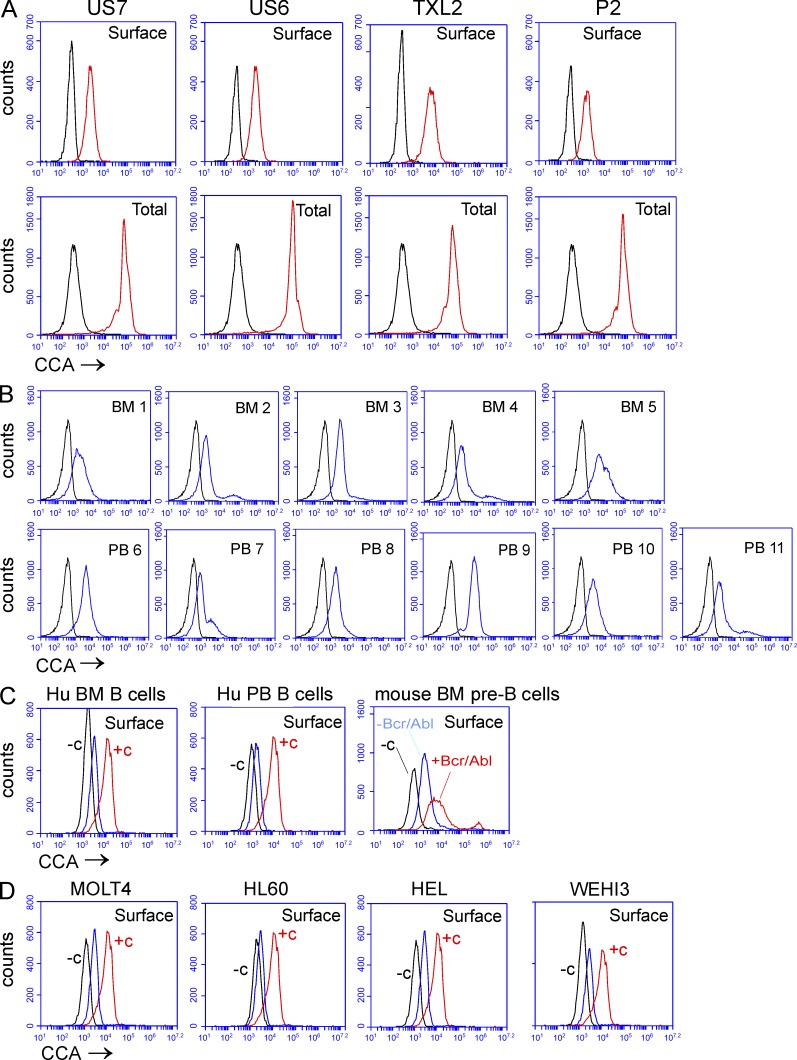
Though elevated 9-O-Ac on the terminal Neu5Ac in GD3 has been the most intensively studied in cancers including lung cancer, breast cancer, glioblastoma, melanoma, and basaliomas (Fuentes et al., 1997; Gocht et al., 1998; Birklé et al., 1999, 2000; Fahr and Schauer, 2001; Malisan et al., 2002; Erdmann et al., 2006), other sialoglycolipids and sialoglycoproteins can also be modified by 9-O-acetylation (Krishna and Varki, 1997; Schauer, 2009). We used the lectin from C. antennarius (CCA), which is able to detect O-acetylated Neu5Ac in a wider range of structures including glycoproteins and lipids, to examine this in the ALL cells. Compared with unstained controls, there was a clear presence of O-acetylated Neu5Ac–bearing structures on the surface of different human ALLs. Moreover, O-Ac Neu5Ac was also detected intracellularly in all ALL cells tested (Fig. 4 A, compare surface with total). We also confirmed the presence of O-acetylated Neu5Ac-bearing structures on the surface of CD19+ CD10+ pre-B BM and PB cells from different ALL patient samples (Fig. 4 B). Compared with US7 ALL cells, normal human BM CD19+ B cells overall had low expression of O-Ac Neu5Ac. In agreement with Krishna and Varki (1997), the expression on human CD19+ B cells from normal PB was low (Fig. 4 C, left two panels). Interestingly, whereas pre-B cells from normal mouse BM also contained low levels of CCA lectin–binding cell surface Neu5Ac, this was markedly increased (transformed/normal mean fluorescent intensity [MFI] ratio of 4.32) upon transformation by Bcr/Abl (Fig. 4 C, right). Surface expression of CCA lectin–reacting Neu5Ac on US7 ALL cells was significantly higher than that detected in other malignant hematopoietic cell lines, including the T cell leukemia MOLT-4, the myeloid leukemia HL-60, erythroleukemia HEL cells, and mouse myeloid leukemia WEHI-3 cells (Fig. 4 D). This suggests that these structures are characteristic of malignant pre-B cells.
Figure 4.
Human ALL cells express O-acetylated Neu5Ac detected by FITC–CCA lectin. FACS analysis showing cell surface binding of FITC–CCA lectin. (A) human ALL cells co-cultured with mouse stroma (black, control signal; red, CCA lectin signal). (B) CD19+, CD10+ pre-B cells from BM and PB of ALL patients (black, control; blue, CCA lectin). (C) Normal human BM B cells (left), normal human PB B cells (middle), or WT pre-B cells (red) with or (blue) without BCR/ABL transduction. (D) Non-ALL leukemia cells. In C and D, −c (black) indicates controls without CCA lectin, and +c indicates US7 staining (red) as positive reference sample; CCA lectin binding is shown in blue.
Cell surface expression of O-acetylated Neu5Ac correlates with development of drug resistance
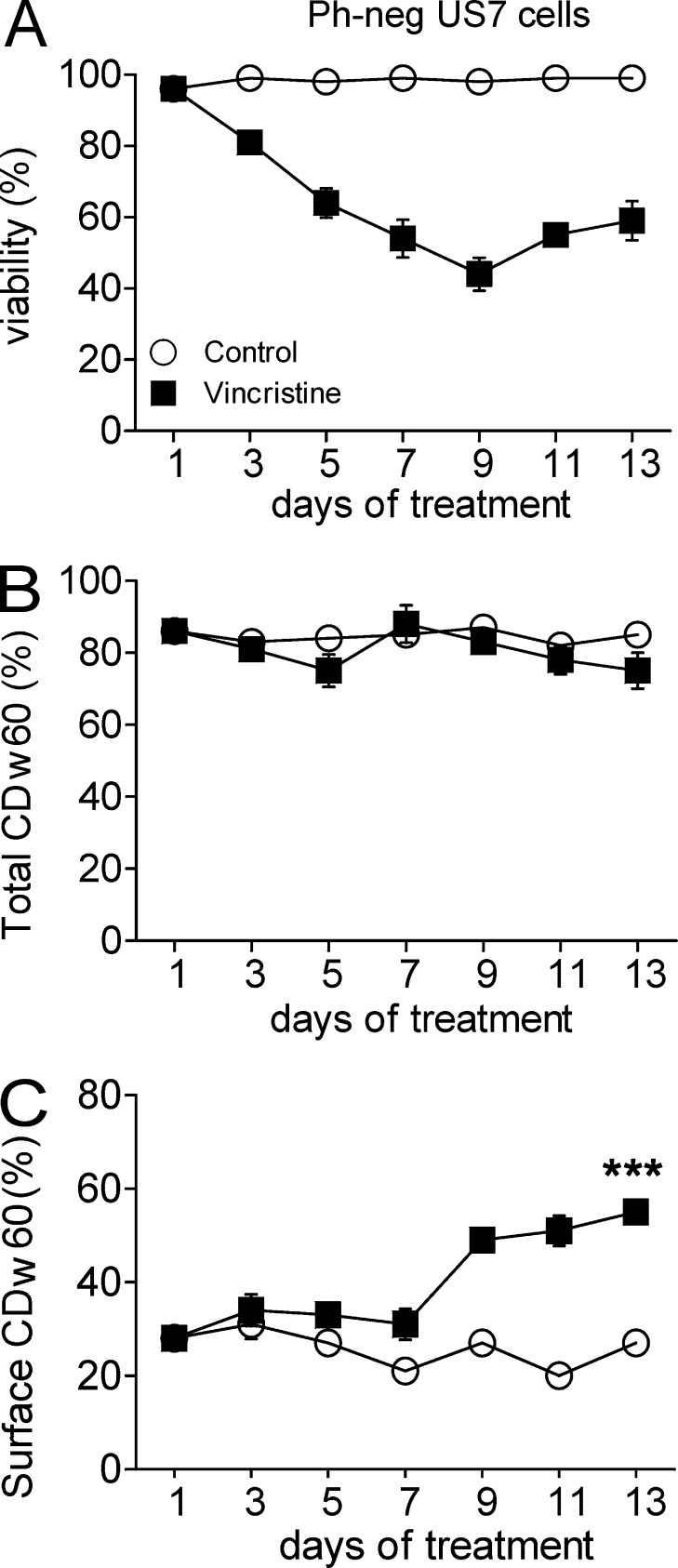
We next examined 9-O-Ac–GD3 expression and CCA lectin binding in ALL cells as they develop drug resistance. US7 is an ALL lacking the Ph-chromosome, and first-line treatment of such leukemias typically includes the chemotherapeutic drug vincristine. As shown in Fig. 5 A, in the course of 13 d of 2.5-nM vincristine treatments, viability of US7 cells stabilized and then improved after an initial drop. During this process, the total expression of 9-O-Ac–GD3 did not change in either vehicle- or drug-treated cells (Fig. 5 B). Interestingly however, the expression of the CDw60 epitope on the surface of cells increased (from 27 to 55% positive cells) in the phase of development of drug tolerance in the vincristine-treated US7 cells (Fig. 5 C).
Figure 5.
Increased cell surface expression of 9-O-Ac–GD3 correlates with the development of drug resistance. US7 cells on stroma treated with vehicle or 2.5 nM vincristine. Fresh drug was added every second day with fresh medium. (A) Viability of the culture. (B and C) Percentage of cells positive for total CDw60 (B) or cell surface CDw60 (C) as measured by FACS. The experiment was performed twice independently on triplicate samples. Surface CDw60 on day 13 control compared with vincristine: ***, P < 0.001. Error bars show the standard deviation of triplicate samples.
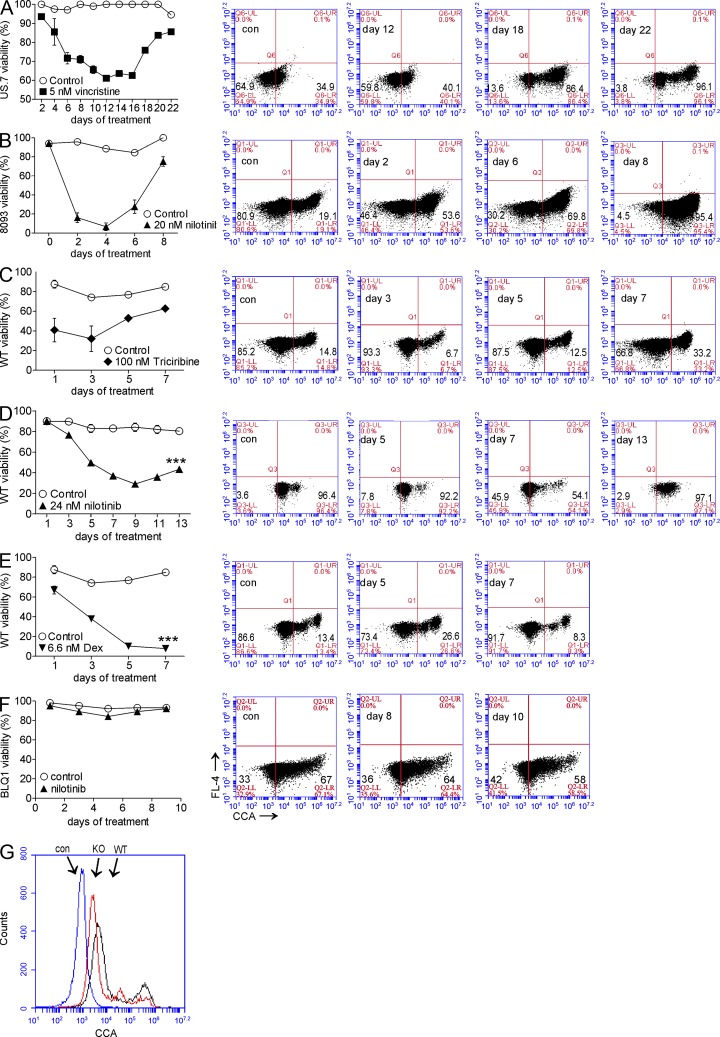
A similar increase in CCA lectin–reactive Neu5Ac structures on the cell surface was measured (MFI ratio US7 day 22 vincristine/US7 control = 5.4) in an experiment using US7 ALL cells and 5 nM vincristine (Fig. 6 A). We then assessed CCA lectin binding on mouse Bcr/Abl-expressing pre-B ALL cells and used different drugs to exclude the possibility that this increase is cell, drug, or species specific. BCR/ABL transgenic 8093 ALL cells that developed resistance to nilotinib similarly exhibited a marked increase in CCA lectin cell surface reactivity (MFI ratio 8093 day 8/8093 control = 4.81; Fig. 6 B). We extended these observations by drug treatment of the pre-B ALL cells generated by retroviral transduction of normal mouse pre-B cells with the Bcr/Abl tyrosine kinase. Fig. 6 (C and D) illustrates that both emerging tolerance to nilotinib and to the Akt inhibitor triciribine, drugs with very different mechanisms of action, correlated with increased CCA lectin–reacting cell surface expression. In contrast, resistance to dexamethasone did not develop under these conditions, and no increased CCA lectin signal was measured (Fig. 6 E). We further confirmed this by treating relapse human Ph-positive ALL cells, which express a T315I-mutated Bcr/Abl, with 24 nM nilotinib. These cells neither responded to the drug nor showed any increase in CCA signal (Fig. 6 F). These results show that there is a significant increase in expression of one or more O-acetylated Neu5Ac–bearing macromolecules, including GD3, associated with drug resistance.
Figure 6.
CCA lectin binding to ALL cells increases during development of drug resistance. (A–F, left) Percent viability. (right) FACS dot plot analysis showing surface CCA lectin binding of US7 cells treated with 5 nM vincristine (A), transgenic mouse 8093 pre-B ALL cells treated with 20 nM nilotinib (B), WT BCR/ABL pre-B ALL cells treated with 100 nM triciribine (C), 24 nM nilotinib (D), or 6.6 nM dexamethasone (E). ***, P < 0.001. (F) BLQ1 Ph-positive human ALL cells containing Bcr/Abl with a T315I mutation treated with 24 nM nilotinib. (G) Cell surface CCA lectin binding to GD3 synthase KO BCR/ABL-transduced cells, WT BCR/ABL-transduced cells, and unstained control cells as indicated. All experiments were performed two to three times. Error bars show the standard deviation of triplicate samples.
The availability of pre-B ALL cells lacking GD3 and thus 9-O-Ac–GD3 enabled us to evaluate to what extent the signal generated by the CCA lectin on the surface of pre-B ALL cells can be attributed to modified GD3. Interestingly, as shown in Fig. 6 G, cells lacking GD3 synthase still retained a strong CCA lectin signal on their surface, indicating that other glycoproteins or lipids, modified by 9-O-acetylation of Neu5Ac, are detected by this lectin.
9-O-acetylation of Neu5Ac is involved in pre-B ALL cell survival
One approach to address the significance of 9-O-acetylation on Neu5Ac is to use enzymes that cleave this linkage. Influenza C virus encodes a 9-O-acetylesterase that has been widely used to specifically remove 9-O-Ac from Neu5Ac-containing glycans and glycoconjugates in mouse and human cells (Varki et al., 1991; Malisan et al., 2002). We therefore used a CHE-Fc 9-O-acetylesterase to further examine the significance of 9-O-acetylated Neu5Ac to ALL cell survival. Interestingly, as shown in Fig. 7 A, esterase treatment over a 7-d period affected both GD3 synthase WT and KO pre-B ALL cells, with inhibition of proliferation in the more slowly growing WT cells.
Figure 7.
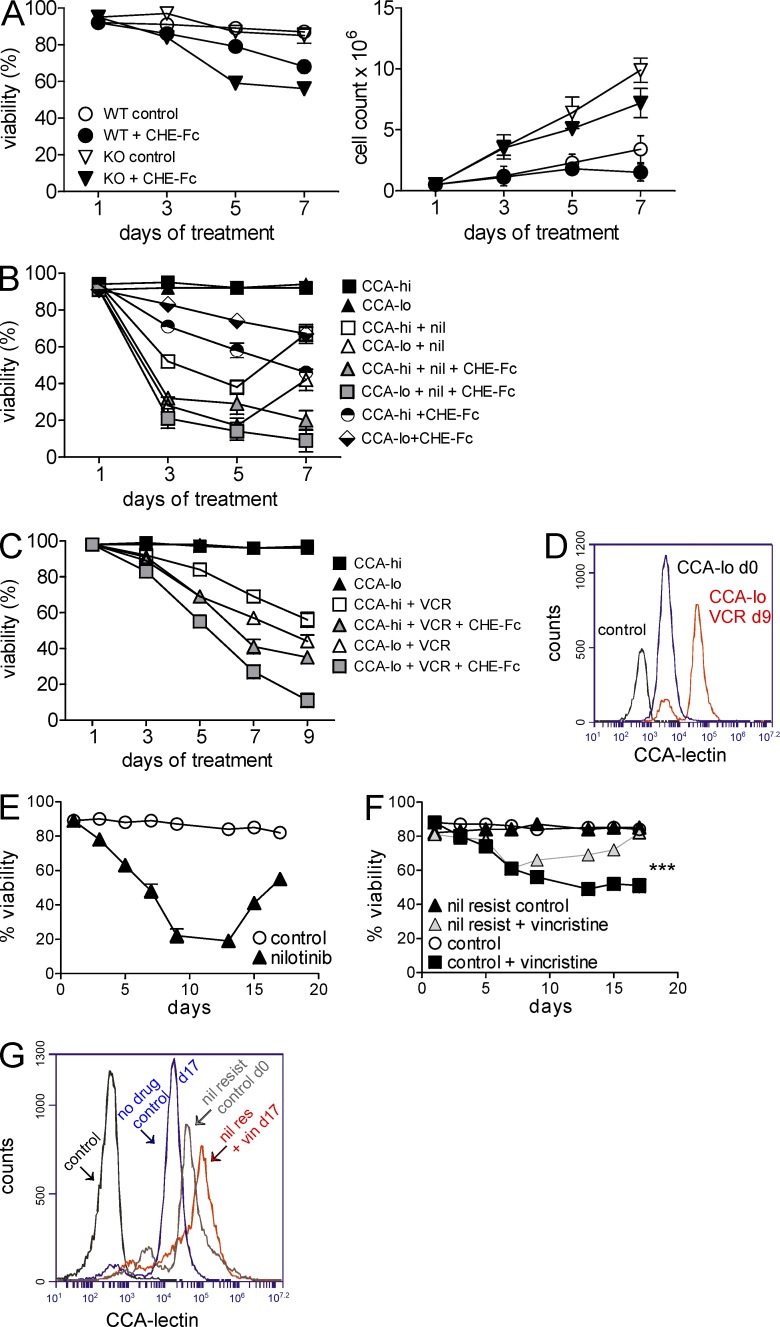
CHE-Fc acetylesterase treatment sensitizes ALL cells to drugs. (A) Percent viability (left) and cell counts (right) of WT and GD3 KO BCR/ABL-transduced cells treated with 25 µg/ml CHE-Fc. Viability WT versus WT + CHE-Fc: P = 0.0008; KO versus KO-CHE-Fc: P = 0.0009. The experiment was performed twice independently. (B) Percent viability of flow-sorted CCAhi and CCAlo 8093 cells co-cultured with OP9 stroma in the presence or absence of 20 nM nilotinib (nil), 10 µg/ml CHE-Fc, or both. P < 0.001 nil only, CHE-Fc only, and nil + CHE-Fc for CCAhi versus CCAlo on day 9 by two-way ANOVA with Bonferroni post-test. (C) Percent viability of CCAhi and CCAlo US7 cells treated with 5 nM vincristine (VCR) or 5 nM vincristine plus 10 mg/ml CHE-Fc. CCAhi and CCAlo US7 cells were sorted after 22 d of 5-nM vincristine treatment. In both B and C, treatment was continued 24 h after sorting. A single experiment was performed with triplicate samples. Day 9 vincristine and vincristine + CHE-Fc for CCAhi versus CCAlo: P < 0.001 by two-way ANOVA with Bonferroni post-test. (D) CCA lectin detection on CCAlo US7 cells treated with vincristine on day 9 from C, compared with day 0 and unstained cells as indicated. (E and F) Viability of BCR/ABL-transduced WT pre-B cells treated with 24 nM nilotinib every alternate day for 17 d (E) and viability of the nilotinib-resistant cells (on day 17 from E) subsequently treated with 2.5 nM vincristine for 17 d (F). ***, P < 0.001 day 17 control + vincristine versus nil resis + vincristine. (G) CCA lectin–binding cell surface structures detected on cells including nilotinib-resistant controls (in E, day 17 treated = day 0 cells in panel F), vincristine-resistant cells from F on day 17, untreated control cells from day 17 from F, and unstained cells as indicated. Error bars show the standard deviation of triplicate samples.
This result indicates that 9-O-acetylated Neu5Ac–containing glycoconjugates different from GD3 also affect survival of ALL cells. To further investigate the contribution of this modification to ALL drug resistance, we flow-sorted mouse BCR/ABL transgenic ALL cells that had developed tolerance to 20 nM nilotinib (from Fig. 6 B) into a CCAhi and a CCAlo fraction and observed their proliferation over a period of 7 d. Their viability (Fig. 7 B) and growth (not depicted) were comparable. However, the CCAhi population developed tolerance to renewed exposure to nilotinib at a rate more rapid than that of the CCAlo population and was more sensitive to esterase monotreatment compared with CCAlo cells. Also, combined treatment with nilotinib and the esterase to remove 9-O-acetylated Neu5Ac affected CCAhi and CCAlo populations more than nilotinib monotreatment, indicating that the 9-O-Ac–Neu5Ac provided continued protection to the cells (Fig. 7 B). We also flow-sorted human US7 ALL cells that developed vincristine resistance around day 22 (from Fig. 6 A) into CCAhi and CCAlo subpopulations. As shown in Fig. 7 C, the CCAhi cells lost less viability when treated with vincristine and the esterase than the CCAlo subpopulation.
The increased detection of CCA lectin–reacting molecules during drug resistance development could be either the result of selective outgrowth of an already existing CCAhi population or the result of increased expression. To analyze this, we monitored CCA reactivity on the CCAlo population as they were treated with vincristine and observed that CCAlo cells become CCAhi during the process of drug resistance (Fig. 7 D). This supports the concept that these cells gain CCA-detected structures as they adapt to the stress of the presence of vincristine. We also generated nilotinib-resistant WT pre-B ALL cells by culture for extended periods with nilotinib (Fig. 7 E). Next, these nilotinib-resistant cells with high CCA detection were treated with a second drug, vincristine. Surprisingly, the nilotinib-resistant cells attained resistance to vincristine significantly faster than the control cells (Fig. 7 F), and also the CCA lectin detection was further increased in these cells (Fig. 7 G).
De-O-acetylation of Neu5Ac structures leads to ALL cell death
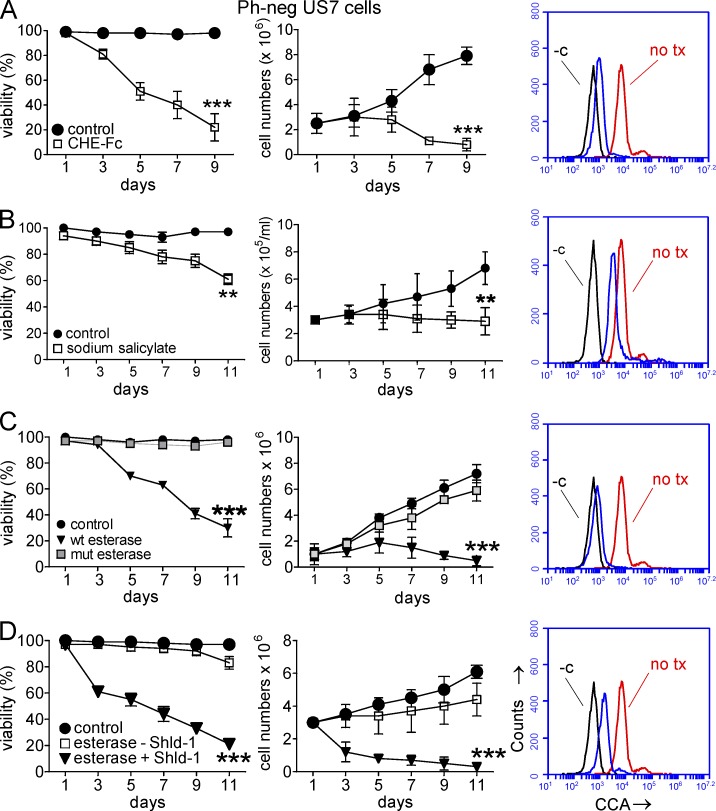
We next investigated the effect of de-O-acetylation of Neu5Ac in more detail in human ALL cells. Fig. 8 A shows that removal of the 9-O-acetyl moiety from Neu5Ac on the surface of human US7 ALL cells by treatment with the CHE-Fc esterase, similar to mouse WT pre-B ALL cells, stopped ALL cell growth and caused progressive loss of viability of the culture over 9 d.
Figure 8.
Removal of 9-O-acetyl groups from Neu5Ac leads to death of ALL cells protected by stroma. (A–D) US7 cells exposed to 20 µg/ml CHE-Fc (A), 5 mM sodium salicylate (B), lentivirally transduced WT and mutant (mut) esterase (C), or inducible esterase lentiviral construct (pTuner-esterase-Puro) cultured in the presence or absence of Shield-1 (D). Left panels show viability, middle panels show total viable cell counts, and right panels show FACS histogram analysis for CCA lectin binding. CCA binding in control US7 cells indicated with no tx; −c indicates unstained US7 cells on day 9. Unmarked curve in A shows CHE-Fc–treated cells, in B shows sodium salicylate–treated cells, in C shows WT esterase-transduced cells, and in D shows Shield-1–treated esterase-transduced cells. Sodium salicylate and CHE-Fc esterase were added on every alternate day during the treatment period. For Shield-1, cells were left untreated or treated on every second day starting on day 1. ***, P < 0.001 for A, C, and D at the indicated time points; **, P < 0.01 for B, treated cells compared with controls (A and B) or active esterase compared with control (C and D). All cells were grown on irradiated OP9 stroma. All experiments were independently repeated four to five times. Error bars show the standard deviation of triplicate samples.
Treatment with 5 mM sodium salicylate was reported to result in de-O-acetylation on T cell lines MOLT-4 and Jurkat and was proposed to occur through a chemical reaction involving the nucleophilic activity of salicylate (Kniep et al., 2006; Mukherjee et al., 2008). The treatment resulted in a block in proliferation and increased death of the ALL cells (Fig. 8 B). We verified that sodium salicylate treatment of ALL cells decreases Neu5Ac O-acetylation, as indicated by decreased binding of CCA lectin using FACS (Fig. 8 B, right, compare red line representing no treatment with blue line representing sodium salicylate–treated cells). However, coincubation of 9-O-Ac–GD3 with salicylate did not result in chemical 9-O-deacetylation of 9-O-Ac–GD3 in vitro, suggesting that the deacetylation observed in cells does not involve a chemical reaction on the O-acetyl moieties associated with Neu5Ac (not depicted).
These methods remove cell surface O-acetyl moieties on Neu5Ac. To also remove 9-O-acetyl moieties from intracellular Neu5Ac-bearing structures, we expressed the O-acetylesterase (Malisan et al., 2002) in a lentiviral vector. As a control, we also generated a lentivirus encoding an enzymatically inactive S57A mutant O-acetylesterase. Fig. 8 C shows that expression of the WT esterase in ALL cells had a very pronounced effect on their viability as well as on cell division. Based on BrdU staining, proliferation of these cells was severely inhibited (not depicted).
The effect of O-acetylesterase activity on the ALL cells was so marked that it was not feasible to perform long-term experiments with the cells because the cultures died out after lentiviral transduction with the WT O-acetylesterase. For this reason, we generated a lentiviral construct with the O-acetylesterase linked to a 12-kD degradation domain from FKBP, resulting in continuous degradation of the enzyme. Stable production of the esterase protein can be induced by addition of a synthetic ligand for FKBP12, called “Shield-1” (Banaszynski et al., 2008). Fig. 8 D shows that the leakiness of the system in transduced human US7 ALL cells was small, with cultures of non–Shield-1–induced cells showing only minor decreases in viability and growth. In contrast, induction of the O-acetylesterase protein had a dramatic effect on the cells, with loss of viability measurable as early as day 3 and with total lack of cell proliferation. Similar experiments were performed, and results obtained, using the human Ph-positive ALL TXL2 (not depicted).
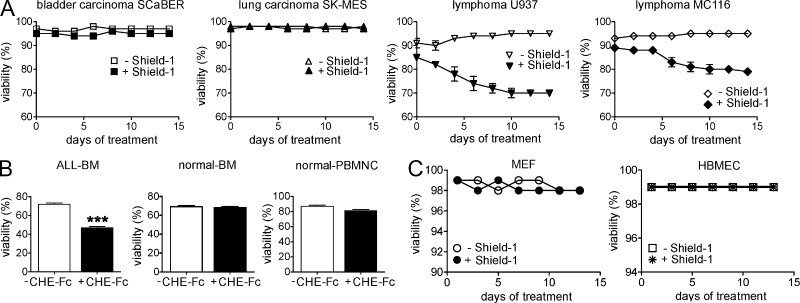
Because expression of the esterase had dramatic negative effects on the survival of ALL cells, we tested the impact of esterase treatment on other malignant as well as normal cells, including bladder carcinoma, lymphoma, lung carcinoma (Fig. 9 A), primary human PB, and BM cells (Fig. 9 B), MEFs, and human endothelial cells (Fig. 9 C). Esterase treatment had no effect on any of these cells tested, with the exception of the two lymphomas in which it caused a small decrease in viability.
Figure 9.
Esterase treatment has minimal effect on cells other than ALL. (A) Viability of malignant cells as indicated transduced with the inducible esterase lentiviral construct (pTuner-esterase-Puro) cultured in the presence or absence of Shield-1. (B) Viability of BM from an ALL patient after an overnight incubation with 20 µg/ml CHE-Fc compared with BM and PB from normal donors. Untreated cells (−CHE-Fc) serve as controls. (C) Viability of primary MEFs and HBMECs transduced with an inducible esterase lentiviral construct (pTuner-esterase-GFP). More than 90% of transduced cells were EGFP positive. ***, P < 0.001 for ALL BM −CHE-Fc compared with +CHE-Fc. Experiments were performed once. Error bars show the standard deviation of triplicate samples.
Removal of 9-O-acetyl moieties from Neu5Ac in vivo causes ALL cell death despite BM microenvironment protection
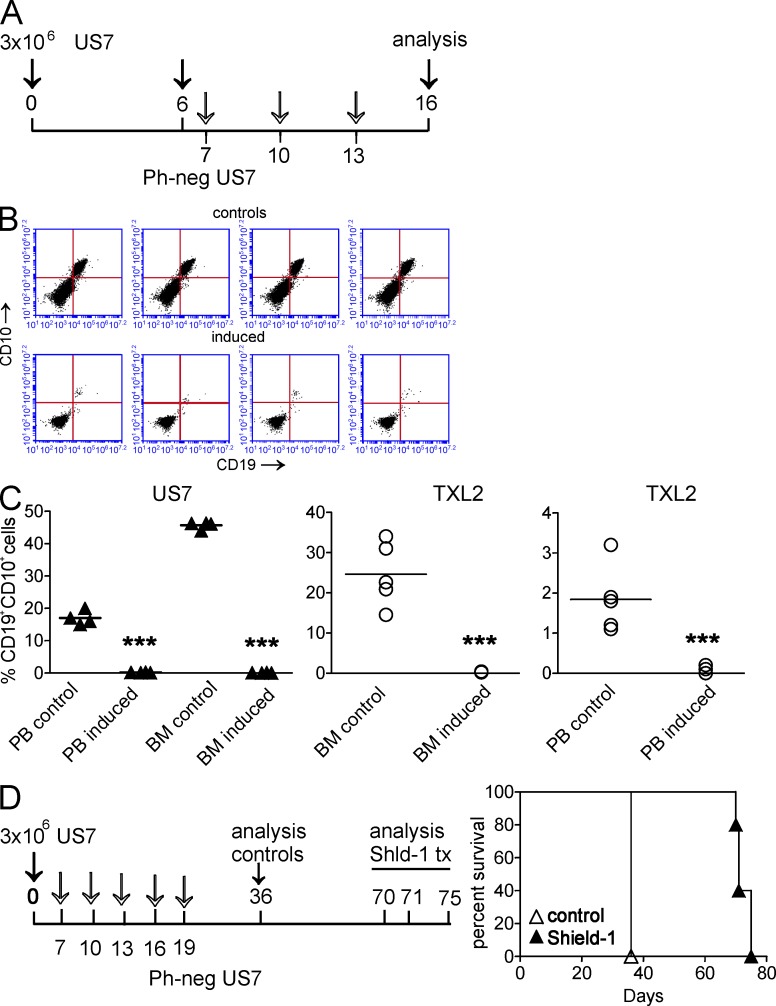
These experiments clearly showed that removal of O-acetyl moieties from Neu5Ac has a drastic lethal effect on ALL cells, even when these cells are protected by direct contact with stroma. The optimal environment for ALL cells with maximal protection is the BM microenvironment, and BM recurrence after first-line therapy signals poor outcome in childhood ALL (Rivera et al., 2005). Moreover, additional protective mechanisms beyond those provided by ex vivo stromal co-culture are available in vivo to promote ALL cell survival. To investigate whether ALL cells would survive de-O-acetylation when they are growing in the most protective location, we transplanted NSG mice with US7 cells transduced with the Shield-1–inducible esterase and allowed the cells to home to the BM and proliferate in that location for 6 d. PB samples taken from the animals at day 6 (Fig. 10 A, arrow) showed, as expected, that human CD19+, CD10+ ALL cells were already detectable in the circulation (not depicted). Animals were examined for the presence of ALL cells in BM and blood 3 d after the last induction of the esterase with Shield-1. BMs of the control animals, as expected, contained a high percentage of CD19+, CD10+ human ALL cells (Fig. 10, B and C). Remarkably, we could detect no human ALL cells in the PB or BMs of the animals in which the O-acetylesterase had been induced (Fig. 10 B, induced). This was a stunning result and led us to repeat the experiment with TXL2, which belongs to the subclass of Ph-positive ALL with poor prognosis. As shown in Fig. 10 C (right two panels), we observed significantly reduced ALL cells in BM and PB of esterase-induced mice as well. To examine the effect of de-O-acetylation of Neu5Ac on survival of ALL-bearing mice, we induced esterase expression by five repeated Shield-1 injections in mice transplanted with esterase-inducible US7 cells (Fig. 10 D). Control mice died around 36 d after transplant, whereas mice with induction of the esterase in the ALL cells survived twice as long as the controls.
Figure 10.
Induced expression of 9-O-acetylesterase in vivo causes ALL cell death. (A) Schematic illustration of treatment of US7-transplanted mice. Induction of 9-O-acetylesterase was on days 7, 10, and 13 with 10 mg/kg Shield-1. (B) FACS analysis showing human CD19 and CD10 expression on BM cells isolated from control mice or Shield-1–injected mice, transplanted with US7 cells transduced with the inducible O-acetylesterase gene. (C) Graphs showing the percentage of human CD19, CD10 double-positive cells in PB and BM of control as well as esterase-induced mice. Each in vivo experiment was performed once with four to five mice/group. For details on the TXL2 experiment, see Materials and methods. Horizontal bars indicate mean. ***, P < 0.001 for BM and PB control compared with induced. (D, left) Schematic illustration of treatment of US7-transplanted mice for long-term survival. Induction of 9-O-acetylesterase was as indicated by open arrows on days 7, 10, 13, 16, and 19 with 10 mg/kg Shield-1. (right) Survival of mice treated with vehicle or Shield-1. Significance was calculated by Log-rank Mantel-Cox test. P = 0.0027 for controls compared with Shield-1–treated mice. The experiment was performed once with five mice/group.
DISCUSSION
In the current study, we have shown that increased O-acetylation of Neu5Ac-bearing glycoconjugates is a hallmark of oncogenic transformation in pre-B ALL. Chowdhury et al. (2008) have used a different lectin commonly referred to as Achatinin H, which detects terminal 9-O-acetylated Neu5Ac that is α2,6-linked to N-acetylgalactosamine, to examine BMs and PB of 109 children with ALL for cell surface expression of 9-O-acetylated Neu5Ac using FACS. They further confirmed the specificity of detection by treatment of childhood ALL lymphoblasts with influenza C virus O-acetylesterase, which reduced Achatinin H reactivity from 90 to 12%. They concluded that this could be used as a marker for childhood ALL at presentation and after treatment to detect minimal residual disease. The C. antennarius lectin used in our experiments is not sensitive to the Neu5Ac linkage and identifies 9-O-acetylated gangliosides such as GD3 which contain α2,8-linked disialic acids as well as O-Ac Neu5Ac linked to glycoproteins in human melanoma cells (Ravindranaths et al., 1988). Using this broader-specificity lectin, we show for the first time that O-Ac Neu5Ac is a universal hallmark for ALL of both mouse and human origin: we detected it on human adult ALL cells including both Ph-negative and -positive subgroups, on presentation and relapsed samples, and on Ph-positive ALLs with and without the Bcr/Abl T315I mutation. Moreover, because Neu5Ac O-acetylation as detected by the C. antennarius lectin was induced by BCR/ABL oncogenic transformation of normal mouse pre-B cells (Fig. 4 C), we showed that this is truly a cancer-specific marker. Although we could demonstrate, using the CDw60 antibody, that ALL cells were positive for 9-O-acetylated GD3, our results using pre-B ALL cells lacking GD3 synthase and thus deficient of GD3 clearly show that there are one or more other structures in ALL modified by O-Ac Neu5Ac.
Importantly, our results also show that the O-acetylated Neu5Ac modification is not merely a tumor-specific marker. Because GD3 and its modification by 9-O-acetylation have been studied in other cell types, we first examined the contribution of GD3 to ALL cell survival. Our results are in agreement with those of Malisan et al. (2002) who have shown that HEK-293 cells were protected against the proapoptotic effects of GD3 by its 9-O-acetylation. Mukherjee et al. (2008) also have shown that exogenous addition of GD3 induces apoptosis in childhood ALL lymphoblasts. In contrast to our study, this was performed in the absence of stroma and with a higher concentration of GD3. We have shown that even in the presence of stroma, which has the capacity to protect ALL cells against many drugs, GD3 can lead to apoptosis, and its effects were in fact reminiscent of those of chemotherapeutic drugs.
Interestingly, our experiments showed that the ALL cells fail to 9-O-acetylate exogenously added GD3. In CHO cells, exposure to exogenous GD3 was shown to result in increased levels of 9-O-Ac GD3, and the authors concluded that in these cells, GD3 induces sialyl O-acetyltransferase (SOAT) production or activation (Satake et al., 2003). Because ALL cells contain endogenous 9-O-Ac–GD3, it is reasonable to assume that the SOAT enzyme, which is directly or indirectly responsible for 9-O-Ac–GD synthesis, must be expressed in pre-B ALL cells. We can offer two explanations for the failure of ALL cells to react similarly to CHO cells. One is that the exogenously added GD3 is inaccessible to the SOAT, for example as the result of rapid internalization and transport to the mitochondria. Alternatively, if 9-O-Ac–GD3 is not synthesized from GD3 by a SOAT but by St8sia1 using GM3 and 9-O-Ac–Sia, exogenous GD3 will not be converted to 9-O-Ac–GD3 in these ALL cells.
Our experiments with st8sia1-null mutant pre-B ALL cells clearly show that GD3 is not required for ALL survival because the BCR/ABL-transduced GD3 synthase KO cells survive and in fact proliferate much faster than WT cells. Interestingly, it has been hypothesized that GD3 may function to restrict neural progenitor cell proliferation (Lanctot et al., 2007; Yanagisawa and Yu, 2007). Thus, the reason that loss of GD3 increases ALL cell proliferation is of interest but is outside the focus of the current study and will require further investigation. Additionally, we found that the absence of GD3 also makes these cells less sensitive to drug treatment. We therefore considered the possibility that low levels of GD3 would correlate with increased ability to withstand drug treatment in human ALL cells, but we did not observe a correlation between the amount of cell surface GD3 expression and degree of nilotinib sensitivity in five Ph-positive ALLs that lack the Bcr/Abl T315I mutation (unpublished data).
Unexpectedly, we found that increased expression of 9-O-Ac–GD3, and that of O-acetylated Neu5Ac in general, was induced on the surface of pre-B ALL cells by drugs that have very different modes of action, in both human and mouse pre-B ALL cells. This correlated with the emergence of tolerance to drug treatment in the absence of changes in total cellular levels of O-acetylated Neu5Ac. This implies that enzymatic activity of the SOAT is not responsible for these increases, but rather that increased cell surface transport of existing structures, reduced endocytosis, or the balance between these two causes these net increases. Our analysis of flow-sorted CCAhi and CCAlo ALL cells further does not support the idea that the measured increase is caused by the selective proliferation of a preexisting clone of ALL cells with a higher level of 9-O-acetylated Neu5Ac expression.
9-O-acetylation of Neu5Ac, and in particular that of GD3 which has been most elaborately studied, is not restricted to cancer cells. 9-O-acetylated Neu5Ac–containing glycoconjugates and GD3 are expressed on CD4 T cells, on TPA-activated PB B cells, and activated tonsillar B lymphocytes (Krishna and Varki, 1997; Vater et al., 1997; Wipfler et al., 2011). GD3 and oxidized GD3 were shown to be proapoptotic for ALL cells, and O-acetylation of Neu5Ac is thought to delay oxidation and degradation of the underlying Neu5Ac, as well as create new or mask existing ligands (Schauer et al., 2011). Based on this, we speculate that the extracellular increase in 9-O-acetylated Neu5Ac seen upon transformation of pre-B cells and upon drug treatment of pre-B ALL cells are protective extracellular manifestations of the intracellular stress such cells experience as they successfully adapt to a radically changed cellular physiology.
We are the first to demonstrate the high potential of de-O-acetylation to treatment of ALL. First, our data indicate that O-acetylation, presently considered a largely obscure modification of glycolipids and glycoproteins, correlates with the development of drug resistance in ALL cells. Importantly, our data show that 9-O-acetylated Neu5Ac not only signifies, but also actually supports drug resistance development. Finally, using different classes of ALL with different relapse risk, including Ph-positive and -negative ALL cells, in vitro and in vivo in mice, we show that removal of O-acetyl moieties from Neu5Ac kills ALL cells.
In a survival experiment, mice transplanted with ALL cells containing the inducible esterase, which was induced five times over a period of 12 d, did relapse, but only after significantly prolonged survival compared with controls. We believe that relapse may have been caused by failure of the Shield-1 compound to adequately reach residual ALL cells in the BM, a typical site of relapse of human ALL patients treated with different drugs. Indeed, we were able to kill the ALL cells from these relapsed mice by in vitro treatment with fresh Shield-1 (unpublished data). This indicates that the cells that gave rise to relapse contained the inducible esterase and moreover were still sensitive to esterase activity, which supports the possibility that drug delivery, as opposed to esterase insensitivity, caused the relapse in these mice.
We further found that esterase treatment had little effect on other cells tested here, including normal endothelial cells and fibroblasts. This suggests that toxicity of esterase treatment may be limited to specific cell types, an important consideration for a possible future therapeutic application. However, de-O-acetylation will require further exploration before it can be used as a workable therapy for the treatment of pre-B ALL. Other opportunities for novel drug therapies for ALL based on our findings include inhibition of the Neu5Ac 9-O-acetyltransferase that is responsible for the 9-O-acetylation of Neu5Ac and which remains largely elusive (Arming et al., 2011). Alternatively, activation of the endogenous 9-O-acetylesterase SIAE (Cariappa et al., 2009) specifically in ALL cells would similarly be expected to kill ALL cells.
As mentioned above, the postglycosylation modification of GD3 by 9-O-acetylation has been previously reported for melanoma and glioblastoma cell lines (Malisan et al., 2002). Recently, Birks et al. (2011) treated glioblastoma cell lines with SF9-produced recombinant esterase. Interestingly, they reported that increased GD3 and decreased 9-O-Ac–GD3 levels resulted in increased cell death in the glioblastoma cells, with little effect on normal astrocytes. As our data show that increased postglycosylation O-acetylation of Neu5Ac correlates with malignant transformation, the results described in the current study may have relevance to a more extended spectrum of malignancies such as breast cancer and melanomas, as well as their resistance to therapeutic drugs.
MATERIALS AND METHODS
Cells.
ALL cells were co-cultured with irradiated OP-9 cells in αMEM + 20% FBS, 1% l-glutamine, and 1% penicillin. Mouse OP9 cells were purchased from the American Type Culture Collection (ATCC; CRL-2749). The 8093 Bcr/Abl P190-expressing mouse lymphoblastic leukemia cells were grown on irradiated MEFs as previously described (Kaur et al., 2007). Human ALL cells have been previously described (Fei et al., 2010a,b; Parameswaran et al., 2010; Duy et al., 2011; Park et al., 2011) and include US7, P2, BLQ1, UCSFO1, UCSFO2, US6, US9, US7R, BLQ5, US6, TXL3, TXL2, and BLQ11. HL-60, HEL, Wehi-3, and MOLT4 cells as well as 293 cells producing the CHE-Fc esterase, the bladder carcinoma (Scaber), lung carcinoma (SK-MES), and lymphomas (U937 and MC116) were obtained from the ATCC. MEFs were isolated from embryonic day (E) 14.5 embryos using standard procedures. Primary human brain microvascular endothelial cells (HBMECs) were obtained from A. Epstein (Children’s Hospital Los Angeles, Los Angeles, CA). Viably frozen BM from a normal donor and a patient with ALL were purchased from the National Disease Research Interchange. BM and PB cells from ALL patients and normal donors were obtained after approval of the committee on Clinical Investigations of Children’s Hospital Los Angeles.
Reagents, antibodies, and flow cytometry.
Shield-1 was obtained from Cheminpharma LLC. The inducible ProteoTuner vector system was from Takara Bio Inc. FITC–C. antennarius (CCA) lectin was obtained from EY Laboratories. BD was the source of CD19, CD10, and IgM antibodies, the PI/Annexin V kit, and the cell fixation/permeabilization kit. Antibodies against CDw60 (M-T6004) and GD3 (R24) were obtained from Abcam. Anti–human CD4 antibodies were obtained from P. Ramakrishnan (California Institute of Technology, Pasadena, CA) and BioLegend, anti-GAPDH antibodies from EMD Millipore, and anti-BCR antibodies from Santa Cruz Biotechnology, Inc. Purified GD3 and sodium salicylate were obtained from Matreya LLC and Sigma-Aldrich. Flow sorting was performed on a FACSort (BD). For detection of binding to CCA lectin, cells were incubated with CCA-FITC for 30 min at room temperature and washed two times with PBS−/− before analysis on an Accuri flow cytometer. For human leukemia cell detection, after RBC lysis, PB and BM cells were incubated with anti–human CD19 and CD10 antibodies for 30 min at room temperature.
For FACS analysis of viably frozen human BM and PB samples, the mononuclear cell fraction was separated using Ficoll. Separation of CD19-positive B cells was performed using CD19 magnetic beads (Miltenyi Biotec). For CCA lectin binding and CDw60 total staining, cells were fixed and permeabilized using a fixation and permeabilization kit (BD). 9-O-Ac–GD3 was synthesized from GD3 in-house according to our previous published acetylation methodology (Kiefel et al., 1996).
Treatments for de-O-acetylation.
Treatment with 5 mM sodium salicylate to remove acetyl groups was performed according to a published protocol (Kniep et al., 2006). Isolation of the CHE-Fc chimeric protein from 293 cells stably expressing the construct (ATCC) was performed as described previously (Klein et al., 1994). A hemagglutination assay was performed to confirm the functionality of the purified CHE-Fc (Klein et al., 1994). In brief, twofold serial dilutions of CHE-Fc were combined with defined numbers of RBCs. RBCs that are not bound by the CHE-Fc form a button at the bottom of the well, whereas RBCs cross-linked by CHE-Fc form a lattice that coats the well.
Retroviral and lentiviral transduction.
Esterase WT and mutant constructs were obtained from F. Malisan (University of Rome Tor Vergata, Rome, Italy; Malisan et al., 2002). The 2.6-kb esterase gene was subcloned into a lentiviral vector pCL-6-eGFP, digested with KpnI and XhoI. HEK 293-FT cells were cotransfected with pCL6-esterase-GFP, pCDNLBH packaging and VSVG envelope vectors. 48 h after transfection, conditioned medium containing the virus was collected, filtered, and concentrated by centrifugation. Concentrated virus was then added to the ALL cells. After 48 h of incubation, GFP-positive transduced cells were sorted on a FACSort. For the inducible expression of the esterase, the WT esterase gene was also subcloned into the pTuner Puro vector (Takara Bio Inc.). The pTuner GFP esterase lentiviral construct was made by deleting the Puromycin gene and adding GFP to the parent vector system. In these plasmids, a mutant of the FKBP protein (the destabilization domain, or DD) is expressed as a tag on the esterase gene. In the presence of the small, membrane-permeant, stabilizing ligand Shield-1, the DD-tagged esterase is stabilized (protected from proteasomal degradation) and accumulates inside the cell (Banaszynski et al., 2008). For retroviral transduction of pre-B cells, BM was pooled from three GD3 KO mice as well as from age-matched, sex-matched WT mice. HEK 293FT cells were cotransfected with plasmids BCR/ABL (P190)-IRES-neo or BCR/ABL T315I (a gift from N. Shah, University of California, San Francisco, San Francisco, CA) together with pHIT60 and pHIT123 (ecotropic envelope; Soneoka et al., 1995) using Lipofectamine 2000 (Invitrogen). After 24 h, the viral supernatant was collected and transferred to a 6-well plate coated with retronectin (Takara Bio Inc.). After centrifugation of the viral supernatant at 2,000 g for 90 min, pre-B cells were added onto the plates and again centrifuged at 600 g for 30 min. To select for pre-B cells, BM cells were cultured in the presence of 10 ng/ml recombinant mouse IL-7 for 2 wk. After 48 h of infection, cells were washed with medium and cultured further in the absence of IL-7. Cells were grown in IMDM with GlutaMAX (Invitrogen) supplemented with 20% FBS and β-mercaptoethanol. The sensitivity of BCR/ABL-transduced WT and KO cells to nilotinib was determined using a Cell Counting kit-8 (Dojindo Laboratories), according to the specifications of the manufacturer. In brief, 100 µl of a cell suspension (5,000 cells/well) was added to a 96-well plate. Plates were preincubated for 24 h in a humidified incubator. 10 µl of various concentrations of nilotinib were then added and incubation continued for 48 h. 10 µl of a CCK-8 solution was added to each well. After 1–4 h, absorbance was measured at 450 nm using a GENios multifunction microplate reader (Tecan).
Xenografts and in vivo treatment with Shield-1.
All animal experiments were performed in concordance with Children’s Hospital Los Angeles Institutional Animal Care and Use Committee and National Institutes of Health guidelines. We used NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice (NSG; The Jackson Laboratory) for transplants of 3 × 106 US7 cells or TXL2 cells transduced with the inducible lentiviral O-acetylesterase construct. On day 6, PB samples were taken to assess the development of leukemia, and mice were then randomly assigned to a treatment or a control group (n = 4 or 5 per group). Treatment consisted of an i.p. injection with 10 mg/kg Shield-1 in 50% DMA, 45% PEG-40, and 5% Tween-80. For US7, injections were performed on days 7, 10, and 13, with analysis on day 16. For TXL2, injections were on days 6, 8, and 10 with analysis on day 12 for the presence of ALL cells in the BM and PB by FACS using human anti-CD19 and CD10 antibodies. The treatment schedule of US7-transplanted mice to measure survival is indicated in Fig. 10 D.
Resistance experiments.
Mouse 8093 ALLs were co-cultured with MEFs. Human ALLs including US7 were co-cultured with OP9 except where indicated. BCR/ABL-transduced pre-B cells were cultured in the absence of stroma. All cells were treated either with nilotinib (AMN107) or 2.5 nM vincristine for several days as indicated in the respective graphs. Every alternate day, medium was refreshed (fresh media with drug), and viable cell counts were performed using Trypan blue. In experiments using a combination treatment of GD3 with 9-O-Ac–GD3, both reagents were added every alternate day to US7 cell cultured in the presence of stroma. For Annexin V/PI staining, an FITC Annexin V Apoptosis Detection kit from BD was used according to the manufacturer’s instructions.
Statistics.
Student’s t test was performed to assess statistical significance. All experiments were performed on triplicate samples. A value of P < 0.05 was considered significant. Significance of survival was calculated using the Log-rank Mantel-Cox test.
Acknowledgments
We thank Dr. Florence Malisan for the generous gift of the WT and mutant esterase constructs. The Children’s Hospital Los Angeles Vector Core is acknowledged for making the inducible esterase LV construct. We also thank Dr. Parameswaran Ramakrishnan for providing antibodies, Dr. Anat Epstein for the HBMECs, and Dr. Neil Shah for the Bcr/Abl T315I retroviral construct.
This work was supported by Public Health Service grant CA090321 with an American Recovery and Reinvestment Act competitive supplement (to N. Heisterkamp and J. Groffen). M. Müschen is supported by National Institutes of Health (NIH) National Cancer Institute grants R01CA137060, R01CA139032, and R01CA157644. R.K. Yu is supported in part by grants from the NIH (grants NS11853 and NS26994) and a Veterans Affairs Merit Review Award. M. von Itzstein gratefully acknowledges the financial support of the Australian Research Council through the award of a Federation Fellowship and Griffith University. This project was initiated during the sabbatical of J. Groffen at the Institute for Glycomics, Griffith University (Gold Coast, Australia) with support from the University of Southern California.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- ALL
- acute lymphoblastic leukemia
- CHO
- Chinese hamster ovary
- HBMEC
- human brain microvascular endothelial cell
- MFI
- mean fluorescent intensity
- Neu5Ac
- N-acetylneuraminic acid
- PB
- peripheral blood
- PI
- propidium iodide
- SOAT
- sialyl O-acetyltransferase
References
- Arming S., Wipfler D., Mayr J., Merling A., Vilas U., Schauer R., Schwartz-Albiez R., Vlasak R. 2011. The human Cas1 protein: a sialic acid-specific O-acetyltransferase? Glycobiology. 21:553–564 10.1093/glycob/cwq153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski L.A., Sellmyer M.A., Contag C.H., Wandless T.J., Thorne S.H. 2008. Chemical control of protein stability and function in living mice. Nat. Med. 14:1123–1127 10.1038/nm.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birklé S., Ren S., Slominski A., Zeng G., Gao L., Yu R.K. 1999. Down-regulation of the expression of O-acetyl-GD3 by the O-acetylesterase cDNA in hamster melanoma cells: effects on cellular proliferation, differentiation, and melanogenesis. J. Neurochem. 72:954–961 10.1046/j.1471-4159.1999.0720954.x [DOI] [PubMed] [Google Scholar]
- Birklé S., Gao L., Zeng G., Yu R.K. 2000. Down-regulation of GD3 ganglioside and its O-acetylated derivative by stable transfection with antisense vector against GD3-synthase gene expression in hamster melanoma cells: effects on cellular growth, melanogenesis, and dendricity. J. Neurochem. 74:547–554 10.1046/j.1471-4159.2000.740547.x [DOI] [PubMed] [Google Scholar]
- Birks S.M., Danquah J.O., King L., Vlasak R., Gorecki D.C., Pilkington G.J. 2011. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro-oncol. 13:950–960 10.1093/neuonc/nor108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariappa A., Takematsu H., Liu H., Diaz S., Haider K., Boboila C., Kalloo G., Connole M., Shi H.N., Varki N., et al. 2009. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J. Exp. Med. 206:125–138 10.1084/jem.20081399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.Y., Varki A. 2002. O-acetylation of GD3: an enigmatic modification regulating apoptosis? J. Exp. Med. 196:1529–1533 10.1084/jem.20021915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.Y., Challa A.K., Varki A. 2006. 9-O-acetylation of exogenously added ganglioside GD3. The GD3 molecule induces its own O-acetylation machinery. J. Biol. Chem. 281:7825–7833 10.1074/jbc.M512379200 [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Mandal C. 2009. O-acetylated sialic acids: multifaceted role in childhood acute lymphoblastic leukaemia. Biotechnol. J. 4:361–374 10.1002/biot.200800253 [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Bandyopadhyay S., Mandal C., Chandra S., Mandal C. 2008. Flow-cytometric monitoring of disease-associated expression of 9-O-acetylated sialoglycoproteins in combination with known CD antigens, as an index for MRD in children with acute lymphoblastic leukaemia: a two-year longitudinal follow-up study. BMC Cancer. 8:40 10.1186/1471-2407-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy C., Hurtz C., Shojaee S., Cerchietti L., Geng H., Swaminathan S., Klemm L., Kweon S.M., Nahar R., Braig M., et al. 2011. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 473:384–388 10.1038/nature09883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann M., Wipfler D., Merling A., Cao Y., Claus C., Kniep B., Sadick H., Bergler W., Vlasak R., Schwartz-Albiez R. 2006. Differential surface expression and possible function of 9-O- and 7-O-acetylated GD3 (CD60 b and c) during activation and apoptosis of human tonsillar B and T lymphocytes. Glycoconj. J. 23:627–638 10.1007/s10719-006-9000-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahr C., Schauer R. 2001. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J. Invest. Dermatol. 116:254–260 10.1046/j.1523-1747.2001.01237.x [DOI] [PubMed] [Google Scholar]
- Fei F., Stoddart S., Groffen J., Heisterkamp N. 2010a. Activity of the Aurora kinase inhibitor VX-680 against Bcr/Abl-positive acute lymphoblastic leukemias. Mol. Cancer Ther. 9:1318–1327 10.1158/1535-7163.MCT-10-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei F., Stoddart S., Müschen M., Kim Y.M., Groffen J., Heisterkamp N. 2010b. Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia. Leukemia. 24:813–820 10.1038/leu.2009.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhahn N., Arutyunyan A., Stoddart S., Zhang B., Schmidhuber S.M., Yi S.-J., Kim Y.M., Groffen J., Heisterkamp N. 2012. Environment-mediated drug resistance in Bcr/Abl-positive acute lymphoblastic leukemia. Oncoimmunology. 1:618–629 10.4161/onci.20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes R., Allman R., Mason M.D. 1997. Ganglioside expression in lung cancer cell lines. Lung Cancer. 18:21–33 10.1016/S0169-5002(97)00049-4 [DOI] [PubMed] [Google Scholar]
- Fukuda M., Rutishauser U., Schnaar R.L., 2005. Neuroglycobiology. Oxford University Press, Oxford; New York: 229 pp [Google Scholar]
- Gaynon P.S., Angiolillo A.L., Carroll W.L., Nachman J.B., Trigg M.E., Sather H.N., Hunger S.P., Devidas M.; Children’s Oncology Group 2010. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children’s Oncology Group Report. Leukemia. 24:285–297 10.1038/leu.2009.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocht A., Rutter G., Kniep B. 1998. Changed expression of 9-O-acetyl GD3 (CDw60) in benign and atypical proliferative lesions and carcinomas of the human breast. Histochem. Cell Biol. 110:217–229 10.1007/s004180050284 [DOI] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N. 1997. The chimeric BCR-ABL gene. Baillieres Clin. Haematol. 10:187–201 10.1016/S0950-3536(97)80002-9 [DOI] [PubMed] [Google Scholar]
- Kaur P., Feldhahn N., Zhang B., Trageser D., Müschen M., Pertz V., Groffen J., Heisterkamp N. 2007. Nilotinib treatment in mouse models of P190 Bcr/Abl lymphoblastic leukemia. Mol. Cancer. 6:67 10.1186/1476-4598-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefel M.J., Beisner B., Bennett S., Holmes I.D., von Itzstein M. 1996. Synthesis and biological evaluation of N-acetylneuraminic acid-based rotavirus inhibitors. J. Med. Chem. 39:1314–1320 10.1021/jm950611f [DOI] [PubMed] [Google Scholar]
- Klein A., Krishna M., Varki N.M., Varki A. 1994. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc. Natl. Acad. Sci. USA. 91:7782–7786 10.1073/pnas.91.16.7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniep B., Kniep E., Ozkucur N., Barz S., Bachmann M., Malisan F., Testi R., Rieber E.P. 2006. 9-O-acetyl GD3 protects tumor cells from apoptosis. Int. J. Cancer. 119:67–73 10.1002/ijc.21788 [DOI] [PubMed] [Google Scholar]
- Krishna M., Varki A. 1997. 9-O-Acetylation of sialomucins: a novel marker of murine CD4 T cells that is regulated during maturation and activation. J. Exp. Med. 185:1997–2013 10.1084/jem.185.11.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot P.M., Gage F.H., Varki A.P. 2007. The glycans of stem cells. Curr. Opin. Chem. Biol. 11:373–380 10.1016/j.cbpa.2007.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Thompson J.E., Wang E.S., Wetzler M. 2011. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 117:1583–1594 10.1002/cncr.25690 [DOI] [PubMed] [Google Scholar]
- Malisan F., Franchi L., Tomassini B., Ventura N., Condò I., Rippo M.R., Rufini A., Liberati L., Nachtigall C., Kniep B., Testi R. 2002. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 196:1535–1541 10.1084/jem.20020960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman A.V. 2012. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 26:123–135 10.1016/j.blre.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Mukherjee K., Chava A.K., Mandal C., Dey S.N., Kniep B., Chandra S., Mandal C. 2008. O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. J. Cell. Biochem. 105:724–734 10.1002/jcb.21867 [DOI] [PubMed] [Google Scholar]
- Parameswaran R., Müschen M., Kim Y.M., Groffen J., Heisterkamp N. 2010. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 70:4346–4356 10.1158/0008-5472.CAN-10-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran R., Yu M., Lim M., Groffen J., Heisterkamp N. 2011. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 25:1314–1323 10.1038/leu.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran R., Yu M., Lyu M.A., Lim M., Rosenblum M.G., Groffen J., Heisterkamp N. 2012. Treatment of acute lymphoblastic leukemia with an rGel/BLyS fusion toxin. Leukemia. 26:1786–1796 10.1038/leu.2012.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Gang E.J., Hsieh Y.T., Schaefer P., Chae S., Klemm L., Huantes S., Loh M., Conway E.M., Kang E.S., et al. 2011. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 118:2191–2199 10.1182/blood-2011-04-351239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G., Hochhaus A., Schultheis B., Hehlmann R. 1998. Chronic myelogenous leukemia: molecular and cellular aspects. J. Cancer Res. Clin. Oncol. 124:643–660 10.1007/s004320050228 [DOI] [PubMed] [Google Scholar]
- Pui C.H., Robison L.L., Look A.T. 2008. Acute lymphoblastic leukaemia. Lancet. 371:1030–1043 10.1016/S0140-6736(08)60457-2 [DOI] [PubMed] [Google Scholar]
- Ravindranath M.H., Higa H.H., Cooper E.L., Paulson J.C. 1985. Purification and characterization of an O-acetylsialic acid-specific lectin from a marine crab Cancer antennarius. J. Biol. Chem. 260:8850–8856 [PubMed] [Google Scholar]
- Ravindranaths M.H., Paulson J.C., Irie R.F. 1988. Human melanoma antigen O-acetylated ganglioside GD3 is recognized by Cancer antennarius lectin. J. Biol. Chem. 263:2079–2086 [PubMed] [Google Scholar]
- Ren R. 2005. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer. 5:172–183 10.1038/nrc1567 [DOI] [PubMed] [Google Scholar]
- Ren S., Scarsdale J.N., Ariga T., Zhang Y., Klein R.A., Hartmann R., Kushi Y., Egge H., Yu R.K. 1992. O-acetylated gangliosides in bovine buttermilk. Characterization of 7-O-acetyl, 9-O-acetyl, and 7,9-di-O-acetyl GD3. J. Biol. Chem. 267:12632–12638 [PubMed] [Google Scholar]
- Rippo M.R., Malisan F., Ravagnan L., Tomassini B., Condo I., Costantini P., Susin S.A., Rufini A., Todaro M., Kroemer G., Testi R. 2000. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J. 14:2047–2054 10.1096/fj.99-1028com [DOI] [PubMed] [Google Scholar]
- Rivera G.K., Zhou Y., Hancock M.L., Gajjar A., Rubnitz J., Ribeiro R.C., Sandlund J.T., Hudson M., Relling M., Evans W.E., Pui C.H. 2005. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 103:368–376 10.1002/cncr.20743 [DOI] [PubMed] [Google Scholar]
- Salzer W.L., Devidas M., Carroll W.L., Winick N., Pullen J., Hunger S.P., Camitta B.A. 2010. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children’s oncology group. Leukemia. 24:355–370 10.1038/leu.2009.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake H., Chen H.Y., Varki A. 2003. Genes modulated by expression of GD3 synthase in Chinese hamster ovary cells. Evidence that the Tis21 gene is involved in the induction of GD3 9-O-acetylation. J. Biol. Chem. 278:7942–7948 10.1074/jbc.M210565200 [DOI] [PubMed] [Google Scholar]
- Schauer R. 2009. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19:507–514 10.1016/j.sbi.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R., Srinivasan G.V., Wipfler D., Kniep B., Schwartz-Albiez R. 2011. O-Acetylated sialic acids and their role in immune defense. Adv. Exp. Med. Biol. 705:525–548 10.1007/978-1-4419-7877-6_28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrappe M., Hunger S.P., Pui C.H., Saha V., Gaynon P.S., Baruchel A., Conter V., Otten J., Ohara A., Versluys A.B., et al. 2012. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N. Engl. J. Med. 366:1371–1381 10.1056/NEJMoa1110169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y., Cannon P.M., Ramsdale E.E., Griffiths J.C., Romano G., Kingsman S.M., Kingsman A.J. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628–633 10.1093/nar/23.4.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Hooshmand F., Diaz S., Varki N.M., Hedrick S.M. 1991. Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell. 65:65–74 10.1016/0092-8674(91)90408-Q [DOI] [PubMed] [Google Scholar]
- Varki N.M., Varki A. 2007. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab. Invest. 87:851–857 10.1038/labinvest.3700656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater M., Kniep B., Gross H.J., Claus C., Dippold W., Schwartz-Albiez R. 1997. The 9-O-acetylated disialosyl carbohydrate sequence of CDw60 is a marker on activated human B lymphocytes. Immunol. Lett. 59:151–157 10.1016/S0165-2478(97)00116-8 [DOI] [PubMed] [Google Scholar]
- Wipfler D., Srinivasan G.V., Sadick H., Kniep B., Arming S., Willhauck-Fleckenstein M., Vlasak R., Schauer R., Schwartz-Albiez R. 2011. Differentially regulated expression of 9-O-acetyl GD3 (CD60b) and 7-O-acetyl-GD3 (CD60c) during differentiation and maturation of human T and B lymphocytes. Glycobiology. 21:1161–1172 10.1093/glycob/cwr050 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Yu R.K. 2007. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 17:57R–74R 10.1093/glycob/cwm018 [DOI] [PubMed] [Google Scholar]
- Yu R.K., Ledeen R. 1969. Configuration of the ketosidic bond of sialic acid. J. Biol. Chem. 244:1306–1313 [PubMed] [Google Scholar]