Anti–4-1BB treatment of tumor-bearing or intracellular pathogen infected mice generates a population of Eomes+KLRG1+ tumor infiltrating T cells that have enhanced cytotoxic activity.
Abstract
4-1BB agonist antibody treatment induces a population of KLRG1+ T cells that infiltrate melanoma tumors. We investigated the origin and function of these cells, as well as their place within established T cell paradigms. We find that these T cells, particularly the CD4 lineage, represent a novel phenotype characterized by enhanced, multipotent cytotoxicity. Distinct from described polarities, this T cell phenotype is driven by the T-box transcription factor Eomesodermin. Formation of this phenotype requires 4-1BB signaling on both T and antigen-presenting cells and the resulting production of the cytokines IL-27, IL-15, and IL-10. Furthermore, we find CD4+ T cells bearing the signature features of this phenotype in the livers of mice infected with both bacterial and viral intracellular pathogens, suggesting a role for these cells in infectious immunity. These T cells constitute a novel phenotype that resolves multiple questions associated with 4-1BB activation, including how 4-1BB enhances tumor-specific cytotoxicity and how 4-1BB can promote tumor immunity while repressing autoimmunity.
T cell activation leads to transient up-regulation of the TNF receptor (TNFR) family member 4-1BB (CD137) in both the CD4 and CD8 subsets (Shuford et al., 1997). When engaged by its ligand or by agonistic antibodies, 4-1BB acts as a T cell costimulatory molecule promoting survival, expansion, and Th1 type cytokine production (Wang et al., 2009). Consistent with these costimulatory properties, 4-1BB promotes both antiviral (Tan et al., 1999; Kwon et al., 2002; Lin et al., 2009) and antitumor (Kocak et al., 2006; Li et al., 2007; Lynch, 2008; Curran et al., 2011) T cell responses. Paradoxically, 4-1BB has also been found to ameliorate autoimmunity in an array of animal models including collagen-induced arthritis (Seo et al., 2004), experimental autoimmune uveoretinitis (Choi et al., 2006), experimental autoimmune encephalomyelitis (Sun et al., 2002b), inflammatory bowel disease (Lee et al., 2005a), and systemic lupus erythematosus (Sun et al., 2002a). Part of the resolution of these seemingly incongruous effects seems to result from the tendency of 4-1BB activation to antagonize Th17 T cell polarization (Kim et al., 2011).
4-1BB agonist antibodies may also increase cytotoxicity, although a detailed mechanism behind these observations remains to be described. 4-1BB activation has been reported to increase in vivo killing of peptide-pulsed targets in a B16 melanoma model (Li et al., 2007). In both mice and humans, increases in T cell Granzyme B and Perforin expression after 4-1BB activation have been observed (Lin et al., 2010; Hernandez-Chacon et al., 2011). In a recent manuscript, the ability of 4-1BB and OX40 (CD134) agonists to polarize CD4 HA-specific T cells toward a Th1-type cytotoxic phenotype dependent on T-box transcription factors was also described (Qui et al., 2011). Another study has shown that OX-40 agonist antibody in conjunction with cyclophosphamide treatment and adoptive transfer of tumor-specific CD4+ T cells can produce cytotoxic CD4 T cells dependent on both Eomesodermin (Eomes) and T-bet (Hirschhorn-Cymerman et al., 2012). A detailed biological context or full pathway leading from 4-1BB activation to enhanced cellular cytotoxicity, however, remains to be elucidated.
Because of their potent activity in murine tumor models, agonist antibodies targeting 4-1BB have entered clinical trials for melanoma and lymphoma. A potentially limiting side effect of this therapy was described in a murine colon carcinoma therapy study in which liver pathology was observed after 4-1BB antibody treatment (Kocak et al., 2006). Although liver inflammation may be manageable in the clinic at therapeutically effective doses, the root cause of this liver pathology has yet to be discovered (Dubrot et al., 2010).
In a previous manuscript, we reported an unexpected population of T cells infiltrating B16 melanoma tumors of 4-1BB agonist antibody-treated mice (Curran et al., 2011). These T cells expressed the inhibitory receptor KLRG1 on the surface of nearly all of the CD8 and half of the CD4 compartments and appeared to be active effectors, as greater numbers of these cells correlated with superior tumor rejection. Here, we report that these KLRG1+ T cells constitute a novel phenotype/polarity which addresses the aforementioned unresolved questions regarding 4-1BB function.
We find that these KLRG1+ T cells, in both the CD4 and CD8 lineages, express highly elevated levels of cytotoxicity-associated genes relative to their KLRG1− counterparts from the tumors of mice not receiving 4-1BB agonist antibody. Contrary to Th1 cells, the induction of this genetic killing program is fully dependent on the master regulatory transcription factor Eomes and independent of changes to T-bet. Unlike other TNFR family members, 4-1BB is expressed on myeloid cells and these cells respond to its activation by producing cytokines such as IL-27 and IL-15, which are critical to development of this phenotype. These KLRG1+Eomes+ CD4 T cells do not fit any established T cell paradigm and may have a role in physiological antiviral immunity, as we find them in the livers of Listeria monocytogenes and lymphocytic choriomeningitis virus (LCMV)–infected mice. We have termed this cytotoxic Eomes-driven CD4 T cell phenotype ThEO and the corresponding CD8 T cells phenotype TcEO. These ThEO/TcEO T cells represent an important new class of T cells that explain the seemingly contradictory functions of 4-1BB, are present during the immune clearance of intracellular pathogens, and may provide a template for the in vitro polarization of highly cytotoxic T cells for adoptive transfer therapy.
RESULTS
Tumor-infiltrating KLRG1+ T cells elicited by 4-1BB agonist antibody treatment express a broad spectrum of cytotoxicity-associated genes
Previously we have shown that therapeutic vaccination against preimplanted B16 melanoma with a combination of irradiated Flt3-ligand–expressing B16 cells (B16 fms-related tyrosine kinase 3 ligand [FVAX]) and 4-1BB agonist antibody treatment results in nearly all of the tumor-infiltrating CD8 T cells and half of the CD4 T cells becoming positive for the E-cadherin receptor KLRG1 (Curran et al., 2011). Adding antibodies that block the T cell co-inhibitory receptor cytotoxic T lymphocyte antigen 4 (CTLA-4) to this therapy increased the number of melanoma-infiltrating CD4 KLRG1+ cells and resulted in greater tumor rejection than either antibody alone.
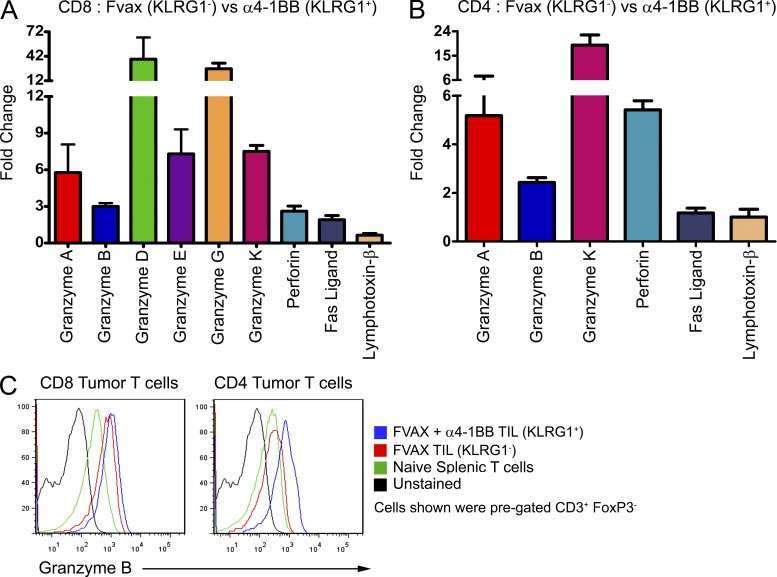
To determine the phenotype of these KLRG1+ T cells, we isolated them from the tumors of mice treated with FVAX and either α4-1BB or a combination of α4-1BB and αCTLA-4 (Combo). We compared gene expression by these KLRG1+ cells to that of isolated KLRG1− T cells from mice treated with only FVAX. KLRG1+ CD4 and CD8 T cells were the dominant populations in the tumors of 4-1BB agonist antibody-treated mice and were qualitatively different in terms of Granzyme B and Eomes expression from either KLRG1+ T cells from mice which didn’t receive α4-1BB or KLRG1− T cells from mice that did (unpublished data). In the tumor-infiltrating CD8 T cells of mice receiving α4-1BB, a broad cytotoxicity program is strongly induced (Fig. 1 A). Six different granzyme genes are induced, ranging from 3-fold up to 38-fold, coupled with 2- to 3-fold increases in perforin and fas-ligand (fasL) expression. More surprisingly, we found significant induction of granzymes A, B, and K, as well as perforin in the CD4 KLRG1+ T cells from 4-1BB agonist-treated mice (Fig. 1 B). This induction of Granzyme B was confirmed at the protein level by flow cytometry (Fig. 1 C). Similar results were obtained from mice receiving the antibody combination, except that mice receiving CTLA-4 blockade in addition to α4-1BB also showed fivefold induction of fasL in the CD4 compartment in addition to the other cytotoxicity-associated genes (unpublished data).
Figure 1.
Cytotoxic gene expression by tumor-infiltrating KLRG1+ T cells. Tumor-infiltrating KLRG1+ T cells from mice receiving FVAX and α4-1BB were isolated on day 16 and compared with KLRG1− T cells from mice treated with only FVAX. Gene expression from each population was calculated using real-time PCR analysis with hprt as the endogenous control. Relative gene expression in KLRG1+ CD8 (A) and CD4 (B) T cells compared with their KLRG1− counterparts was calculated using Taqman primers by the ΔΔCt method. Values shown are the mean (±SEM) of five to six individual experiments with 20–30 pooled mice per group. (C) Flow cytometry histograms comparing Granzyme B expression by tumor-infiltrating KLRG1− and KLRG1+ T cells from single, representative mice.
KLRG1+ CD8 and CD4 T cells exhibit enhanced killing of B16 melanoma cells compared with their KLRG1− counterparts
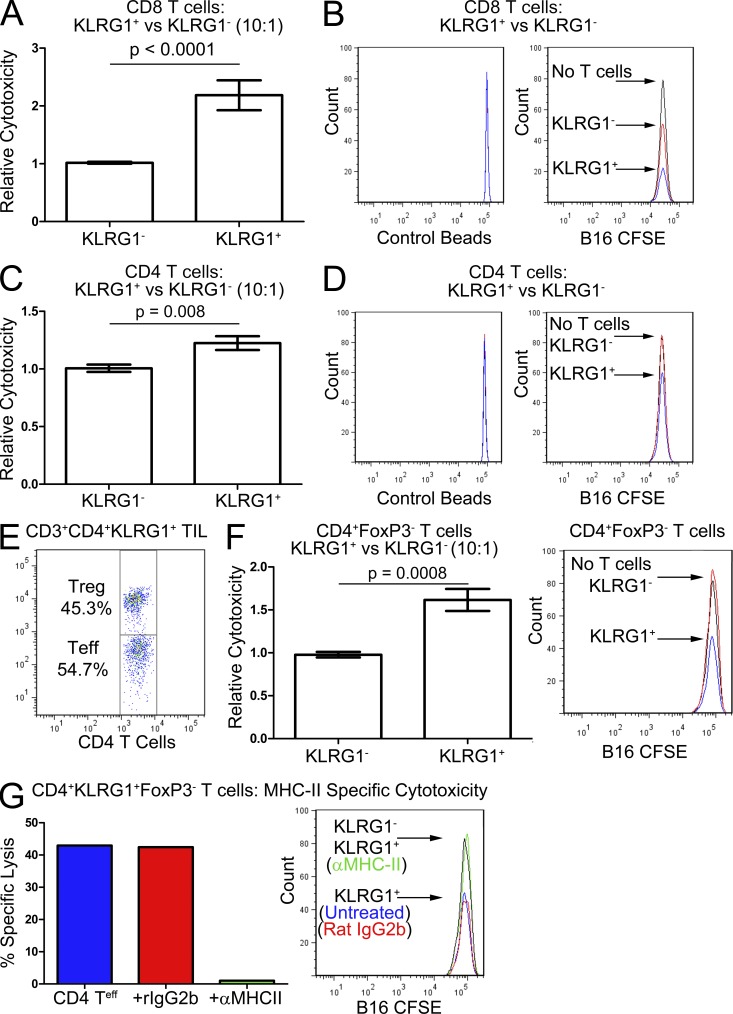
Induction of such a broad range of genes associated with cytolysis suggested these KLRG1+ T cells might be responsible for the enhanced tumor killing associated with 4-1BB agonist antibody. We therefore assayed whether or not these CD8 and/or CD4 KLRG1+ T cells could kill B16 melanoma cells better than their KLRG1− counterparts. Because of the large numbers of tumor-infiltrating lymphocytes (TILs) required for in vitro killing assays, we isolated KLRG1+ T cells from Combo-treated mice rather than mice treated with α4-1BB alone. We performed triplicate 10:1 effector-to-target killing assays for CD8 T cells, and as many as 10:1 replicate wells for CD4 T cells as the total numbers of isolated cells allowed.
Compared with CD8 KLRG1− T cells from FVAX-treated mice, the CD8 KLRG1+ T cells from Combo-treated mice demonstrated more than double the in vitro killing capacity against B16 melanoma cells (Fig. 2 A). The histograms from representative wells show the increased killing of dye-labeled B16 cells by the KLRG1+ cells relative to the KLRG1− ones (Fig. 2 B). The CD4 KLRG1+ cells from Combo-treated mice also exhibited 1.2-fold the cytotoxicity against B16 melanoma of their KLRG1− counterparts which had no significant killing activity over background (Fig. 2 C). Although the histograms show that these KLRG1+CD4 T cells had killing capacity (Fig. 2 D), these results were unimpressive in light of the high Granzyme expression of these cells observed by real-time PCR. Further analysis revealed that this sorted CD4 KLRG1+ population consisted of ∼50% FoxP3+ regulatory T cells, which could be obscuring the killing capacity of the effector CD4s in this assay (Fig. 2 E). To test this hypothesis, we repeated this experiment in FoxP3-GFP transgenic mice from which we isolated CD4 GFP− cells. The CD4 KLRG1+ T cells from these mice were nearly 1.6-fold more cytotoxic than their KLRG1− counterparts, confirming the enhanced killing capacity of these cells (Fig. 2 F). CD4+ T cells can kill via a variety of mechanisms including direct cytolysis, ligation of death receptors on target cells, or cytokine release. When MHC-II blocking antibody was added to the in vitro cytotoxicity assay, we found that all killing activity of these CD4 KLRG1+ cells was lost, confirming the need for specific TCR–MHC-II recognition (Fig. 2 G).
Figure 2.
Improved killing of B16 melanoma by KLRG1+ versus KLRG1− tumor-infiltrating T cells. Tumor-infiltrating KLRG1+ T cells from mice receiving FVAX and α4-1BB/αCTLA-4 were isolated on day 16 and compared with KLRG1− T cells from mice treated with only FVAX. In vitro killing assays were performed by incubating 105 T cells with 104 dye-labeled B16-BL6 melanoma cells for 12 h at 37°C. Samples were harvested in flow cytometry buffer with a fixed concentration of Pacific blue fluorescent beads so that values could be normalized for the fraction analyzed. (A) Relative cytotoxicity of CD8 KLRG1+ T cells compared with their KLRG1− counterparts is shown for four independent experiments with 20–30 pooled mice per group with 3 wells per experiment. (B) Representative histograms showing the number of B16-CFSE cells remaining alive after a 12-h incubation with each CD8 T cell population or no T cells. (C) Relative cytotoxicity of CD4 KLRG1+ T cells compared with their KLRG1− counterparts is shown for two experiments of 20–30 pooled mice per group with 3 wells (KLRG1−) or 1–3 wells (KLRG1+) per experiment. (D) Representative histograms showing the number of B16-CFSE cells remaining alive after a12-h incubation with each CD4 T cell population or no T cells. (E) Flow cytometry plot showing the percentage of the CD4 KLRG1+ tumor-infiltrating T cells composed of FoxP3+ regulatory cells from a representative mouse. (F) Relative cytotoxicity of CD4 KLRG1+FoxP3− T cells compared with their KLRG1−FoxP3− counterparts from FoxP3-GFP mice is shown for two experiments of 25–35 pooled mice per group with 1–3 wells per experiment, including histograms of the number of B16-CFSE cells alive after incubation with each T cell population. (G) Absolute cytotoxicity of CD4 KLRG1+FoxP3− T cells against B16-CFSE–labeled targets is shown from a single experiment of 25–35 pooled mice per group without antibody, with Rat IgG2b added, or with the M5/114 MHC-II blocking antibody added. (A, C, and F) Student’s t tests were performed to determine statistical significance between samples and the specific p-values are indicated where relevant.
Enhanced cytotoxicity of KLRG1+ TIL is driven by high expression of the T-box transcription factor Eomes
We sought to learn the molecular mechanism driving the highly cytotoxic phenotype of these KLRG1+ T cells. Previously, a Th1-type CD8 T cell population with enhanced effector function had been described as the outcome of IL-12 production eliciting high levels of the T-box transcription factor T-bet, which in turn induced KLRG1 and higher Granzyme B expression (Rao et al., 2010). In addition, T-bet has previously been described as controlling the formation of a cytotoxic CD4 melanoma antigen-specific T cell line (Xie et al., 2010).
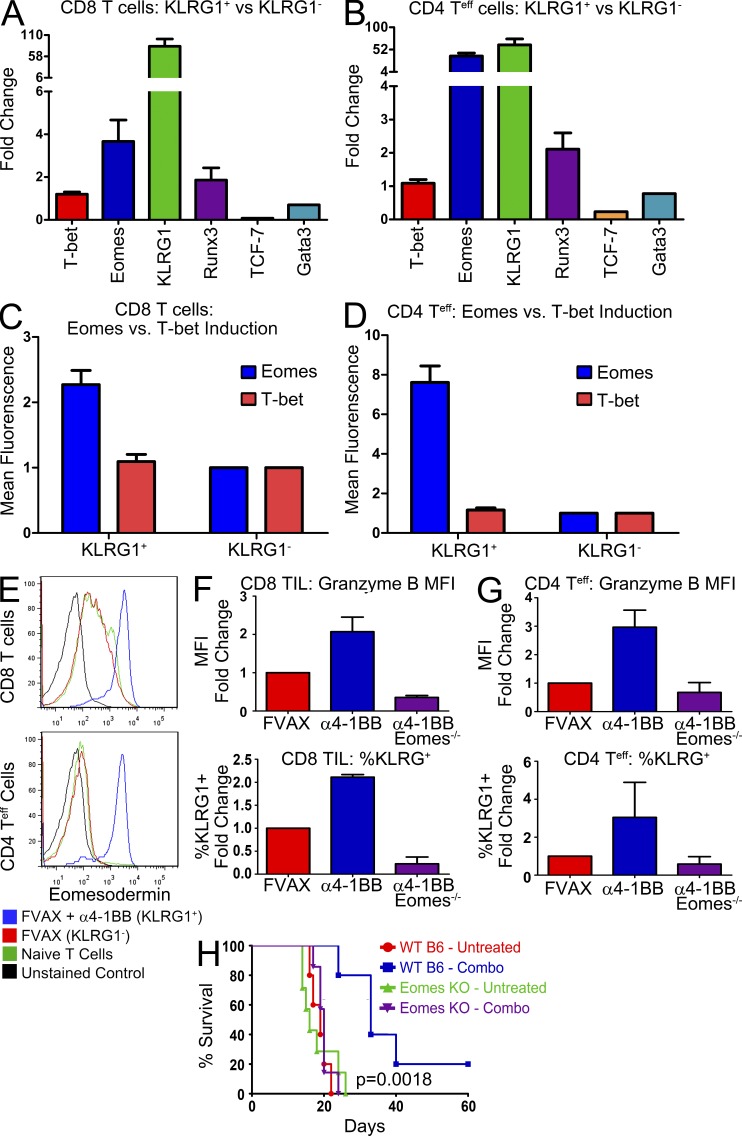
We compared the KLRG1+ T cells infiltrating the tumors of mice treated with FVAX and α4-1BB or Combo to the KLRG1− T cells from the tumors of mice receiving FVAX alone. CD8 KLRG1+ T cells from mice receiving α4-1BB showed heightened levels of the T-box transcription factor eomes and no substantial changes in levels of t-bet (Fig. 3 A). Interestingly, we also observed increased runx3 expression, which has been implicated as a cofactor for Eomes in inducing ifn-γ expression (Collins et al., 2010).
Figure 3.
Eomes drives the cytotoxic KLRG1+ T cell phenotype. Tumor-infiltrating KLRG1+ T cells from mice receiving FVAX and α4-1BB were isolated on day 16 and compared with KLRG1− T cells from mice treated with only FVAX. Gene expression from each population was calculated using real-time PCR analysis with hprt as the endogenous control. Relative gene expression in KLRG1+ CD8 (A) and CD4 (B) T cells compared with their KLRG1− counterparts was calculated using Taqman primers by the ΔΔCt method. Values shown are the mean (±SEM) of two to three individual experiments of 20–30 mice per group. Tumors from individual mice were also isolated at day 16 and analyzed by flow cytometry. Mean fluorescence intensity of Eomes and T-bet antibody staining in CD8 CD3+KLRG1+ (C) and CD3+CD4 FoxP3−KLRG1+ (D) T cells compared with their KLRG1-negative counterparts is shown for 8–10 experiments with 3–10 mice per group. (E) Representative flow cytometry histograms are shown from a representative experiment comparing Eomes expression in these KLRG1+ T cells to their KLRG1− counterparts. Tumor-infiltrating T cells from CD4-CRE,Eomesflox/flox mice treated with Fvax and α4-1BB were isolated on day 16 and analyzed by flow cytometry. Mean fluorescence intensity of Granzyme B antibody staining and percentage of cells positive for KLRG1 expression in CD8 (F) and CD4 (G) FoxP3− tumor-infiltrating T cells is shown for two to three experiments with three to five mice per group. Means are shown ± SEM. (H) Survival of five to seven mice per group of C57BL/6 or Eomesflox/flox × CD4-CRE mice challenged with 1.5 × 105 B16-BL6 cells and vaccinated on days 3, 6, and 9 with 106 Fvax intradermally and α4-1BB/ αCTLA-4 i.p. Lack of survival was defined as death or tumor size > 1,500 mm3. Statistical significance was assessed using the Mantel-Cox test.
CD4 T cells generally express little to no Eomes. Like the CD8 KLRG1+ cells, however, these CD4 KLRG1+ T cells expressed >35-fold higher eomes than their KLRG1− counterparts (Fig. 3 B). As in the CD8 lineage, we see no change in t-bet expression and some concomitant induction of runx3. This enhanced eomes expression is also reflected at the protein level as measured by flow cytometry for both the CD8 (Fig. 3 C) and CD4 T cells (Fig. 3 D). Representative raw flow cytometry data illustrates that the levels of Eomes expression in these CD4+KLRG1+ T cells nearly equal those of the CD8+KLRG1+ CD8s (Fig. 3 E).
To confirm the central role of Eomes as the driver of this phenotype, we vaccinated tumor-bearing CD4-CRE,Eomesflox/flox transgenic mice with either FVAX + α4-1BB with or without αCTLA-4 and analyzed their tumor-infiltrating T cells at day 16 (Intlekofer et al., 2008). In the absence of Eomes, neither CD8 T cells (Fig. 3 F) nor CD4 effector T cells (Fig. 3 G) up-regulate Granzyme B or KLRG1 in response to 4-1BB agonist antibody treatment. Combo-treated mice showed the same dependence on Eomes in both the CD4 and CD8 compartments as those receiving α4-1BB alone (not depicted).
Our earlier manuscript showed that these KLRG1+ tumor-infiltrating T cells were the majority of effector cells responding to α4-1BB treatment, and that the addition of aCTLA-4 increased the numbers of CD4s with this phenotype and in turn enhanced tumor rejection (Curran et al., 2011). To confirm the necessity of Eomes expression for the anti-melanoma effect of 4-1BB agonist treatment, we challenged C57BL/6 or CD4-CRE,Eomesflox/flox mice with a large 1.5 × 105 B16-BL6 tumor challenge and treated them with Fvax + α4-1BB and αCTLA-4. Combination therapy had a significant benefit even with this high tumor challenge in wild-type mice, but no effect at all in the mice lacking Eomes in their T cells (Fig. 3 H). A recent manuscript showed no induction of Eomes as a result of CTLA-4 blockade (Hirschhorn-Cymerman et al., 2012), nor have we observed any dependence of CTLA-4 blockade on Eomes induction or expression (not depicted); thus, any observed differences should be attributable to the efficacy of α4-1BB.
This T cell polarity driven by Eomes and specialized for enhanced target lysis has not been previously described but is likely emblematic of cytotoxic CD4+ T cells described in other recent manuscripts generated using TNF-receptor agonist antibodies (Qui et al., 2011; Hirschhorn-Cymerman et al., 2012). We therefore termed this CD4 phenotype ThEO and the Eomes-driven CD8 phenotype TcEO.
Of the major TNFR family agonists, only 4-1BB generates this KLRG1+Eomes+ phenotype in both CD4 and CD8 T cells
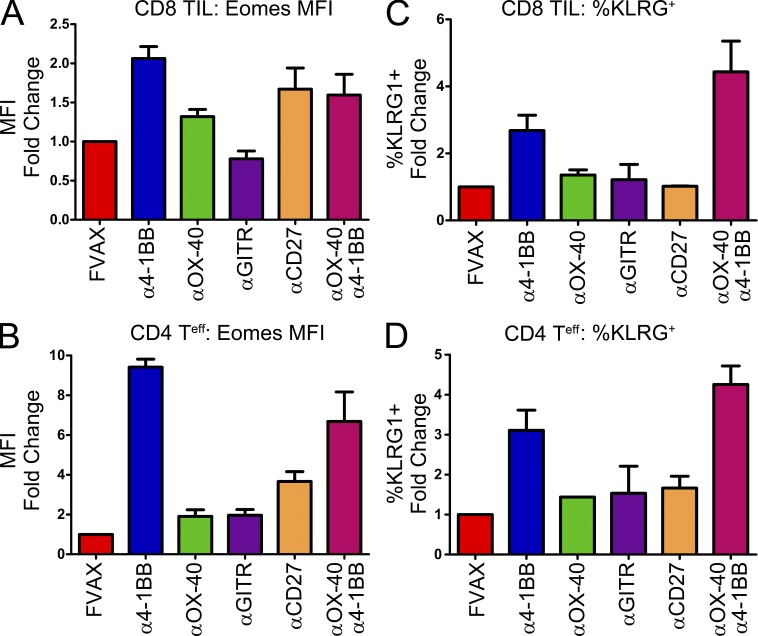
Having established the role of Eomes as the master regulator in these KLRG1+ cytotoxic T cells, we sought to determine whether activation of other TNFR family receptors could also generate this phenotype. Mice were challenged with B16 melanoma, vaccinated with FVAX and the indicated antibody on days 4, 7, 10, and 13, and then analyzed by flow cytometry on day 16. In tumor-infiltrating CD8 T cells, 4-1BB activation was the strongest inducer of both Eomes (Fig. 4 A) and KLRG1 (Fig. 4 B). αOX-40 and αCD27 were also able to induce Eomes to a moderate degree but without concomitant induction of KLRG1. Combining αOX-40 and α4-1BB did not yield higher levels of Eomes expression than α4-1BB alone but did promote greater induction of KLRG1.
Figure 4.
Of the TNFR family, only 4-1BB activation induces both Eomes and KLRG1 in CD4 and CD8 T cells. Mice were challenged with B16 melanoma and vaccinated with FVAX and the indicated TNFR agonist antibody. Tumor-infiltrating T cells were harvested at day 16 and analyzed by flow cytometry. Mean fluorescence intensity of Eomes antibody staining and percentage of cells positive for KLRG1 expression in (A and B) CD8 and (C and D) CD4 FoxP3− tumor-infiltrating T cells is shown for two to three experiments with 4–10 mice per group. Means are shown ± SEM.
In the CD4 compartment, α4-1BB was the dominant inducer of Eomes expression (Fig. 4 C). αCD27 was able to promote a small up-regulation of Eomes but had little to no effect on KLRG1 (Fig. 4 D). As in the CD8 compartment, α4-1BB was the only strong single agent inducer of KLRG1; however, αOX-40 was able to enhance KLRG1 levels when given in combination (Fig. 4 D).
4-1BB is expressed by myeloid cells which produce cytokines that support development of these KLRG1+Eomes+ T cells
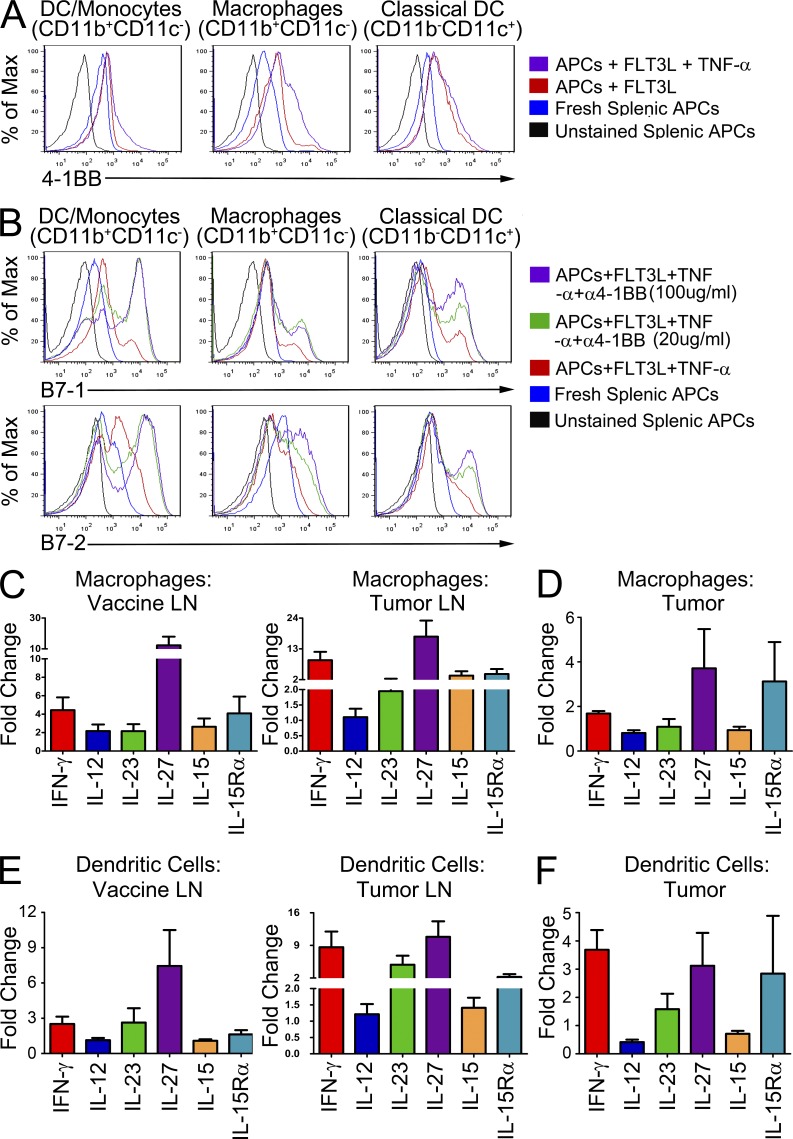
As 4-1BB appeared uniquely capable of inducing these KLRG1+Eomes+ T cells in both the CD4 and CD8 lineages, we investigated what properties of 4-1BB might functionally distinguish it from other TNFR family members. One unique property of 4-1BB is its expression on myeloid APCs in addition to T cells (Futagawa et al., 2002). We found that isolated myeloid cells cultured in the presence of Flt3-ligand expressed moderate amounts of 4-1BB in multiple lineages capable of antigen presentation (Fig. 5 A). Previously, we showed that 4-1BB agonist antibody treatment elicits high levels of IFN-γ and TNF production from tumor-specific T cells in the lymph nodes and tumor (Curran et al., 2011). Interestingly, we observed that exposing the Flt3-ligand cultured myeloid cells to TNF triggered further up-regulation of 4-1BB. Although TNF increased 4-1BB expression, its addition was not required for these cells to express measurable levels of 4-1BB. Up-regulation of the costimulatory ligand B7-1 by APCs in response to 4-1BB-ligand had been previously observed (Futagawa et al., 2002); however, we expanded on these observations, showing that these cells responded to 4-1BB agonist antibody in vitro by increasing their expression of both B7-1 and B7-2 (Fig. 5 B).
Figure 5.
Myeloid cells up-regulate costimulatory ligands and production of inflammatory cytokines in response to 4-1BB activation. Splenic myeloid cells were cultured for 72 h in complete RPMI with Flt3-ligand and TNF and either 20 µg/ml or 100 µg/ml α4-1BB antibody as indicated. Fresh splenic myeloid cells from naive mice were isolated at the end of the 72-h culture period for use as controls. (A) Cells were divided into three populations based on their CD11b and CD11c expression and assayed by flow cytometry to determine their expression of 4-1BB. (B) Cells were cultured and analyzed as above to measure their expression of B7-1 and B7-2 in response to in vitro activation of 4-1BB by the agonist antibody. Vaccine and tumor-draining lymph node and tumor-infiltrating myeloid cells from mice receiving FVAX and α4-1BB were isolated on day 16 and compared with their counterparts from mice treated with only FVAX. Gene expression from each population was calculated using real-time PCR analysis with gapdh as the endogenous control. Gene expression in CD11b+c− macrophages in the nodes (C) and tumor (D) and CD11c+b+/− dendritic cells in the nodes (E) and tumor (F) relative to the FVAX alone group was calculated using Taqman primers by the ΔΔCt method. Values shown are the mean (±SEM) of four to five individual experiments of 20–30 pooled mice per group.
We compared CD11c+CD11b+/− DC/monocytes and CD11b+CD11c− macrophages from mice receiving FVAX and α4-1BB or Combo with those from mice receiving FVAX alone. In both the vaccine and tumor-draining lymph nodes, we observed that CD11b+ macrophages responded to 4-1BB activation by up-regulating il-27, il-15/il-15ra, and ifn-γ (Fig. 5 C). Even in the suppressive tumor microenvironment there is evidence of a proinflammatory cytokine response after 4-1BB activation from these CD11b+ cells (Fig. 5 D). In the CD11c+ DC-like population, il-27 again appears to be the major cytokine induced by 4-1BB activation, although there is also evidence for up-regulation of il-23, ifn-γ, and il-15/il-15ra (Fig. 5 E). As in the macrophage population, some proinflammatory activity appears to be elicited even from CD11c+ cells in the tumor microenvironment itself (Fig. 5 F). We measured detectable levels of IL-12β as well as Ebi3 in these samples, but the changes in their expression were not significant enough to merit inclusion here (unpublished data).
4-1BB activation elicits IL-27 production by myeloid cells in the liver which may contribute to hepatitis
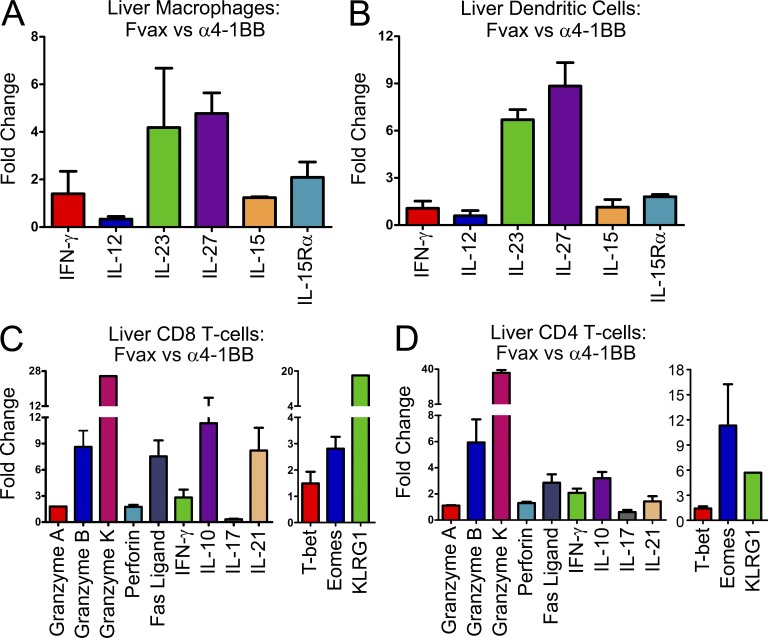
IL-27 production in the liver has been linked to pathological hepatic inflammation (Siebler et al., 2008). We compared gene expression by myeloid cells from FVAX + α4-1BB or Combo-vaccinated mice to those of mice receiving only FVAX. Similar to our prior observations in the nodes and tumor, there was strong induction of il-27 by both CD11b+c− (Fig. 6 A) and CD11c+b+/− (Fig. 6 B) myeloid cells in the liver. There also appeared to be stronger induction of il-23 than we had observed in other sites but less induction of ifn-γ. As before, we saw no evidence for il-12 up-regulation.
Figure 6.
The livers of α4-1BB–treated mice are infiltrated by IL-27–producing myeloid cells and Eomes+KLRG1+ T cells. Liver-infiltrating myeloid and T cells from mice receiving FVAX and α4-1BB were isolated on day 16 and compared with their counterparts from mice treated with only FVAX. Gene expression from each population was calculated using real-time PCR analysis with gapdh (myeloid cells) or hprt (T cells) as the endogenous control. Gene expression in CD11b+c− macrophages (A) and CD11c+b+/− dendritic cells (B) in the liver relative to the FVAX alone group was calculated using Taqman primers by the ΔΔCt method. Values shown are the mean (±SEM) of two individual experiments of 20–30 pooled mice per group. Gene expression in CD8 T cells (C) and CD4 T cells (D) in the liver relative to the FVAX alone group was calculated using Taqman primers by the ΔΔCt method. Values shown are the mean (±SEM) of two to three individual experiments of 20–30 pooled mice per group.
We also examined T cells in the livers of these treated mice to determine if their activation state was affected by 4-1BB agonist antibody treatment in a manner that could impact hepatic homeostasis. We found that both CD8 T cells (Fig. 6 C) and CD4 T cells (Fig. 6 D) in the livers of mice receiving α4-1BB were expressing an activated cytotoxicity program similar to that of the Eomes+KLRG1+ cells we described in tumors. One potentially significant difference, however, was the up-regulation of il-10 by these cells in the liver which was not observed in the tumor. These cells were not presorted based on KLRG1; therefore, the il-10 expressing population may represent a separate population from the KLRG1+Eomes+ cells or the influence of the liver microenvironment on the known capacity of IL-27 to elicit IL-10 production in certain settings (Murugaiyan et al., 2009). Consistent with the capacity of both Eomes and IL-27 to repress Th17 polarization, in all cases we observed 1.6-fold to 8-fold decreases in il-17 expression in the mice receiving 4-1BB agonist antibody (Diveu et al., 2009; Ichiyama et al., 2011).
IL-10, IL-15, and IL-27 contribute to the development of Eomes+KLRG1+ cytotoxic T cells in vivo
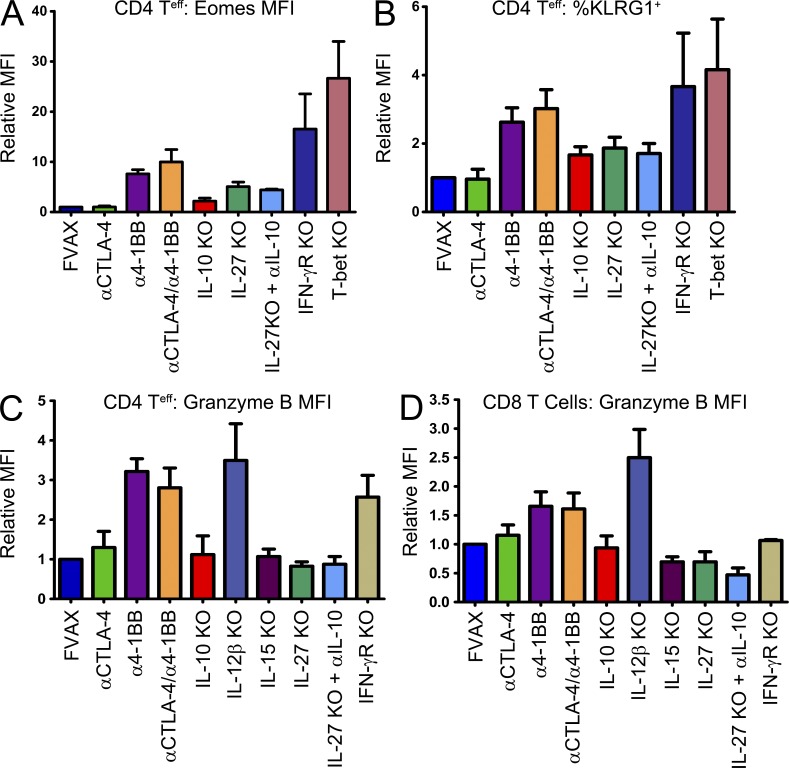
To ascertain whether these cytokines influenced the induction of Eomes on tumor-infiltrating T cells, we examined the impact of relevant cytokine and transcription factor deficiencies on Eomes, KLRG1, and Granzyme B up-regulation after 4-1BB agonist or Combo treatment. Induction of Eomes was impaired in the CD4 lineage in both IL-10 and IL-27 knockout mice and increased in mice lacking the IFN-γ receptor or T-bet (Fig. 7 A). Our earlier Taqman and Eomes knockout mouse studies suggest that KLRG1 is either a direct or a downstream target of Eomes, and here we find that KLRG1 induction shares nearly the same cytokine dependencies as Eomes (Fig. 7 B). None of the mice we tested exhibited a strong defect in Eomes up-regulation in CD8 T cells, but, as in the CD4 compartment, higher levels of Eomes were seen in the T-bet knockouts (unpublished data). The enhanced cytotoxicity emblematic of this phenotype appeared to depend on IL-10, IL-27, and IL-15 for both CD4 T cells (Fig. 7 C) and CD8 T cells as measured by Granzyme B induction (Fig. 7 D). These same cytokines also appear to influence Fas-ligand up-regulation in response to 4-1BB agonist antibody (unpublished data). Although IFN-γR knockout mice exhibited increased Eomes expression, they failed to fully induce Granzyme B in the CD8 compartment, illustrating the functional role of IFN-γ (which is produced in large quantities by these T cells) in this system. Interestingly, the IL-12β knockout mice which lack both IL-12 and IL-23 exhibited higher levels of cytotoxic protein expression after α4-1BB treatment compared with wild-type mice.
Figure 7.
IL-27, IL-10, and IL-15 knockout mice are defective in developing the cytotoxic Eomes+KLRG1+ T cell phenotype. Wild-type or knockout mice of the indicated phenotype were challenged with B16 melanoma and vaccinated with FVAX + α4-1BB with or without αCTLA-4. Tumor-infiltrating T cells were isolated on day 16 and analyzed by flow cytometry. For CD4 FoxP3+ T cells the MFI of Eomes staining (A) and the percentage of KLRG1+ cells (B) are shown. Also, the MFI of Granzyme B staining for these CD4 cells (C), as well as for CD8 T cells (D), is shown. Knockout mouse data are shown as the mean ± SEM for 3–10 experiments with 3–10 mice per group.
ThEO phenotype CD4 T cells may have a physiological role in pathogen-specific immunity
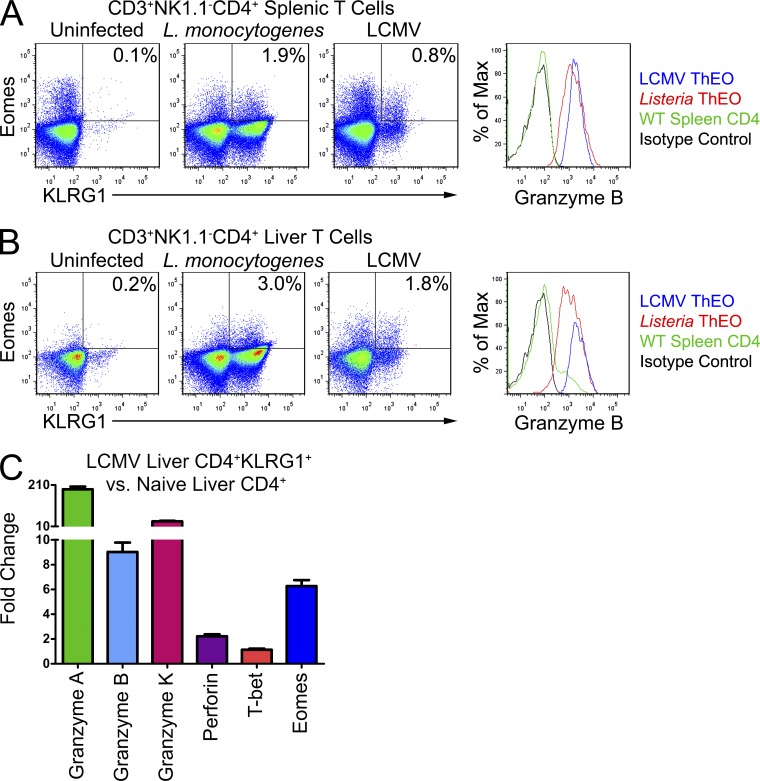
The pathway we revealed leading from 4-1BB activation to formation of these KLRG1+Eomes+ T cells could be beneficial in clearance of pathogens that require extensive cellular cytotoxicity to eradicate. Although CD8 Eomes+KLRG1+ T cells are difficult to distinguish from Th1-type terminal effectors by flow cytometry alone, no CD4 KLRG1+Eomes+ T cells have been previously described nor do they fit into any existing polarity paradigm. We examined the spleens, lymph nodes, and livers of mice infected with either Listeria monocytogenes or LCMV. We chose to examine these pathogens in particular because mice lacking 4-1BB are known to exhibit defects in their clearance (Tan et al., 1999; Lee et al., 2005b). In both the spleens (Fig. 8 A) and livers (Fig. 8 B) of infected mice, there was a clear population of CD3+NK1.1−CD4 KLRG1+Eomes+ cells which also expressed Granzyme B. We sorted these CD4 KLRG1+ cells from the livers of LCMV-infected mice on day 8 after infection and analyzed their gene expression compared with CD4 T cells from the livers of uninfected mice. This sorted population contains all of the CD4 KLRG1+Eomes+ cells but also a contaminating population of KLRG1+Eomes− cells. Much like the tumor-infiltrating CD4 T cells we described after systemic 4-1BB agonist treatment, these sorted cells express elevated levels of eomes but not t-bet and express significantly elevated levels of granzymes A, B, and K, as well as twofold elevated perforin (Fig. 8 C). These data suggest that further study of this novel T cell population and its impact on immune responses to viruses and intracellular bacteria is warranted.
Figure 8.
CD4 KLRG1+Eomes+ T cells are active during L. monocytogenes and LCMV infections. Mice were challenged with 2 × 105 PFU of LCMV Armstrong strain or 2 × 103 cells of Listeria monocytogenes and 8 d later lymphocytes were isolated from their livers and spleens. T cells were first gated on the CD3+CD4+NK1.1− cells. Flow cytometry analysis of the spleens (A) and livers (B) of infected versus naive mice reveals a population of Eomes+KLRG1+ T cells expanded in the infected animals. These CD4 T cells from infected mice also express a high level of Granzyme B. (C) Liver CD4 KLRG1+ T cells from 8-d LCMV-infected mice were isolated and compared with liver CD4 KLRG1− T cells from naive mice. Livers from five mice were pooled for each group. Gene expression from each population was calculated using real-time PCR analysis with hprt as the endogenous control. Gene expression in CD4 KLRG1+ T cells from LCMV livers relative to CD4 KLRG1− T cells from naive livers was calculated using Taqman primers by the ΔΔCt method. All cells were CD3+NK1.1−. Values shown are the mean (±SEM) of 3 replicate wells from one experiment.
DISCUSSION
The data presented here suggests that the cytotoxic CD4 and CD8 KLRG1+ T cells generated in vivo after systemic 4-1BB activation comprise a novel T cell phenotype. In both lineages, this phenotype is dependent on high expression of the T-box transcription factor Eomes and forms even in the absence of T-bet. Among the accepted T cell polarities, these cells most resemble Th1 (O’Shea and Paul, 2010); however, they fail to conform to the defining criteria of Th1 cells in so many respects that we consider them an entirely novel polarity. In the classical Th1 setting, IL-12 activates Stat4 which activates the transcription factor T-bet which in turn executes the bulk of the Th1 transcriptional program. We find no evidence for induction of il-12 on relevant APCs, no up-regulation of t-bet gene or protein expression, and a complete dependence of the phenotype we observe on Eomes. In contrast to existing paradigms, these cells appear to require IL-15, IL-27, and IL-10 for their formation, they rely on Eomes as the master regulator of the phenotype, and they produce multiple Granzymes, Perforin, and IFN-γ once polarized. The unique function of these cells appears to be multipotent cytotoxicity, involving high expression of multiple Granzymes of varying specificities as well as Fas-ligand. In lieu of STAT4 activation, they may depend on some subset of STAT1, STAT5, and particularly STAT3 activation based on the cytokines involved (Shuai et al., 1993; Johnston et al., 1995; Lucas et al., 2003; Owaki et al., 2008). Furthermore, the combination of Eomes and IL-27 in this system acts to stabilize this phenotype and block polarization to other phenotypes through inhibition of RORγT, FoxP3, and possibly also GATA3 (Huber et al., 2008; Diveu et al., 2009; Ichiyama et al., 2011). This dual inhibition of Th17 polarization by both Eomes in cis and IL-27 in trans resolves the paradox of how α4-1BB can repress autoimmunity while potentiating antiviral and antitumor immunity. We have termed this CD4 T cell phenotype ThEO and the corresponding CD8 T cell phenotype TcEO.
A recently published manuscript provides strong support for our observations, as they show that Trp1 melanoma-specific CD4 T cells can be rendered capable of eradicating large established tumors when adoptively transferred into mice treated with cyclophosphamide and αOX-40 (Hirschhorn-Cymerman et al., 2012). They show that the enhanced cytotoxicity of these cells is dependent on Eomes and that they up-regulate KLRG1, demonstrating that they have taken on the ThEO polarity described here. The use of Trp1 transgenic cells is potentially confounding here, however, as they express very high basal levels of T-bet on which their efficacy also depends. One apparent conflict between our studies is that we find little capacity of αOX-40 to induce Eomes in CD4+ T cells, whereas they find that it can. A major difference between OX-40 and 4-1BB is the expression of 4-1BB by both T cells and myeloid cells, whereas OX-40 is expressed only by T cells. They only observe this enhanced Trp1 phenotype when cyclophosphamide is used in addition to αOX-40, suggesting that cytokines contributed by the lymphopenic environment are important. We hypothesize that these cytokines, which are known to include IL-15 which we show as being important for the generation of this phenotype, are substituting for the myeloid response elicited by α4-1BB. This in fact shows that our observations are quite similar and the ThEO phenotype is likely a more broadly accessible program which yields highly tumoricidal T cells.
Although a complete signaling cascade leading from surface receptor activation to activation of the eomes gene has yet to be described, some extracellular inducers of eomes have been discovered in other systems. IL-27, in particular, has proven capable of triggering eomes induction and appears particularly relevant in this setting (Morishima et al., 2005). Activators of the Wnt pathway have also been described as inducers of eomes expression by T cells; however, we found the T cell downstream mediator of Wnt signaling, tcf7, to be down-regulated by up to 13-fold in the CD8 and fivefold in the CD4 KLRG1+Eomes+ T cells (Zhao et al., 2010). This suggests that eomes maintenance is likely independent of Wnt signaling in our system; however, it remains possible that Wnt agonists could play a role in induction of this phenotype, leading to feedback down-regulation of tcf7. Our knockout studies also implicated IL-10 as being important for Eomes up-regulation in CD4 but not CD8 T cells. IL-10 and IL-27 are often described to transmit antiinflammatory signals via Stat3; however, Herrero et al. (2003) have described a system by which Stat3 signals are converted to proinflammatory signals in the presence of IFN-γ. As all of these cytokines seem to play a role in Granzyme induction in the ThEO/TcEO phenotype, we believe this signal conversion pathway may be relevant here.
Previously, it has been established that activation of 4-1BB leads to extended survival of both T cells and APCs, as well as facilitating expansion of antigen-specific T cells, particularly in the CD8 compartment (DeBenedette et al., 1997; Hurtado et al., 1997; Choi et al., 2009). Here, we demonstrate that systemic α4-1BB antibody polarizes tumor-infiltrating T cells toward a highly cytotoxic phenotype and activates a supportive cytokine program in myeloid cells residing in the draining lymph node and tumor. Taken in sum, these mechanistic insights explain the potent antitumor activity of α4-1BB antibody against a wide variety of malignancies. Although Th1 polarized T cells are capable of expressing high levels of Granzyme B and Perforin, tumors may resist Granzyme B–mediated cytolysis via the expression of serine protease inhibitors such as Spi6 in the mouse and PI9 in man (Bots et al., 2005). The broad spectrum of cytolytic effector mechanisms induced in these KLRG1+Eomes+ T cells, however, would be difficult to fully resist, likely contributing to the enhanced antitumor killing we observe.
Tumor-specific CD4 killer T cells can mediate regressions of even large established tumors as demonstrated by the TRP-1-specific T cells used in the B16 melanoma model (Quezada et al., 2010; Xie et al., 2010). The phenotype and activity of these cells seems to depend on T-bet rather than Eomes (Xie et al., 2010) and has yet to be replicated in polyclonal T cell populations relevant for human adoptive cell therapy. Qui et al. (2011) recently described a protocol for in vitro polarizing CD4 T cells to this cytotoxic Th1 phenotype in which Eomes plays an important role in Granzyme induction; however, the overall phenotype they describe is primarily a classical Th1 T cell in which T-bet plays the critical role in specifying polarity. The tumor-infiltrating CD4 KLRG1+Eomes+ T cells we find after α4-1BB treatment express multiple cytotoxicity-associated genes and are efficient killers of B16 melanoma tumor cells in vitro and in vivo. Furthermore, we describe cytokines relevant to the generation of this novel T cell polarity in vivo which may be relevant for inducing this phenotype in vitro in T cells for future adoptive transfer applications.
It has previously been observed that 4-1BB agonist treatment induces a population of CD8+ T cells which express the dendritic cell marker CD11c and produce high levels of IFN-γ (Ju et al., 2007). We found that ∼50% of our KLRG1+Eomes+ T cells also expressed CD11c in both the CD8 and CD4 lineages and that expression of both KLRG1 and CD11c by these TILs was absent when 4-1BB knockout mice were used (unpublished data). In the future, we will explore the relationship between the factors inducing Eomes and KLRG1 and those inducing CD11c downstream of 4-1BB activation.
Given that 4-1BB plays a role in the clearance of natural LCMV and L. monocytogenes infections (Tan et al., 1999; Lee et al., 2005b), we investigated whether KLRG1+Eomes+ T cells might be present at relevant sites of anti-pathogen immunity. Using available murine antibodies, it was difficult to discriminate Th1-type terminal effector CD8 T cells from Eomes-dependent CD8 T cells like those in our B16 tumors; however, we did find CD4 Eomes+KLRG1+ T cells in both the livers and spleens of infected mice which resemble the ThEO phenotype in many ways. These observations evoke the possibility that this novel T cell polarity may be important for clearance of these infections; however, further investigation will be required to firmly establish the role, if any, played by these KLRG1+Eomes+ T cells in immunity to intracellular pathogens.
The potential of these Eomes+KLRG1+ T cells to contribute to the memory pool remains to be elucidated. They are a poor fit for existing memory paradigms, as Eomes expression tends to be positively correlated with memory formation, whereas KLRG1 marks terminal effector populations lacking memory potential (Rutishauser and Kaech, 2010). Within the tumors of 4-1BB agonist antibody-treated mice, we do observe that a small percentage of these KLRG1+ T cells also express CD127 (unpublished data) which are generally described as mutually exclusive in models of T cell memory precursors. Future studies to address the memory potential and phenotype of these Eomes+KLRG1+ T cells will expand our understanding of the populations contributing to the memory T cell pool.
The novel Eomes+KLRG1+ T cell phenotype described here answers many questions associated with the function of 4-1BB on both T cells and myeloid cells, as well as solving the paradox of how 4-1BB could both promote antitumor immunity and inhibit Th17-dependent autoimmunity. These cells do not conform to any established T cell paradigm and open new avenues of study in antitumor immunity, antipathogen immunity, and T cell memory.
MATERIALS AND METHODS
Mice.
All mouse procedures were performed in accordance with institutional protocol guidelines at Memorial Sloan-Kettering Cancer Center (MSKCC). Mice were maintained according to National Institutes of Health Animal Care guidelines, under protocol 04–07-019 approved by the MSKCC Institutional Animal Care Committee. C57BL/6, IL-10−/−, IL-12β−/−, IFN-γR−/−, Ebi3−/−, T-bet−/−, OT-II, Pmel, and CD4-Cre mice were obtained from The Jackson Laboratory. IL15−/− mice were obtained from Taconic. Eomesflox/flox mice were provided by S.L. Reiner. FoxP3-GFP reporter mice were contributed by A.Y. Rudensky (MSKCC, New York, NY). 4-1BB knockout mice were provided by R. Mittler (Emory University, Atlanta, GA) with permission from B. Kwon (University of Ulsan, Ulsan, South Korea).
Antibodies.
Anti-CTLA-4 (9D9), α4-1BB (LOB12.3; Taraban et al., 2002), αOX-40 (OX-86), α4-1BB (DTA-1), αIL-10 (JES5-2A5), and αIFN-γ (XMG1.2) used in vivo were produced by BioXcell. αCD27 (AT126-1) was provided by A. Al-Shamkhani. Dosing per injection was 100 µg 9D9 and 350 µg per injection for all others.
Staining antibodies included CD4-Q605, CD8–Pacific Orange, CD3-APCAlexa750, and Granzyme B–APC (Invitrogen); CD4-APC, Eomes-PE, Tbet-PerCP-Cy5.5, FasL-PE, FoxP3-ef450, KLRG1-FITC, KLRG1-PE-Cy7, CD3-APC-ef780, and 4-1BB-PE (eBioscience); CD8-PE and Ki67–Alexa Fluor 700 (BD); B7-1-BV421 (BioLegend); and Runx3-APC (R&D Systems). Some clones were conjugated using monoclonal antibody conjugation kits (Invitrogen) to Alexa Fluor 532, Alexa Fluor 594, or Qdot 655.
Cell lines.
B16/BL6 cells, as well as B16-sFlt3L-Ig (FVAX), have been described previously (Curran and Allison, 2009).
Tumor-infiltrating T cell analysis.
Mice receiving a 1.5 × 105 B16-BL6 challenge in 30% growth factor–reduced matrigel (BD) were vaccinated on days 4, 7, 10, and 13 and sacrificed on day 16. Vaccination consisted of 106 FVAX cells intradermally on the contralateral flank and antibody i.p. as indicated. Tumors were measured immediately before sacrifice. Excised tumors were digested using Collagenase H (Sigma-Aldrich) and DNAase (Roche) and lymphocytes were enriched on a ficoll gradient (Histopaque 1119; Sigma-Aldrich). Cells were stained using the FoxP3 staining kit (eBioscience). Stained samples were run on an LSRII (BD) cytometer.
Taqman gene expression analysis.
Mice were vaccinated as above except that mice being treated with both α4-1BB and αCTLA-4 received a 2.5 × 105 B16-BL6 challenge to ensure recovery of sufficient numbers of tumor-infiltrating lymphocytes for analysis. Lymph nodes, livers, and tumors of mice were digested and lymphocytes purified as above. Cells were stained for CD8, CD4, CD11c, CD11b, CD3, and KLRG1 and then enriched using anti-PE and anti-APC-positive selection beads (Miltenyi Biotec). The enriched cells were then sorted on a FACSAria (BD), counted, and then their RNA was isolated using the RNeasy kit (QIAGEN). cDNA was then produced from RNA using the Superscript II Reverse Transcription kit (Invitrogen). Relative gene expression was then determined using the ABI 7500 Real-Time PCR System using VIC- and FAM-conjugated primer probes (Applied Biosystems). Primers for multimeric cytokines and receptors presented here included IL-12-p35, IL-23-p19, IL-27-p28, and IL-15 receptor α chain.
In vitro cytotoxicity assays.
20–35 mice per group were vaccinated as above with either FVAX alone or FVAX + αCTLA-4/α4-1BB. Tumor-infiltrating T cells were isolated and purified as above except that after FACS sorting, T cells were plated with labeled B16 melanoma cells to measure target cell lysis. Naive splenic T cells from wild-type mice were sorted as controls. B16-melanoma cells labeled with either CFSE (Invitrogen) or Cellvue Burgundy (eBioscience) were plated at 104 cells/well in a round-bottom 96-well plate. Sorted CD8 and CD4 T cells were added at 1:1, 5:1, 10:1, and 20:1 if sufficient cells were obtained after purification. Otherwise only 10:1 and 1:1 incubations were performed. Cells were cultured for 12 h in complete RPMI with 20 U/ml IL-2. Cells were resuspended in HBSS with 3% fetal calf serum and a fixed concentration of 460-nm 6-µm Peakflow beads (Invitrogen) to standardize for the amount of each well harvested and analyzed. Cytotoxicity was measured by running the cells on an LSRII flow cytometer and assessing the number of surviving B16 melanoma cells relative to controls.
In vitro T cell polarization.
Naive splenic OT-II or Pmel T cells were purified using Dynal CD4 and CD8 negative selection beads (Invitrogen). These T cells were then activated in vitro by co-culture with 30 IU/ml IL-2 and DCs isolated from C57BL6 spleens using CD11c positive selection beads (Miltenyi Biotec) which were loaded with Gp100 25–33 (EGSRNQDWL) or Ovalbumin 323–339 (ISQAVHAAHAFINEAGR) peptides (Biosynthesis Inc.). These cells were then either maintained in IL-2 alone or cultured in a mix of IL-2 (R&D Systems), IL-15:IL-15Rα (eBioscience), IL-27 (eBioscience), IFN-γ (PeproTech), Wnt3a (R&D Systems), and 100 µg/ml α4-1BB.
Infectious disease models.
Mice were either challenged i.p. with 2 × 105 PFU of LCMV or i.v. with 2 × 103 L. monocytogenes cells. On day 8 after infection, mice were sacrificed for analysis.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (J.P. Allison).
Dr. Allison is a paid consultant for Bristol-Myers Squib and is the primary inventor on the Patent “Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling.” The authors have no additional financial interests.
Footnotes
Abbreviations used:
- CTLA-4
- cytotoxic T lymphocyte antigen 4
- Eomes
- Eomesodermin
- FVAX
- B16 fms-related tyrosine kinase 3 ligand
- LCMV
- lymphocytic choriomeningitis virus
- TIL
- tumor-infiltrating lymphocyte
- TNFR
- TNF receptor
References
- Bots M., Kolfschoten I.G., Bres S.A., Rademaker M.T., de Roo G.M., Krüse M., Franken K.L., Hahne M., Froelich C.J., Melief C.J., et al. 2005. SPI-CI and SPI-6 cooperate in the protection from effector cell-mediated cytotoxicity. Blood. 105:1153–1161 10.1182/blood-2004-03-0791 [DOI] [PubMed] [Google Scholar]
- Choi B.K., Asai T., Vinay D.S., Kim Y.H., Kwon B.S. 2006. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine. 34:233–242 10.1016/j.cyto.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Choi B.K., Kim Y.H., Kwon P.M., Lee S.C., Kang S.W., Kim M.S., Lee M.J., Kwon B.S. 2009. 4-1BB functions as a survival factor in dendritic cells. J. Immunol. 182:4107–4115 10.4049/jimmunol.0800459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.L., Chang S., Henderson M., Soutto M., Davis G.M., McLoed A.G., Townsend M.J., Glimcher L.H., Mortlock D.P., Aune T.M. 2010. Distal regions of the human IFNG locus direct cell type-specific expression. J. Immunol. 185:1492–1501 10.4049/jimmunol.1000124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M.A., Allison J.P. 2009. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 69:7747–7755 10.1158/0008-5472.CAN-08-3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M.A., Kim M., Montalvo W., Al-Shamkhani A., Allison J.P. 2011. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE. 6:e19499 10.1371/journal.pone.0019499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedette M.A., Shahinian A., Mak T.W., Watts T.H. 1997. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J. Immunol. 158:551–559 [PubMed] [Google Scholar]
- Diveu C., McGeachy M.J., Boniface K., Stumhofer J.S., Sathe M., Joyce-Shaikh B., Chen Y., Tato C.M., McClanahan T.K., de Waal Malefyt R., et al. 2009. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 182:5748–5756 10.4049/jimmunol.0801162 [DOI] [PubMed] [Google Scholar]
- Dubrot J., Milheiro F., Alfaro C., Palazón A., Martinez-Forero I., Perez-Gracia J.L., Morales-Kastresana A., Romero-Trevejo J.L., Ochoa M.C., Hervás-Stubbs S., et al. 2010. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol. Immunother. 59:1223–1233 10.1007/s00262-010-0846-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagawa T., Akiba H., Kodama T., Takeda K., Hosoda Y., Yagita H., Okumura K. 2002. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14:275–286 10.1093/intimm/14.3.275 [DOI] [PubMed] [Google Scholar]
- Hernandez-Chacon J.A., Li Y., Wu R.C., Bernatchez C., Wang Y., Weber J.S., Hwu P., Radvanyi L.G. 2011. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J. Immunother. 34:236–250 10.1097/CJI.0b013e318209e7ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero C., Hu X., Li W.P., Samuels S., Sharif M.N., Kotenko S., Ivashkiv L.B. 2003. Reprogramming of IL-10 activity and signaling by IFN-gamma. J. Immunol. 171:5034–5041 [DOI] [PubMed] [Google Scholar]
- Hirschhorn-Cymerman D., Budhu S., Kitano S., Liu C., Zhao F., Zhong H., Lesokhin A.M., Avogadri-Connors F., Yuan J., Li Y., et al. 2012. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J. Exp. Med. 209:2113–2126 10.1084/jem.20120532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Steinwald V., Guralnik A., Brüstle A., Kleemann P., Rosenplänter C., Decker T., Lohoff M. 2008. IL-27 inhibits the development of regulatory T cells via STAT3. Int. Immunol. 20:223–234 10.1093/intimm/dxm139 [DOI] [PubMed] [Google Scholar]
- Hurtado J.C., Kim Y.J., Kwon B.S. 1997. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 158:2600–2609 [PubMed] [Google Scholar]
- Ichiyama K., Sekiya T., Inoue N., Tamiya T., Kashiwagi I., Kimura A., Morita R., Muto G., Shichita T., Takahashi R., Yoshimura A. 2011. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity. 34:741–754 10.1016/j.immuni.2011.02.021 [DOI] [PubMed] [Google Scholar]
- Intlekofer A.M., Banerjee A., Takemoto N., Gordon S.M., Dejong C.S., Shin H., Hunter C.A., Wherry E.J., Lindsten T., Reiner S.L. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 321:408–411 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J.A., Bacon C.M., Finbloom D.S., Rees R.C., Kaplan D., Shibuya K., Ortaldo J.R., Gupta S., Chen Y.Q., Giri J.D., et al. 1995. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc. Natl. Acad. Sci. USA. 92:8705–8709 10.1073/pnas.92.19.8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S.A., Park S.M., Lee S.C., Kwon B.S., Kim B.S. 2007. Marked expansion of CD11c+CD8+ T-cells in melanoma-bearing mice induced by anti-4-1BB monoclonal antibody. Mol. Cells. 24:132–138 [PubMed] [Google Scholar]
- Kim Y.H., Choi B.K., Shin S.M., Kim C.H., Oh H.S., Park S.H., Lee D.G., Lee M.J., Kim K.H., Vinay D.S., Kwon B.S. 2011. 4-1BB triggering ameliorates experimental autoimmune encephalomyelitis by modulating the balance between Th17 and regulatory T cells. J. Immunol. 187:1120–1128 10.4049/jimmunol.1002681 [DOI] [PubMed] [Google Scholar]
- Kocak E., Lute K., Chang X., May K.F., Jr, Exten K.R., Zhang H., Abdessalam S.F., Lehman A.M., Jarjoura D., Zheng P., Liu Y. 2006. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 66:7276–7284 10.1158/0008-5472.CAN-05-2128 [DOI] [PubMed] [Google Scholar]
- Kwon B.S., Hurtado J.C., Lee Z.H., Kwack K.B., Seo S.K., Choi B.K., Koller B.H., Wolisi G., Broxmeyer H.E., Vinay D.S. 2002. Immune responses in 4-1BB (CD137)-deficient mice. J. Immunol. 168:5483–5490 [DOI] [PubMed] [Google Scholar]
- Lee J., Lee E.N., Kim E.Y., Park H.J., Chang C.Y., Jung D.Y., Choi S.Y., Lee S.K., Lee K.W., Kwon G.Y., et al. 2005a. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol. Lett. 101:210–216 10.1016/j.imlet.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Lee S.C., Ju S.A., Pack H.N., Heo S.K., Suh J.H., Park S.M., Choi B.K., Kwon B.S., Kim B.S. 2005b. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect. Immun. 73:5144–5151 10.1128/IAI.73.8.5144-5151.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lin J., Vanroey M., Jure-Kunkel M., Jooss K. 2007. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin. Immunol. 125:76–87 10.1016/j.clim.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Lin G.H., Sedgmen B.J., Moraes T.J., Snell L.M., Topham D.J., Watts T.H. 2009. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J. Immunol. 182:934–947 [DOI] [PubMed] [Google Scholar]
- Lin G.H., Liu Y., Ambagala T., Kwon B.S., Ohashi P.S., Watts T.H. 2010. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS ONE. 5:e11003 10.1371/journal.pone.0011003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S., Ghilardi N., Li J., de Sauvage F.J. 2003. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA. 100:15047–15052 10.1073/pnas.2536517100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D.H. 2008. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol. Rev. 222:277–286 10.1111/j.1600-065X.2008.00621.x [DOI] [PubMed] [Google Scholar]
- Morishima N., Owaki T., Asakawa M., Kamiya S., Mizuguchi J., Yoshimoto T. 2005. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 175:1686–1693 [DOI] [PubMed] [Google Scholar]
- Murugaiyan G., Mittal A., Lopez-Diego R., Maier L.M., Anderson D.E., Weiner H.L. 2009. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 183:2435–2443 10.4049/jimmunol.0900568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea J.J., Paul W.E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 327:1098–1102 10.1126/science.1178334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki T., Asakawa M., Morishima N., Mizoguchi I., Fukai F., Takeda K., Mizuguchi J., Yoshimoto T. 2008. STAT3 is indispensable to IL-27-mediated cell proliferation but not to IL-27-induced Th1 differentiation and suppression of proinflammatory cytokine production. J. Immunol. 180:2903–2911 [DOI] [PubMed] [Google Scholar]
- Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A., et al. 2010. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207:637–650 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui H.Z., Hagymasi A.T., Bandyopadhyay S., St Rose M.C., Ramanarasimhaiah R., Ménoret A., Mittler R.S., Gordon S.M., Reiner S.L., Vella A.T., Adler A.J. 2011. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J. Immunol. 187:3555–3564 10.4049/jimmunol.1101244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., Shrikant P.A. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser R.L., Kaech S.M. 2010. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol. Rev. 235:219–233 [DOI] [PubMed] [Google Scholar]
- Seo S.K., Choi J.H., Kim Y.H., Kang W.J., Park H.Y., Suh J.H., Choi B.K., Vinay D.S., Kwon B.S. 2004. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 10:1088–1094 10.1038/nm1107 [DOI] [PubMed] [Google Scholar]
- Shuai K., Stark G.R., Kerr I.M., Darnell J.E., Jr 1993. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 261:1744–1746 10.1126/science.7690989 [DOI] [PubMed] [Google Scholar]
- Shuford W.W., Klussman K., Tritchler D.D., Loo D.T., Chalupny J., Siadak A.W., Brown T.J., Emswiler J., Raecho H., Larsen C.P., et al. 1997. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 186:47–55 10.1084/jem.186.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler J., Wirtz S., Frenzel C., Schuchmann M., Lohse A.W., Galle P.R., Neurath M.F. 2008. Cutting edge: a key pathogenic role of IL-27 in T cell-mediated hepatitis. J. Immunol. 180:30–33 [DOI] [PubMed] [Google Scholar]
- Sun Y., Chen H.M., Subudhi S.K., Chen J., Koka R., Chen L., Fu Y.X. 2002a. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat. Med. 8:1405–1413 10.1038/nm1202-796 [DOI] [PubMed] [Google Scholar]
- Sun Y., Lin X., Chen H.M., Wu Q., Subudhi S.K., Chen L., Fu Y.X. 2002b. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J. Immunol. 168:1457–1465 [DOI] [PubMed] [Google Scholar]
- Tan J.T., Whitmire J.K., Ahmed R., Pearson T.C., Larsen C.P. 1999. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 163:4859–4868 [PubMed] [Google Scholar]
- Taraban V.Y., Rowley T.F., O’Brien L., Chan H.T., Haswell L.E., Green M.H., Tutt A.L., Glennie M.J., Al-Shamkhani A. 2002. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur. J. Immunol. 32:3617–3627 [DOI] [PubMed] [Google Scholar]
- Wang C., Lin G.H., McPherson A.J., Watts T.H. 2009. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol. Rev. 229:192–215 10.1111/j.1600-065X.2009.00765.x [DOI] [PubMed] [Google Scholar]
- Xie Y., Akpinarli A., Maris C., Hipkiss E.L., Lane M., Kwon E.K., Muranski P., Restifo N.P., Antony P.A. 2010. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 207:651–667 10.1084/jem.20091921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.M., Yu S., Zhou X., Haring J.S., Held W., Badovinac V.P., Harty J.T., Xue H.H. 2010. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 184:1191–1199 10.4049/jimmunol.0901199 [DOI] [PMC free article] [PubMed] [Google Scholar]