Lung-resident antigen-presenting macrophages promote tolerance to inhaled antigens via the induction of regulatory T cells.
Abstract
Airway tolerance is the usual outcome of inhalation of harmless antigens. Although T cell deletion and anergy are likely components of tolerogenic mechanisms in the lung, increasing evidence indicates that antigen-specific regulatory T cells (inducible Treg cells [iTreg cells]) that express Foxp3 are also critical. Several lung antigen-presenting cells have been suggested to contribute to tolerance, including alveolar macrophages (MØs), classical dendritic cells (DCs), and plasmacytoid DCs, but whether these possess the attributes required to directly promote the development of Foxp3+ iTreg cells is unclear. Here, we show that lung-resident tissue MØs coexpress TGF-β and retinal dehydrogenases (RALDH1 and RALDH 2) under steady-state conditions and that their sampling of harmless airborne antigen and presentation to antigen-specific CD4 T cells resulted in the generation of Foxp3+ Treg cells. Treg cell induction in this model depended on both TGF-β and retinoic acid. Transfer of the antigen-pulsed tissue MØs into the airways correspondingly prevented the development of asthmatic lung inflammation upon subsequent challenge with antigen. Moreover, exposure of lung tissue MØs to allergens suppressed their ability to generate iTreg cells coincident with blocking airway tolerance. Suppression of Treg cell generation required proteases and TLR-mediated signals. Therefore, lung-resident tissue MØs have regulatory functions, and strategies to target these cells might hold promise for prevention or treatment of allergic asthma.
Exposure to environmental antigens via the airways can lead to a state of tolerance thereby preventing lung disease such as asthma. Even though deletion and anergy of antigen-reactive T cells are likely to play a significant role in promoting airway tolerance, studies in mice and humans have suggested that regulatory T cells (Treg cells) are critical for controlling inflammation (Hawrylowicz and O’Garra, 2005; Akdis, 2006; Umetsu and Dekruyff, 2006; Larché, 2007; Lloyd and Hawrylowicz, 2009). Treg cells expressing Foxp3 or IL-10, or both molecules, have been described to associate with suppression of lung inflammation in humans and to increase in numbers in individuals responding to allergen immunotherapy. In mouse models, the majority of data suggest that a peripherally inducible antigen-specific CD4+ Treg cell (iTreg cell) is required for generating or maintaining a state of airway tolerance (Ostroukhova et al., 2004; Mucida et al., 2005; Curotto de Lafaille et al., 2008; Duan et al., 2008, 2011; Josefowicz et al., 2012). Furthermore, in naive, unsensitized mice, it has readily been demonstrated that inhalation of soluble antigen promotes tolerogenic mechanisms that prevent susceptibility to developing Th2-driven allergic inflammation in the lung (Tsitoura et al., 1999; Ostroukhova et al., 2004; Duan et al., 2008), and from variants of this type of model, Foxp3+ iTreg cells have been proposed to be crucial (Ostroukhova et al., 2004; Mucida et al., 2005; Curotto de Lafaille et al., 2008; Duan et al., 2008).
How these airway iTreg cells are generated is not fully understood, but this has potential implications for therapy of lung disease. TGF-β was found to be key to the conversion of naive CD4 T cells into Foxp3+ iTreg cells from an in vitro study (Chen et al., 2003), and we and others in several models of lung tolerance showed that neutralizing TGF-β allowed the development of Th2-driven eosinophilia in the airway and blocked the generation of antigen-specific Foxp3+ iTreg cells (Mucida et al., 2005; Duan et al., 2008). More recently, we described another iTreg cell that developed after i.n. exposure to soluble antigen and could suppress lung inflammation. This CD4+ T cell expressed membrane LAP (latency-associated peptide) and was Foxp3 negative, but similar to Foxp3+ iTreg cells, it also relied on endogenously produced TGF-β for its development (Duan et al., 2011). A new study of a mouse deficient in an intronic Foxp3 enhancer, CNS1, which specifically lacks Foxp3+ iTreg cells, further supports these conclusions. These mice spontaneously displayed Th2 inflammatory activity in mucosal tissues including the lungs (Josefowicz et al., 2012). Significantly, CNS1 contains a binding site for Smad3 that is critical for TGF-β–dependent induction of Foxp3 (Tone et al., 2008; Zheng et al., 2010). CNS1 also binds the nuclear retinoic acid receptor (RAR; Zheng et al., 2010), which mediates the ability of retinoic acid to synergize with TGF-β and enhance the induction of Foxp3 (Benson et al., 2007; Mucida et al., 2007). Although it is presently not clear whether retinoic acid is required for induction of iTreg cells that accumulate in the lung, these data collectively suggest that an APC within the airway environment that either makes TGF-β alone or TGF-β with retinoic acid might critically contribute to tolerance.
Several years ago, both lung-resident CD11c+ classical DCs (cDCs) and plasmacytoid DCs (pDCs) were suggested to participate in tolerance in the airways and shown to block priming of CD4 T cells (Akbari et al., 2001; de Heer et al., 2004), but their activity was either centered on the production of IL-10 and induction of IL-10–producing Treg cells or was undefined. Two major lung cDC populations are now recognized, CD103−CD11bhi and CD103+CD11blo, but recent results suggest that both are stimulatory rather than tolerogenic, although the exact type of T cell response they favor may be variable (Beaty et al., 2007; Furuhashi et al., 2012; Nakano et al., 2012). In addition, older data suggested that cells obtained from lung lavages, and thought to consist primarily of alveolar macrophages (MØs), were suppressive for T cell proliferation. In vitro studies showed that these alveolar MØs from mice or from humans functioned by producing soluble molecules like nitric oxide, prostaglandins, and, interestingly, TGF-β, leading to an anergic phenotype in T cells (Thepen et al., 1992; Holt et al., 1993; Lipscomb et al., 1993; Roth and Golub, 1993; Upham et al., 1995; Strickland et al., 1996; Blumenthal et al., 2001). However, no studies to date have identified a lung APC that might intrinsically have the ability to promote the efficient generation of iTreg cells via TGF-β. In the present study, our data reveal that tissue-resident lung MØs in unsensitized mice constitutively express TGF-β and retinal dehydrogenases (RALDH1 and RALDH2), the enzymes which regulate retinoic acid production. These MØs can take up inhaled antigen and present to naive and activated T cells in a tolerogenic manner without any exogenous stimuli, resulting in the development of Foxp3+ iTreg cells. We also show that these MØs retain expression of TGF-β and RALDH when allergens are inhaled, but they lose their antiinflammatory activity and ability to induce iTreg cells, correlating with a loss of tolerance. Clinical therapy for allergic asthma is limited at present, and insight into mechanisms that induce tolerance to allergens could lead to new treatment strategies. Our data suggest that knowledge of ways to specifically target this lung MØ and maintain its Treg cell–inducing activity or promote the accumulation of these MØs may be applicable for therapy of allergic airway disease.
RESULTS
Phenotypic characterization of tissue-resident MØs and DCs in the naive murine lung
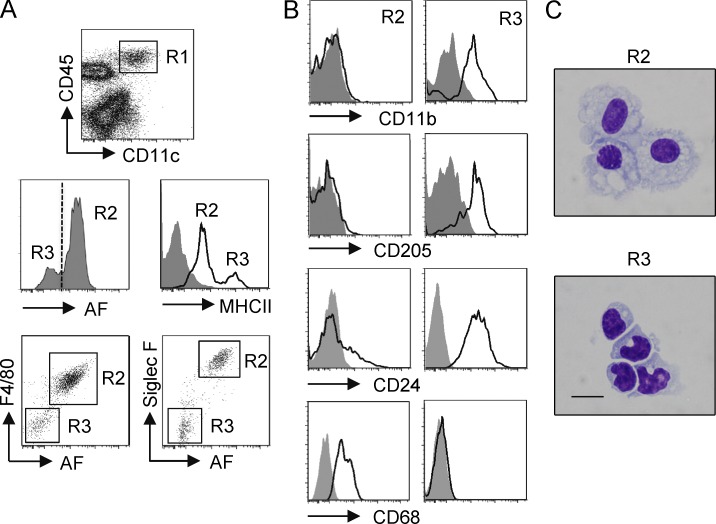
As our prior data identified TGF-β–inducible Foxp3+ Treg cells as mediating tolerance after inhalation of soluble antigen in naive mice (Duan et al., 2008, 2011), we sought to identify a resident APC in the noninflamed lung that might possess the ability to promote these antigen-specific iTreg cells. We focused on pulmonary tissue MØs and DCs, reasoning they would be immediately available to take up and process inhaled antigens. We gated on CD11c+CD45+ cells that should encompass all DC and MØ populations and assessed samples from naive murine lungs that were perfused and lavaged to exclude circulating cells and bronchoalveolar cells including alveolar MØs (Fig. 1 A). CD11c+CD45+ cells contained two distinct subpopulations in terms of autofluorescence (AF), with the majority being high AF. CD11c+ high-AF cells were MHC IIlo, whereas CD11c+ low-AF cells were MHC IIhi. In addition, CD11c+ high-AF cells expressed high levels of F4/80 and Siglec F (a sialic acid–recognizing lectin), whereas the low-AF cells were negative for these markers (Fig. 1 A). This combined phenotype corresponds to previous designations used to discriminate pulmonary-resident MØs and DCs (Vermaelen and Pauwels, 2004; Stevens et al., 2007; Zasłona et al., 2009). Further phenotypic analysis showed that CD11c+ high-AF F4/80+ Siglec F+ cells had little expression of CD11b and the C-type lectin CD205 and low-level expression of CD24 (HSA) but were positive for CD68, an MØ/monocyte marker. In contrast, CD11c+ low-AF F4/80− Siglec F− cells highly expressed CD11b, CD205, and CD24 but did not express CD68 (Fig. 1 B). Sorting of these cells from perfused and lavaged naive lungs into CD11c+ high-AF Siglec F+ MHC IIlo versus CD11c+ low-AF Siglec F− MHC IIhi populations confirmed morphological features of MØs and DCs, respectively (Fig. 1 C).
Figure 1.
Phenotypic characterization of lung-resident MØs and DCs in naive mice. Lungs from naive, unmanipulated, unsensitized mice were lavaged and perfused, and lung tissue was digested to make single-cell suspensions. (A) Cells were stained for CD45 and CD11c and further gated (R1) as CD45+CD11c+ for analysis of AF. CD11c+CD45+ cells contained AF-high (R2) and AF-low (R3) cells, which were further characterized as MHC IIlo, F4/80+, Siglec F+ versus MHC IIhi, F4/80−, Siglec F−, respectively. (B) Lung MØs (R2) and DCs (R3) gated as in A were stained for CD11b, CD205, CD24, and CD68. (C) Lung MØs (CD11c+, AFhi, Siglec F+, MHC IIlo, F4/80+) and lung DCs (CD11c+, AFlo, Siglec F−, MHC IIhi, F4/80−) were sorted from enriched Siglec F+ or CD11c+ cells as described in Materials and methods and visually assessed in cytospins. Bar, 50 µm. The purity of these two populations after sorting was typically >99% as measured by flow cytometry. Data are representative of two (B and C) or three (A) independent experiments.
Lung tissue MØs and DCs differentially generate Foxp3+ iTreg cells in vitro
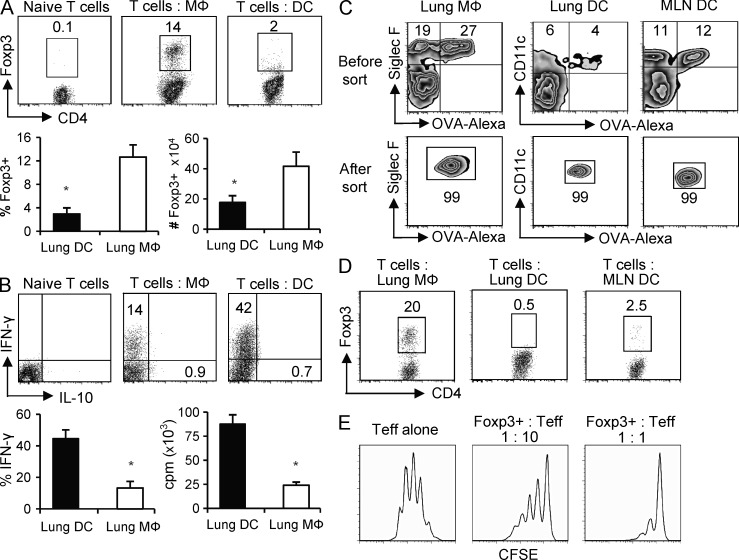
To test whether these lung-resident tissue MØs and DCs had the potential to act as APCs and promote the generation of Foxp+ iTreg cells, they were isolated from a naive, unmanipulated, unsensitized mouse and cultured in vitro with naive CD25−Foxp3− OT-II TCR transgenic CD4+ T cells, lacking preexisting Foxp3+ natural Treg cells, in the presence of OVA peptide but in the absence of exogenous TGF-β. After 5 d of culture at a 1:25 APC/T ratio, the lung MØs induced 12–16% of naive T cells to express Foxp3, whereas parallel cultures with DCs resulted in fewer cells with Foxp3 expression, and those cells that were positive had extremely weak expression (Fig. 2 A). The total number of Foxp3+ T cells generated by lung MØs was also significantly greater than that generated by lung DCs (Fig. 2 A). Assessing the development of effector T cells in these cultures additionally showed that lung MØs had very weak activity compared with lung DCs in driving T cell proliferation and differentiation into IFN-γ–secreting effector cells (Fig. 2 B). Foxp3+ T cells induced by MØs did not coexpress IFN-γ (not depicted). Neither MØs nor DCs induced IL-10–secreting T cells (Fig. 2 B).
Figure 2.
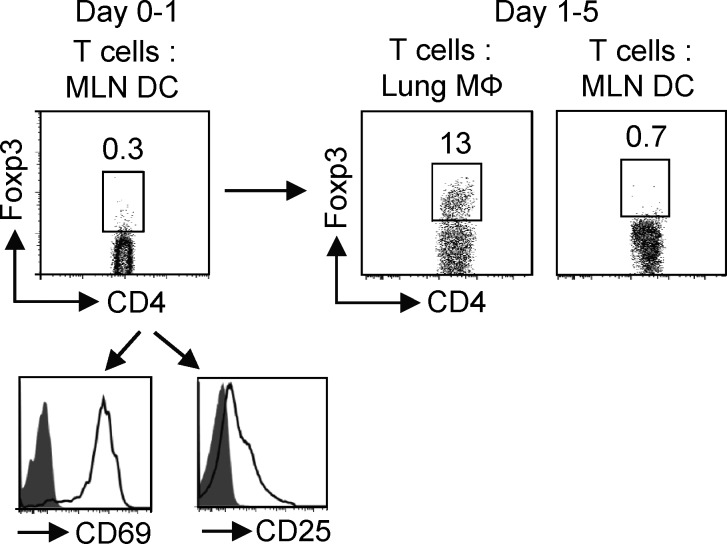
Lung tissue MØs induce iTreg cell differentiation in vitro. (A and B) Foxp3− OT-II CD4 T cells were stimulated with purified lung tissue MØs or DCs at a 1:25 APC/T ratio in the presence of OVA peptide for 5 d. (A, top) Foxp3 intracellular staining before and after APC culture. (bottom) Mean percentage (left) and mean number (right) ± SD of Foxp3+ OT-II cells generated by lung MØs and DCs from three to four independent experiments. (B, top) Intracellular IFN-γ and IL-10 expression before and after APC culture. (bottom) Mean percentage ± SD of T cells expressing IFN-γ (left) and mean proliferation (tritiated thymidine incorporation) ± SD of CD4 T cells (right) from three to four independent experiments. (A and B) *, P < 0.01. (C and D) Naive mice were administered OVA-conjugated Alexa Fluor 647 i.n. 24 h later, lung MØs (Alexa Fluor 647+ CD11c+ Siglec F+ AFhi), lung DCs (Alexa Fluor 647+ CD11c+ Siglec F− AFlo), and MLN DCs (Alexa Fluor 647+ CD11c+ B220−) that had taken up OVA were then sorted (see Materials and methods). (C) Representative profile of Alexa Fluor–OVA versus Siglec F expression in gated CD11c+ cells for lung MØs and Alexa Fluor–OVA versus CD11c expression in gated Siglec F− cells for lung and MLN DCs before and after sorting. (D) Purified OVA-loaded MØs and DCs were prepared as in A and cultured with Foxp3− OT-II CD4 T cells at a 1:25 APC/T ratio for 5 d, and induction of Foxp3+ CD4 T cells was analyzed. Data are representative of three independent experiments with APCs purified from groups of 8–10 mice. (E) CFSE-labeled naive OT-II CD4 T cells were stimulated with OVA-pulsed T-depleted splenocytes (APCs) for 4 d in the absence (Teff alone) or presence (Foxp3+: Teff) of 1:10 or 1:1 ratios of Foxp3+ T cells generated from lung MØ cultures as in A. Data are representative of two experiments.
To address the potential role of lung tissue MØs and DCs to process and present inhaled antigen in a tolerogenic manner in vivo, mice were exposed to i.n. administered OVA protein conjugated to a fluorochrome to allow the fate of inhaled antigen to be tracked in lung tissue and the draining mediastinal LNs (MLNs). Within 24 h, 60–70% of lung tissue MØs took up inhaled antigen, and 30–40% of lung tissue DCs also captured antigen (Fig. 2 C and not depicted). Approximately 50% of MLN DCs were additionally found to bear OVA, likely reflecting both DCs that migrated from the lung to the LN as well as DCs that directly captured OVA in the LN, corresponding to results reported in a previous study (Wikstrom et al., 2010). Pulmonary tissue MØs and DCs and MLN DCs were then isolated and specifically sorted to purity based on being positive for OVA expression (Fig. 2 C). These ex vivo derived APCs were then co-cultured with Foxp3− OT-II CD4 T cells for 5 d. Consistent with our prior observations, OVA-capturing lung tissue MØs induced significant numbers of Foxp3+ CD4 T cells, whereas OVA-capturing DCs from either lung tissue or MLN only weakly generated Foxp3+ T cells (Fig. 2 D). Finally, the Foxp3+ T cells generated by the MØs were recultured with naive T cells and found to display regulatory activity suppressing T cell proliferation (Fig. 2 E). These data suggest that tissue MØs are a resident lung APC population with the intrinsic ability to promote the generation of Foxp3-expressing iTreg cells.
TGF-β and retinoic acid are constitutively expressed by lung tissue MØs and control differentiation of Foxp3+ iTreg cells
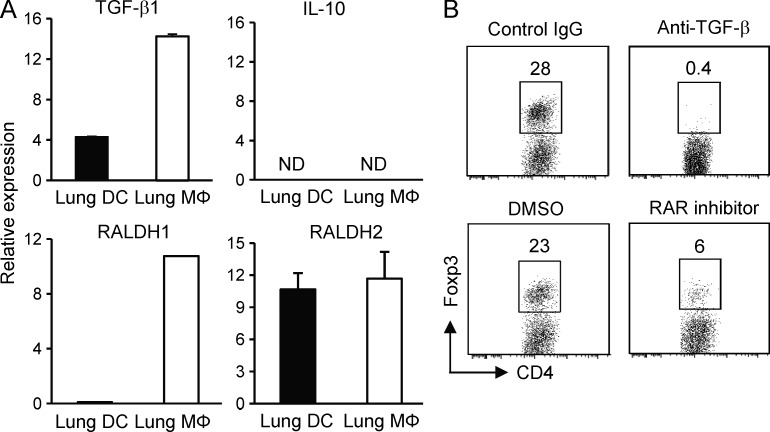
To understand the mechanisms underlying the differential ability of lung tissue MØs and DCs to generate Foxp3+ iTreg cells, we examined the expression of TGF-β, RALDH1 and RALDH2, and IL-10. Directly ex vivo isolated MØs from naive unmanipulated murine lungs displayed significantly higher expression of mRNA for TGF-β1 than lung tissue DCs, but neither population expressed IL-10 mRNA (Fig. 3 A). Moreover, mRNA for RALDH1 and RALDH2 was also found highly expressed in tissue MØs, whereas DCs only expressed RALDH2 (Fig. 3 A). This unique coexpression of high levels of TGF-β1, RALDH1, and RALDH2 in lung tissue MØs directly correlated with the intrinsic ability of these cells to induce Foxp3 expression (Fig. 2). Directly showing they were active, neutralization of TGF-β with an anti–TGF-β antibody or retinoic acid with the synthetic RAR antagonist LE540 significantly diminished induction of Foxp3 in CD4 T cells when cultured with antigen-presenting lung MØs (Fig. 3 B). Therefore, TGF-β and retinoic acid are both constitutively expressed in lung tissue MØs and control the development of Foxp3+ iTreg cells when these cells present antigen.
Figure 3.
TGF-β and retinoic acid are coexpressed by lung tissue MØs. (A) Lung tissue MØs and DCs were isolated ex vivo from naive, unmanipulated, unsensitized mice, and mRNA expression of TGF-β1, IL-10, RALDH1, and RALDH2 was measured by qPCR. Results are depicted as relative expression compared with L32 ± SD from three independent experiments. ND, not detectable. (B) Lung tissue MØs were cultured with Foxp3− OT-II CD4 T cells at a ratio of 1:5 APCs/T cells in the presence of OVA peptide. After 5 d, the expression of Foxp3 in gated CD4 T cells was analyzed. Neutralizing anti–TGF-β1, isotype control IgG, RAR antagonist LE540 in DMSO, or vehicle control DMSO was added as indicated. Data are representative of three independent experiments.
Antigen-bearing lung MØs can induce Foxp3+ Treg cells within lung tissue
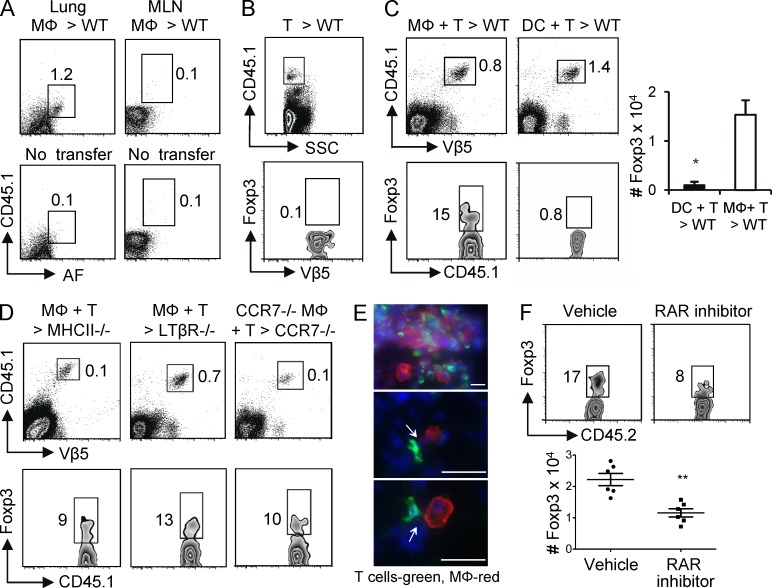
To further substantiate the view that lung tissue MØs may be a primary iTreg cell–generating APC, we isolated these cells, pulsed them with OVA protein, and transferred them into the lungs of recipient mice to determine whether they could induce Foxp3+ Treg cells in vivo. After intratracheal (i.t.) transfer of purified MØs into intact CD45 congenic mice, we detected these cells in lung tissue with few if any in MLNs (Fig. 4 A). To monitor the induction of iTreg cells, naive Foxp3− OT-II CD4 T cells were also adoptively transferred into the congenic mice. The transferred T cells were detected in the lung, but none of them expressed Foxp3 in mice that did not receive antigen-bearing MØs (Fig. 4 B). In contrast, when both T cells and antigen-pulsed MØs were cotransferred, a large percentage of donor T cells were found that expressed Foxp3 (Fig. 4 C). Corresponding to our in vitro data (Fig. 2), when the same experiment was performed with antigen-pulsed lung DCs, little conversion to Foxp3+ Treg cells was observed (Fig. 4 C). To address the possibility that the donor antigen-bearing MØs may have released or transferred OVA such that it was presented by endogenous APCs, we performed the same experiment in an MHC class II–deficient recipient whose APCs would not be able to present antigen. The number of donor T cells visualized in this scenario was considerably lower than in a WT recipient (Fig. 4 D), suggesting that released and endogenously presented antigen did contribute to expansion of the T cell population. However, a similar percentage of the T cells were induced to express Foxp3, implying that the donor lung MØs presented antigen directly and that these MØs played a role in promoting most of the iTreg cells that were generated.
Figure 4.
Lung tissue MØs induce iTreg cell differentiation in vivo. (A) 5 × 105 purified lung-resident tissue MØs from CD45.1 mice were transferred i.t. into WT CD45.2 mice. After 24 h, CD45.1+ AFhi MØs were analyzed in lung (left) and MLN (right). Controls were mice that did not receive transferred MØs (bottom). (B) 106 purified Foxp3− CD45.1+ Vβ5+ OT-II T cells were transferred i.v. into WT CD45.2 mice. After 24 h, CD45.1+ T cells were visualized in the lung (top) and analyzed for expression of Foxp3 and Vβ5 (bottom). (C and D) Foxp3− CD45.1+ OT-II T cells were transferred i.v. into WT, MHC II−/−, LTβR−/−, or CCR7−/− CD45.2 mice, and 24 h later, OVA-pulsed lung tissue MØs or DCs from WT CD45.2 mice were transferred i.t. into the same recipients. On day 5 after APC transfer, the accumulation of donor OT-II T cells in the lungs was assessed (top), and the expression of Foxp3 was analyzed in the gated CD45.1+ Vβ5+ cells (bottom). The absolute number of Foxp3+ Vβ5+ donor T cells in lung tissue of WT mice transferred with OVA-pulsed lung MØs or DCs was also calculated (C). (C) Data are mean ± SD from six individual mice per group. *, P < 0.01. All data in A–D are representative of three to four independent experiments with cells pooled from groups of four to six mice. (E) Naive WT CD45.2 mice were administered 100 µg of soluble OVA i.n. 8 h later, purified CD45.1+ OT-II T cells were transferred i.v. into the same recipient mice. After 16 h, lung sections were analyzed by fluorescent microscopy for transferred T cells (anti-CD45.1, green) and endogenous lung MØs (anti–Siglec F, red). Cell nuclei were visualized with DAPI (blue). Arrows indicate direct contacts between T cells and MØs. Representative lung sections are shown from two experiments. Bars, 10 µm. (F) Foxp3− CD45.2+ OT-II T cells and OVA-pulsed lung tissue MØs from CD45.1 mice were transferred into WT CD45.1 mice as in C and D. Recipient mice were orally treated with the RAR antagonist LE540 (50 µg/mouse) or soybean oil (vehicle) every day. On day 5 after APC transfer, the expression of Foxp3 was analyzed in gated CD45.2+ Vβ5+ cells from lungs (top), and the numbers of Foxp3+ donor OT-II T cells in the lungs were calculated (bottom). Data from six individual hosts are shown with mean ± SD. **, P < 0.001.
Although we did not detect any lung tissue MØs in the draining LN after i.t. transfer, a few studies have suggested that alveolar MØs can migrate to the LN in some circumstances (Thepen et al., 1993; Kirby et al., 2009). To partly address this, purified OT-II T cells and OVA-pulsed MØs were cotransferred into congenic lymphotoxin β receptor–deficient mice (LTβR−/−) that do not possess peripheral LNs (Fütterer et al., 1998). Similar induction of Foxp3+ T cells was seen in LTβR−/− recipients as in WT recipients (Fig. 4, D compared with C). Although this rules out an obligate activity for the MØs to travel to the LN to promote iTreg cell generation, this does not rule out a role for the spleen in contributing to the overall T cell response as reported previously (Gajewska et al., 2001a). Next, WT OT-II T cells were transferred into CCR7−/− recipient mice together with OVA-pulsed MØs also isolated from CCR7−/− mice. CCR7 regulates migration of lymphoid cells into LN, thus without CCR7, the transferred MØs were not able to traffic from the lung tissue to the LN, and endogenous APCs such as DCs in the lungs were also unable to migrate to the LN. In this scenario, the donor T cells did not expand efficiently compared with the response in WT recipients; however, again a similar percentage of Foxp3+ T cells were visualized in the lungs (Fig. 4 D). Collectively, these data suggest that even though antigen presentation in the lung-draining LNs contributed to the expansion of antigen-specific T cells, induction of Foxp3 occurred in the lung and/or was programmed in the lung and at least in part was driven by the lung tissue MØs. In line with this, immunofluorescent microscopy revealed that donor T cells distributed throughout the whole lung, lying within the parenchymal tissue, and T cell and MØ clusters were observed with direct contact between donor T cells and local MØs (Fig. 4 E). Lastly, we addressed the role of retinoic acid in promoting iTreg cell development in vivo and treated mice with the RAR inhibitor at the time of transfer of antigen-pulsed lung MØs and naive T cells. Similar to the in vitro data (Fig. 3), blocking retinoic acid substantially reduced the percentage and total number of Foxp3+ T cells that developed in vivo (Fig. 4 F).
CD4 T cells primed on MLN DCs can differentiate into Foxp3+ iTreg cells after encounter with lung tissue MØs
It is commonly thought that APCs in the lung-draining LNs contribute to the priming of T cells that accumulate in the lungs after inhalation of antigen under either inflammatory or tolerogenic conditions (Gajewska et al., 2001b; Hintzen et al., 2006; Bakocević et al., 2010), and our data in MHC II−/− and CCR7−/− recipients lead to the same conclusion. But the sequence in which a T cell will encounter an APC in the lung or draining LNs is not clear and may vary depending on the antigen load and other factors. Naive T cells have a preference for migrating through lymphoid organs, but a low number have been shown to traffic into parenchymal tissues of nonlymphoid organs including the lung (Cose et al., 2006; Harp and Onami, 2010). However, short-term stimulation of naive T cells via the T cell receptor quickly changes their trafficking program, allowing them to enter tissues such as the lung (Hamann et al., 2000). Thus, it is possible that lung tissue MØs may be the primary APC or a secondary APC for a responding T cell.
As our prior experiments had largely tested the effects of lung tissue MØs as a primary APC for resting T cells, we assessed whether a short-term activated T cell that initially encountered a stimulatory LN DC could still be influenced by lung tissue MØs to become a Foxp3+ iTreg cell. WT mice were administered fluorochrome-conjugated OVA i.n. as before (Fig. 2 C), and then 24 h later, OVA-capturing DCs from MLN were isolated and co-cultured with Foxp3− OT-II T cells for 1 d. This short time of stimulation resulted in activation of the T cells but did not induce expression of Foxp3 (Fig. 5). Another group of WT mice were administrated fluorochrome-conjugated OVA i.n. for isolation of lung tissue MØs and more MLN DCs, and these were then co-cultured with the purified T cells that had been preactivated with MLN DCs. Significantly, a high percentage of Foxp3+ T cells were evident after 4 d in the MØ cultures, whereas the short-term primed T cells recultured with OVA-loaded DCs from MLN were not induced to express Foxp3. Therefore, resident lung tissue MØs from naive mice can present antigen to both naive and activated T cells in a tolerogenic manner. When the naive T cells were preactivated by DCs for 3 d, lung tissue MØs were however incapable of promoting significant Foxp3 expression (not depicted), suggesting a window of opportunity may exist when their regulatory ability can manifest.
Figure 5.
Activated CD4 T cells primed by MLN DCs become iTreg cells after encounter with lung tissue MØs. WT mice were administered OVA–Alexa Fluor 647 i.n., and after 24 h, OVA-loaded DCs from MLNs were sorted as in Fig. 2 (C and D). These DCs were cultured with Foxp3− OT-II T cells for 1 d and assessed for activation markers and induction of Foxp3 (left). Another group of WT mice were given OVA–Alexa Fluor 647 i.n., and OVA-loaded lung tissue MØs and MLN DCs were isolated. These secondary APCs were then co-cultured with purified OT-II T cells that had been activated with MLN DCs at a 1:10 APC/T ratio. Induction of Foxp3 was assessed after 4 d of secondary culture (right). Data are representative of three independent experiments.
Lung tissue MØs suppress asthmatic lung inflammation and airway hyperreactivity
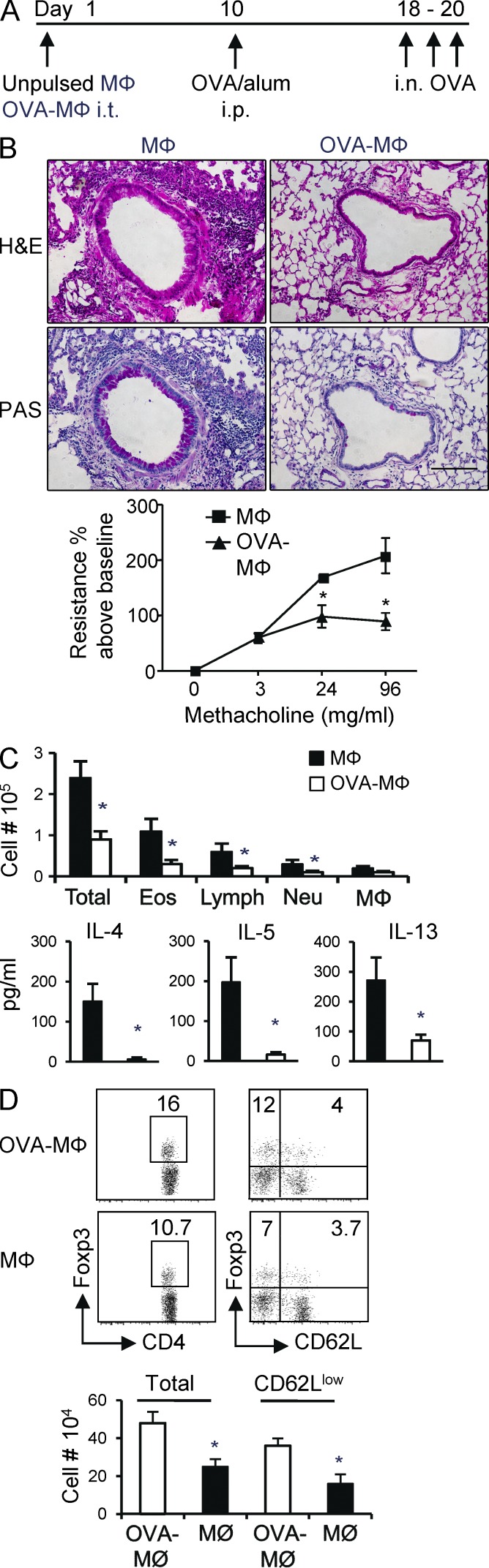
To more formally address the tolerogenic activity of lung tissue MØs, OVA-pulsed or unpulsed MØs were injected into the airways of naive mice. To test whether a state of tolerance was induced, the mice were immunized 9 d later with OVA/alum, followed by serial recall challenges with soluble OVA given i.n. after another 9 d to assess lung inflammation (Fig. 6 A). This is a protocol we have previously used to show that inhalation of soluble antigen results in iTreg cell generation and airway tolerance (Duan et al., 2008, 2011). Corresponding to our prior results above showing the induction of Foxp3+ iTreg cells, administration of OVA-loaded MØs strongly suppressed the subsequent induction of lung inflammation as assessed by tissue infiltration, mucus production, and airway hyperreactivity (Fig. 6 B). In contrast, mice receiving MØs not pulsed with antigen developed severe lung inflammation (Fig. 6 B). Analysis of bronchoalveolar lavages (BALs) revealed reduced numbers of total BAL cells including eosinophils and lymphocytes and reduced production of IL-4, IL-5, and IL-13 (Fig. 6 C). As we did not adoptively transfer OT-II T cells to track antigen-specific Treg cells in these experiments, we assessed the accumulation of all Foxp3+ Treg cells (natural Treg and iTreg cells) in the lungs of treated mice. The number of Foxp3+ Treg cells was significantly elevated in mice receiving OVA-loaded MØs compared with those receiving unpulsed MØs. This largely was caused by an increase in CD62Llo Foxp3+ Treg cells, which likely reflected the antigen-reactive cells (Fig. 6 D). Thus, antigen presentation by lung tissue MØs can limit the development of airway inflammation upon subsequent encounter with immunogenic antigen.
Figure 6.
Antigen-pulsed lung tissue MØs suppress asthmatic lung inflammation. (A) Groups of WT mice were instilled i.t. with OVA-pulsed MØs (OVA-MØ) or 5 × 105 MØs alone. 9 d later, the mice were sensitized with OVA/alum and subsequently challenged with i.n. OVA on day 18 for three consecutive days to induce lung inflammation. Samples were collected 24 h after the last OVA challenge. (B) Representative H&E (top) and periodic acid-Schiff (PAS; bottom) staining of lung sections. Airway hyperresponsiveness to methacholine was assessed by invasive measurement of airway resistance. Bar, 100 µm. (C) Total BAL cells and numbers of eosinophils (Eos), neutrophils (Neu), lymphocytes (Lymph), and MØs from cytospin analysis. Cytokines in BAL were measured by ELISA (bottom). (D, top left) Expression of Foxp3 in CD4+ T cells from lungs analyzed by flow cytometry. (top right) Expression of Foxp3 versus CD62L in gated CD4+ T cells. (bottom) Absolute number of total Foxp3+ Treg cells and CD62Llo Foxp3+ Treg cells in lungs. All results are the mean ± SD from four to five individual mice per group and representative of two independent experiments. *, P < 0.001.
Allergens induce inflammatory cytokines in lung tissue MØs, block Treg cell–inducing activity, and antagonize airway tolerance
We have previously shown that inhalation of purified pattern recognition receptor ligands, like the TLR4 ligand LPS, or inhalation of allergen extracts such as from house dust mite (HDM) suppresses the generation of lung Foxp3+ iTreg cells while promoting CD4 effector T cell generation and thereby blocks airway tolerance (Duan et al., 2008, 2010, 2011). This activity may derive from induction in the lung of several proinflammatory cytokines and cell membrane–expressed co-stimulatory ligands (Duan et al., 2008, 2010, 2011) and has been suggested to reflect direct activities of the pattern recognition receptor ligand or allergen on both lung structural cells as well as lung-resident APCs (Hammad et al., 2009). As the ability of allergens to antagonize airway tolerance is highly relevant to clinical allergic asthma, we hypothesized that allergens might in part act by stimulating the lung tissue MØs, leading to reduced expression of TGF-β and RALDH/retinoic acid and a blocked ability of these MØs to induce iTreg cells.
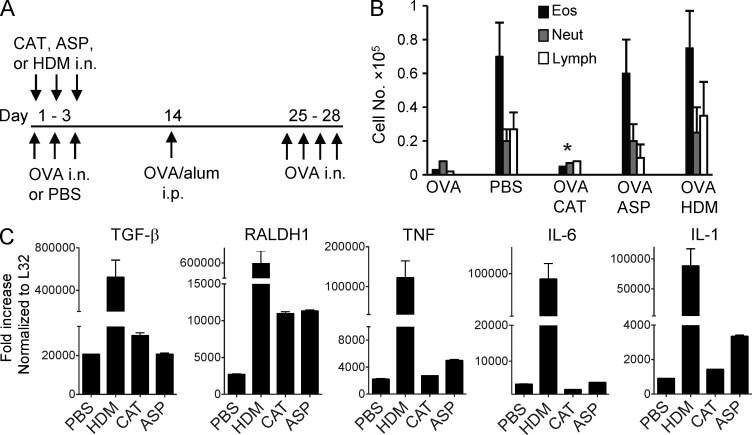
As an initial test of this, mice were exposed to soluble OVA given in a tolerogenic manner i.n. once a day for 3 d (Fig. 7 A). Separate groups of mice were administered several different clinically used allergen extracts from HDM, Aspergillus fumigatus (ASP), and cat dander (CAT), mixed with the soluble OVA. The activity of the extracts on lung tolerance was assessed by subsequently immunizing and challenging the animals with OVA using a protocol that normally promotes functional Th2 development and eosinophilic lung inflammation in naive mice (Fig. 7 A). HDM extract can activate TLR4 and contains Der p2, an antigen which mimics MD-2, a component of the TLR4 signaling complex (Hammad et al., 2009; Trompette et al., 2009). Aspergillus extract has also been found to activate TLR4 as well as TLR2 (Mambula et al., 2002; Braedel et al., 2004). These allergens additionally express protease activity that has been linked to triggering inflammation (Kheradmand et al., 2002; Kauffman et al., 2006). In contrast, there is no significant literature suggesting that cat dander extract has either strong protease activity or TLR ligand activity. Inhalation of HDM extract completely abrogated lung tolerance, as reported previously (Hammad et al., 2009; Duan et al., 2011), and ASP extract displayed the same activity. In contrast, cat dander extract had no effect on tolerance, providing a useful internal control (Fig. 7 B).
Figure 7.
Inhaled allergens block airway tolerance and induce inflammatory cytokines in lung MØs. (A) Groups of mice were exposed to i.n. PBS or 100 µg of soluble OVA or OVA mixed with allergen extracts (100 µg each) for three consecutive days. On day 14, all mice were sensitized with OVA/alum and subsequently challenged with i.n. OVA on day 25 for four consecutive days to induce lung inflammation. (B) Differential cell counts of inflammatory cells in BAL collected 24 h after the last OVA challenge. *, P < 0.01 relative to PBS. (C) Mice were exposed i.n. to PBS or CAT, ASP, or HDM extracts for three constitutive days. Lung tissue MØs were isolated on day 5, and cells were assessed for mRNA expression of TGF-β, RALDH1, TNF, IL-1, and IL-6 by qPCR. All results are the mean ± SD from four individual mice per group and are representative of two independent experiments.
To assess whether the allergens affected the activity of the lung tissue MØs, these cells were isolated after inhalation of the extracts and mRNA expression assessed by quantitative PCR (qPCR; Fig. 7 C). Most interestingly, inhalation of the extracts did not suppress expression of TGF-β or RALDH. CAT and ASP had little effect on TGF-β but enhanced mRNA for RALDH, whereas HDM strongly augmented TGF-β as well as RALDH mRNA. We then assessed several proinflammatory cytokines, TNF, IL-1, and IL-6, each of which can promote effector T cell development either directly or indirectly. Cat dander had no activity on promoting the expression of these cytokines, whereas HDM and ASP to varying degrees enhanced expression of two or all three, at least in part correlating with their effects on lung tolerance. Although this did not address the activity of the allergens on other lung-resident cell types, the data implied that some allergens might block tolerance partly by promoting the expression of inflammatory mediators in lung MØs rather than suppressing the expression of the iTreg cell–inducing molecules TGF-β and retinoic acid.
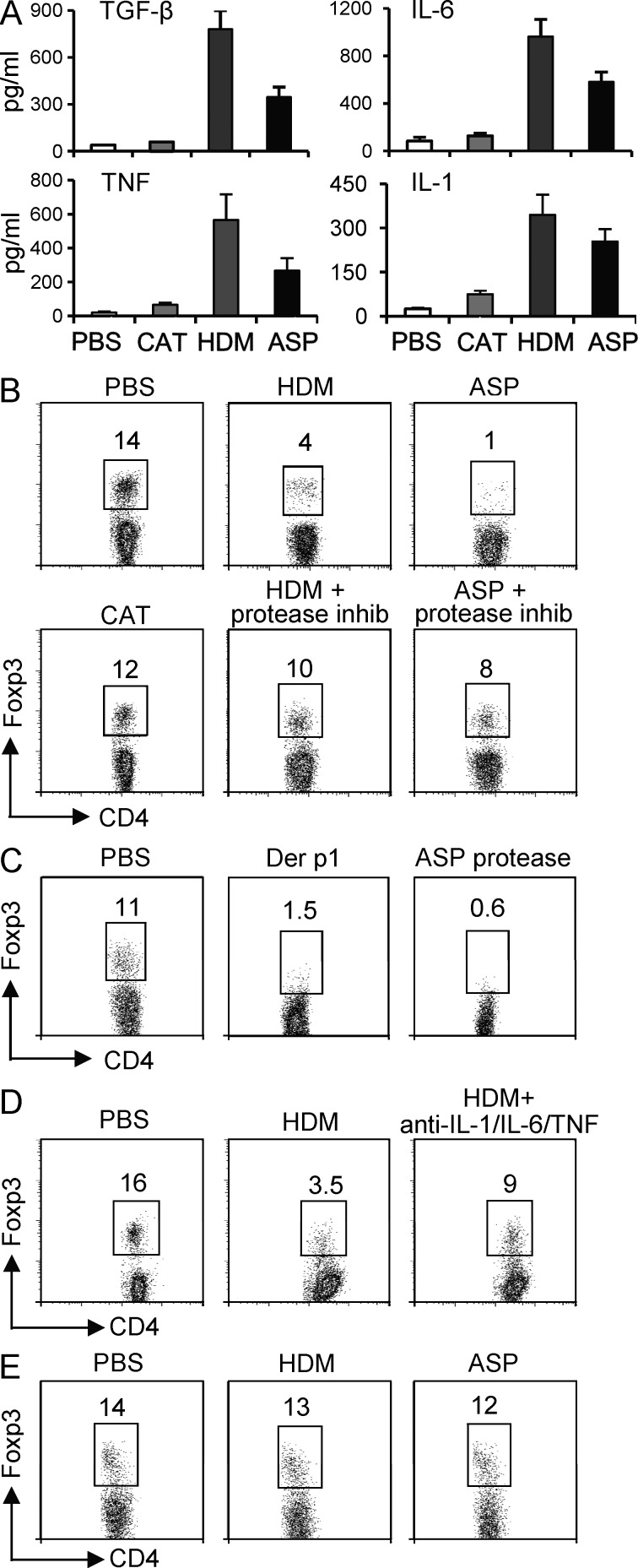
To further pursue this and directly assess the effect of allergens on lung tissue MØs, these cells were isolated from naive animals and cultured in vitro with PBS, HDM, ASP, and CAT extract, and supernatants were then assayed for cytokine release. IL-1, TNF, and IL-6 were strongly up-regulated by HDM and ASP, whereas cat dander had no appreciable effect (Fig. 8 A). Again, most interestingly, TGF-β secretion was promoted by HDM and ASP along with the other cytokines, rather than TGF-β being down-regulated. Given the caveats in terms of the concentration of active allergen-derived products encountered by MØs in vitro versus direct exposure after inhalation of the allergens, these results provided a reasonable correlate to the in vivo data. We then analyzed the effect of the allergens on the ability of lung MØs to induce Foxp3+ Treg cells. Purified tissue MØs were first treated with HDM, ASP, or CAT extract and then washed and co-cultured with Foxp3− OT-II CD4 T cells and OVA peptide for 4 d. ASP and HDM exposure resulted in MØs being impaired in driving Foxp3 expression, whereas cat dander extract did not appreciably alter the intrinsic activity of the MØs (Fig. 8 B). Therefore, even though TGF-β production was not suppressed and was actually enhanced, the iTreg cell–promoting ability of the MØs was lost, correlating in part with the proinflammatory cytokine phenotype induced by HDM and ASP.
Figure 8.
Allergen-induced activation of lung tissue MØs inhibits generation of Foxp3+ iTreg cells. (A) 5 × 104 lung MØs were isolated from naive mice and cultured in vitro with PBS, CAT, ASP, or HDM extracts. Supernatant was collected on day 2 for ELISA. Results are means ± SD from four mice per group and are representative of two independent experiments. (B–D) Lung MØs were exposed to PBS or CAT, ASP, or HDM extract or recombinant purified protease from HDM (Der p1) or protease from Aspergillus overnight. The next day, MØs were washed and co-cultured with Foxp3− OT-II CD4 T cells at a 1:25 APC/T ratio in the presence of OVA peptide for 5 d before analysis of intracellular Foxp3 expression. In some cases, protease inhibitor was added to the extracts or neutralizing antibodies to IL-1, IL-6, and TNF were added into the MØ/T cultures. (E) Lung MØs were isolated from MyD88/TRIF−/− mice and exposed to PBS or ASP or HDM extract overnight and assessed for Treg cell–inducing ability as in B. Results are representative of two independent experiments.
We then tested whether these effects were driven by TLR and/or protease activities contained in the allergen extracts. Neutralizing protease activity with a pan-serine/cysteine protease inhibitor partially, but not fully, prevented HDM and ASP from blocking the iTreg cell–inducing ability of lung MØs (Fig. 8 B). Treatment of lung tissue MØs with recombinant proteases from HDM (Der p1) and ASP also suppressed the ability to induce Foxp3+ iTreg cell generation (Fig. 8 C) but did not induce secretion of any inflammatory cytokines (not depicted). Additionally, combined blockade of IL-1, IL-6, and TNF partially restored the induction of iTreg cells by HDM-exposed MØs (Fig. 8 D), suggesting the total activity of the extracts was likely mediated through several mechanisms. In accordance, lung tissue MØs from naive MyD88/TRIF double knockout (MyD88/TRIF−/−) mice, which are unresponsive to multiple TLR ligands, displayed a normal ability to promote Foxp3+ Treg cells but were strongly refractory to the effects of HDM and ASP in blocking this Treg cell–inducing activity (Fig. 8 E). Altogether, these observations indicate that some allergens through protease- and TLR-dependent mechanisms, which do not involve down-regulation of TGF-β or RALDH expression, can antagonize the tolerogenic function of lung tissue MØs for inducing Foxp3+ iTreg cell development.
DISCUSSION
We show that tissue-resident MØs that are present in the steady-state lung of unmanipulated mice constitutively express TGF-β and RALDH and display an intrinsic ability to promote the generation of iTreg cells that contribute to tolerance in the airways. Moreover, these MØs lose their ability to induce Treg cells when exposed to allergens that mediate lung inflammation and can block tolerance. These data suggest that a greater understanding of why these MØs are present in the steady-state lung, how they may be generated, how they respond to stimuli, and how they lose or maintain their Treg cell–inducing ability, may offer new insights into therapy of asthmatic disease.
Prior studies have suggested that lung cDCs expressing IL-10, lung pDCs, and alveolar MØs all exert suppressive function and might contribute to maintaining tolerance in the lung (Thepen et al., 1992; Bilyk and Holt, 1993; Lipscomb et al., 1993; Roth and Golub, 1993; Akbari et al., 2001; de Heer et al., 2004). However, none of these populations have been described to promote the development of Foxp3+ iTreg cells, which recent results have shown are indispensable for preventing inflammation in this mucosal tissue (Ostroukhova et al., 2004; Mucida et al., 2005; Curotto de Lafaille et al., 2008; Duan et al., 2008, 2011; Josefowicz et al., 2012). The constitutive expression of TGF-β that drives Foxp3 expression and of RALDH1 and RALDH2 that results in retinoic acid production that synergizes with TGF-β identifies the lung tissue MØs as a central component of the tolerogenic mechanism within the lung.
Our data do not argue against the participation of cDCs, pDCs, or alveolar MØs in the tolerance process. It is likely that multiple APCs are involved in promoting and/or maintaining tolerance in all tissues and that the induction of Foxp3+ iTreg cells by lung tissue MØs is only one, albeit critical, component of the mechanism by which tolerance is perpetuated, with other phenomenon such as deletion and anergy of antigen-reactive T cells also contributing. Currently, it is not possible to specifically neutralize or delete the lung tissue MØs in vivo to evaluate their relative importance to the overall tolerogenic phenotype that results after inhalation of soluble antigen. However, several studies have used i.t. administered clodronate-containing liposomes as a means of depleting lung phagocytes, with the result that significantly greater lung and systemic inflammatory responses were observed upon antigen challenge (Thepen et al., 1992; Holt et al., 1993; Bang et al., 2011). Although the focus of these studies was the alveolar MØs, clodronate liposomes most likely also depleted at least a proportion of lung tissue MØs. There are several caveats to the use of clodronate in terms of selective activity; however, these studies potentially demonstrate a role for both tissue and alveolar MØs in mediating tolerance.
We did not address pDCs or alveolar MØs in our study of Treg cell generation. However, analysis of lung tissue cDCs failed to reveal any strong activity in promoting Foxp3+ Treg cells in the steady-state, but rather an ability to promote proliferation and effector T cell differentiation. This is in line with recent data showing that lung cDCs subdivided into CD103−CD11bhi and CD103+CD11blo induce the development of effector T cells (Beaty et al., 2007; Furuhashi et al., 2012; Nakano et al., 2012). The lung tissue cDCs we isolated did however express RALDH2, and thus it is still possible that if a sufficient amount of TGF-β was produced locally from another cell type, these DCs could participate in promoting the generation of Foxp3+ iTreg cells. We found that addition of exogenous TGF-β added into culture with these DCs and naive T cells did result in Foxp3 expression, but the number of Foxp3+ T cells was still approximately fourfold less than in parallel cultures with tissue MØs (unpublished data). The lung tissue MØs and cDCs did not express IL-10 mRNA, and we found no evidence for induction of IL-10–producing Treg cells, either in this study or our previous studies (Duan et al., 2008, 2011). Similarly, analysis of lungs from IL-10/GFP reporter mice did not reveal any constitutive IL-10 expression in lung MØs or DCs (unpublished data). Therefore, an IL-10–producing cDC may play a role at a later time in either perpetuating an existing tolerogenic program or limiting ongoing lung inflammation. We did find that IL-10 is active at some stage during the initial development of lung Foxp3+ iTreg cells, as shown in a blocking study in which anti–IL-10R prevented the generation of tolerance to inhaled antigen (Duan et al., 2011), but whether this IL-10 is required in the lung or lung-draining LNs is presently not clear, nor its source.
Sequential interactions with APCs and several antigen-presenting events may need to occur both within the lung tissue itself and the draining LNs to afford full development of airway tolerance. However, we found that both naive and DC-activated T cells could be induced to express Foxp3 when stimulated by lung tissue MØs, suggesting the MØs could be the initial APC or a secondary APC and still exert suppressive function. Additionally, our transfer experiments in CCR7−/− and MHC II−/− mice showed similar levels of conversion of T cells into Foxp3+ Treg cells (Fig. 4), but their accumulation was impaired, implying that both migration to the LNs and antigen presentation on other APCs, likely cDCs, are required for the overall tolerogenic response. This is further substantiated by a study showing that CCR7−/− mice could not be tolerized efficiently with inhaled antigen, although the interpretation of results in these animals is complicated as they generated lower asthmatic inflammatory responses under nontolerizing conditions (Hintzen et al., 2006). Additionally or alternatively, separate antigen presentation events might contribute. As well as iTreg cells developing after inhalation of soluble antigen, a substantial degree of deletion and anergy appears to occur, based on tracking T cell reactivity and the proportion of naive T cells that respond but do not become iTreg cells (Duan et al., 2008, 2011). From older studies with alveolar MØs, it is likely that deletion and/or anergy may largely result from antigen being presented on these cells (Holt et al., 1993; Upham et al., 1995; Strickland et al., 1996; Blumenthal et al., 2001). Within the gut, another mucosal tissue that may be very similar to the lung, both MØs and CD103+ cDCs in mesenteric LNs and in lamina propria have been suggested to afford tolerance and promote Foxp3+ iTreg cells, and again sequential interactions among these APCs in different compartments may be critical for maintaining overall homeostasis (Coombes et al., 2007; Denning et al., 2007; Sun et al., 2007; Hadis et al., 2011). CD103+ mesenteric LN DCs express RALDH2 but not appreciable RALDH1. They can produce TGF-β but only at low levels and have a weaker ability to promote Foxp3 iTreg cells than the lung tissue MØs in the absence of exogenous TGF-β (Coombes et al., 2007; Sun et al., 2007; unpublished data). The gut regulatory MØs originally reported were CD11c− and F4/80+ CD11b+ CD24+ and spontaneously made IL-10 (Kamada et al., 2005; Denning et al., 2007). They also express RALDH1 and RALDH2 mRNA and mRNA for TGF-β but are only weakly able to promote Foxp3+ iTreg cells without exogenous TGF-β. Thus, they are likely not the same as the lung tissue MØs or they are at a different stage of differentiation. CD11c+ F4/80+ MØs have recently been visualized in the gut (Denning et al., 2011). They expressed RALDH2 at similar levels as CD11c− F4/80+ gut MØs and lower levels of RALDH1 but expressed TGF-β mRNA at comparable levels. The gut CD11c+ MØs also supported Foxp3+ iTreg cell generation in the presence of exogenous TGF-β, but it was not tested whether they could promote significant numbers of Foxp3+ Treg cells without adding TGF-β (Denning et al., 2011). This MØ population expressed IL-10 mRNA directly ex vivo, again distinguishing them from the resident lung tissue MØs that we describe here.
In summary, we demonstrate that CD11c+ F4/80+ MØs that are present in lung tissue of naive, unmanipulated mice possess an intrinsic capacity to promote development of Foxp3+ iTreg cells through constitutive expression of TGF-β and retinoic acid/RALDH. These MØs are likely to represent an integral component of the mechanism by which tolerance and homeostasis is afforded in the lung. Although they maintain expression of TGF-β and retinoic acid/RALDH upon exposure to allergens that cause lung disease, the tissue MØs lose the capacity to promote Foxp3+ iTreg cells and instead take on an inflammatory phenotype. Not only may the inflammatory cytokines such as IL-1 and IL-6 made by these MØs contribute to asthmatic disease (Doganci et al., 2005; Neveu et al., 2009; Willart et al., 2012), but continued expression of TGF-β potentially drives aspects of lung inflammation. Activated/differentiated MØs have been implicated in lung-remodeling disease, including that seen in severe asthmatics, which is characterized by tissue fibrosis, and it has been suggested that they contribute to this process through production of TGF-β (Doherty and Broide, 2007; Halwani et al., 2011; Lekkerkerker et al., 2012). It is possible that the MØs associated with airway remodeling represent a differentiated state of the regulatory MØs that promote iTreg cell development. Understanding how these tissue MØs develop and are maintained in the resting noninflamed lung, and whether they can be targeted and modulated to retain regulatory activity in the face of allergen insults, may provide significant insights into strategies attempting to induce tolerance in patients with lung disease.
MATERIALS AND METHODS
Mice.
6–8-wk-old female WT C57BL/6 (CD45.2+), C57BL/6-SJL (CD45.1+), and BL/6 MHC II–deficient (MHC II−/−) mice were purchased from the Jackson Laboratory. CCR7-deficient (CCR7−/−), lymphotoxin β receptor–deficient (LTβR−/−), and MyD88/TRIF double-deficient mice on the BL/6 background were bred in-house at the La Jolla Institute for Allergy and Immunology (LIAI). OT-II TCR transgenic mice (CD45.2+) were used as a source of Vα2+Vβ5+ CD4 T cells responsive to the OVA 323–339 peptide. CD45.1+ OT-II TCR transgenic mice were generated by backcrossing OT-II mice with C57BL/6 CD45.1+ mice. All mice were backcrossed at least six times. The experiments reported here were approved by the LIAI Animal Care Committee and conform to the principles outlined by the animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Antibodies and flow cytometry.
The following antibodies were purchased from BD: anti–CD4-allophycocyanin, anti–CD4-PerCp, anti–CD4-FITC, anti–CD11c-FITC or -allophycocyanin, anti–CD11b-FITC or -allophycocyanin, anti-CD24–Alexa Fluor 647, anti–CD45-PerCp, anti–CD45.2-FITC or -allophycocyanin, anti–CD45.1-FITC or -allophycocyanin, anti–CD62L-FITC, anti-CD68–Alexa Fluor 647, anti-CD205–Alexa Fluor 647, anti–I-A/I-E–PE, anti–Vα2-PE, anti–Vβ5-FITC, and anti–Siglec F–PE. Streptavidin-allophycocyanin (BD) was used to visualize biotin-labeled antibodies. Anti–F4/80-PE, anti–IFN-γ, anti–IL-4, anti–IL-5, anti–IL-10 (all PE or allophycocyanin conjugated), and anti–Foxp3-PE were obtained from eBioscience. Reagents for cell fixation and permeabilization for detecting intracellular cytokines and Foxp3 were obtained from eBioscience, and staining was performed according to the manufacturer’s instructions. Cells were examined by flow cytometry using the FACSCalibur or FACSCanto II (BD) and analyzed with FlowJo software (Tree Star). Blocking antibodies to mouse IL-1β, TNF, and IL-6 were from BD.
Allergen extracts, proteases, and antigens.
Extracts from Dermatophagoides pteronyssinus (HDM), A. fumigatus (ASP), or cat dander (CAT) were purchased from GREER Laboratories. Recombinant or purified protease from HDM (Der p1) and from Aspergillus oryzae was obtained from Indoor Biotechnologies and Sigma-Aldrich, respectively. OVA (LPS low/free) was obtained from Worthington Biochemical Corp. Endotoxin contamination was as follows: HDM, 8.5 EU/µg; Aspergillus, 2.4 EU/µg; cat dander, 7.3 EU/µg; Der p1 protease, <0.01 EU/µg; Aspergillus protease, <0.01 EU/µg; and OVA, <0.01 EU/µg.
MØ and DC isolation.
To exclude nontissue-resident cells, lungs were perfused with cold PBS, and then bronchoalveolar cells were removed by lavage with extensive washing with PBS. After digestion of lung tissues and after incubation with mouse Fc-blocking antibody (2.4G2), recovered cells were stained with PE-conjugated anti–Siglec F antibody followed by anti-PE MicroBeads (Miltenyi Biotec). Siglec F+ cells were enriched on an AutoMACS Pro cell separator (Miltenyi Biotec), followed by further isolation on a FACSAria II cell sorter (BD) to purify Siglec F+ CD11c+ AFhi lung-resident tissue MØs. To isolate DCs, CD11c+ cells were enriched on an AutoMACS Pro cell separator from Siglec F–depleted lung or MLN cell suspensions. Siglec F− MHC IIhi, AFlo cells were further sorted as DCs with additional sorting on B220− cells for MLN DCs. To purify antigen bearing MØs and DCs, OVA protein conjugated to Alexa Fluor 647 (Invitrogen) was given to mice i.n. 24 and 48 h later, Alexa Fluor 647+ Siglec F+ CD11c+ AFhi cells (MØs) and Alexa Fluor 647+ Siglec F− CD11c+ AFlo cells (DCs) were sorted from single cell suspensions of digested lung and MLNs with additional sorting on B220− cells for MLNs.
Stimulation of T lymphocytes.
Foxp3−CD25− CD62L+CD4+ T cells were purified from spleen and peripheral LNs of OT-II TCR transgenic mice as before (Duan et al., 2008, 2011). For in vitro stimulation, purified MØs or DCs were cultured with T cells and 0.1 µM OVA peptide (ISQVHAAHAEINEAGR) in 200 µl complete RPMI medium in 96-well round-bottomed plates. In some experiments, MØs and DCs loaded with OVA–Alexa Fluor 647 in vivo were sorted and co-cultured with OT-II CD4+ T cells. In other experiments, naive OT-II were first activated with OVA-loaded DCs for 24 h and then co-cultured with OVA-loaded MØs. Intracellular Foxp3 expression in T cells was assessed after 4–5 d of culture. In co-culture experiments, APCs were added to T cells at a ratio of 1:25 or 1:5. Supernatants were analyzed after 3 d, or cells were restimulated with PMA/ionomycin to detect intracellular cytokines at day 5. For measurement of proliferation, 3H-TdR (GE Healthcare) was added to cells during the final 16 h of culture. In some experiments, neutralizing anti–TGF-β (R&D Systems) or rat IgG isotype control antibody was added (10 µg/ml). RAR pan-antagonist LE540 was added to some cultures at a concentration of 1 µM.
For assessing suppressive activity, Foxp3− OT-II CD4 T cells were labeled with 2.5 µM CFSE (Molecular Probes, Invitrogen) and stimulated with OVA-pulsed T depleted splenocytes in the absence or presence of Foxp3+ T cells generated in cultures with lung MØs at 1:10 or 1:1 ratios. 4 d later, CFSE dilution was monitored by flow cytometry.
Real-time qPCR.
MØs were isolated ex vivo or from in vitro culture, and their total RNA was isolated using TRIzol reagent (Invitrogen). Single-strand cDNA was prepared by reverse transcribing 5 µg of total RNA using the SuperScript III kit (Invitrogen). The oligonucleotide primer sequences were: TGF-β forward primer, 5′-CCCTATATTTGGAGCCTGGA-3′; TGF-β reverse primer, 5′-GTTGGTTGTAGAGGGCAAGG-3′; RALDH1 forward primer, 5′-ATGGTTTAGCAGCAGGACTCTTC-3′; RALDH1 reverse primer, 5′-CCAGACATCTTGAATCCACCGAA-3′; RALDH2 forward primer, 5′-GACTTGTAGCAGCTGTCTTCACT-3′; RALDH2 reverse primer, 5′-TCACCCATTTCTCTCCCATTTCC-3′; TNF forward primer, 5′-AACTAGTGGTGCCAGCCGAT-3′; TNF reverse primer, 5′-CTTCACAGAGCAATGACTCC-3′; IL-6 forward primer, 5′-GACAAAGCCAGAGTCCTTCAGAGAG-3′; IL-6 reverse primer, 5′-CTAGGTTTGCCGAGTAGATCTC-3′; IL-1β forward primer, 5′-CTCCATGAGCTTTGTACAAGG-3′; IL-1β reverse primer, 5′-TGCTGATGTACCAGTTGGGG-3′; IL-1α forward primer, 5′-GCCAGTTGAGTAGGATAAAGG-3′; and IL-1α reverse primer, 5′-CAGTCTGTCTCCTTCTTGAGG-3′. Real-time PCR assay was performed with LightCycler (Roche) using LightCycler 480 SYBR Green I master (Roche). Data are presented as fold increase to ribosomal protein housekeeping gene L32. All results were representative of at least two experiments.
Adoptive transfer experiments.
106 Foxp3−CD25− CD4 T cells purified from CD45.1+ OT-II mice (Duan et al., 2008, 2011) were adoptively transferred i.v. into CD45.2+ mice. Purified lung tissue MØs were cultured with or without 100 µg/ml OVA protein (Worthington Biochemical Corp.) in complete RPMI for 18 h. 5 × 105 MØs were washed with cold PBS to remove additional OVA protein and then instilled i.t. into CD45.2+ mice that had or had not already received naive OT-II CD4 T cells. For in vivo retinoic acid inhibition, purified naive OT-II CD4 T cells and OVA-pulsed MØs were transferred into congenic hosts as above. Recipient mice were orally treated with the RAR antagonist LE540 (50 µg/mouse) or soybean oil (vehicle) every day for a total of 6 d.
Airway tolerance and allergic airway inflammation.
C57BL/6 mice were instilled i.t. with OVA-loaded MØs or 5 × 105 MØs alone or exposed to 100 µg of soluble OVA protein (Worthington Biochemical Corp.) in PBS or to PBS alone, given i.n. for three consecutive days. Some mice were exposed to soluble OVA mixed with 100 µg (based on protein) of HDM, ASP, or CAT extracts (GREER Laboratories) in PBS for three consecutive days. After 9–12 d, mice were sensitized by i.p. injection of 50 µg OVA protein (Worthington Biochemical Corp.) adsorbed to 2 mg of aluminum hydroxide (alum; Thermo Fisher Scientific). On day 18 or 25, mice were challenged with 50 µg of soluble OVA in PBS, given i.n. for three consecutive days.
Mice were sacrificed 1 d after the last i.n. OVA challenge, and BAL fluid, lungs, and lung-draining LNs were obtained. BAL was performed by i.t. insertion of catheter, lavaging with 0.8–0.9 ml of 2% filtered BSA (Sigma-Aldrich). Total cell counts were performed using a hemocytometer (Hausser Scientific). BAL differential cell counts, ELISA for BAL cytokines, and FACS analysis of cells from lung and MLNs were performed as described previously (Duan et al., 2008, 2011). The right hilum was tied off, and right lungs were isolated and digested for cellular analysis as above. The left lung was instilled with 0.4 ml of 4% paraformaldehyde for histology. Fixed lung sections were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff for mucus secretion. In some experiments, invasive pulmonary function testing was performed using the Flexivent system (Scireq) and airway resistance analyzed by Flexivent 5.1 software (Scireq) as previously described (Duan et al., 2011).
Stimulation of MØs with allergens and co-culture with T cells.
5 × 104 purified CD45+ Siglec F+ CD11c+ AFhi lung tissue MØs from naive mice were cultured in 200 µl complete RPMI medium in 96-well round-bottomed plates in the presence of 50 µg/ml (based on protein) of HDM, ASP, and CAT extracts (GREER Laboratories) or PBS alone. 48 h later, cytokines were measured by ELISA in supernatants. Isolated MØs were also cultured with recombinant Der p1 (Indoor Biotechnologies) and Aspergillus (Sigma-Aldrich) protease (10 µg/ml each) for 24 h. In some experiments, allergen-stimulated or unstimulated MØs were washed several times with cold PBS and co-cultured with Foxp3−CD25− OT-II CD4 T cells and OVA peptide for an additional 4–5 d for analysis of intracellular Foxp3 induction. In other cases, the protease activity of HDM and ASP was blocked with a serine/cysteine protease inhibitor (Roche).
Acknowledgments
We thank D. Mucida and M. Murai for critical discussion.
This work was supported by National Institutes of Health grants CA91837 and AI70535 to M. Croft. This is manuscript number #1534 from the La Jolla Institute for Allergy and Immunology.
The authors have no competing financial interests related to this work.
Footnotes
Abbreviations used:
- AF
- autofluorescence
- BAL
- bronchoalveolar lavage
- cDC
- classical DC
- HDM
- house dust mite
- i.t.
- intratracheal(ly)
- iTreg cell
- inducible Treg cell
- MLN
- mediastinal LN
- MØ
- macrophage
- pDC
- plasmacytoid DC
- qPCR
- quantitative PCR
- RAR
- retinoic acid receptor
References
- Akbari O., DeKruyff R.H., Umetsu D.T. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731 10.1038/90667 [DOI] [PubMed] [Google Scholar]
- Akdis M. 2006. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 18:738–744 10.1016/j.coi.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Bakocević N., Worbs T., Davalos-Misslitz A., Förster R. 2010. T cell-dendritic cell interaction dynamics during the induction of respiratory tolerance and immunity. J. Immunol. 184:1317–1327 10.4049/jimmunol.0902277 [DOI] [PubMed] [Google Scholar]
- Bang B.R., Chun E., Shim E.J., Lee H.S., Lee S.Y., Cho S.H., Min K.U., Kim Y.Y., Park H.W. 2011. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp. Mol. Med. 43:275–280 10.3858/emm.2011.43.5.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty S.R., Rose C.E., Jr, Sung S.S. 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 178:1882–1895 [DOI] [PubMed] [Google Scholar]
- Benson M.J., Pino-Lagos K., Rosemblatt M., Noelle R.J. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774 10.1084/jem.20070719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk N., Holt P.G. 1993. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 177:1773–1777 10.1084/jem.177.6.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R.L., Campbell D.E., Hwang P., DeKruyff R.H., Frankel L.R., Umetsu D.T. 2001. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. J. Allergy Clin. Immunol. 107:258–264 10.1067/mai.2001.112845 [DOI] [PubMed] [Google Scholar]
- Braedel S., Radsak M., Einsele H., Latgé J.P., Michan A., Loeffler J., Haddad Z., Grigoleit U., Schild H., Hebart H. 2004. Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br. J. Haematol. 125:392–399 10.1111/j.1365-2141.2004.04922.x [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cose S., Brammer C., Khanna K.M., Masopust D., Lefrançois L. 2006. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 36:1423–1433 10.1002/eji.200535539 [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Kutchukhidze N., Shen S., Ding Y., Yee H., Lafaille J.J. 2008. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 29:114–126 10.1016/j.immuni.2008.05.010 [DOI] [PubMed] [Google Scholar]
- de Heer H.J., Hammad H., Soullié T., Hijdra D., Vos N., Willart M.A., Hoogsteden H.C., Lambrecht B.N. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98 10.1084/jem.20040035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086–1094 10.1038/ni1511 [DOI] [PubMed] [Google Scholar]
- Denning T.L., Norris B.A., Medina-Contreras O., Manicassamy S., Geem D., Madan R., Karp C.L., Pulendran B. 2011. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 187:733–747 10.4049/jimmunol.1002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganci A., Eigenbrod T., Krug N., De Sanctis G.T., Hausding M., Erpenbeck V.J., Haddad B., Lehr H.A., Schmitt E., Bopp T., et al. 2005. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 115:313–325 (published erratum appears in J. Clin. Invest. 2005. 115:1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T., Broide D. 2007. Cytokines and growth factors in airway remodeling in asthma. Curr. Opin. Immunol. 19:676–680 10.1016/j.coi.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Duan W., So T., Croft M. 2008. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J. Immunol. 181:8650–8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Mehta A.K., Magalhaes J.G., Ziegler S.F., Dong C., Philpott D.J., Croft M. 2010. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J. Allergy Clin. Immunol. 126:1284–1293: e10 10.1016/j.jaci.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., So T., Mehta A.K., Choi H., Croft M. 2011. Inducible CD4+LAP+Foxp3- regulatory T cells suppress allergic inflammation. J. Immunol. 187:6499–6507 10.4049/jimmunol.1101398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi K., Suda T., Hasegawa H., Suzuki Y., Hashimoto D., Enomoto N., Fujisawa T., Nakamura Y., Inui N., Shibata K., et al. 2012. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am. J. Respir. Cell Mol. Biol. 46:165–172 10.1165/rcmb.2011-0070OC [DOI] [PubMed] [Google Scholar]
- Fütterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 9:59–70 10.1016/S1074-7613(00)80588-9 [DOI] [PubMed] [Google Scholar]
- Gajewska B.U., Alvarez D., Vidric M., Goncharova S., Stämpfli M.R., Coyle A.J., Gutierrez-Ramos J.C., Jordana M. 2001a. Generation of experimental allergic airways inflammation in the absence of draining lymph nodes. J. Clin. Invest. 108:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewska B.U., Swirski F.K., Alvarez D., Ritz S.A., Goncharova S., Cundall M., Snider D.P., Coyle A.J., Gutierrez-Ramos J.C., Stämpfli M.R., Jordana M. 2001b. Temporal-spatial analysis of the immune response in a murine model of ovalbumin-induced airways inflammation. Am. J. Respir. Cell Mol. Biol. 25:326–334 [DOI] [PubMed] [Google Scholar]
- Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Müller W., Sparwasser T., Förster R., Pabst O. 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 34:237–246 10.1016/j.immuni.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Halwani R., Al-Muhsen S., Al-Jahdali H., Hamid Q. 2011. Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 44:127–133 10.1165/rcmb.2010-0027TR [DOI] [PubMed] [Google Scholar]
- Hamann A., Klugewitz K., Austrup F., Jablonski-Westrich D. 2000. Activation induces rapid and profound alterations in the trafficking of T cells. Eur. J. Immunol. 30:3207–3218 [DOI] [PubMed] [Google Scholar]
- Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. 2009. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15:410–416 10.1038/nm.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp J.R., Onami T.M. 2010. Naïve T cells re-distribute to the lungs of selectin ligand deficient mice. PLoS ONE. 5:e10973 10.1371/journal.pone.0010973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz C.M., O’Garra A. 2005. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5:271–283 10.1038/nri1589 [DOI] [PubMed] [Google Scholar]
- Hintzen G., Ohl L., del Rio M.L., Rodriguez-Barbosa J.I., Pabst O., Kocks J.R., Krege J., Hardtke S., Förster R. 2006. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J. Immunol. 177:7346–7354 [DOI] [PubMed] [Google Scholar]
- Holt P.G., Oliver J., Bilyk N., McMenamin C., McMenamin P.G., Kraal G., Thepen T. 1993. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177:397–407 10.1084/jem.177.2.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S.Z., Niec R.E., Kim H.Y., Treuting P., Chinen T., Zheng Y., Umetsu D.T., Rudensky A.Y. 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 482:395–399 10.1038/nature10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Hisamatsu T., Okamoto S., Sato T., Matsuoka K., Arai K., Nakai T., Hasegawa A., Inoue N., Watanabe N., et al. 2005. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J. Immunol. 175:6900–6908 [DOI] [PubMed] [Google Scholar]
- Kauffman H.F., Tamm M., Timmerman J.A., Borger P. 2006. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin. Mol. Allergy. 4:5 10.1186/1476-7961-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand F., Kiss A., Xu J., Lee S.H., Kolattukudy P.E., Corry D.B. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169:5904–5911 [DOI] [PubMed] [Google Scholar]
- Kirby A.C., Coles M.C., Kaye P.M. 2009. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 183:1983–1989 10.4049/jimmunol.0901089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larché M. 2007. Regulatory T cells in allergy and asthma. Chest. 132:1007–1014 10.1378/chest.06-2434 [DOI] [PubMed] [Google Scholar]
- Lekkerkerker A.N., Aarbiou J., van Es T., Janssen R.A. 2012. Cellular players in lung fibrosis. Curr. Pharm. Des. 18:4093–4102 10.2174/138161212802430396 [DOI] [PubMed] [Google Scholar]
- Lipscomb M.F., Pollard A.M., Yates J.L. 1993. A role for TGF-beta in the suppression by murine bronchoalveolar cells of lung dendritic cell initiated immune responses. Reg. Immunol. 5:151–157 [PubMed] [Google Scholar]
- Lloyd C.M., Hawrylowicz C.M. 2009. Regulatory T cells in asthma. Immunity. 31:438–449 10.1016/j.immuni.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula S.S., Sau K., Henneke P., Golenbock D.T., Levitz S.M. 2002. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 277:39320–39326 10.1074/jbc.M201683200 [DOI] [PubMed] [Google Scholar]
- Mucida D., Kutchukhidze N., Erazo A., Russo M., Lafaille J.J., Curotto de Lafaille M.A. 2005. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Invest. 115:1923–1933 10.1172/JCI24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Nakano H., Free M.E., Whitehead G.S., Maruoka S., Wilson R.H., Nakano K., Cook D.N. 2012. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 5:53–65 10.1038/mi.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu W.A., Allard J.B., Dienz O., Wargo M.J., Ciliberto G., Whittaker L.A., Rincon M. 2009. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J. Immunol. 183:1732–1738 10.4049/jimmunol.0802923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroukhova M., Seguin-Devaux C., Oriss T.B., Dixon-McCarthy B., Yang L., Ameredes B.T., Corcoran T.E., Ray A. 2004. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Invest. 114:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M.D., Golub S.H. 1993. Human pulmonary macrophages utilize prostaglandins and transforming growth factor beta 1 to suppress lymphocyte activation. J. Leukoc. Biol. 53:366–371 [DOI] [PubMed] [Google Scholar]
- Stevens W.W., Kim T.S., Pujanauski L.M., Hao X., Braciale T.J. 2007. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 327:63–74 10.1016/j.jim.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D., Kees U.R., Holt P.G. 1996. Regulation of T-cell activation in the lung: alveolar macrophages induce reversible T-cell anergy in vitro associated with inhibition of interleukin-2 receptor signal transduction. Immunology. 87:250–258 10.1046/j.1365-2567.1996.459542.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepen T., McMenamin C., Girn B., Kraal G., Holt P.G. 1992. Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin. Exp. Allergy. 22:1107–1114 10.1111/j.1365-2222.1992.tb00137.x [DOI] [PubMed] [Google Scholar]
- Thepen T., Claassen E., Hoeben K., Brevé J., Kraal G. 1993. Migration of alveolar macrophages from alveolar space to paracortical T cell area of the draining lymph node. Adv. Exp. Med. Biol. 329:305–310 10.1007/978-1-4615-2930-9_51 [DOI] [PubMed] [Google Scholar]
- Tone Y., Furuuchi K., Kojima Y., Tykocinski M.L., Greene M.I., Tone M. 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9:194–202 10.1038/ni1549 [DOI] [PubMed] [Google Scholar]
- Trompette A., Divanovic S., Visintin A., Blanchard C., Hegde R.S., Madan R., Thorne P.S., Wills-Karp M., Gioannini T.L., Weiss J.P., Karp C.L. 2009. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 457:585–588 10.1038/nature07548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitoura D.C., DeKruyff R.H., Lamb J.R., Umetsu D.T. 1999. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J. Immunol. 163:2592–2600 [PubMed] [Google Scholar]
- Umetsu D.T., Dekruyff R.H. 2006. Immune dysregulation in asthma. Curr. Opin. Immunol. 18:727–732 10.1016/j.coi.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Upham J.W., Strickland D.H., Bilyk N., Robinson B.W., Holt P.G. 1995. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 84:142–147 [PMC free article] [PubMed] [Google Scholar]
- Vermaelen K., Pauwels R. 2004. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 61A:170–177 10.1002/cyto.a.20064 [DOI] [PubMed] [Google Scholar]
- Wikstrom M.E., Batanero E., Judd S.R., Wiqvist K., Holt P.G., Stumbles P.A. 2010. Lung homing T-cell generation is dependent on strength and timing of antigen delivery to lymph nodes. Immunol. Cell Biol. 88:658–666 10.1038/icb.2010.18 [DOI] [PubMed] [Google Scholar]
- Willart M.A., Deswarte K., Pouliot P., Braun H., Beyaert R., Lambrecht B.N., Hammad H. 2012. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 209:1505–1517 10.1084/jem.20112691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasłona Z., Wilhelm J., Cakarova L., Marsh L.M., Seeger W., Lohmeyer J., von Wulffen W. 2009. Transcriptome profiling of primary murine monocytes, lung macrophages and lung dendritic cells reveals a distinct expression of genes involved in cell trafficking. Respir. Res. 10:2 10.1186/1465-9921-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Josefowicz S., Chaudhry A., Peng X.P., Forbush K., Rudensky A.Y. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 463:808–812 10.1038/nature08750 [DOI] [PMC free article] [PubMed] [Google Scholar]