Abstract
Plant sterols, or phytosterols, are very similar in structure to cholesterol and are abundant in typical diets. The reason for poor absorption of plant sterols by the body is still unknown. Mutations in the ABC transporters G5 and G8 are known to cause an accumulation of plant sterols in blood and tissues (sitosterolemia). To determine the significance of phytosterol exclusion from the body, we fed wild-type and ABCG5/G8 knockout mice a diet enriched with plant sterols. The high-phytosterol diet was extremely toxic to the ABCG5/G8 knockout mice but had no adverse effects on wild-type mice. ABCG5/G8 knockout mice died prematurely and developed a phenotype that included high levels of plant sterols in many tissues, liver abnormalities, and severe cardiac lesions. This study is the first to report such toxic effects of phytosterol accumulation in ABCG5/G8 knockout mice. We believe these new data support the conclusion that plant sterols are excluded from the body because they are toxic when present at high levels.
One of the longstanding mysteries of sterol metabolism is the need for discrimination between plant sterols (or phytosterols) and cholesterol. It has long been observed that although a typical diet generally contains similar amounts of cholesterol and phytosterols, intestinal cholesterol absorption rates are 40% to 60%, whereas phytosterols are mostly excluded from the body due to intestinal absorption rates of less than 5%.1,2 The discrimination against phytosterols has a basis in chemical structure. Campesterol, with a single-carbon side chain at carbon 24, typically has a plasma concentration 500 times less than cholesterol; whereas, the concentration of sitosterol, with a 2-carbon side chain at carbon 24, is about 20,000 times lower than cholesterol.3
The need for discrimination among sterols is not fully understood. In 1974, Bhattacharyya and Connor4 described a new lipid storage disease, termed β-sitosterolemia, in two sisters with plant sterol accumulation in blood and tissues. Subsequently, several other patients with sitosterolemia have been identified.5 Patients accumulate plant sterols in many tissues and often present with tendinous and cutaneous xanthomas and hypercholesterolemia.5,6 There are also indications that plasma plant sterol accumulation has negative effects on platelet biology.7 Elimination of plant sterols from their diet is an effective treatment for patients.7,8
Subsequent work has identified genetic mutations to the heterodimeric ABC transporters, ABCG5 and ABCG8, as the cause of sitosterolemia.9–11 Both ABCG5 and ABCG8 must be expressed for their cotransport from the endoplasmic reticulum to the cell surface.12,13 Therefore, mutations to either or both of these proteins can result in a loss of functionality. These proteins are mainly expressed in the intestine and liver on the apical surface of enterocytes and hepatocytes.12,14 They apparently function to transport cholesterol and phytosterols into bile in the liver and into the lumen of the small intestine,15 although their full functional significance is yet to be determined.
The identification of ABCG5 and ABCG8 as important players in sterol transport has led to the discovery and creation of several mouse models that lack one or both of the proteins. By genetic manipulations, ABCG5 single-knockout (KO), ABCG8 single-KO, and ABCG5/G8 double-KO mice have been created. Additionally, one mouse model with a spontaneous mutation of ABCG5 has been previously described in the literature. The trac mutant mouse, which develops thrombocytopenia and cardiomyopathy, has a spontaneous point mutation at base 1435 of Abcg5 that results in a premature stop codon.16 The trac mutant mice and the genetically modified KO mice have subtle differences among the models, but all have the fundamental accumulation of plant sterols in plasma and tissues.15,17–20 This phenotype is similar to that of human patients with sitosterolemia. Clearly, ABCG5 and ABCG8 play an essential role in excluding plant sterols from the body, although the purpose underlying this function is not well understood.
To determine the significance of phytosterol exclusion from the body, wild-type (WT) and ABCG5/G8 KO mice were fed a diet with 0.2% w/w phytosterols. The high-phytosterol diet had no adverse effects on WT mice, but surprisingly, was extremely toxic to ABCG5/G8 KO mice. High levels of tissue plant sterol accumulation cause premature death, highly complex cardiac lesions, liver damage, and hepatosplenomegaly, which have not been previously reported in this model.
Materials and Methods
Animals and Diet
ABCG5/G8 KO mice have been described previously.15 WT and ABCG5/G8 KO mice on a mixed background of 81.5% C57Bl/6, 12.5% SvJae, and 6% 129SvEv were housed in a specific pathogen–free animal facility in plastic cages in a temperature-controlled room (22°C) with a 12-hour daylight cycle. The mice were fed ad libitum a standard rodent chow diet (5P00Prolab; LabDiet, St. Louis, MO) before the start of the high-phytosterol diet and had free access to water. At 6 to 8 weeks of age, female WT and ABCG5/G8 KO mice were fed a low-fat (10% of energy as palm oil–enriched fat) synthetic diet containing 0.2% (w/w) phytosterols and 0.001% (w/w) cholesterol (Supplemental Table S1) for 6 weeks. The diet was made at the Wake Forest University Primate Center Diet Laboratory. Body weights were measured weekly throughout the study. All animal procedures were approved by the institutional animal care and use committee at Wake Forest University Health Sciences.
Measurement of Plasma and Tissue Lipid Concentrations
After 4-hour fasting, blood was collected from mice that had been fed the synthetic diets for 6 weeks. Tissues were flushed with saline, removed, weighed, and either snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin (NBF) for later analyses.
To determine total plasma cholesterol and phytosterol concentrations, 25 μL of plasma was placed into a glass tube containing 21 μg of 5α-cholestane and extracted in 2:1 CHCl3:methanol overnight. Erythrocytes were separated from plasma and rinsed with saline before an overnight extraction in 2:1 CHCl3:methanol. 5α-Cholestane was used as an internal standard. For tissue analysis of cholesterol and phytosterol concentrations, approximately 50 mg of frozen tissue was placed into a glass tube containing 135 μg of 5α-cholestane. Lipids were extracted in 2:1 CHCl3:methanol at room temperature overnight. Total sterol mass was measured for all samples by gas chromatography as described previously.21
Hematological Measurements
After 4-hour fasting, a blood sample was obtained biweekly via submandibular puncture after the beginning of a high-phytosterol diet. Separate tubes were used for blood collection of serum and plasma samples. Samples were spun to separate erythrocytes. Serum chemistry values were determined by ANTECH Diagnostics (Lake Success, NY). Hematocrit values were determined by collection of blood into hematocrit tubes and spinning to pack red cells (VWR International, West Chester, PA).
Real-Time PCR Analysis of Gene Expression in Livers and Spleens
Approximately 50 mg of frozen pieces of liver and spleen were homogenized into 1 mL of Trizol Reagent (Invitrogen, Carlsbad, CA), and total RNA was extracted according to the manufacturer’s instructions. Recovery and integrity of RNA was determined, and cDNA was made using Omniscript Reverse Transcriptase (Qiagen, Valencia, CA) and 1 μg of RNA according to the manufacturer’s instructions. Relative gene expression was determined in each cDNA sample using forward and reverse primers designed for each gene of interest (Table 1) and SYBR-Green PCR master mix (Applied Biosciences, CA). Reactions were monitored on an ABI Prism 7000 Sequence Detection System using the cycling parameters: 50°C for 2 minutes, 95°C for 10 minutes, (95°C for 15 seconds, 60°C for 1 minute) for 40 cycles, 60°C for 1 minute, and 60°C dissociation. Relative expression of each gene was normalized to expression of a housekeeping gene (cyclophilin or 18S ribosomal RNA) to control for intra-/intersample variation and expressed as arbitrary units (AU).
Table 1.
Real-Time PCR Primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ABCA1 | 5′-CGTTTCCGGGAAGTGTCCTA-3′ | 5′-GCTAGAGATGACAAGGAGGATGGA-3′ |
| ABCB11 | 5′-GACATGCTTGCGAGGACCTT-3′ | 5′-AGCGTTGCCGGATGGA-3′ |
| BLVRA | 5′-CTTGATGGAAGAGTTCGAATTCCT-3′ | 5′-GTGAAGCGAAGAGATCCTTTTAGC-3′ |
| CD68 | 5′-CCTCCACCCTCGCCTAGTC-3′ | 5′-TTGGGTATAGGATTCGGATTTGA-3′ |
| CYP7A1 | 5′-AGCAACTAAACAACCTGCCAGTACTA-3′ | 5′-GTCCGGATATTCAAGGATGCA-3′ |
| PPIA (cyclophilin A) | 5′-TGGAGAGCACCAAGACAGACA-3′ | 5′-TGCCGGAGTCGACAATGAT-3′ |
| HMGCS | 5′-GCCGTGAACTGGGTCGAA-3′ | 5′-GCATATATAGCAATGTCTCCTGCAA-3′ |
| HO1 | 5′-CAACAGTGGCAGTGGGAATTTA-3′ | 5′-CCAGGCAAGATTCTCCCTTACC-3′ |
| IL-6 | 5′-CTGCAAGAGACTTCCATCCAGTT-3′ | 5′-AGGGAAGGCCGTGGTTGT-3′ |
| PLTP | 5′-TGGGACGGTGTTGCTCAA-3′ | 5′-CCCACGAGATCATCCACAGA-3′ |
| SCD1 | 5′-CCGGAGACCCCTTAGATCGA-3′ | 5′-TAGCCTGTAAAAGATTTCTGCAAACC-3′ |
| SR-BI | 5′-TCCCCATGAACTGTTCTGTGAA-3′ | 5′-TGCCCGATGCCCTTGA-3′ |
| SREBP1C | 5′-GGAGCCATGGATTGCACATT-3′ | 5′-GGCCCGGGAAGTCACTGT-3′ |
| UGT1A | 5′-CACCACACCTGCGCCC-3′ | 5′-CCAATCACATCCAAGGAGTGGTA-3′ |
Histology
Hearts were preserved in 10% neutral buffered formalin for at least 24 hours, processed routinely for histology, cut at 6 μm, and sections were stained with H&E, Masson’s trichrome, and von Kossa. Snap-frozen liver samples were preserved for 48 hours in 10% neutral buffered formalin, followed by cryopreservation in 20% sucrose, before routine histology processing and staining with H&E. Tissues were examined by light microscopy in a blinded fashion by a board-certified anatomical veterinary pathologist (N.D.K.). To determine Kupffer cell infiltration, cells were counted in a single microscopic field for each liver section. Kupffer cell number was normalized to the total number of hepatocytes in each field. To quantitate cardiac fibrosis, trichrome-stained sections were used. An outline of the heart chambers was traced and subtracted from the total heart area. Areas of fibrosis were then traced and normalized to the calculated heart area.
Statistical Analyses and Data Presentation
Data are expressed as the means ± SEM. Significance of differences was determined for each group of values by analysis of variance (Tukey-Kramer honestly significant difference) or unpaired t-test. A P value <0.05 was considered significant. All analyses were performed using GraphPad Prism software version 4 (GraphPad Software, La Jolla, CA).
Results
ABCG5/G8 KO Mice Fail to Gain Weight and Develop Hepatosplenomegaly When Fed a High-Phytosterol Diet
When fed a diet rich in plant sterols, WT mice maintained a normal phenotype, whereas ABCG5/G8 KO mice showed generally poor health, characterized by weight loss, hunching, lethargy, and decreased life span (A.L. McDaniel and H.M. Alger, unpublished observations). Figure 1A shows that WT mice gained weight over the 6-week time-course of the high-phytosterol diet, whereas the ABCG5/G8 KO mice did not gain weight relative to their baseline measurement. This phenotype was not seen in ABCG5/G8 KO mice fed a diet containing an identical amount of cholesterol (Supplemental Figure S1 and Supplemental Table S1). This observation suggests that these effects were not caused by a general accumulation of sterols in the body, but rather were caused specifically by the accumulation of plant sterols.
Figure 1.
Measurement of body and tissues weights. Animals were fed a diet containing 0.2% plant sterols for 6 weeks. WT (n = 5), ABCG5/G8 KO (n = 10). Weekly bodyweight in grams (A), terminal liver to bodyweight ratio multiplied by 100 (B), terminal organ mass in grams (C), and percentage of total bodyweight as individual tissue (D). *P < 0.05 by analysis of variance and Tukey-Kramer posttest.
Further investigation revealed that by 6 weeks, the ABCG5/G8 KO mice fed the high-phytosterol diet had a high liver-to-bodyweight ratio (Figure 1B). Measurement of organ weights showed significantly higher liver mass and spleen mass (Figure 1C) in ABCG5/G8 KO mice compared to WT mice. This organ enlargement translated into significantly increased percentages of total bodyweight for liver and spleen (Figure 1D). By contrast, there was no change in the mass or percentage of bodyweight of the kidneys or lungs of ABCG5/G8 KO mice (Figure 1, C and D).
In ABCG5/G8 KO Mice Plant Sterol Accumulation Causes Liver Damage
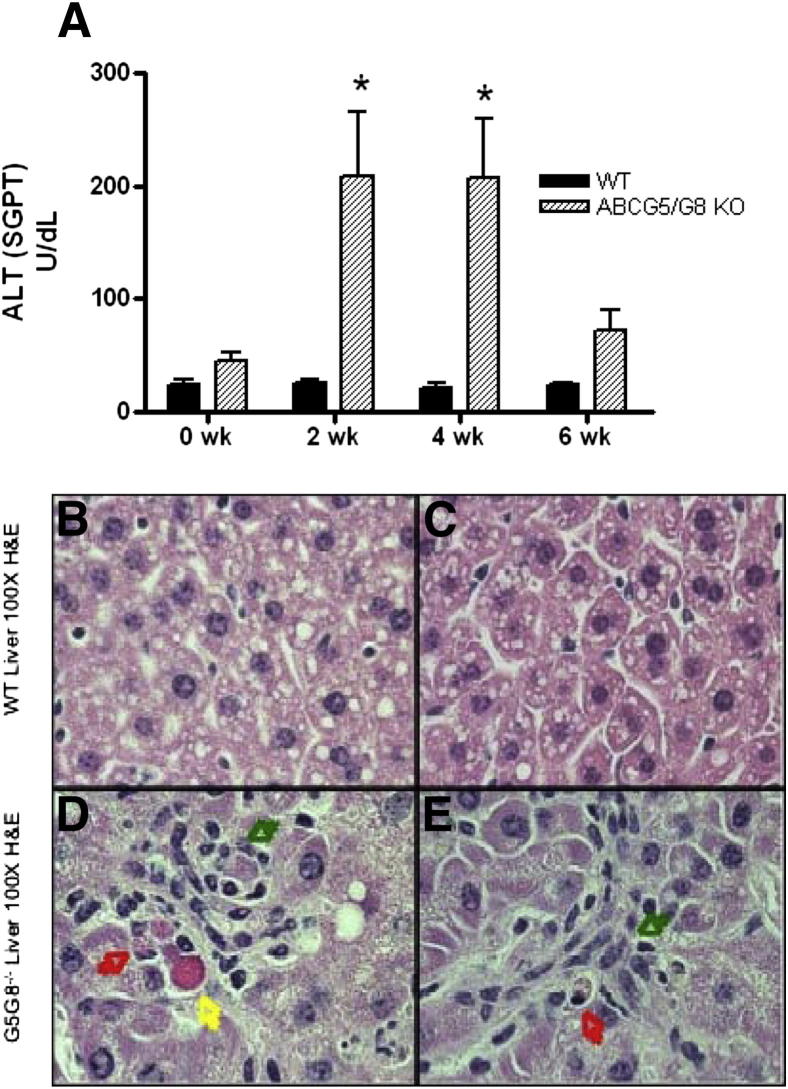
To further characterize the health of the phytosterol-fed ABCG5/G8 KO mice, serum chemistry values were assessed. Serum alanine transaminase/glutamic-pyruvic transaminase concentrations were found to be elevated in ABCG5/G8 KO mice at all time-points, in contrast to concentrations in WT mice that remained in the normal range (Figure 2A). ABCG5/G8 KO mice showed no changes in serum glucose, urea nitrogen, creatinine, total protein, alkaline phosphatase, calcium, or phosphorus when compared to WT mice over the 6-week dietary time-course (data not shown).
Figure 2.
Assessment of liver pathology. A: Biweekly measures of serum alanine transaminase ALT (glutamic-pyruvic transaminase [SGPT]), expressed as U/dL, showed significant increases in the phytosterol-fed ABCG5/G8 KO mice. B–E: Photomicrographs of liver from WT mice (B and C) and ABCG5/G8 KO mice (D and E). Liver from the WT mice was considered within normal limits. Liver from the ABCG5/G8 KO mice shows piecemeal hepatocellular necrosis with cellular rounding and detachment (red and yellow arrows), nuclear pyknosis, hepatocellular swelling, and Kupffer cell hyperplasia (green arrows). H&E stain, original magnification, ×100. *P < 0.05 as measured by analysis of variance and Tukey-Kramer posttest.
Sections of liver from WT and ABCG5/G8 KO mice stained with H&E were reviewed by an anatomical veterinary pathologist (N.D.K.) (Figure 2, B–E). ABCG5/G8 KO mouse livers (Figure 2, D and E) had piecemeal necrosis and hepatocellular degeneration, cellular rounding and detachment, and prominent diffuse Kupffer cell hyperplasia. Quantification of Kupffer cells in these livers revealed a 2.19-fold increase in the number of Kupffer cells present in ABCG5/G8 KO mouse livers as compared to WT. This increase in Kupffer cells was also reflected by a higher ratio of Kupffer cells to hepatocytes (WT = 0.68, KO = 2.01). Further confirming the histological increase in Kupffer cells, a significant 2.3-fold increase in the hepatic macrophage marker, CD68, was measured by mRNA levels in ABCG5/G8 KO mice (Table 2). WT mouse livers (Figure 2, B and C) were considered normal.
Table 2.
Hepatic and Splenic Gene Expression
| WT | ABCG5/G8 KO | P value | |
|---|---|---|---|
| Hepatic gene expression | |||
| Bilirubin metabolism | |||
| BLVRA | 12.38 ± 0.94 | 10.86 ± 1.84 | 0.5656 |
| HO-1 | 7.73 ± 0.79 | 24.93 ± 4.06 | 0.0061∗ |
| UGT1a | 38.29 ± 4.14 | 18.34 ± 2.77 | 0.0069∗ |
| Inflammatory markers | |||
| CD68 | 12.7 ± 2.68 | 29.8 ± 3.29 | 0.007∗ |
| IL-6 | 3.1 ± 0.65 | 3.4 ± 0.66 | 0.7672 |
| Lipid metabolism | |||
| HMGCS | 27.90 ± 2.68 | 14.62 ± 2.82 | 0.0145∗ |
| SR-BI | 8.8 ± 1.86 | 9.2 ± 1.45 | 0.8670 |
| ABCA1 | 4.3 ± 0.40 | 5.3 ± 0.76 | 0.2924 |
| SCD1 | 145.5 ± 18.53 | 305.5 ± 45.01 | 0.0167∗ |
| PLTP | 1.36 ± 0.31 | 1.18 ± 0.11 | 0.6046 |
| SREBP1C | 2.3 ± 0.27 | 3.1 ± 0.32 | 0.1271 |
| Bile acid metabolism | |||
| CYP7A1 | 22.9 ± 8.61 | 18.1 ± 2.66 | 0.6104 |
| ABCB11 | 9.3 ± 0.97 | 4.4 ± 0.34 | 0.0029∗ |
| Splenic gene expression | |||
| BLVRA | 10.61 ± 0.62 | 13.86 ± 0.97 | 0.0369∗ |
| HO-1 | 26.31 ± 1.66 | 31.19 ± 6.83 | 0.5059 |
| HMGCS | 8.53 ± 0.97 | 8.25 ± 0.86 | 0.6891 |
mRNA was extracted from terminal tissues. Gene expression was normalized to cyclophilin or 18s rRNA and expressed as average units (AU) ± SEM
∗P < 0.05 as measured by unpaired t-test.
To examine the effects of phytosterol accumulation on hepatic lipid and bile acid metabolism, a variety of genes were selected and mRNA levels were measured by real-time PCR. As previously reported by Yu et al,15 ABCG5/G8 KO mice had a significant decrease in hepatic hydroxymethylglutaryl CoA synthase (HMGCS) mRNA when compared to WT mice. Other significant changes to hepatic mRNA levels included an increase in stearoyl-CoA desaturase-1 (SCD1) and a decrease in ATP-binding cassette transporter B11 (ABCB11). Expression levels of scavenger receptor class B member I (SR-BI), ATP-binding cassette transporter A1 (ABCA1), phospholipid transfer protein (PLTP), sterol regulatory element binding protein 1C (SREBP1C), and cholesterol 7α-hydroxylase (CYP7A1) were not significantly different between WT and ABCG5/G8 KO mice. Preliminary data were obtained suggesting that in these mice, phytosterols are not converted into C21 bile acids as had been suggested to occur in rats22 (H.M. Alger and I. Bjorkhem, unpublished results). More work, however, needs to be done to determine the specific roles phytosterols may play in hepatic lipid and bile acid metabolism.
ABCG5/G8 KO Mice Accumulate Plant Sterols in a Variety of Tissues
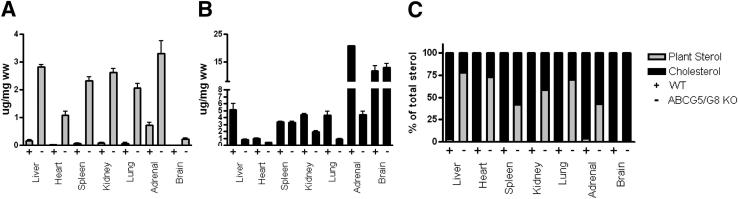
Because ABCG5/G8 KO mice accumulate plant sterols in liver and plasma on a chow-based diet with only natural phytosterol levels,15 we measured the total sterol concentrations across several tissues. Liver, heart, spleen, kidney, lung, and adrenal tissues from ABCG5/G8 KO mice all have greatly increased levels of phytosterols, relative to WT mice, but minimal changes in the amount of total sterol present—with the exception of adrenal tissue (Figure 3, A and B). As a result, phytosterols account for a large percentage of the total sterol amounts in the tissues of ABCG5/G8 KO mice. In fact, tissue total sterol composition reached as high as 77% phytosterol in the liver (Figure 3C). Other plant sterol–containing tissues had a range of total sterol composition from 41% to 72% phytosterol (Figure 3C). As previously reported in chow-fed ABCG5/G8 KO mice,19 plant sterols did not accumulate in the brain to the same extent as in other tissues (Figure 3, A and C).
Figure 3.
Tissue sterol concentrations. Sterols from terminal tissues were extracted and analyzed by gas chromatography. Plant sterol values are shown in grey and cholesterol values are shown in black. A: Plant sterol mass expressed as μg/mg of tissue wet weight. B: Cholesterol mass expressed as μg/mg of tissue wet weight. C: Tissue plant sterol and cholesterol values expressed as percentages of the total sterol concentration.
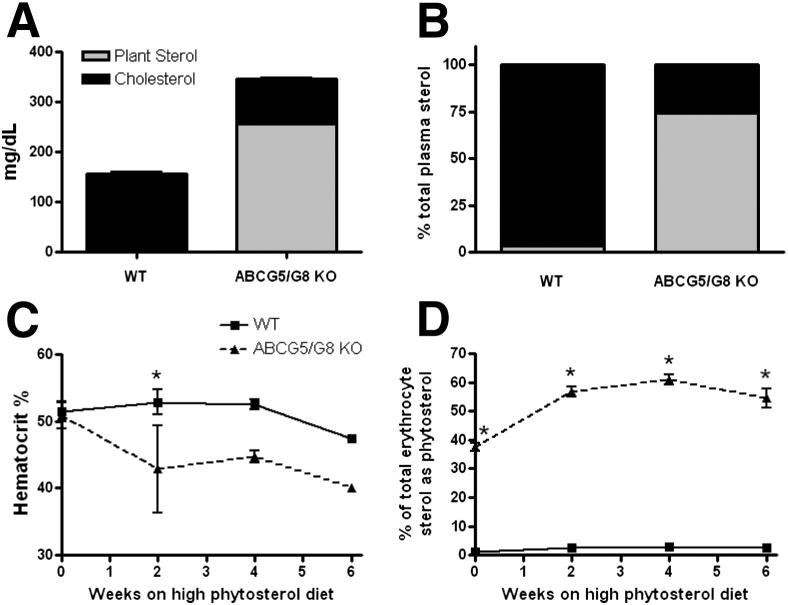
Total plasma sterol concentration was over twofold higher in ABCG5/G8 KO mice compared to WT mice (Figure 4A). This increase was due to a plasma phytosterol concentration of 250 mg/dL (Figure 4A), which resulted in almost 75% of total plasma sterol to be plant sterol in ABCG5/G8 KO mice (Figure 4B). As previously reported in chow-fed ABCG5/G8 KO mice, plasma cholesterol concentrations decreased in the phytosterol-fed ABCG5/G8 KO mice as compared to WT mice15 (Figure 4A).
Figure 4.
Plasma characterization. Terminal plasma samples were collected for analysis. A: Sterols were extracted and expressed as mg/dL of plasma. Plant sterol values are shown in grey, and cholesterol values are shown in black. B: Plasma plant sterol and cholesterol values expressed as percentages of the total sterol concentration. C: Biweekly hematocrit measurements are expressed as % of blood that is erythrocytes. D: Biweekly erythrocyte plant sterol content is shown as a percentage of the total sterol concentration. *P < 0.05 by analysis of variance and Tukey-Kramer posttest.
Hematocrit and erythrocyte sterol composition were also measured because macrothrombocytopenia has been observed in both human patients with sitosterolemia and in ABCG5/G8 KO mice.7,20 After receiving the phytosterol-enriched diet for only 2 weeks, ABCG5/G8 KO mice had lower hematocrits compared to that of WT mice (Figure 4C), which persisted at weeks 4 and 6. Additionally, the erythrocyte sterol composition of ABCG5/G8 KO mice, which began at 38% phytosterol at baseline, increased to almost 60% phytosterol over the 6-week dietary time-course (Figure 4D).
Erythrocyte Clearance Gene Expression Is Elevated in ABCG5/G8 KO Mice
The liver and spleen both play an important role in erythrocyte removal from the plasma. Because the liver and spleen were significantly enlarged and the erythrocytes of ABCG5/G8 KO mice contained a large amount of plant sterol that could affect their function, real-time PCR was used to measure the expression of several hepatic and splenic genes involved in erythrocyte clearance. These genes were selected based on previous studies by Meurs et al23 describing the erythrocyte clearance pathway and the genes involved in the controlled breakdown of erythrocyte bilirubin. These genes included biliverdin reductase A (BLVRA), hemeoxygenase 1 (HO-1), diphosphoglucuronate glucuronosyltransferase 1A (UGT1A), and HMG-CoA synthase (HMGCS). Relative hepatic and splenic gene expression was normalized to cyclophilin and compared between WT and ABCG5/G8 KO mice on the high-phytosterol diet. Consistent with the findings of hepatosplenomegaly and reduced hematocrit, expression of hepatic HO-1, UGT1-a, and HMGCS along with splenic BLVRA was significantly altered in mice lacking ABCG5/G8 (Table 2).
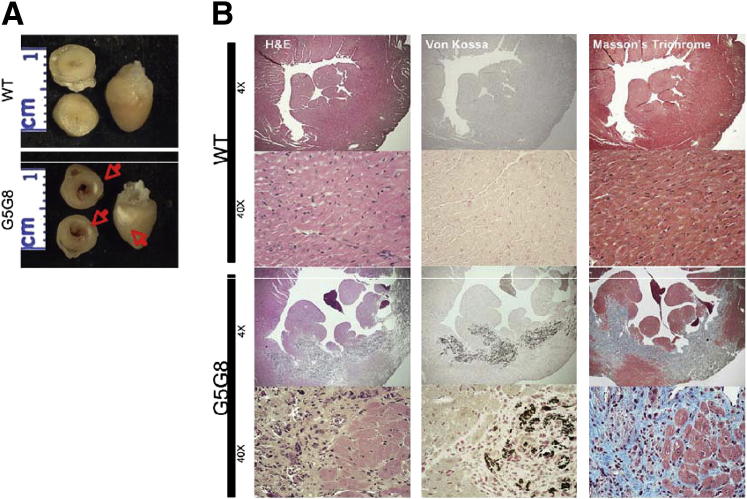
ABCG5/G8 KO Mice Develop Complex Cardiac Lesions
During necropsy, an unusual cardiac phenotype was detected in the ABCG5/G8 KO mice. Approximately half of the phytosterol-fed ABCG5/G8 KO mice had irregularly shaped and sized areas of pallor in the left ventricle (Figure 5A). To characterize these cardiac lesions, hearts fixed in neutral buffered formalin were sectioned and stained with H&E, von Kossa, and Masson’s trichrome. H&E staining confirmed that the WT hearts did not have lesions (Figure 5B). H&E-stained hearts from ABCG5/G8 KO mice, however, had extensive myocardiocyte loss with replacement by fibrosis and calcification (Figure 5B). von Kossa and Masson’s trichrome stains confirmed the presence of calcium and collagen in these cardiac lesions (Figure 5B). Morphometric quantification of the fibrosis revealed a >20-fold increase in the amount of myocardial fibrosis detected in ABCG5/G8 KO mice compared to WT mice. When cross-sectional fibrosis was measured, approximately 20% of the area was fibrotic in ABCG5/G8 KO mouse heart, but less than 1% of the area was fibrotic in WT mouse heart.
Figure 5.
Cardiac histology. A: Gross images of hearts from WT (top panel) and ABCG5/G8 KO mice (bottom panel, labeled G5G8 in the figure). The ABCG5/G8 KO mice had areas of pallor on the left ventricular free wall (red arrows). Lesions were absent in the WT hearts. B: Photomicrographs of WT and ABCG5/G8 KO mouse hearts. WT hearts were considered within normal limits. Hearts from ABCG5/G8 KO mice had extensive left ventricular myocardial loss (H&E) with replacement by fibrous connective tissue (Masson’s trichrome) and extensive mineralization (von Kossa). Lesions were absent in the WT mice.
Discussion
This study shows for the first time the specific toxicities that occur when phytosterols accumulate at high levels in the body. ABCG5/G8 KO mice, which hyperabsorb plant sterols, fail to gain weight, become lethargic and hunched, and die prematurely when fed a high-phytosterol diet. This phenotype is specific to plant sterol accumulation, as we did not see signs of poor health in ABCG5/G8 KO mice fed a high (0.2% w/w) cholesterol diet. Toxic side effects have not been reported in other studies where ABCG5/G8 double-KO mice were fed chow-based diets containing relatively lower levels of phytosterols.15,19
In agreement with other studies,15,19 campesterol and sitosterol were the main plant sterol species that accumulated in ABCG5/G8 KO mice that were fed the plant sterol-rich diet. Plant sterols accumulated to very high levels in the tissues of phytosterol-fed ABCG5/G8 KO mice. Phytosterols constituted 77% of all hepatic sterols in our mice and ranged from 41% to 72% of sterols in spleen, heart, kidney, lung, and adrenal glands. These values are much higher than the reported values in ABCG5/G8 KO mice fed a chow diet.15 On a chow diet, plasma phytosterol concentrations in ABCG5/G8 KO mice averaged 30 to 50 mg/dL.15,19 On the high-phytosterol diet we used, however, plasma phytosterol levels reached five times that amount.
The extreme levels of phytosterol accumulation caused damage in several tissues. ABCG5/G8 KO mice had hepatosplenomegaly and liver damage. Most strikingly, ABCG5/G8 KO mice developed extensive myocardial calcification and fibrosis with loss of myocardiocytes. By contrast, cardiomyopathy with multifocal myocardial fibrosis was reported in the trac mutant mouse, but cardiac lesions were not described.16
The trac mutant mice have a naturally occurring spontaneous mutation only in ABCG5 that causes them to accumulate plant sterols. Interestingly, this particular mutation seems to be associated with a less severe phenotype than seen here in phytosterol-fed ABCG5/G8 KO mice. The trac mutant mice, however, were not fed the high-phytosterol diet. Trac mutant mice become hunched and have a shortened life span of 4-6 months on a regular chow diet.16 By comparison, ABCG5/G8 KO mice died as early as 3 months of age when fed the high-phytosterol diet (5 weeks of diet feeding). Other gross indicators of poor health found in our ABCG5/G8 KO mice, such as lethargy and failure to gain weight, were not reported in trac mutant mice.
The differences between these mouse models could be attributed to the amount of phytosterols that accumulate in the body. Trac mutant mice have plasma phytosterol concentrations of approximately 130 mg/dL, which fall in between 30 and 50 mg/dL chow-fed ABCG5/G8 KO mice and >250 mg/dL phytosterol-fed ABCG5/G8 KO mice. This would support the idea that the level of toxicity of phytosterols depends on the total amount of accumulation in the body.
Studies in both humans and mouse models have revealed macrothrombocytopenia as a marker of sitosterolemia.7,20 The plasma phenotype of ABCG5/G8 KO mice fed a high-phytosterol diet was consistent with those findings. Erythrocyte phytosterol content increased by 20%, and significant decreases in hematocrit were seen as early as 2 weeks of phytosterol-enriched diet feeding. Hepatic HO-1, UGT-1A, HMGCS, and splenic BLVRA expression levels were significantly altered in ABCG5/G8 KO mice. These data indicate that the erythrocyte clearance pathway is up-regulated, which could be an attempt to clear altered red blood cells.
Other studies have not reported large accumulations of phytosterols in the brain of ABCG5/G8 KO mice.19 Alternatively, Jansen et al24 measured a small, but significant, amount of phytosterols, 600-1000 ng/mg dry weight, in the brain of chow-fed ABCG5 or ABCG8 KO mice. In our study, we also found small, but significant, amounts of plant sterols that averaged 230 ng/mg in the brain of ABCG5/G8 KO mice fed a high-phytosterol diet. These levels of phytosterols in the brain were miniscule (<2%) compared to the amounts that accumulated in other tissues.
The findings of this study are useful in relation to dietary phytosterol supplementation, which is currently used to reduce the risk of coronary heart disease (CHD).25,26 Plant sterols are thought to reduce plasma cholesterol concentrations by inhibiting cholesterol absorption.27,28 Most individuals are able to exclude plant sterols from the body via ABCG5/G8, and the Food and Drug Administration allows phytosterol-enriched foods to be classified as “generally recognized as safe.” In several species of animals, presumably with ABCG5/G8 intact, that have been administered high doses of plant sterols, there have been no reports of toxicity.2 Some debate still exists, however, about the safety of dietary phytosterol supplementation, specifically concerning their potential cytotoxic effects on endothelial cells in arteries.
Our study shows that an extremely high concentration of phytosterols in plasma and tissues is widespread and toxic. This phenotype seems to depend specifically on the amount of phytosterols that accrue. The presence of intact ABCG5/G8 in an individual appears to offer full protection against dietary phytosterol accumulation, and there is no indication that long-term exposure could cause levels of phytosterols to build up to toxic levels. Consequently, dietary supplementation with phytosterols appears safe for the general population.
Our findings also lend novel insight into the need of the body to discriminate between plant sterols and cholesterol. We have shown for the first time that high levels of tissue plant sterol accumulation cause premature death, extensive cardiac lesions, liver damage, and hepatosplenomegaly. These toxicities appear related to accumulation of high levels of unesterified phytosterols that cannot be efficiently metabolized into bile acids nor esterified for storage in lipid droplets. The free sterol probably results in accumulation in membranes with subsequent disruption of membrane integrity. In conclusion, our data show that the role of ABCG5/G8 in phytosterol exclusion from the body is to protect from the toxic effects of plant sterols.
Footnotes
Supported by NIH grant P01-HL49373 (L.L.R.)
A.L.M. and H.M.A. contributed equally to this work.
Current address of H.M.A., The Pew Charitable Trusts, Washington, District of Columbia.
Supplemental Data
Bodyweight measurement. Animals were fed a high-cholesterol diet of 0.2% w/w for 6 weeks. WT (n = 5), ABCG5/G8 KO (n = 8). Bodyweights were measured weekly.
References
- 1.Weihrauch J.L., Gardner J.M. Sterol content of foods of plant origin. J Am Diet Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- 2.Schoenheimer R. New contributions in sterol metabolism. Science. 1931;74:579–584. doi: 10.1126/science.74.1928.579. [DOI] [PubMed] [Google Scholar]
- 3.Ostlund R.E., Jr., McGill J.B., Zeng C.M., Covey D.F., Stearns J., Stenson W.F., Spilburg C.A. Gastrointestinal absorption and plasma kinetics of soy Delta(5)-phytosterols and phytostanols in humans. Am J Physiol Endocrinol Metab. 2002;282:E911–E916. doi: 10.1152/ajpendo.00328.2001. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya A.K., Connor W.E. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M.H., Lu K., Patel S.B. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hidaka H., Nakamura T., Aoki T., Kojima H., Nakajima Y., Kosugi K., Hatanaka I., Harada M., Kobayashi M., Tamura A., Fujii T., Shigeta Y. Increased plasma plant sterol levels in heterozygotes with sitosterolemia and xanthomatosis. J Lipid Res. 1990;31:881–888. [PubMed] [Google Scholar]
- 7.Rees D.C., Iolascon A., Carella M., O’marcaigh A.S., Kendra J.R., Jowitt S.N., Wales J.K., Vora A., Makris M., Manning N., Nicolaou A., Fisher J., Mann A., Machin S.J., Clayton P.T., Gasparini P., Stewart G.W. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br J Haematol. 2005;130:297–309. doi: 10.1111/j.1365-2141.2005.05599.x. [DOI] [PubMed] [Google Scholar]
- 8.Belamarich P.F., Deckelbaum R.J., Starc T.J., Dobrin B.E., Tint G.S., Salen G. Response to diet and cholestyramine in a patient with sitosterolemia. Pediatrics. 1990;86:977–981. [PubMed] [Google Scholar]
- 9.Berge K.E., Tian H., Graf G.A., Yu L., Grishin N.V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.H., Lu K., Hazard S., Yu H., Shulenin S., Hidaka H., Kojima H., Allikmets R., Sakuma N., Pegoraro R., Srivastava A.K., Salen G., Dean M., Patel S.B. Identification of a gene. ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu K., Lee M.H., Hazard S., Brooks-Wilson A., Hidaka H., Kojima H., Ose L., Stalenhoef A.F., Mietinnen T., Bjorkhem I., Bruckert E., Pandya A., Brewer H.B., Jr., Salen G., Dean M., Srivastava A., Patel S.B. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69:278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graf G.A., Li W.P., Gerard R.D., Gelissen I., White A., Cohen J.C., Hobbs H.H. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graf G.A., Yu L., Li W.P., Gerard R., Tuma P.L., Cohen J.C., Hobbs H.H. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 14.Repa J.J., Berge K.E., Pomajzl C., Richardson J.A., Hobbs H., Mangelsdorf D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 15.Yu L., Hammer R.E., Li-Hawkins J., von Bergmann K., Lutjohann D., Cohen J.C., Hobbs H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase T.H., Lyons B.L., Bronson R.T., Foreman O., Donahue L.R., Burzenski L.M., Gott B., Lane P., Harris B., Ceglarek U., Thiery J., Wittenburg H., Thon J.N., Italiano J.E., Jr., Johnson K.R., Shultz L.D. The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: a spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood. 2010;115:1267–1276. doi: 10.1182/blood-2009-05-219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plosch T., Bloks V.W., Terasawa Y., Berdy S., Siegler K., Van Der Sluijs F., Kema I.P., Groen A.K., Shan B., Kuipers F., Schwarz M. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 18.Klett E.L., Lu K., Kosters A., Vink E., Lee M.H., Altenburg M., Shefer S., Batta A.K., Yu H., Chen J., Klein R., Looije N., Oude-Elferink R., Groen A.K., Maeda N., Salen G., Patel S.B. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L., von Bergmann K., Lutjohann D., Hobbs H.H., Cohen J.C. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res. 2004;45:301–307. doi: 10.1194/jlr.M300377-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Kruit J.K., Drayer A.L., Bloks V.W., Blom N., Olthof S.G., Sauer P.J., de Haan G., Kema I.P., Vellenga E., Kuipers F. Plant sterols cause macrothrombocytopenia in a mouse model of sitosterolemia. J Biol Chem. 2008;283:6281–6287. doi: 10.1074/jbc.M706689200. [DOI] [PubMed] [Google Scholar]
- 21.Temel R.E., Gebre A.K., Parks J.S., Rudel L.L. Compared with Acyl-CoA:cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase. ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J Biol Chem. 2003;278:47594–47601. doi: 10.1074/jbc.M308235200. [DOI] [PubMed] [Google Scholar]
- 22.Boberg K.M., Lund E., Olund J., Bjorkhem I. Formation of C21 bile acids from plant sterols in the rat. J Biol Chem. 1990;265:7967–7975. [PubMed] [Google Scholar]
- 23.Meurs I., Hoekstra M., van Wanrooij E.J., Hildebrand R.B., Kuiper J., Kuipers F., Hardeman M.R., van Berkel T.J., Van Eck M. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp Hematol. 2005;33:1309–1319. doi: 10.1016/j.exphem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Jansen P.J., Lutjohann D., Abildayeva K., Vanmierlo T., Plosch T., Plat J., von Bergmann K., Groen A.K., Ramaekers F.C., Kuipers F., Mulder M. Dietary plant sterols accumulate in the brain. Biochim Biophys Acta. 2006;1761:445–453. doi: 10.1016/j.bbalip.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Wu T., Fu J., Yang Y., Zhang L., Han J. The effects of phytosterols/stanols on blood lipid profiles: a systematic review with meta-analysis. Asia Pac J Clin Nutr. 2009;18:179–186. [PubMed] [Google Scholar]
- 26.AbuMweis S.S., Barake R., Jones P.J. Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr Res. 2008 doi: 10.3402/fnr.v52i0.1811. http://dx.doi.org/10.3402/fnr.v52i0.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozner S., Garti N. The activity and absorption relationship of cholesterol and phytosterols. Colloid Surf A Physicochem Eng Asp. 2006;282:435–456. [Google Scholar]
- 28.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bodyweight measurement. Animals were fed a high-cholesterol diet of 0.2% w/w for 6 weeks. WT (n = 5), ABCG5/G8 KO (n = 8). Bodyweights were measured weekly.