Abstract
Osteoarthritis (OA) is the most common joint disease and involves progressive degeneration of articular cartilage. The aim of this study was to investigate if chondrocytes from human articular cartilage express gap junction proteins called connexins (Cxs). We show that human chondrocytes in tissue express Cx43, Cx45, Cx32, and Cx46. We also find that primary chondrocytes from adults retain the capacity to form functional voltage-dependent gap junctions. Immunohistochemistry experiments in cartilage from OA patients revealed significantly elevated levels of Cx43 and Cx45 in the superficial zone and down through the next approximately 1000 μm of tissue. These zones corresponded with regions damaged in OA that also had high levels of proliferative cell nuclear antigen. An increased number of Cxs may help explain the increased proliferation of cells in clusters that finally lead to tissue homeostasis loss. Conversely, high levels of Cxs in OA cartilage reflect the increased number of adjacent cells in clusters that are able to interact directly by gap junctions as compared with hemichannels on single cells in normal cartilage. Our data provide strong evidence that OA patients have a loss of the usual ordered distribution of Cxs in the damaged zones and that the reductions in Cx43 levels are accompanied by the loss of correct Cx localization in the nondamaged areas.
The surfaces of articulating bones are covered by articular cartilage, which is required for the smooth and painless movement of the skeleton. Osteoarthritis (OA) is a condition that is characterized by the progressive degradation of matrix components that leads to a loss of joint mobility and function accompanied by chronic pain. OA is the most common joint disorder in Western populations, and its incidence increases with age. The molecular mechanisms regulating the pathogenesis and progression of OA, however, are poorly understood, and no proven disease-modifying therapy is currently available.
In adult cartilage, the chondrocytes remain resting in a nonproliferating state, but display moderate metabolic activity and the ability to maintain the surrounding matrix. Chondrocytes are isolated inside their lacunae and the communication between chondrocytes in the superficial zone and chondrocytes in the middle and deeper layers occurs through diffusion, although it has been reported that cultured animal primary chondrocytes and the chondrocytes located in the superficial zone of adult cartilage both express Cx43,1–6 a protein that forms gap junction (GJ) channels and is implicated in cell-to-cell communication.
Maintenance of the homeostatic tissue balance is controlled by communication between the extracellular and intercellular networks. GJs are membrane channels that often assemble as large membrane rafts and are identified as plaques at the membrane surface. Vertebrate GJ channels are composed of proteins encoded by the connexin (Cx) gene family and consist of intercellular channels that directly connect the cytoplasm of adjacent cells.7 In addition to their role in providing a pathway for direct intercellular communication, hemichannels are also involved in the release and exchange of small molecules between cells and the surrounding matrix.8 Cells can interchange small molecules, such as metabolites (eg, ATP), nutrients (eg, glucose), second messengers (eg, IP3, Ca2+, or AMPc,), and even synthetic oligonucleotides with a molecular weight of approximately 2 to 4 kDa. These small molecules can ultimately regulate transcription in the cell. Historically speaking, GJs were first described as those responsible for the electrical synapses in the central nervous system, however, they were subsequently found in most tissues. Actually, GJ intercellular communication is required for normal cellular functioning and for tissue development and differentiation.9 There are 21 expressed Cxs in humans, and each connexin may play unique roles that are not interchangeable.10 For example, in the liver, GJs serve metabolic functions and in the heart they conduct electrical signals. Cxs have been implicated in multiple cellular functions and the impairment or loss of Cx expression has been implicated in the pathogenesis of several diseases.9
OA is characterized by multiple molecular alterations, and most changes that occur in OA cartilage are directly related to functions controlled by Cx channels. Correct cell-matrix and cell-cell communication is essential to maintain the structural integrity and function of any tissue. In addition, GJ proteins interact with many other types of proteins in the cell, including catenins and claudins, and activate multiple molecular pathways. Actually, changes in Cx protein levels, assembly state, or localization are characteristic of a variety of Cx-dysregulated diseases.11–16 The aim of this work was to investigate if adult human chondrocytes express Cx proteins and contain functional gap junctions. The results presented here demonstrate that normal human adult articular chondrocytes express Cx43, Cx45, Cx46, and Cx32 in all zones of adult cartilage. Primary chondrocytes retain the capacity to form functional Cx43 gap junctions. Remarkably, we have found that cartilage from OA patients has higher levels of Cx43 and Cx45 protein in the damaged proliferative zones and that Cx43 localization is lost in the mid and deep nondamaged zones.
Materials and Methods
Cartilage Collection and Processing
Human knee and femoral head articular cartilage from adult donors was obtained after joint surgery. All patients signed the informed consent form and the Institutional Ethics Committee (Galicia, Spain) approved the study. Cartilage from healthy persons who suffered a knee or hip fracture was obtained following the same procedure. Histological samples (from healthy and OA subjects with radiological diagnoses) were graded using a modified Mankin score17 (mean age, 70.6 years; range, 60 to 89 years; Mankin score, 0 to 13 points). A higher Mankin score corresponds to a lower structural integrity of the tissue. The samples (patients and age-matched healthy subjects) were divided into 4 groups: i) 0 to 1 indicates normal/healthy, ii) 2 to 3 indicates grade 1 early OA, iii) 4 to 5 indicates grade 2 mild OA, and iv) 6 to 13 indicated grades 3 (6 to 7) and 4 (8 to 13) severe OA. Samples for RNA isolation were immediately frozen in liquid nitrogen and stored at −80°C. Primary chondrocytes were isolated as previously described.18
Primary Culture of Chondrocytes
Cells were seeded onto 100 or 162 cm2 flasks for gene expression assays. Chondrocytes were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies Ltd, Madrid, Spain) supplemented with 100 μg/mL Primocin (Nucliber, Madrid, Spain) and 15% fetal calf serum (Life Technologies Ltd). Cells were grown to approximately 90% confluence.
RNA Isolation and Real-Time PCR Assays
Cultured primary chondrocytes were recovered by trypsinization and stored at −80°C. Frozen articular cartilage samples were digested in digestion buffer (200 mmol/L Tris-HCl, 200 mmol/L NaCl, 5% SDS, and 100 mmol/L sodium citrate) containing proteinase K. TRIzol Reagent (Invitrogen, Paisley, UK) was added to the sample according to the manufacturer’s instructions. Whole RNA was treated with DNase (RNase-free DNase; Invitrogen, Madrid, Spain) according to the manufacturer’s instructions to ensure total degradation of the DNA in the sample.
IHC Assays
In situ cartilage was frozen immediately in a Cryomold Standard using Tissue-Tek OCT Compound (Sakura, Japan) and isopentanol (BDH, Poole, UK) in liquid nitrogen and stored at −80°C. Cartilage sections were serially sectioned (4 μm) at −20°C in a Cryostat (Leica CM1510). Tissue sections were fixed with acetone (BDH) for 10 minutes at 4°C, dried at room temperature, and washed for 10 minutes with PBS with 0.1% Tween 20, pH7.6 (PBST). Before staining, endogenous peroxidase was inhibited by H2O2 and methanol for 10 minutes and was washed one time with PBST. Primary antibody was applied 1 hour at room temperature. After three washes of 10 minutes with PBST, sections were incubated with a peroxidase-labeled polymer conjugated to goat anti-mouse/rabbit Ig (Dako, Glostrup, Denmark) for 1 hour. After three washes with PBST, peroxidase activity was developed using a substrate chromogen solution prepared freshly and containing 3,3 diaminobenzidine tetrahydrochloride and H2O2, (Dako). Sections were then washed in distilled water and counterstained with Gill’s hematoxylin, gradually dehydrated with graded alcohols, and mounted in xylene with DePeX (SERVA, Heidelberg, Germany).
Cultured cells were seeded onto chamber slides and fixed with acetone for 10 minutes at 4°C, dried at room temperature, and washed for 10 minutes with PBST before performing the procedure previously described. Negative controls (omitting primary antibody) were performed to test the specificity of the antibody. To study cartilage morphology and verify the grade of the disease, cartilage sections were stained with H&E, Safranin O, Fast Green, Masson’s trichrome, Alcian Blue-PAS, Sirius red polarization method, and toluidine blue. The slides were imaged using an Olympus BX61 microscope and a DP71 digital camera (Olympus Biosystems, Hamburg, Germany). The antibodies were procured as followed: Cx32 antibody (ab66020) from Abcam (Cambridge, UK), anti-Cx46 (sc-365394) from Santa Cruz Biotechnology (Santa Cruz, CA), anti-Cx45 (MAB3101), and anti–collagen-II (MAB1330) from Millipore Iberica (Madrid, Spain), anti-Cx43 (610062) from BD Transduction Laboratories (Madrid, Spain), and anti-proliferating cell nuclear antigen (PCNA) (NA03) from Calbiochem (Madrid, Spain). Calibration and quantification of the images was performed with AnalySISD software version 5.0 (Olympus Biosystems, Hamburg, Germany).
E-Northerns
Expression of different genes related to cell cycle and ion transport in cartilage was analyzed by electronic Northerns (E-Northerns). E-Northerns retrieved expression data from DNA sequences stored in the dbEST (Database of Expressed Sequence Tags) division of GenBank through identification using the BLAST software (National Center for Biotechnology Information, Bethesda, MD). To search for genes expressed in normal or OA cartilage, BLAST analyses were performed using reference sequences of query genes in the GenBank dbEST database, limiting the results to matches unequivocally identified as either normal or OA cartilage. The best-characterized libraries were obtained from pools of RNA with approximately 5000 sequence clones from normal and OA cartilage.19 Putative differences in gene expression between normal and osteoarthritic cartilage were detected for several genes related to cell cycle and ion transport. The number of transcripts corresponding to p21 and p27 were significantly elevated in normal cartilage compared to OA. In regard to ion and transmembrane transport, overexpression of GRID2 (glutamate receptor) was detected in normal cartilage. GRIA2 was also detected in normal cartilage, albeit at relatively low levels. DPP10 (a known regulator of Kv4 channels) was detected in normal cartilage but not in OA. Several cell cycle and ion channel genes are expressed in normal and OA. The most representative differences found between normal and OA cartilage corresponded to CDKN1A (p21), CDKN1B (p27), DPP10, GRIA4, GRID2, GABARAP, GABARAPL2, and GABRG2.
Western Blot
Cell lysates were prepared from confluent monolayer cells. Equal amounts of cellular protein were resolved by 10% SDS-PAGE and transferred to nitrocellulose (Bio-Rad, Madrid, Spain) membranes.18
Electrophysiological Measurements and Dye Injections
Experiments were performed on cell pairs. A dual voltage-clamp method and whole-cell and/or perforated patch recording were used to control the membrane potential of both cells and to measure currents as previously described.20,21 Dye transfer through gap junctions was investigated using cell pairs and Lucifer yellow (Molecular Probes; Life Technologies Ltd).21
Statistical Analysis
The data were analyzed using the GraphPad Prism software version 5 (La Jolla, CA). Statistical differences between sample groups were assessed using the Student’s t-test and Kruskal-Wallis test with Dunn’s Multiple Comparison test. Significant differences are represented as P < 0.05 and P < 0.01. Data are represented as the means ± SEM.
Results
Adult Human Primary Chondrocytes Express High Levels of Cx43 and Form Functional GJs
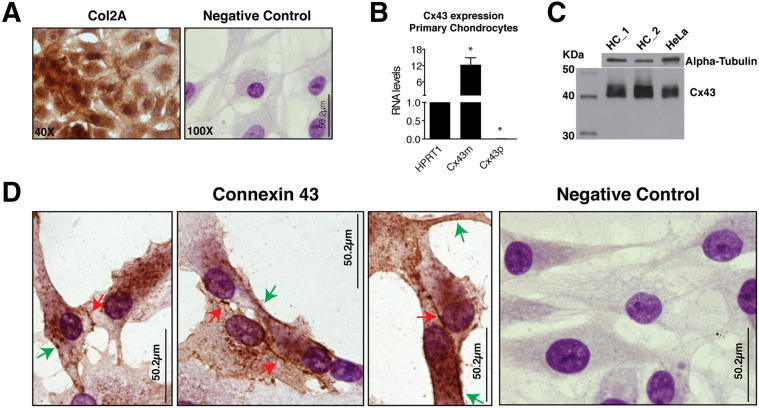
Despite the limitations of monolayer chondrocyte cultures, these cells have served as a useful model for the study of cartilage. Chondrocytes synthesize high levels of collagen type 2. With time, cultured chondrocytes become more fibroblastic and down-regulate type 2 collagen synthesis. To test the hypothesis that these cells maintained their original phenotype in culture at the time of our assays, collagen type 2 levels and overall cellular structure were assayed by immunohistochemistry (IHC) (Figure 1A). Quantitative gene expression analysis using real-time RT-PCR revealed that human primary chondrocytes express very high levels of Cx43 mRNA (Figure 1B). We analyzed both primary transcription (intron-exon junctions; primary transcripts; Cx43p) and the processed RNA (exon-exon; mRNA; Cx43m) (Figure 1B). Western blot analysis also showed high levels of Cx43 protein in comparison with a cell line expressing Cx43 (Figure 1C).
Figure 1.
Human primary chondrocytes express high levels of Cx43. A: Primary chondrocytes cultured for 3 weeks keep their original phenotype and express high levels of collagen type 2, as detected by IHC analysis using a monoclonal anti-human type II collagen antibody. Cells were seeded onto 100 cm2 flasks until confluence before being seeded onto chamber slides for 2 days and fixed with acetone. B: Expression analyses of hypoxanthine phosphoribosyltransferase 1 (HPRT1) and Cx43 genes by real-time RT-PCR in primary chondrocytes isolated from the cartilage of healthy individuals. Data were normalized to HPRT1 levels. Note that cells accumulate high levels of Cx43 mRNA. Very low levels were detected (0.005 times relative to HPRT1), however, of primary transcription. Data are represented as means ± SEM with n = 4. *P < 0.05; U-test, HPRT1 versus Cx43m, and HPRT1 versus Cx43p. C: Western blot analysis of primary chondrocytes from two healthy individuals (ie, HC-1 and HC-2). A control cell line (HeLa Cx43H10) expressing Cx43 was used. D: IHC analysis using the monoclonal anti-Cx43 antibody. As is shown in the negative control, this technique can differentiate the nucleus (dark purple) from the cytoplasm (clear purple). Cx43 spots in the edges of the cell surface (green arrows) and at the plasma membrane in cell-to-cell contact areas (red arrows).
To test if Cx43 is found in the membrane, we used IHC coupled with Gill’s hematoxylin counterstaining using a monoclonal anti-Cx43 antibody (Figure 1D). Cx43 was mainly localized in the cytoplasm, but we could detect Cx43 between the cytoplasms of 2 adjacent cells. We also detected multiple positive spots at the edge of the cytoplasm.
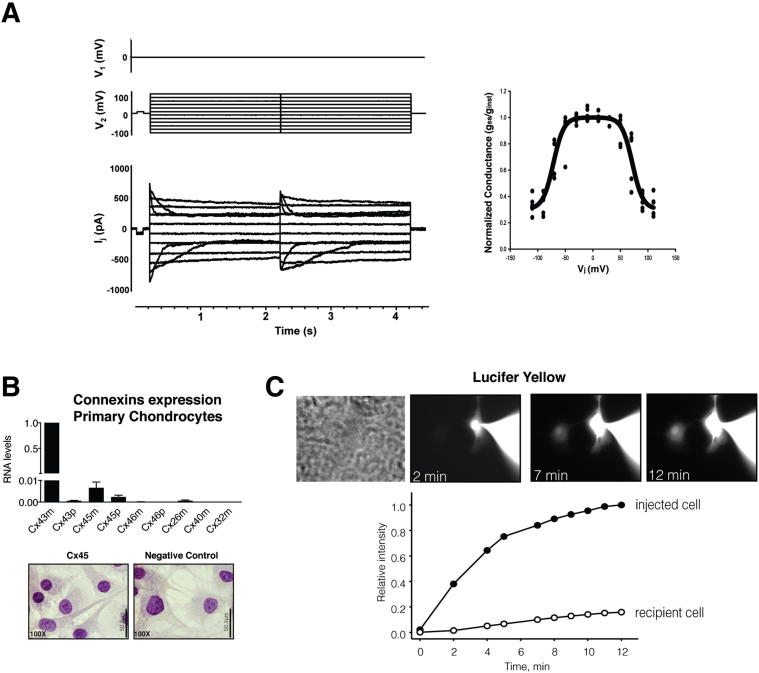
To test if human primary chondrocytes are electrically coupled to each other by voltage-dependent GJ channels, we conducted a dual voltage-clamp method and whole-cell/perforated patch recording to control the membrane potential of both cell pairs and to measure currents. The results displayed in Figure 2A show the junctional current recordings corresponding to the typical voltage-dependent behavior pattern described for GJ channels containing Cx43. When we investigated the expression of different Cxs using primary chondrocytes from healthy joints, we only detected the expression of Cx43 and Cx45 (Figure 2B). We could detect low levels of Cx45 mRNA, although we did not observe any corresponding Cx45 protein signal in our IHC experiments. Analysis of the typical gap-junction-permeable dye Lucifer yellow revealed that primary chondrocytes communicate and transfer molecules through Cx channels (Figure 2C).
Figure 2.
Human primary adult chondrocytes form functional GJs. A: Voltage-gating properties of human chondrocytes. Junctional currents (bottom panel) recorded from primary chondrocyte pairs excised from adult cartilage in response to a voltage step protocol (Vj) (upper panel). Cell 2 was stepped, whereas cell 1 was held at 0 mV. Summary plot of normalized junctional conductance (gss/ginst) versus transjunctional voltage (Vj) obtained from five cell pairs (right panel). The measures gss and ginst are the conductance measured at the end and at the beginning of the voltage pulse, respectively. The smoothed curve indicates the best fit of data to the Boltzmann equation using the following values: Vj,0 = 71mV, gj,min = 0.3, and z = 2.8. The recording and data analysis showed typical voltage-dependent behavior for GJ channels containing Cx43 (means ± SEM). B: The expression analysis of several Cxs by real-time RT-PCR in primary chondrocytes isolated from the cartilage of healthy individuals (n = 6). Data were normalized to hypoxanthine phosphoribosyltransferase 1 and then to Cx43m (means ± SEM; n = 6). Primer sequences are shown in Table 1. C: Lucifer yellow flux in human chondrocyte cell pairs. A pipette containing 2 mmol/L Lucifer yellow was attached to the cell on the right in the whole cell configuration (upper panel). Epifluorescent micrographs were taken at 2, 7, and 12 minutes after dye injection into the right-sided cell. The micrographs showed a progressive fluorescence intensity increase in the recipient cell. Fluorescence intensity plots versus time for the injected cell (black circles) and the recipient cell (white circles) (lower panel). Data are represented as means ± SEM.
Cx43 Protein Is Overexpressed in OA Cartilage
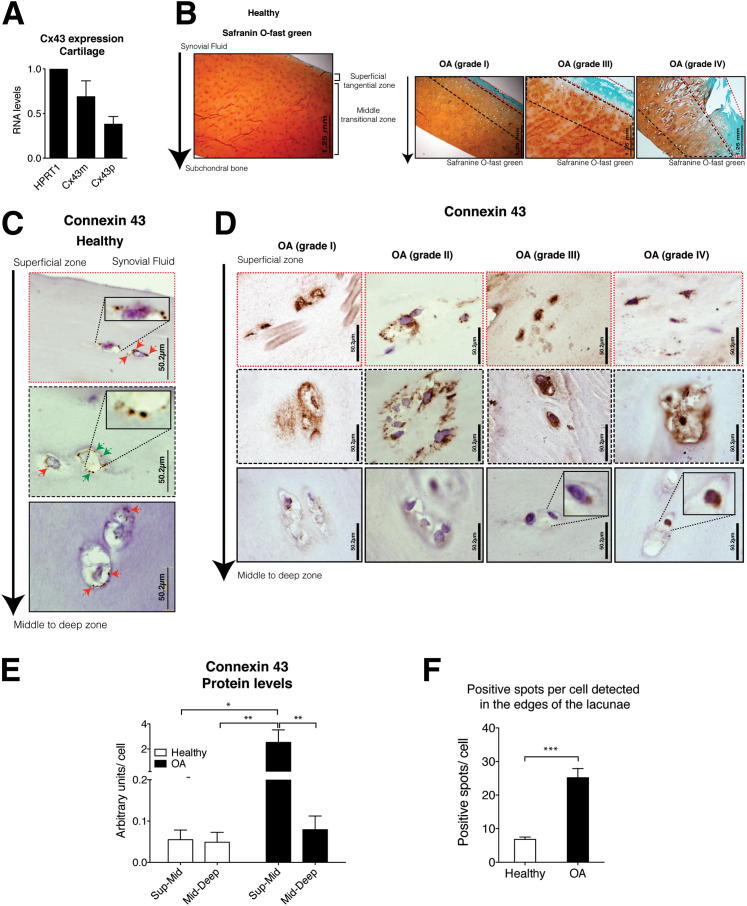
RNA was isolated from frozen sections of cartilage tissue. Quantification using real-time PCR demonstrated that chondrocytes within cartilage expresses Cx43 mRNA (both mRNA and primary transcript) (Figure 3A). The pattern of expression differs from cultured chondrocytes (Figure 1B), however, as in the case of tissue, we could detect both mRNA and primary transcript (Cx43p). To rule out alternative splicing, the primer sequences (Table 1) were compared against the GenBank Expressed Sequence Tag database. When we examined protein localization by IHC using an anti-Cx43 antibody, we observed multiple positive Cx43 spots in all layers of the healthy cartilage, including the deep zone (Figure 3, B and C). The population of immunopositive chondrocytes in the superficial and mid-layers of healthy cartilage was similar to that of the deeper layers (at 90% to 100% and 80% to 90% of the cell population, respectively). We found that Cx43 is localized at the margin of cells. We also detected positive spots that might correspond to connections between chondrocytes located in the same lacunae. In case of healthy cartilage, the number of positive spots per cell that were located in the edges of the lacunae varied between 4 and 10 (mean, 6.8).
Figure 3.
OA cartilage has high levels of Cx43 protein. A: RNA levels were determined by real-time RT-PCR and normalized to hypoxanthine phosphoribosyltransferase 1 (means ± SEM n = 4). B: Healthy cartilage showed homogeneous uptake of the Safranin O–fast green. The superficial zone (dashed red line). The red-orange zone (dashed black line) down to approximately 1000 μm. In contrast, reduction of the staining and ulceration was observed in the most severe OA cartilage sections. Mankin score analyses were conducted to verify the morphology and grade of the disease. Samples were divided into 4 groups (see Materials and Methods). Cartilage explants were cut in the operating room immediately after surgery, and therefore all did not have identical depth. C: IHC analysis using an anti-Cx43 antibody. The cartilage showed here corresponds to a healthy donor. Magnifications (×10) of positive spots are shown. D: IHC analysis using an anti-Cx43 antibody. The cartilage showed corresponds to OA patients with different grades. Negative control (top row). E: Quantitative image analysis of IHC staining revealed that OA cartilage showed significantly more intense Cx43 staining in the superficial layer and down through the top 1000 μm (Sup-mid). *P < 0.05, **P < 0.01 by Kruskal-Wallis test with Dunn’s multiple comparison test (n = 4 for healthy; n = 10 for OA; n = 4 for knee OA; and n = 6 for hip OA). F: Representation of the number of positive spots per cell (n = 17 for healthy; n = 17 for OA). ***P < 0.0001; Student’s t-test, healthy versus OA (means ± SEM).
Table 1.
Sequences of the Primers Used for Quantitative Gene Expression Analysis
| Gene | Target | Primer sequence |
|---|---|---|
| GJA1 | Cx43m | 5′-CAATCACTTGGCGTGACTTC-3′ |
| 5′-AACGAAAGGCAGACTGCTCA-3′ | ||
| Cx43p | 5′-TTGCAATCTGTGATCCTTGAA-3′ | |
| 5′-AACGAAAGGCAGACTGCTCA-3′ | ||
| GJC1 | Cx45m | 5′-ATCTGGAAAAATTGCAATCAAAA-3′ |
| 5′-CTGTAAGGACGATCCGGAAG-3′ | ||
| Cx45p | 5′-TGGGTAACCGAAGTTCTGGA-3′ | |
| 5′-TCCAGGATCCAGGTAAAAACC-3′ | ||
| GJA3 | Cx46m | 5′-GCCGGCCAGTACTTTCTGTA-3′ |
| 5′-CCTGCTTGAGCTTCTTCCAG-3′ | ||
| Cx46p | 5′-ATGCCTGTCCTGTGGAGAAG-3′ | |
| 5′-GAAGATGAACAGCACGGTCA-3′ | ||
| GJB2 | Cx26m | 5′-CTTCCTCCCGACGCAGAG-3′ |
| 5′-GCTCATCTCCCCACACCTC-3′ | ||
| GJA5 | Cx40m | 5′-AAGTCCAGGGAGGAGGAAAA-3′ |
| 5′-TCGTATCACACCGGAAATCA-3′ | ||
| GJB1 | Cx32m | 5′-TGCAGACATTCTCTGGGAAA-3′ |
| 5′-TCTCATCACCCCACACACTC-3′ | ||
| HPRT-1 | 5′-TTGAGTTTGGAAACATCTGGAG-3′ | |
| 5′-GCCCAAAGGGAACTGATAGTC-3′ |
The PCR products size is approximately 200 bp.
m, mRNA; p, primary transcript.
In the case of cartilage from OA patients, Cx43 was found in both the nucleus and the cytoplasm, with positive spots around the edges of the lacunae (Figure 3D). Analysis of Cx43 using IHC followed by quantification of positive signals using the AnalySISD software version 5.0 (Olympus Biosystems) (Figure 3E) revealed that cartilage from OA donors showed very high levels of Cx43 positive staining (approximately 40 times more than healthy) in the 80% to 90% of cells in the superficial layer as well as in 100% of the cells in the mid-region down through 1000 μm.
The protein localization was the same in both the superficial and damaged zones, however, in the intermediate and deeper zones (where the tissue was less damaged), we detected positive spots in the edges of the lacuna in only 20% to 30% of the cell population. In the deeper zones, when the cells did show positive signals, the Cx43 was mainly localized in the cytoplasm instead of the membrane (Figure 3D).
Cartilage from OA donors showed a different grade of injury (Figure 3, B and D). Cartilage damage severity was rated (see Materials and Methods). The area around the articular surface and down to 100 to 1000 μm is predisposed to be damaged in early and later grades of the disease (Figure 3B). These zones either showed severe damage or, in the case of patients with grade 1 disease, bigger lacunae. High levels of positive staining were already detected in the first stage of OA disease (grade 1) (Figure 3D). Overall, in case of OA cartilage, the number of positive spots per cell that were located in the edges of the lacunae varied between 8 and 36 (mean, 25.1) (Figure 3F).
Increased Levels of Cx43 Correlate with Nuclear PCNA
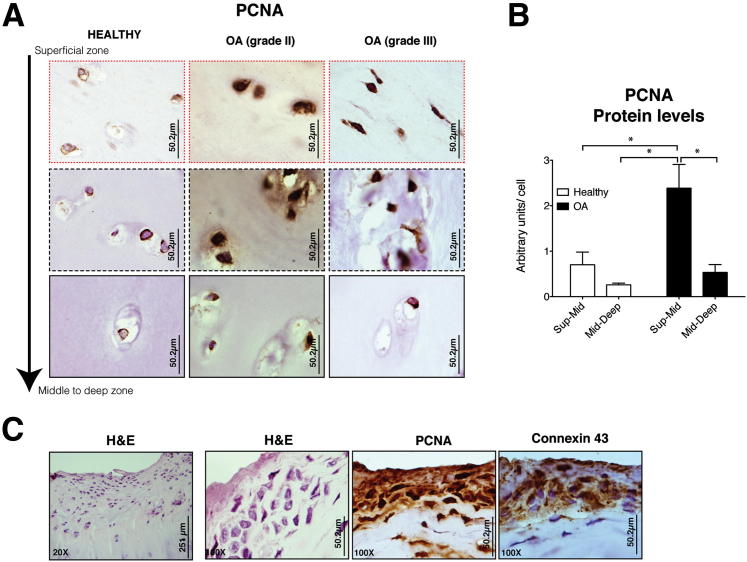
It is important to note that we observed low levels of cytoplasmic PCNA in cartilage from healthy donors (Figure 4A). In contrast, cartilage from OA patients showed high levels of nuclear PCNA in the damaged areas, covering the superficial layer and down through ∼1000 μm (Figure 4A). Compared to tissue from healthy donors, the levels of Cx43 and PCNA in OA tissue were 30-fold and 4-fold higher, respectively (Figure 3E and 4B). In contrast, the less damaged intermediate-deep zone cartilage showed a PCNA staining pattern similar to healthy cartilage (ie, lower levels and a more cytoplasmic PCNA distribution) (Figure 4A). Very high levels of Cx43 were detected in proliferating cells that are exclusively found in the superficial layer of OA cartilage22 (Figure 4C).22
Figure 4.
High levels of Cx43 protein and PCNA are observed in OA cartilage since early stage to late grades of the disease. A: Representative patterns of staining with PCNA. Cartilage sections from healthy joints (n = 4) and cartilage from OA patients (n = 4 for knee OA; n = 6 for hip OA). Negative control. B: Quantitative image analysis of IHC staining with PCNA is shown. *P < 0.05, **P < 0.01 by a Kruskal-Wallis test with a Dunn’s multiple comparison test. C: IHC coupled with Gill's haematoxylin counterstaining using anti-Cx43 and PCNA antibody. Superficial layer grouped cells found in some of the cartilage sections from OA patients were highly positive for PCNA and Cx43 (H&E; original magnification, ×20 and ×100, respectively).
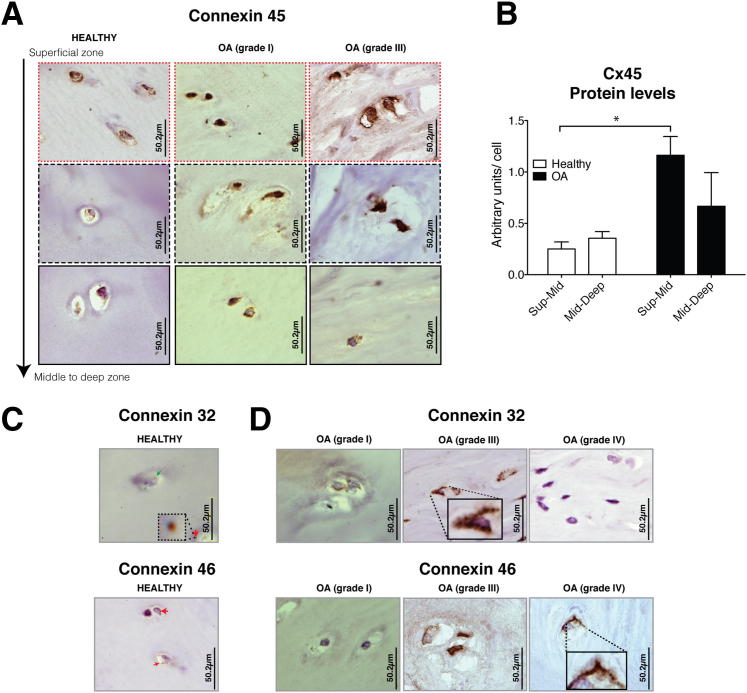
Cx45, Cx32, and Cx46 Show Altered Levels in Cartilage from OA Patients
In cultured chondrocytes, we detected Cx45 mRNA, but no corresponding protein (Figure 2B). IHC of cartilage from healthy joints, however, showed positive signals for Cx45, Cx32, and Cx46 in all zones (Figure 5). Cx45 protein was detected in all healthy cartilage zones, especially in the superficial layer through the top 1000 μm (Figure 5A). Cx45 formed petite positive spots around the margin of the cells, but it was diffuse in the cytoplasm (Figure 5A). In contrast, Cx45 in OA cartilage showed significantly more positive staining in the superficial and damaged areas (Figure 5, A and B), similar to what was previously observed for Cx43 and PCNA (Figure 3E and 4B). In case of OA joints, Cx45 localized in positive spots around the margin of the cells and in the nucleus but was diffuse in the cytoplasm (Figure 5A).
Figure 5.
Human cartilage expresses Cx32, Cx46, and Cx45, all of which have altered levels in cartilage from OA joints. A: IHC coupled with Gill’s hematoxylin counterstaining using anti-Cx45 antibody. We have observed positive signals for Cx45 in healthy cartilage and OA. B: Quantitative image analysis (n = 3 for healthy; n = 10 for OA) *P < 0.05. Kruskal-Wallis test with a Dunn’s multiple comparison test. C: IHC using anti-Cx32 and anti-Cx46 antibodies (normal cartilage, n = 3). D: Cx32 and Cx46 were detected in cartilage from patients (n = 4 for knee OA; n = 6 for hip OA).
In the case of Cx32 and Cx46, these proteins localized around the edges of the cell and were possibly incorporated into the membrane (Figure 5C). In cartilage from patients, Cx32 was mainly found in the damaged zones and in the intermediate cartilage (Figure 5D). Cx46, however, was mainly detected in the superficial zone and in the 1000 μm of tissue immediately beneath. Nevertheless, some OA patients showed higher levels of Cx32 and/or Cx46, whereas others were negative for both Cxs (Figure 5D). Unlike Cx43 and Cx45, we could not find distinctive patterns for either Cx32 or Cx46.
Discussion
This study demonstrates for the first time that normal human adult articular chondrocytes express several GJ proteins that are altered in OA. We have also demonstrated that human articular chondrocytes from adults retain the capacity to form functional GJs with voltage-dependent gating. The characteristic junctional current pattern obtained using electrophysiological techniques demonstrate that adult chondrocytes form functional GJs composed by Cx43. Studies conducted in tissue demonstrate that Cxs are well localized around the cell and form structures that look like pearl necklaces around the edge of the lacunae. Within tissue, chondrocytes are immersed in lacunae and because there is usually one cell per lacuna, most of these positive spots could therefore correspond to hemichannels. The potential presence of tubular canals (termed canaculi tracks) in the tissue,23 however, together with unpublished results from our group suggests that it is also possible that GJs are being formed. Direct cell–cell communication between paired chondrocytes have already been suggested.24
Single hemichannels allow cells to interchange components with the matrix, and Cx43 has been reported to form functional hemichannels in a wide variety of mammalian cell lines.25 Cx43 hemichannels were involved in the release of ATP, glutamate, or NAD+ in the extracellular space,26,27 and they have actually been implicated in the response of rabbit and bovine articular chondrocytes to mechanical loading.1,2,28 Hemichannels and GJs formed by Cx43 are very permissive and are therefore less selective than channels formed by Cx45 or Cx32.29 Conversely, while chondrocytes found in healthy cartilage also contain Cx45, Cx32, and Cx46 in the cytoplasm, these proteins also form multiple positive spots localized around the edges of the lacunae. When cells express multiple Cxs, the Cxs can co-oligomerize into homomeric or heteromeric Cxs, although only certain combinations of the 21-member family are permitted.30 Most cells express more than one Cx, and heteromeric connexons are common in vivo. This heterogeneity produces variations in the permeability and conductance of GJs that may allow cells to dynamically regulate their intercellular communication properties and molecular selectivity.31 The functional data strongly demonstrated a Cx43-dominated coupling (Figure 2), although it is possible that other functional forms are present in the tissue and provide additional methods for regulating cell–cell and cell-matrix communication.
Examining cartilage from OA donors, we found that OA patients have alterations in all studied proteins. The alterations of Cx43 and Cx45 protein levels, however, appeared to occur post-transcriptionally, as Cx transcript levels were unaffected in OA. Increases of the Cx43 protein without corresponding increases in the transcript levels has been previously observed in synovial cells treated with IL-1.32 In the case of human articular chondrocytes, we found that the Cx43 and Cx45 proteins were already overrepresented in cartilage from patients at the very early disease stage, especially in the superficial zone and down through the top approximately 1000 μm of tissue. Both Cxs were found in the cytoplasm and formed small spots around the cells on the edges of the lacunae. In the mid-deep zones, the cells were positive for Cx43, but the localization was mainly cytoplasmic around the nucleus. These results suggest that mid-deep OA chondrocytes found in nondamaged zones express these proteins but may lack hemichannels and/or GJs composed of Cx43. Deeper cells probably communicate through Cx45 channels, as shown by our detection of Cx45 in both the cytoplasm and cell membrane. The functional deletion of a Cx isoform produces a distinct pathology and the genetic replacement of one Cx by another fails to fully compensate.10,33 Previous results have shown that pharmacological inhibition of connection channels reduces IL-1ß-induced metalloprotease production by synovial cells, implying that the expression of catabolic factors destroying the matrix, such as metalloproteases, are dependent on the presence of Cx43 channels.34 The results here demonstrate that high levels of Cxs coincide with cartilage damage in OA.
Our results suggest a close relationship between Cx43 and proliferation. Cx expression is differently regulated between healthy proliferative primary chondrocytes and resting healthy chondrocytes in cartilage (cultured chondrocytes showed approximately 12 times more Cx43 expression than chondrocytes in tissue) (Figures 1B and 3A). Similarly, more proliferative chondrocytes and higher levels of Cxs are both found in OA cartilage. Cartilage from OA patients showed the most radical changes in Cx protein expression in the superficial zone and down through the top approximately 1000 μm of tissue, areas in which OA changes are associated with a significant catabolic activation and upregulation of chondrocytes proliferation.35 This is evidenced by increased PCNA staining in mid-region and superficial zones (Figure 4). Furthermore, although Cx43 can regulate cell cycle progression, this ability differs between cell types.36,37 Cx43 also regulates gene expression, and this includes the regulation of p21 and p27.38,39 Interestingly, the over-representation of the cyclin-dependent kinase inhibitors p21 and p27 in normal cartilage (see Materials and Methods), suggests that healthy chondrocytes in tissue might be found mostly in the G0/G1 stage, paused likely through active inhibition of the cell cycle via p21 (WAF1/CIP1/p21) and/or p27 (Kip1). The close connection between p21/p27 and Cx43 might help to elucidate the role of Cx43 in articular chondrocyte proliferation and dedifferentiation. Conversely, the C-terminal domain of Cx43 interacts with different cell-cycle proteins,40 making the study of the effect of Cx43 on proliferation more difficult. More studies will be necessary to fully understand the role of Cx43 in chondrocytes.
Voltage-dependent GJs are acutely regulated in response to various stimuli. Hemichannels are normally closed, but they open in response to decreases in extracellular calcium, strong membrane depolarization, mechanical stimulation, and metabolic inhibition. All of these conditions are altered in OA cartilage.41–46 Moreover, articular chondrocytes express several types of potassium, calcium, sodium, and N-methyl-D-aspartate channels47 that regulate the potential membrane and/or the ratio of intracellular and extracellular ion concentrations. The functions of these channels, however, have not been extensively studied in either normal or OA cartilage. For example, transcripts coding for different GABA receptor-related proteins were found in the cartilage (see Materials and Methods), and an altered response to mechanical stimulation with increased levels of glutamate has been previously reported to occur in OA.46,48–50 Hyperpolarization and depolarization processes affect the activity of voltage-dependent Cx gating channels. Some of these effects might be related to upstream mechanisms that lead to the alteration of the Cxs observed in OA. Others might be related to the distant downstream responses of early or late events involved in cell transformation.
This study demonstrates that adult chondrocytes from humans are able to communicate with each other through GJ channels specifically formed by Cx43. Normal human adult articular chondrocytes within tissue express Cx43, Cx45, Cx46, and Cx32 in all zones of adult cartilage. Our results show a pathological increase of Cx43 and PCNA in superficial and damaged cartilage from OA donors, and it is tempting to speculate that cell proliferation may be regulated via p21/p27. The important finding of this study, however, was that Cxs are altered in OA cartilage, with Cx43 and Cx45 overrepresented in the damaged zones and Cx43 delocalized from the membrane in the nondamaged zones. The overexpression and/or loss of localization of these proteins would affect the structural and functional integrity of chondrocytes and may help explain the degeneration of the matrix that is observed in OA patients.11
Acknowledgments
We thank Purificación Filgueira-Fernández and Noa Goyanes for collecting and storing cartilage samples, preparing the samples, and for IHC experiments, Lourdes Sanjurjo and Maria Dolores Salinas Bujan for generously taking cartilage samples after surgery, Maria Jose Sanchez Dopico and Tamara Hermida for isolating articular chondrocytes from cartilage for cell culture, Mar Haz Conde and Maria Vazquez for technical advice regarding RNA isolation from tissue, Emma Rodriguez and Estefania Cives for administrative help during the whole process, and Isacc Fuentes and Moises Blanco for helpful discussions.
All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version for publication. F.J.B and M.D.M. have full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data presented. Study conception and project planning by M.D.M. and F.J.B. Acquisition of data by M.D.M., P.C.-F., R.G.-F., O.M.-d.-I., H.-Z.W., V.V., P.B., and F.J.B. Analysis and interpretation of data by M.D.M., V.V., P.B., and F.J.B. Manuscript writing by M.D.M. and F.J.B.
Footnotes
Supported in part by the Fondo Investigación Sanitaria (CIBER- CB06/01/0040 and PI 08/2028 to F.J.B.) Ministerio Ciencia e Innovación (PLE2009-0144 to F.J.B.), with additional funds from the European Regional Development Fund (European Community, to F.J.B.), the NIH (grants R01 GM088181 to V.V. and PRB-NIHRO1 GM088180 to P.R.B.), R.G.-F. received a CIBBER-BNN fellowship.
M.D.M., P.C.-F., and R.G.-F. contributed equally to this work.
Disclosure: M.D.M. is an Isidro Parga Pondal researcher for Xunta de Galicia.
Contributor Information
Maria D. Mayan, Email: Ma.Dolores.Mayan.Santos@sergas.es.
Francisco J. Blanco, Email: fblagar@sergas.es.
References
- 1.Donahue H.J., Guilak F., Vander Molen M.A., McLeod K.J., Rubin C.T., Grande D.A., Brink P.R. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995;10:1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- 2.Knight M.M., McGlashan S.R., Garcia M., Jensen C.G., Poole C.A. Articular chondrocytes express connexin 43 hemichannels and P2 receptors — a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214:275–283. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab W., Hofer A., Kasper M. Immunohistochemical distribution of connexin 43 in the cartilage of rats and mice. Histochem J. 1998;30:413–419. doi: 10.1023/a:1003220225670. [DOI] [PubMed] [Google Scholar]
- 4.D’Andrea P., Vittur F. Propagation of intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. FEBS Lett. 1997;400:58–64. doi: 10.1016/s0014-5793(96)01356-7. [DOI] [PubMed] [Google Scholar]
- 5.Jones S.J., Gray C., Sakamaki H., Arora M., Boyde A., Gourdie R., Green C. The incidence and size of gap junctions between the bone cells in rat calvaria. Anat Embryol (Berl) 1993;187:343–352. doi: 10.1007/BF00185892. [DOI] [PubMed] [Google Scholar]
- 6.Stains J.P., Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Burra S., Jiang J.X. Regulation of cellular function by connexin hemichannels. Int J Biochem Mol Biol. 2011;2:119–128. [PMC free article] [PubMed] [Google Scholar]
- 9.Laird D.W. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plum A., Hallas G., Magin T., Dombrowski F., Hagendorff A., Schumacher B., Wolpert C., Kim J., Lamers W.H., Evert M., Meda P., Traub O., Willecke K. Unique and shared functions of different connexins in mice. Curr Biol. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- 11.Talhouk R.S., Zeinieh M.P., Mikati M.A., El-Sabban M.E. Gap junctional intercellular communication in hypoxia-ischemia-induced neuronal injury. Prog Neurobiol. 2008;84:57–76. doi: 10.1016/j.pneurobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang C.M., Lincoln J., Cook J.E., Becker D.L. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–2817. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Cobo M., Gingalewski C., De Maio A. Expression of the connexin 43 gene is increased in the kidneys and the lungs of rats injected with bacterial lipopolysaccharide. Shock. 1998;10:97–102. doi: 10.1097/00024382-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Toubas J, Beck S, Pageaud AL, Huby AC, Mael-Ainin M, Dussaule JC, Chatziantoniou C, Chadjichristos CE: Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol 301:F24–F32 [DOI] [PubMed]
- 15.De Vuyst E, Boengler K, Antoons G, Sipido KR, Schulz R, Leybaert L: Pharmacological modulation of connexin-formed channels in cardiac pathophysiology. Br J Pharmacol 163:469–483 [DOI] [PMC free article] [PubMed]
- 16.Green C.R., Nicholson L.F. Interrupting the inflammatory cycle in chronic diseases — do gap junctions provide the answer? Cell Biol Int. 2008;32:1578–1583. doi: 10.1016/j.cellbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Pascual Garrido C., Hakimiyan A.A., Rappoport L., Oegema T.R., Wimmer M.A., Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17:1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-Romero C., Carreira V., Rego I., Remeseiro S., Lopez-Armada M.J., Blanco F.J. Proteomic analysis of human osteoarthritic chondrocytes reveals protein changes in stress and glycolysis. Proteomics. 2008;8:495–507. doi: 10.1002/pmic.200700249. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S., Connor J.R., Dodds R.A., Halsey W., Van Horn M., Mao J., Sathe G., Mui P., Agarwal P., Badger A.M., Lee J.C., Gowen M., Lark M.W. Identification and initial characterization of 5000 expressed sequenced tags (ESTs) each from adult human normal and osteoarthritic cartilage cDNA libraries. Osteoarthritis Cartilage. 2001;9:641–653. doi: 10.1053/joca.2001.0421. [DOI] [PubMed] [Google Scholar]
- 20.Valiunas V., Gemel J., Brink P.R., Beyer E.C. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol. 2001;281:H1675–1689. doi: 10.1152/ajpheart.2001.281.4.H1675. [DOI] [PubMed] [Google Scholar]
- 21.Valiunas V., Beyer E.C., Brink P.R. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res. 2002;91:104–111. doi: 10.1161/01.res.0000025638.24255.aa. [DOI] [PubMed] [Google Scholar]
- 22.Holloway I., Kayser M., Lee D.A., Bader D.L., Bentley G., Knight M.M. Increased presence of cells with multiple elongated processes in osteoarthritic femoral head cartilage. Osteoarthritis Cartilage. 2004;12:17–24. doi: 10.1016/j.joca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez S., Fragoso-Soriano R.J., Kouri J.B. Chondrocytes interconnecting tracks and cytoplasmic projections observed within the superficial zone of normal human articular cartilage – a transmission electron microscopy, atomic force microscopy, and two-photon excitation microscopy studies. Microsc Res Tech. 2007;70:1072–1078. doi: 10.1002/jemt.20516. [DOI] [PubMed] [Google Scholar]
- 24.Chi S.S., Rattner J.B., Matyas J.R. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205:363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV: Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316:2377–2389 [DOI] [PubMed]
- 26.Stout C.E., Costantin J.L., Naus C.C., Charles A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 27.Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 28.D’Andrea P., Calabrese A., Capozzi I., Grandolfo M., Tonon R., Vittur F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology. 2000;37:75–83. [PubMed] [Google Scholar]
- 29.Goldberg G.S., Valiunas V., Brink P.R. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 30.White T.W., Bruzzone R., Wolfram S., Paul D.L., Goodenough D.A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayad W.A., Locke D., Koreen I.V., Harris A.L. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem. 2006;281:16727–16739. doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- 32.Niger C, Howell FD, Stains JP: Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular-signal-regulated kinase pathway. Biol Cell 102:37–49 [DOI] [PMC free article] [PubMed]
- 33.White T.W. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 34.Kolomytkin O.V., Marino A.A., Waddell D.D., Mathis J.M., Wolf R.E., Sadasivan K.K., Albright J.A. IL-1beta-induced production of metalloproteinases by synovial cells depends on gap junction conductance. Am J Physiol Cell Physiol. 2002;282:C1254–C1260. doi: 10.1152/ajpcell.01166.2000. [DOI] [PubMed] [Google Scholar]
- 35.Pfander D., Kortje D., Weseloh G., Swoboda B. [Cell proliferation in human arthrotic joint cartilage] German. Z Orthop Ihre Grenzgeb. 2001;139:375–381. doi: 10.1055/s-2001-17977. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J.X., Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gramsch B., Gabriel H.D., Wiemann M., Grummer R., Winterhager E., Bingmann D., Schirrmacher K. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res. 2001;264:397–407. doi: 10.1006/excr.2000.5145. [DOI] [PubMed] [Google Scholar]
- 38.Herrero-Gonzalez S, Gangoso E, Giaume C, Naus CC, Medina JM, Tabernero A: Connexin43 inhibits the oncogenic activity of c-Src in C6 glioma cells. Oncogene 29:5712–5723 [DOI] [PubMed]
- 39.Zhang Y.W., Morita I., Ikeda M., Ma K.W., Murota S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene. 2001;20:4138–4149. doi: 10.1038/sj.onc.1204563. [DOI] [PubMed] [Google Scholar]
- 40.Giepmans B.N. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury T.T., Appleby R.N., Salter D.M., Bader D.A., Lee D.A. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol. 2006;5:192–201. doi: 10.1007/s10237-006-0032-3. [DOI] [PubMed] [Google Scholar]
- 42.Guzman R.E., Evans M.G., Bove S., Morenko B., Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 43.Konttinen Y.T., Mandelin J., Li T.F., Salo J., Lassus J., Liljestrom M., Hukkanen M., Takagi M., Virtanen I., Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- 44.Millward-Sadler S.J., Wright M.O., Flatman P.W., Salter D.M. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41:567–575. [PubMed] [Google Scholar]
- 45.Millward-Sadler S.J., Wright M.O., Lee H., Caldwell H., Nuki G., Salter D.M. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:272–278. doi: 10.1053/joca.1999.0301. [DOI] [PubMed] [Google Scholar]
- 46.Ramage L., Martel M.A., Hardingham G.E., Salter D.M. NMDA receptor expression and activity in osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2008;16:1576–1584. doi: 10.1016/j.joca.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Barrett-Jolley R., Lewis R., Fallman R., Mobasheri A. The emerging chondrocyte channelome. Front Physiol. 2010;1:135. doi: 10.3389/fphys.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNearney T., Speegle D., Lawand N., Lisse J., Westlund K.N. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27:739–745. [PMC free article] [PubMed] [Google Scholar]
- 49.Salter D.M., Wright M.O., Millward-Sadler S.J. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology. 2004;41:273–281. [PubMed] [Google Scholar]
- 50.Shimazaki A., Wright M.O., Elliot K., Salter D.M., Millward-Sadler S.J. Calcium/calmodulin-dependent protein kinase II in human articular chondrocytes. Biorheology. 2006;43:223–233. [PubMed] [Google Scholar]