Abstract
β-Arrestins are intracellular scaffolding proteins that modulate specific cell signaling pathways. Recent studies, in both cell culture and in vivo models, have demonstrated an important role for β-arrestin-1 in inflammation. However, the role of β-arrestin-1 in the pathogenesis of inflammatory bowel disease (IBD) is not known. Our goal was to investigate the role of β-arrestin-1 in IBD using mouse models of colitis. To this end, we subjected wild-type (WT) and β-arrestin-1 knockout (β-arr-1−/−) mice to colitis induced by trinitrobenzenesulfonic acid or dextran sulfate sodium and examined the clinical signs, gross pathology, and histopathology of the colon, as well as inflammatory components. The β-arr-1−/− mice displayed significantly attenuated colitis, compared with WT mice, in both models. Consistent with the phenotypic observations, histological examination of the colon revealed attenuated disease pathology in the β-arr-1−/− mice. Our results further demonstrate that β-arr-1−/− mice are deficient in IL-6 expression in the colon, but have higher expression of the anti-inflammatory IL-10 family of cytokines. Our results also demonstrate diminished ERK and NFκB pathways in the colons of β-arr-1−/− mice, compared with WT mice. Taken together, our results demonstrate that decreased IL-6 production and enhanced IL-10 and IL-22 production in β-arrestin-1–deficient mice likely lead to attenuated gut inflammation.
Arrestins are scaffolding proteins now classified into α and β arrestin families.1 The β-arrestins were discovered for their role in binding to phosphorylated G-protein coupled receptors (GPCRs) to evoke receptor desensitization. Of the four members of the β-arrestin family, β-arrestin-1 (originally arrestin-2) and β-arrestin-2 (originally arrestin-3) are ubiquitously distributed. Even though their role in receptor desensitization has been well characterized and has been shown to have pharmacological and therapeutic implications, recent studies indicate that β-arrestins have a much broader role in cell signaling related to both GPCRs and non-GPCRs.2 In addition, although several members of the arrestin family are present during development, the crucial ones are β-arrestins 1 and 2, because deletion of both results in embryonic lethality.3 Several studies have shown that β-arrestins, by virtue of regulating cell signaling, are able to modulate a variety of cell biological processes including gene expression, chemotaxis, proliferation, and apoptosis. Although β-arrestin-1 and -2 have been shown to have many overlapping cellular functions, they also have unique roles of their own.2
Because of their critical role in many cellular functions, β-arrestins are crucial in the pathogenesis of many different diseases, including Parkinson's disease, multiple sclerosis, cardiovascular disease, rheumatoid arthritis, sepsis, and allergic asthma.4–8 In this context, we recently showed that β-arrestin-1 and -2 have differential and overlapping roles in endotoxin- and adenovirus-induced inflammatory responses in vivo.9,10 In addition, other researchers have also shown that β-arrestin-2 plays a crucial role in sepsis and arthritis models of inflammatory disease.7,11 Although it is clear that β-arrestins are important modulators of inflammation, nonetheless the role of β-arrestins in gastrointestinal inflammation is not known.
Inflammatory bowel disease (IBD) affects more than 1.4 million people in the United States and accounts for more than $1.7 billion dollars in health care costs.12 IBD broadly includes both Crohn's disease and ulcerative colitis, both of which are characterized by chronic relapsing inflammation of the gastrointestinal tract.13 Importantly, IBD requires lifetime care and currently has no medical cure. Understanding the mechanisms involved in the pathogenesis of IBD is crucial for developing new therapeutic strategies to prevent or cure this inflammatory disease. In this regard, recent studies have shown that activation of Toll-like receptors (TLRs) and production of inflammatory cytokines are important mediators in the pathogenesis of colitis, including in mouse models of chemically induced colitis.14–17 Previous studies from our laboratory have demonstrated that β-arrestin-1 mediates TLR signaling and the consequent inflammatory cytokine production in vivo.9 Based on these data, we hypothesized that β-arrestin-1 is an important and critical regulator of colitis pathogenesis. Using two different colitis models, we demonstrate here that β-arrestin-1 is a crucial mediator of colitis pathogenesis in mice. Deficiency of β-arrestin-1 almost completely abrogated the development of intestinal inflammation in the dextran sulfate sodium (DSS) model of colitis, whereas inflammation was significantly attenuated in the trinitrobenzenesulfonic acid (TNBS) model. Furthermore, our results also demonstrate that lack of β-arrestin-1 leads to diminished production of IL-6 but enhanced production of cytokines IL-10 and IL-22, and in combination these likely result in a favorable outcome for intestinal inflammation. The present study implicates a β-arrestin-1–mediated signaling pathway as a potential molecular target in the treatment of colitis.
Materials and Methods
Animals
Mice deficient in β-arrestin-1 (β-arr-1−/− mice, kindly provided by Dr. Robert Lefkowitz, Duke University) and WT mice were maintained at Michigan State University. These mice have been described previously.9 All animals were housed in a pathogen-free facility with a 12-hour light–dark cycle and were given mouse chow and water ad libitum. Groups of mice were age-matched (8 to 12 weeks) and sex-matched. All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines (8th edition, 2011).
Mouse Models of Colitis
DSS-Induced Colitis
Mice were subjected to 2% (w/v) DSS (reagent-grade DSS salt; molecular mass, 36 to 50 kDa; MP Biomedicals, Solon, OH) in drinking water for 7 days, with a change of DSS every 2 days.18
TNBS-Induced Colitis
Mice were presensitized by epicutaneous application of 1% TNBS (5% stock solution; Sigma, St. Louis, MO) in acetone and oil mixture in a volume of 150 μL. At 7 days after TNBS application, mice were anesthetized by an intraperitoneal injection of xylazine/ketamine, followed by intrarectal administration of 70 μL of TNBS (2.75 mg/mouse) dissolved in 50% ethanol. Control mice received 70 μL of 50% ethanol under the same conditions.18
Determination of Clinical Scores
The clinical colitis score was calculated using a modified scoring system based on body weight, stool consistency, the presence of occult blood, coat appearance, crusty eyes, and hunched posture.19 The baseline clinical score was determined on day 0. Scoring was as follows. Weight loss, relative to baseline: 0 = no weight loss, 1 = 1% to 5% weight loss, 2 = 5% to 10% weight loss, 3 = 10% to 20% weight loss, and 4 = >20% weight loss. Stool consistency: 0 = well-formed pellets, 2 = pasty and semiformed stools, and 4 = liquid stools. Presence of fecal blood: 0 = no blood, 2 = positive fecal occult blood test findings, and 4 = gross bleeding. Coat appearance: 0 = normal, and 1 = ruffled/rough coat. Crusty eyes: 0 = no crusting, 1 = one eye, 2 = both eyes. Posture: 0 = no hunching, and 1 = hunched posture. These scores were summed and divided by 6.
Histopathology
Distal and proximal colonic tissues were collected from mice subjected to DSS or TNBS. Tissues were flushed with phosphate-buffered saline (PBS). Tissues were fixed in 10% formalin overnight, embedded in paraffin, and then were sectioned and stained with H&E. The degree of inflammation on longitudinal sections of the colon was scored by a Board-certified pathologist (P.C.L.) in a masked manner. Specifically, for each colon section, a determination was made of the percentage of the mucosal surface area involved with severe damage (defined as complete mucosal ulceration/epithelial denudation and associated marked inflammation), moderate damage (defined as partial crypt damage/partial epithelial destruction and associated modest inflammation), or minimal damage (defined as slight/focal cryptitis or essentially normal mucosa).
Myeloperoxidase Activity Assay
Tissue myeloperoxidase (MPO) activity was measured as described previously.20 Briefly, snap-frozen colon tissues were homogenized in 50 mmol/L potassium phosphate buffer (pH 6.0). After centrifugation, the pellets were incubated in 50 mmol/L potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide. An aliquot of the supernatant was incubated at 25°C in 50 mmol/L potassium phosphate buffer (pH 6.0) containing 0.0005% H2O2 and 167 μg/mL o-dianisidinehydrochloride. MPO activity was determined spectrophotometrically by measuring the change in absorbance at 450 nm over time using a 96-well plate reader as described previously.20
Cytokine Measurements
To determine relative levels of cytokines from colonic tissue, tissues were snap-frozen in liquid nitrogen and homogenized in PBS. Levels of TNF-α, IFN-γ, IL-6, IL-10, IL-17A, and IL-22 in homogenates were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit from eBioscience (San Diego, CA). Cytokine levels were normalized to the total protein and expressed as picograms per milligram of total protein as described previously.20 Plasma cytokine levels were determined as described previously.21
Western Blot Analysis
Tissue samples as described above were subjected to SDS-PAGE, followed by Western blotting, as described previously.22 Blots were probed for p-ERK1/2, p-IκBα, p-NFκBp65, p-JNK, and p-P38 using an Odyssey infrared imaging system (Li-Cor, Lincoln, NE) as described previously.22
qPCR
For quantitative real-time PCR (qPCR), total RNA was extracted from colonic tissues and cDNA synthesized as described previously.23 cDNA (1 μL) was amplified by PCR in a final volume of 25 μL using iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA) with 10 pmol of each primer (Integrated DNA Technologies, Coralville, IA). IL6 was amplified using the primers 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′ and 5′-TAAGCCTCCGACTTGTGAAGTGGT-3′.23 HPRT was used as a non-modulated control gene and was amplified using the primers 5′-AAGCCTAAGATGAGCGCAAG-3′ and 5′-TTACTAGGCAGATGGCCACA-3′.23 qPCR was performed for 40 cycles (95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds), using an iCycler thermal cycler, and data were evaluated using the manufacturer's iCycler software version 3.1 (Bio-Rad Laboratories). RNA-free samples, a negative control, did not produce amplicons. Melting curve and gel analyses (sizing, isolation, and sequencing) were used to verify single products of the appropriate base-pair size.
Cell Isolation from Lamina Propria
Colons were harvested, rinsed with PBS, and feces and mesenteric fat tissue were removed. The colon was then washed in Hank's balanced salt solution (free of calcium and magnesium) and incubated twice in Hank's balanced salt solution containing 5 mmol/L EDTA for 15 minutes at 37°C. After incubation, the epithelial cell layer and intraepithelial lymphocytes were removed by intensive vortexing and passing through a 100-μm cell strainer. The tissue was then washed in Hank's balanced salt solution, cut into 1-mm pieces, and placed in 10 mL digestion solution containing 10% fetal calf serum, 0.5 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN). Digestion was performed by incubating the pieces at 37°C for 2 hours. After incubation, the solution was passed through a 100-μm cell strainer, and the cell pellet was washed in cold PBS containing 10% fetal calf serum, resuspended in 10 mL of the 40% fraction of a 40:80 Percoll gradient (GE Healthcare, Little Chalfont, UK), and overlaid on 5 mL of the 80% fraction in a 15-mL tube. Lamina propria cells were collected at the interphase of the Percoll gradient, washed once, and resuspended in cell culture medium at a density of 106 cells/mL.
Antibodies and Flow Cytometry
Antibodies against CD3 (145-2C11), CD19 (1D3), and CD4 (GK1.5) were purchased from eBioscience. Antibody against IL-22 (Poly5164) was purchased from BioLegend (San Diego, CA). For intracellular cytokine staining, cells were restimulated with 50 ng/mL phorbol 12-myristate 13-acetate, 1000 ng/mL ionomycin, and GolgiPlug protein transport inhibitor (BD Biosciences, San Jose, CA) for 5 hours. Cells were first stained with fixable viability dye to remove dead cells and stained for surface antigens. Cells were then permeabilized with Cytofix/Cytoperm solution (BD Biosciences) according to the manufacturer's recommendations. Intracellular cytokine staining was performed using anti–IL-22 antibody. A Foxp3 antibody reagent kit (eBioscience) was used for intracellular Foxp3 staining according to the manufacturer's instructions and using the manufacturer's fixation/permeabilization solution and permeabilization buffer. All surface antibody staining was performed in the presence of anti-CD16/32 (2.4G2, eBioscience), to block nonspecific binding of antibody. Flow cytometry was performed using an LSR II system (BD Biosciences), and data were analyzed using Flow Jo software version 9.4 (Tree Star, Ashland, OR).
Statistical Analysis
Data are expressed as means ± SEM. Data were analyzed and statistical tests were performed using GraphPad Prism software version 5.0b (GraphPad Software, La Jolla, CA). The Student's t-test (two-tailed) was used to compare mean values between two experimental groups; analysis of variance with post hoc Bonferroni correction was used for comparing more than two groups. A P value of <0.05 was considered significant.
Results
β-Arrestin-1 Mediates Weight Loss and Clinical Signs during Experimental Colitis
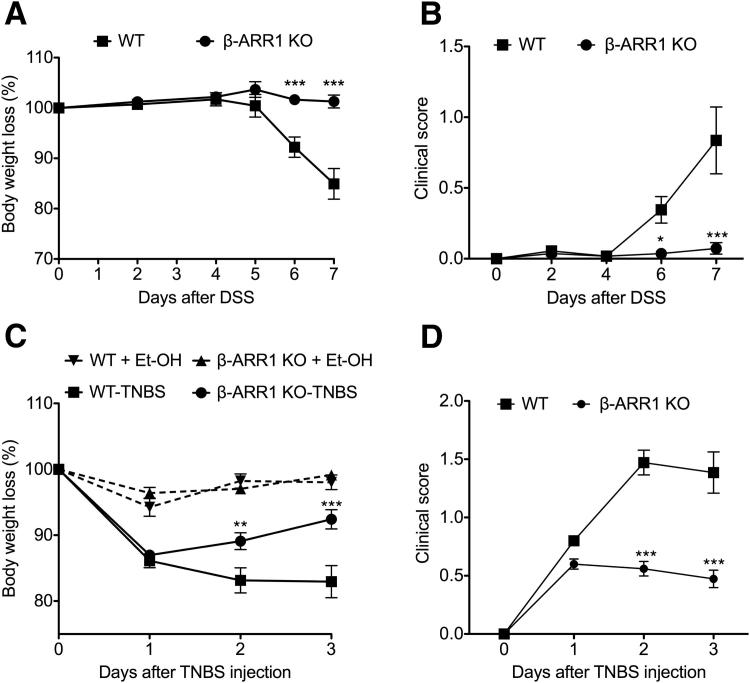
To investigate the role of β-arrestin-1 in the pathogenesis of IBD, we initially tested the effect of DSS-induced colitis on body weight in wild-type (WT) and β-arrestin-1 knockout (β-arr-1−/−) mice. Mice were fed with 2% DSS in drinking water for 7 days, followed by regular drinking water. As expected, the WT mice lost considerable body weight over the course of the study (Figure 1A). The β-arr-1−/− mice, however, were strikingly protected from the body weight loss induced by DSS administration (Figure 1A). To confirm that these effects were not model-dependent and to delineate potential mechanisms, we then induced colitis in mice of both genotypes using TNBS. Previous studies have shown that, whereas DSS-induced colitis can occur in the absence of T and B lymphocyte, TNBS-induced colitis is characterized by T helper 1 (Th1) cytokine patterns.24–27 Consistent with the lack of weight loss in β-arr-1−/− mice observed in the DSS model, β-arr-1−/− mice were significantly protected from weight loss also in the TNBS model (Figure 1C). We further clinically scored the mice based on body weight loss, presence of fecal blood, coat appearance, crusty eyes, and hunched posture. Consistent with the weight loss data, the severity of clinical signs induced by either DSS or TNBS administration was significantly attenuated in β-arr-1−/− mice, compared with WT mice (Figure 1, B and D).
Figure 1.
Diminished weight loss and clinical disease severity in β-arrestin-1–deficient mice after DSS- or TNBS-induced colitis. A and C: Mice were subjected to colitis induced by DSS (A) or TNBS (C), and body weight was monitored daily from 0 to 7 days. B and D: Clinical scores for disease severity in mice subjected to colitis induced by DSS (B) or TNBS (D) were determined as described in Materials and Methods. Data are expressed as means ± SEM, pooled from 2 or 3 independent experiments. n = 11 (DSS); n = 14 or 15 (TNBS). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus corresponding WT treatment and time point. β-ARR1, β-arrestin-1; KO, knockout.
β-Arrestin-1 Mediates Inflammation in the Colon
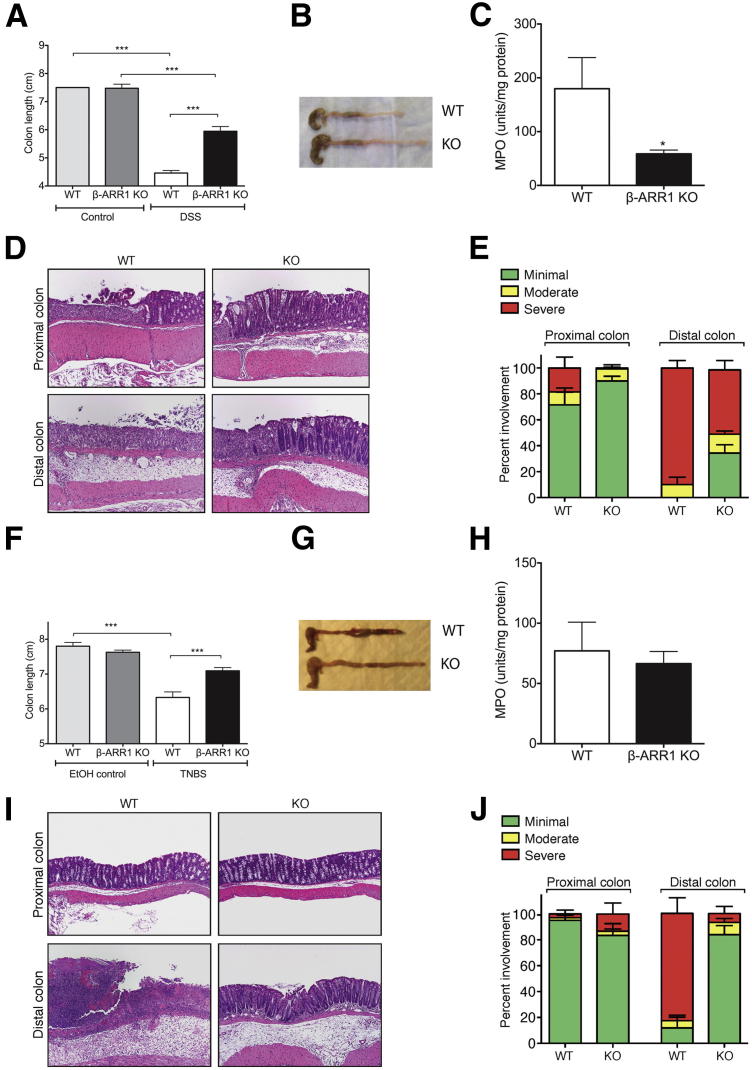
To evaluate the extent and severity of pathological changes in the colon, we measured the length of colons from mice subjected to colitis in both DSS and TNBS models. Even though DSS-induced colitis led to colon shortening in both WT and β-arr-1−/− mice, the colon was still significantly longer in β-arr-1−/− mice than in WT mice (Figure 2, A and B). Similar results were also observed in the TNBS model; that is, colon length shortening due to colitis was significantly attenuated in the β-arr-1−/− mice (Figure 2, F and G).
Figure 2.
Diminished gross and histopathological disease assessment in β-arrestin-1–deficient mice after DSS- or TNBS-induced colitis: A, B, F, and G: Colon length in WT and β-arr-1−/− mice at 8 days after DSS treatment (A) or at 3 days after TNBS treatment (F), with representative gross morphology for DSS treatment (B) and TNBS treatment (G). C and H: MPO activity in the colon of mice subjected to DSS (C) or TNBS (H) colitis. D, E, I, and J: Distal and proximal colon histopathology from WT and β-arr-1−/− mice subjected to DSS (D) or TNBS (I) colitis, with quantitation for histopathological assessment of DSS-treated (E) and TNBS-treated (J) mice. Data are expressed as means ± SEM, pooled from 2 or 3 independent experiments. n = 10 or 11 (DSS, all); n = 8 or 11 (TNBS, H); n = 14 or 15 (TNBS, F and J). ∗P < 0.05, ∗∗∗P < 0.001. Original magnification, ×100 (D and I).
To further verify the clinical assessment, we measured MPO activity and performed histological examination of the colon from both genotypes of mice subjected to colitis. In the DSS model, as expected, MPO activity in the colon was decreased significantly in β-arr-1−/− mice, compared with WT mice (Figure 2C). In the TNBS model, however, MPO activity was similar in the two genotypes (Figure 2H). Healthy mice of either genotype displayed no significant MPO activity (data not shown). Consistent with the overall phenotypic differences in body weight loss, clinical signs, and gross colon morphology, H&E-stained microscopic sections of the colon revealed marked differences between the diseased β-arr-1−/− and WT mice (Figure 2, D, E, I, and J). Specifically, histological analyses showed that β-arr-1−/− mice were significantly protected from colitis, compared with WT mice. Although both strains showed the same overall pattern of chronic mucosal ulceration and associated inflammation, the number and extent of ulcers were significantly reduced in the β-arr-1−/− mice. There were some differences in the extent of severity between the two models. In the DSS model, severity of inflammation was markedly reduced in both the proximal and the distal colon of β-arr-1−/− mice. In the TNBS model, however, severity was significantly reduced only in the distal colon of β-arr-1−/− mice, compared with WT mice. Taken together, these results demonstrate that the pathogenesis of colitis induced by DSS or TNBS is significantly attenuated in β-arrestin-1 deficiency.
Regulation of IL-6 and the IL-10 Family of Cytokines by β-Arrestin-1 in Experimental Colitis
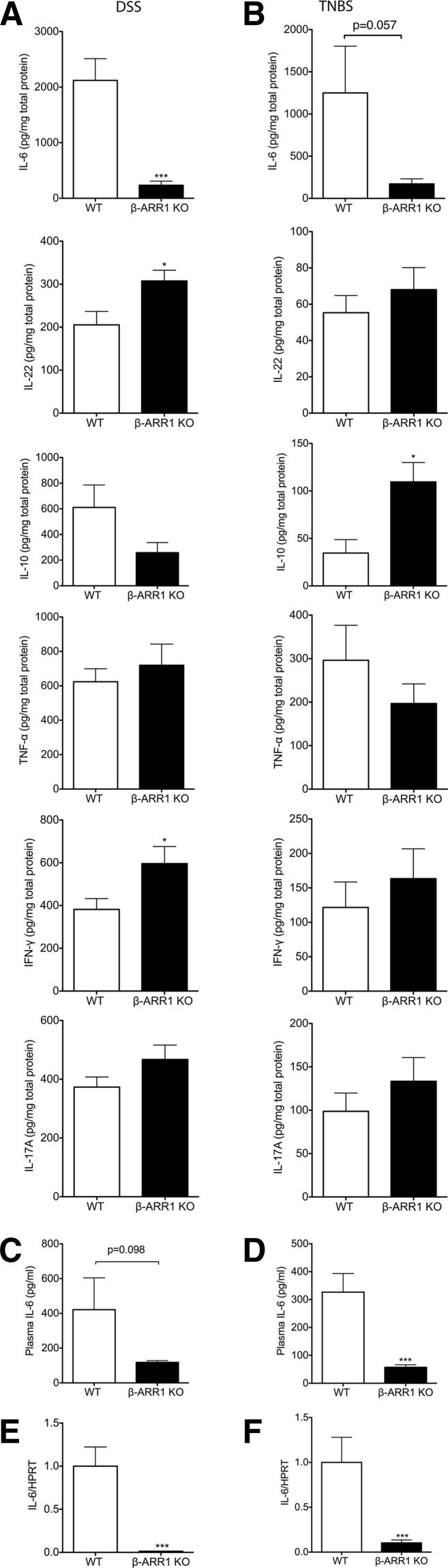
Our results thus far had demonstrated an important role for β-arrestin-1 in the pathogenesis of colitis in the mouse models studied. To further understand the inflammatory mechanisms by which β-arrestin-1 mediates this effect, we examined the cytokines modulated by colitis in the two mouse genotypes. Cytokines are critical mediators of the innate and adaptive immune responses in mucosal inflammation. Although several cytokines have been implicated in both DSS and TNBS models of colitis, studies have shown that TNF-α, IL-6, IL-10, IFN-γ, IL-17A, and IL-22 in particular play critical roles in the pathogenesis of both animal and human colitis.17 To examine whether the mechanism by which β-arrestin-1 deficiency protects mice from colitis is mediated by influence on these cytokines, we measured the tissue levels of these cytokines in the colon from mice of the two genotypes. As predicted, IL-6 levels were elevated in the WT colons in both models of disease (Figure 3, A and B). IL-6 levels were undetectable in healthy mice (data not shown). Interestingly, induction of IL-6 was markedly blocked in the β-arr-1−/− mice subjected to either DSS or TNBS treatment (Figure 3, A and B). Expression of other cytokines was more variable between genotypes in the two models of colitis (Figure 3, A and B). Production of IL-22 (a member of the IL-10 family) was higher in β-arr-1−/− mice with DSS-induced colitis, compared with the corresponding WT mice. Interestingly, IL-10 was enhanced in the colon of β-arr-1−/− mice in the TNBS model. Both of these cytokines (IL-10 and IL-22) have been shown to have protective effects in gut inflammation models.28–32
Figure 3.
Diminished IL-6 levels in β-arrestin-1–deficient mice after experimental colitis. A and B: Distal colonic tissues were collected from mice treated with DSS (A) or TNBS (B), proteins were extracted, and various cytokines were assayed using ELISA. C and D: Plasma was collected from mice treated with DSS (C) and TNBS (D) and ELISA for IL-6 was performed. E and F: Distal colonic tissues were collected from mice treated with DSS (E) and TNBS (F), RNA was extracted, and IL-6 mRNA determined. Data are expressed as means ± SEM, pooled from two (DSS, all; TNBS, B and F) or three independent experiments (TNBS, D). n = 11 (DSS); n = 14 or 15 (TNBS). ∗P < 0.05, ∗∗∗P < 0.001.
To further identify cell types in the gut that likely mediate expression of these cytokines, we obtained lamina propria cells from DSS-treated mice of both genotypes and examined the immune cell populations, as well as expression of IL-6 and IL-22, using flow cytometry. Interestingly, the percentage of CD3+ T cells but not CD19+ B cells was significantly enhanced in the β-arr-1−/− mice treated with DSS (Supplemental Figure S1). Staining for IL-6 was unsuccessful because of high nonspecific staining (data not shown). IL-22 staining, however, clearly demonstrated a significant increase in the percentage of IL-22+CD4+ T cells in the lamina propria of β-arr-1−/− mice, compared with WT mice (Supplemental Figure S2). As predicted from the IL-10 cytokine data for the DSS model, the percentage of FoxP3+CD4+ T cells (ie, regulatory T cells) did not differ between genotypes in the DSS model (Supplemental Figure S3). Because IL-6 levels were inhibited in both models of colitis, and because IL-6 has been shown to be a key regulator of colitis in both of these models as well as in human colitis,33 we subsequently focused on this cytokine. Previous studies have shown that serum IL-6 levels correlate well with clinical activity in IBD, and are also predictive for the risk of relapse in IBD.34 We therefore measured plasma IL-6 levels in the two models of colitis. In the DSS model, plasma IL-6 levels were decreased in β-arr-1−/− mice, compared with WT mice, but the difference did not reach statistical significance (Figure 3C). In the TNBS model, however, plasma IL-6 was significantly inhibited in β-arr-1−/− mice, compared with WT mice (Figure 3D). To further understand the mechanism by which IL-6 levels are regulated in the colon by β-arrestin-1, we determined the mRNA expression of IL-6, to assess whether the regulation occurs at the level of transcription. Levels of IL-6 mRNA were significantly inhibited in β-arr-1−/− mice, compared with WT mice, in both models of colitis (Figure 3, E and F). Taken together, these results suggest that β-arrestin-1 mediates IL-6 expression in the colon in both DSS and TNBS models of colitis.
Diminished ERK and NFκB Activation in β-Arrestin-1 Knockout Mice
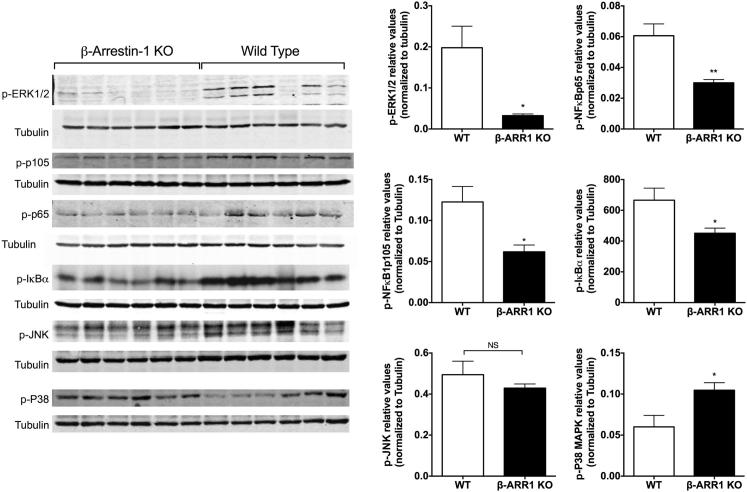
Previous studies in cell-culture models have shown both positive and negative regulatory roles for β-arrestins in MAPK and NFκB signaling.2 Furthermore, studies have also shown that MAPK and NFκB pathways are critical mediators of inflammatory signaling in models of IBD.35,36 We therefore hypothesized that β-arrestin-1 knockout mice may display altered activation of these pathways in the colitis model, which might be associated with the observed changes in IL-6 and IL-22 cytokines. We focused on the DSS model for these studies and examined the phosphorylation levels of the ERK (p-ERK1/2 and p-P105, an upstream regulator of ERK22), NFκB (p-IκBα and p-NFκBp65) pathways, as well as JNK and P38 in the colon tissue lysates, using Western blot analysis. Interestingly, levels of p-ERK1/2, p-P105, p-IκBα, and p-NFκBp65 were markedly inhibited in β-arr-1−/− mice subjected to colitis, compared with the corresponding WT mice (Figure 4). These results suggest deficient activation of the ERK and NFκB pathways in the colon of β-arr-1−/− mice. These results are specific for these pathways, because p-JNK levels did not differ between the two genotypes, and p-P38 levels were enhanced in the β-arr-1−/− mice (Figure 4). Consistent with NFκB activation in the colon (and its role in IL-6 production in macrophages22), lipopolysaccharide-induced NFκB activation in peritoneal macrophages was significantly attenuated in β-arr-1−/− mice, compared with WT mice (Supplemental Figure S4). Further studies will be needed to determine the cell type–specific roles of β-arrestin-1 in colitis, as well as the molecular mechanisms that likely stimulate these signaling pathways in a β-arrestin-1–dependent manner in colitis models.
Figure 4.
Decreased ERK and NFκB pathway in β-arrestin-1–deficient mice after DSS-induced colitis. Distal colonic tissues (as in Figure 3) from β-arr-1−/− and WT mice were subjected to Western blotting for p-ERK, p-p105, p-IκBα, p-NFκBp65, p-JNK, and p-P38, with tubulin as the loading control, and the relative values were quantitated. Each lane represents a sample from different mouse. Data are expressed as means ± SEM. n = 6. ∗P < 0.05, ∗∗P < 0.01.
Discussion
Although the etiology and pathogenesis of IBD in humans have not been fully elucidated, it is clear that the disease might be attributed to complex mucosal immune responses to commensal intestinal bacteria.37 Despite some differences between human IBD and mouse models of colitis, the DSS- and the TNBS-induced colitis models in mice have generally been useful in determining the roles of signaling proteins in the pathogenesis of IBD.38 The DSS-induced colitis model is especially useful in determining the contribution of innate immunity in the development of colitis,24–26 whereas TNBS-induced colitis has been well characterized for its T helper 1 (Th1) cytokine patterns.27 With the present study, we provide the first evidence that the scaffolding cell signaling protein β-arrestin-1 plays an important role in both these models of colitis. Even though β-arrestin-1 was originally discovered for its role in GPCR desensitization, recent studies, including some from our laboratory, demonstrate that β-arrestins have a much broader role in cell signaling, one that is not restricted to GPCR desensitization. In this regard, we recently reported that β-arrestin-1 positively regulates the in vivo inflammatory response after stimulation with bacterial lipopolysaccharide, as well as by adenovirus.9,10 We therefore hypothesized that, in a TLR-dependent disease process such as intestinal inflammation,15 pathogenesis of colitis would be attenuated in β-arrestin-1–deficient mice. As predicted, in two distinctly different models of colitis, β-arrestin-1 deficiency significantly attenuated disease development.
Even though the TNBS and DSS models of colitis involve different pathogenic mechanisms, IL-6 has been shown to play a critical role in disease progression in both models of colitis.39,40 IL-6 deficiency can ameliorate experimental colitis, whereas IL-6 administration in IRF-4–deficient mice (which have reduced mucosal IL-6) can reverse the protective effect of IRF-4 deficiency on colitis.41–43 Recent human studies have also shown an increased presence of IL-6 in colonic tissue from IBD patients.39,44–46 Importantly, IL-6 serum levels have been shown to correlate with clinical and histopathological severity of disease activity.34 In the present study, β-arrestin-1–deficient mice had strikingly lower levels of IL-6 in both colon and plasma. Our studies also suggest that this regulation of IL-6 by β-arrestin-1 may occur at the level of IL-6 transcription, because IL-6 mRNA levels were also significantly lower in the β-arrestin-1–deficient mice in both DSS and TNBS models. It would be of interest from a mechanistic standpoint to test whether β-arrestin-1 regulates IL-6 transcription directly, or indirectly through regulation of other receptor signaling (eg, the NFκB pathway). Our results demonstrate that activation of ERK and NFκB pathways, but not JNK, is regulated by β-arrestin-1 in the colon. Whether this occurs because of interaction of β-arrestin-1 with receptors such as TLRs or via GPCRs remains a subject for future study. However, preliminary work using peritoneal macrophages reveals that β-arrestin-1 is an important mediator of TLR4-induced NFκB activation (Supplemental Figure S4).
Given the complexity of cell types in the in vivo colitis model, it would also be important to determine whether there is a cell type–specific effect of β-arrestin-1. The present study demonstrates that deficiency of β-arrestin-1 enhances a specific T-cell population that produces IL-22, an anti-inflammatory cytokine that has been shown to have protective effects in colitis. How β-arrestin-1 negatively regulates IL-22 remains to be determined. Previous studies have shown that the function of β-arrestin-1 is dependent not only on the disease model being examined, but also on the receptor signaling pathway and the cell type being examined. Thus, β-arrestin-1 has been shown to be a mediator of endotoxemia, pulmonary fibrosis, and autoimmune diseases such as experimental autoimmune encephalomyelitis.9,47,48 Even though previous studies using cell lines have shown a negative regulatory role for β-arrestin-1 in TLR signaling at the cellular level,49,50 in vivo data suggest that this may not be the case (at least not in the models examined to date). One recent study showed that hyaluronic acid–induced IL-6 production in fibroblast-like synoviocytes is mediated by β-arrestin-1.7 Although multiple molecular mechanisms could be at play in how β-arrestin-1 mediates colitis, we demonstrate here that β-arrestin-1 is important in mediating colonic ERK and NFκB phosphorylation. Whether these changes are sufficient or necessary for the observed phenotype remains to be examined in future studies.
IL-6 is a multifunctional cytokine with many different actions, including regulation of hematopoiesis, inflammation, and immune responses.40 Recent studies have identified IL-6 as an important regulator of the differentiation of T helper cells producing IL-17 (Th17 cells).51 The primary function of Th17 cells has been shown to be clearance of pathogens that are not controlled by Th1 and Th2 cells.52,53 However, accumulating evidence also suggests that Th17 cells may be associated with pathogenesis of many autoimmune diseases.54–56 Indeed, IL-17 was increased in patients with active ulcerative colitis or active Crohn's disease, compared with patients with inactive disease.57 We therefore reasoned that, because IL-6 is markedly attenuated in β-arrestin-1–deficient mice, IL-17A expression might be also be inhibited. In the present study, however, we did not observe any difference in IL-17A levels between WT and β-arr-1−/− mice in either the DSS or the TNBS model of colitis. Although the reasons for this finding are not clear, one possibility is that other factors (eg, TGF-β, IL-21, and IL-23) may still be able to drive IL-17.51
Among the other cytokines we examined, regulation of IL-10 and IL-22 (both belonging to the IL-10 family) were differentially regulated in the two models, especially in the β-arr-1−/− mice. Although IL-10 was enhanced in the β-arr-1−/− mice in the TNBS model, it was decreased in the DSS model, compared with the corresponding WT mice. IL-22, however, was enhanced in β-arr-1−/− mice in the DSS model but not in the TNBS model. Both IL-10 and IL-22 have been shown to be protective in the context of colitis development. Thus, it is possible that, in addition to a decrease in IL-6 expression, enhanced expression of IL-10 or IL-22 may contribute to the overall beneficial phenotype of the β-arrestin-1–deficient mice in DSS- and TNBS-induced colitis. Even though IL-10 was enhanced in the β-arrestin-1–deficient mice in the TNBS model, the number of T-regulatory cells (an important source of IL-10) in the colon did not differ between the two genotypes. Previous studies have shown an anti-apoptotic role for IL-22 in gut mucosa.32 In accord with that report, we observed that expression of anti-apoptotic genes in the colon of β-arr-1−/− mice was significantly enhanced, compared with WT mice (data not shown). However, when we examined annexin-V/propidium iodide staining by flow cytometry, we did not observe any difference between the two genotypes (data not shown). Whether this could be related to the kinetics of the disease is not clear and remains to be determined in future studies.
In conclusion, our results demonstrate that β-arrestin-1 is a critical mediator of experimental colitis and that this likely occurs via regulation of IL-6, and possibly also IL-10 family members. Further studies are needed to determine whether β-arrestin-1 regulates these cytokines in a cell type–specific manner in these disease models, as well as to further elucidate the molecular mechanisms by which β-arrestin-1 mediates these functions. Identification of such mechanisms could prove useful for therapeutically targeting β-arrestin-1 and/or IL-6 in the treatment of IBD.
Acknowledgments
We thank the University Laboratory Animal Resources staff (Michigan State University, East Lansing, MI) for excellent care of our animals, the Investigative Histopathology Laboratory staff for excellent service, and Dr. Robert J. Lefkowitz for kindly providing us the β-arrestin-1 knockout mice.
Footnotes
Supported in part by the NIH (grants HL095637, AR055726, and AR056680 to N.P.).
Supplemental Data
Enhanced CD3+ T lymphocytes from colonic tissues in β-arrestin-1–deficient mice after DSS-induced colitis. Single-cell suspensions from colonic tissues of DSS-treated and control mice of both genotypes (WT and Arrb1−/−) were stained with fluorochrome-conjugated antibodies and were analyzed by flow cytometry. Dot plots are shown for B lymphocytes (CD19) and T lymphocytes (CD3); numbers in quadrants indicate the percentage of cells. Cumulative data for CD3+ T cells are presented in graph form, with data expressed as means ± SEM. Data are representative of one of two independent experiments. n =5 per group.
Enhanced IL-22+CD4+ T cells from colonic tissues in β-arrestin-1–deficient mice after DSS-induced colitis. Single-cell suspensions from colonic tissues of WT and Arrb1−/− mice were stained with fluorochrome-conjugated antibodies and were analyzed by flow cytometry. Dot plots are shown for IL-22+CD4+ T cells; numbers in quadrants indicate the percentage of cells. Cumulative data are presented in graph form, with data expressed as means ± SEM. Data are representative of one of two independent experiments. n = 5 per group.
T-regulatory (Treg) cells from colonic tissues are similar after DSS treatment. Single-cell suspensions from colonic tissues of WT and Arrb1−/− mice were stained with CD4 and Foxp3 antibodies and were analyzed by flow cytometry. Data are expressed as the mean percentage (± SEM) of CD4+Foxp3+ T cells in CD4+ T cells and are representative of one of two independent experiments. n = 5 per group.
Diminished NFκBp65 phosphorylation in β-arrestin-1 knockout mouse macrophages. Thioglycollate-induced peritoneal macrophages were obtained as described by Patial et al.21 Cells were treated with 200 ng/mL lipopolysaccharide (LPS) for 0 to 60 minutes. Cell lysates were subjected to Western blotting and probed for p-NFκBp65; tubulin was used as the loading control. n = 5 mice per genotype. *P < 0.05 versus WT.
References
- 1.Patwari P., Lee R.T. An expanded family of arrestins regulate metabolism. Trends Endocrinol Metab. 2012;23:216–222. doi: 10.1016/j.tem.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whalen E.J., Rajagopal S., Lefkowitz R.J. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M., Liu X., Zhang Y., Zhao J. Loss of [beta]arrestin1 and [beta]arrestin2 contributes to pulmonary hypoplasia and neonatal lethality in mice. Dev Biol. 2010;339:407–417. doi: 10.1016/j.ydbio.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Bezard E., Gross C.E., Qin L., Gurevich V.V., Benovic J.L., Gurevich E.V. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol Dis. 2005;18:323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Ohguro H., Chiba S., Igarashi Y., Matsumoto H., Akino T., Palczewski K. Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci USA. 1993;90:3241–3245. doi: 10.1073/pnas.90.8.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J., Zhang L., Peppel K., Wu J.H., Zidar D.A., Brian L., DeWire S.M., Exum S.T., Lefkowitz R.J., Freedman N.J. Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P., Cook J.A., Gilkeson G.S., Luttrell L.M., Wang L., Borg K.T., Halushka P.V., Fan H. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker J.K., Fong A.M., Lawson B.L., Savov J.D., Patel D.D., Schwartz D.A., Lefkowitz R.J. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter K.J., Gonipeta B., Parvataneni S., Appledorn D.M., Patial S., Sharma D., Gangur V., Amalfitano A., Parameswaran N. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. J Cell Physiol. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seregin S.S., Appledorn D.M., Patial S., Bujold M., Nance W., Godbehere S., Parameswaran N., Amalfitano A. beta-Arrestins modulate Adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus Res. 2010;147:123–134. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H., Bitto A., Zingarelli B., Luttrell L.M., Borg K., Halushka P.V., Cook J.A. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananthakrishnan A.N., McGinley E.L., Saeian K., Binion D.G. Trends in ambulatory and emergency room visits for inflammatory bowel diseases in the United States: 1994-2005. Am J Gastroenterol. 2010;105:363–370. doi: 10.1038/ajg.2009.580. [DOI] [PubMed] [Google Scholar]
- 13.Podolsky D.K. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 14.Becker C., Dornhoff H., Neufert C., Fantini M.C., Wirtz S., Huebner S., Nikolaev A., Lehr H.A., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Galle P.R., Karow M., Neurath M.F. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 15.Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis. 2010;16:1583–1597. doi: 10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomariz R.P., Arranz A., Abad C., Torroba M., Martinez C., Rosignoli F., Garcia-Gómez M., Leceta J., Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- 17.Strober W., Fuss I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirtz S., Neufert C., Weigmann B., Neurath M.F. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 19.Vereecke L., Sze M., Mc Guire C., Rogiers B., Chu Y., Schmidt-Supprian M., Pasparakis M., Beyaert R., van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patial S., Shahi S., Saini Y., Lee T., Packiriswamy N., Appledorn D.M., Lapres J.J., Amalfitano A., Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. J Cell Physiol. 2011;226:1323–1333. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvataneni S., Gonipeta B., Packiriswamy N., Lee T., Durairaj H., Parameswaran N. Role of myeloid-specific G-protein coupled receptor kinase-2 in sepsis. Int J Clin Exp Med. 2011;4:320–330. [PMC free article] [PubMed] [Google Scholar]
- 22.Patial S., Saini Y., Parvataneni S., Appledorn D.M., Dorn G.W., 2nd, Lapres J.J., Amalfitano A., Senagore P., Parameswaran N. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. J Cell Physiol. 2011;226:627–637. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motyl K.J., Botolin S., Irwin R., Appledorn D.M., Kadakia T., Amalfitano A., Schwartz R.C., McCabe L.R. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218:575–583. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- 24.Araki A., Kanai T., Ishikura T., Makita S., Uraushihara K., Iiyama R., Totsuka T., Takeda K., Akira S., Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 25.Dieleman L.A., Palmen M.J., Akol H., Bloemena E., Peña A.S., Meuwissen S.G., Van Rees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieleman L.A., Ridwan B.U., Tennyson G.S., Beagley K.W., Bucy R.P., Elson C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 27.te Velde A.A., Verstege M.I., Hommes D.W. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- 28.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay J., Van Montfrans C., Brennan F., Van Deventer S., Drillenburg P., Hodgson H., Te Velde A., Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715–1721. doi: 10.1038/sj.gt.3301841. [DOI] [PubMed] [Google Scholar]
- 30.Cox J.H., Kljavin N.M., Ota N., Leonard J., Roose-Girma M., Diehl L., Ouyang W., Ghilardi N. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K., Blumberg R.S., Xavier R.J., Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., Ouyang W., Neurath M.F., Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mudter J., Neurath M.F. IL-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 34.Umehara Y., Kudo M., Nakaoka R., Kawasaki T., Shiomi M. Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepatogastroenterology. 2006;53:879–882. [PubMed] [Google Scholar]
- 35.Atreya I., Atreya R., Neurath M.F. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 36.Broom O.J., Widjaya B., Troelsen J., Olsen J., Nielsen O.H. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009;158:272–280. doi: 10.1111/j.1365-2249.2009.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nell S., Suerbaum S., Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–577. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 38.Hibi T., Ogata H., Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37:409–417. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 39.Seegert D., Rosenstiel P., Pfahler H., Pfefferkorn P., Nikolaus S., Schreiber S. Increased expression of IL-16 in inflammatory bowel disease. Gut. 2001;48:326–332. doi: 10.1136/gut.48.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimoto N., Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [Erratum appeared in Nat Clin Pract Rheumatol 2006, 2:691] and 691. [DOI] [PubMed] [Google Scholar]
- 41.Mudter J., Amoussina L., Schenk M., Yu J., Brüstle A., Weigmann B., Atreya R., Wirtz S., Becker C., Hoffman A., Atreya I., Biesterfeld S., Galle P.R., Lehr H.A., Rose-John S., Mueller C., Lohoff M., Neurath M.F. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mudter J., Yu J., Amoussina L., Weigmann B., Hoffman A., Rücknagel K., Galle P.R., Neurath M.F. IRF4 selectively controls cytokine gene expression in chronic intestinal inflammation. Arch Immunol Ther Exp (Warsz) 2009;57:369–376. doi: 10.1007/s00005-009-0046-5. [DOI] [PubMed] [Google Scholar]
- 43.Gay J., Kokkotou E., O’Brien M., Pothoulakis C., Karalis K.P. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. Putative effect of antiinflammatory cytokines. Neuroimmunomodulation. 2006;13:114–121. doi: 10.1159/000096656. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuyama K., Sata M., Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn. 1991;26:20–28. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- 45.Murata Y., Ishiguro Y., Itoh J., Munakata A., Yoshida Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. J Gastroenterol. 1995;30(Suppl 8):56–60. [PubMed] [Google Scholar]
- 46.Hosokawa T., Kusugami K., Ina K., Ando T., Shinoda M., Imada A., Ohsuga M., Sakai T., Matsuura T., Ito K., Kaneshiro K. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:987–996. doi: 10.1046/j.1440-1746.1999.01989.x. [DOI] [PubMed] [Google Scholar]
- 47.Lovgren A.K., Kovacs J.J., Xie T., Potts E.N., Li Y., Foster W.M., Liang J., Meltzer E.B., Jiang D., Lefkowitz R.J., Noble P.W. Beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsutsui S., Vergote D., Shariat N., Warren K., Ferguson S.S., Power C. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J. 2008;22:786–796. doi: 10.1096/fj.07-9002com. [DOI] [PubMed] [Google Scholar]
- 49.Parameswaran N., Pao C.S., Leonhard K.S., Kang D.S., Kratz M., Ley S.C., Benovic J.L. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Tang Y., Teng L., Wu Y., Zhao X., Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 51.Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 52.Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J.E., Bagby G.J., Nelson S., Charrier K., Peschon J.J., Kolls J.K. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raffatellu M., Santos R.L., Verhoeven D.E., George M.D., Wilson R.P., Winter S.E., Godinez I., Sankaran S., Paixao T.A., Gordon M.A., Kolls J.K., Dandekar S., Bäumler A.J. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 57.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enhanced CD3+ T lymphocytes from colonic tissues in β-arrestin-1–deficient mice after DSS-induced colitis. Single-cell suspensions from colonic tissues of DSS-treated and control mice of both genotypes (WT and Arrb1−/−) were stained with fluorochrome-conjugated antibodies and were analyzed by flow cytometry. Dot plots are shown for B lymphocytes (CD19) and T lymphocytes (CD3); numbers in quadrants indicate the percentage of cells. Cumulative data for CD3+ T cells are presented in graph form, with data expressed as means ± SEM. Data are representative of one of two independent experiments. n =5 per group.
Enhanced IL-22+CD4+ T cells from colonic tissues in β-arrestin-1–deficient mice after DSS-induced colitis. Single-cell suspensions from colonic tissues of WT and Arrb1−/− mice were stained with fluorochrome-conjugated antibodies and were analyzed by flow cytometry. Dot plots are shown for IL-22+CD4+ T cells; numbers in quadrants indicate the percentage of cells. Cumulative data are presented in graph form, with data expressed as means ± SEM. Data are representative of one of two independent experiments. n = 5 per group.
T-regulatory (Treg) cells from colonic tissues are similar after DSS treatment. Single-cell suspensions from colonic tissues of WT and Arrb1−/− mice were stained with CD4 and Foxp3 antibodies and were analyzed by flow cytometry. Data are expressed as the mean percentage (± SEM) of CD4+Foxp3+ T cells in CD4+ T cells and are representative of one of two independent experiments. n = 5 per group.
Diminished NFκBp65 phosphorylation in β-arrestin-1 knockout mouse macrophages. Thioglycollate-induced peritoneal macrophages were obtained as described by Patial et al.21 Cells were treated with 200 ng/mL lipopolysaccharide (LPS) for 0 to 60 minutes. Cell lysates were subjected to Western blotting and probed for p-NFκBp65; tubulin was used as the loading control. n = 5 mice per genotype. *P < 0.05 versus WT.