Abstract
Neovascularization of the airways occurs in several inflammatory lung diseases, including asthma. Vascular endothelial growth factor (VEGF) plays an important role in vascular remodeling in the asthmatic airways. Fatty acid binding protein 4 (FABP4 or aP2) is an intracellular lipid chaperone that is induced by VEGF in endothelial cells. FABP4 exhibits a proangiogenic function in vitro, but whether it plays a role in modulation of angiogenesis in vivo is not known. We hypothesized that FABP4 promotes VEGF-induced airway angiogenesis and investigated this hypothesis with the use of a transgenic mouse model with inducible overexpression of VEGF165 under a CC10 promoter [VEGF-TG (transgenic) mice]. We found a significant increase in FABP4 mRNA levels and density of FABP4-expressing vascular endothelial cells in mouse airways with VEGF overexpression. FABP4−/− mouse airways showed a significant decrease in neovessel formation and endothelial cell proliferation in response to VEGF overexpression. These alterations in airway vasculature were accompanied by attenuated expression of proinflammatory mediators. Furthermore, VEGF-TG/FABP4−/− mice showed markedly decreased expression of endothelial nitric oxide synthase, a well-known mediator of VEGF-induced responses, compared with VEGF-TG mice. Finally, the density of FABP4-immunoreactive vessels in endobronchial biopsy specimens was significantly higher in patients with asthma than in control subjects. Taken together, these data unravel FABP4 as a potential target of pathologic airway remodeling in asthma.
Vascular remodeling of the airways is a key feature in the pathophysiology of asthma.1 Increased levels of vascular endothelial growth factor A (VEGF), a key mediator of angiogenesis, is detected in bronchoalveolar lavage fluid and in airway mucosa and correlate both with the degree of mucosal vascularity and severity of disease in asthma.2–5 The total number of vessels and vascular area in the lamina propria of the airway mucosa is significantly increased in patients with asthma than in control subjects.6–8 The airway hypervascularity has been proposed to perpetuate the airway obstruction and hyperactivity by increasing trafficking of inflammatory cells, exudation of mediators, and microvascular leakage.9–11 This concept has been supported by studies in a transgenic mouse model of VEGF overexpression, which have provided a link between VEGF-induced neovascularization and type 2 helper T cell type inflammation in the lung.12 Although these studies have suggested that VEGF inhibition could have some translational potential in asthma,13 VEGF has a multitude of effects in the lung, most of which are beneficial to maintaining the integrity of the lung structure.14,15
Fatty acid binding protein 4 (FABP4, adipocyte-FABP, aP2), a small cytosolic lipid-binding protein with a molecular weight of approximately 15 kDa, plays an important role in regulation of glucose and lipid homeostasis as well as inflammation through its actions in adipocytes and macrophages.16,17 Previous studies have also indicated a significant role for this protein in allergic asthma, although the exact mechanism underlying this effect is not clear.18 In recent studies, we have detected FABP4 expression in a subset of endothelial cells in several tissues and identified FABP4 as a target of the VEGF/VEGFR2 signaling pathway.19,20 With the use of in vitro models, we demonstrated that FABP4 plays a proangiogenic role in endothelial cells by promoting cell proliferation, migration, survival, and morphogenesis.21 Furthermore, we found that several angiogenic pathways in endothelial cells are regulated by FABP4, including stem cell factor/c-kit and endothelial nitric oxide synthase (eNOS). Interestingly, endothelial cell FABP4 expression is primarily detected in bronchial circulation-derived vessels in the lung.19,22 Taking advantage of this intriguing expression pattern, we have recently demonstrated that FABP4-expressing bronchial vasculature undergoes an expansion in bronchopulmonary dysplasia, a common chronic lung disease of premature infants, similar to that observed in asthma.22
Collectively, these studies have suggested that FABP4, as a downstream target of VEGF, could play a role in the regulation of pathologic airway angiogenesis that occurs in several inflammatory lung diseases, including asthma. Inhibition of FABP4 could have a key advantage over VEGF blockers because of its restricted expression pattern in the normal lung. Thus, we hypothesized that VEGF-induced bronchial angiogenesis and inflammation may be regulated by endothelial cell FABP4 in vivo. To investigate this hypothesis, we took advantage of the VEGF-TG (transgenic) mouse model that develops airway angiogenesis and inflammation by inducible overexpression of VEGF165 under a Clara cell 10-kDa promoter.12,23 We also explored the clinical relevance by examining the expression of FABP4 in endobronchial biopsy samples obtained from asthmatic subjects.
Materials and Methods
Animals and Human Specimens
Dual transgenic CC10-rtTA-VEGF (VEGF-TG) mice were generated at Yale University as previously described.12 Male VEGF-TG heterozygote mice were bred with female FABP4−/− mice (both on C57BL/6J background as previously described24) to generate the VEGF-TG/FABP4−/− mouse line. Five- to six-week-old transgenic and wild-type (WT) or FABP4−/− littermate control mice were given water that contained 0.5 mg/mL doxycycline hydrochloride (dox-water; Sigma Chemical Co., St. Louis, MO) and were sacrificed at various intervals thereafter. Tracheas were harvested and snap frozen or fixed in 10% buffered formalin. The Harvard Medical Area Standing Committee on Animals approved all animal procedures. Human samples were obtained according to a protocol that was approved by the Cleveland Clinic Institutional Review Board.
Immunohistochemistry and Immunofluorescence Analysis
Immunohistochemistry and immunofluorescence were performed on formalin-fixed, paraffin-embedded tissue sections as previously described.22 All primary antibody incubations were performed overnight at 4°C. The primary antibodies were used at the following concentrations: rabbit polyclonal anti-FABP4 (Abcam, Cambridge, MA; catalog no. 13979), 1:200; rabbit monoclonal anti–Ki-67 (Vector Laboratories Inc., Burlingame CA), 1:200; rat monoclonal anti-mouse CD31 (Dianova, Germany), 1:20; and mouse monoclonal anti-human CD31 (Dako, Carpenteria, CA), 1:50. Antigen retrieval was performed for CD31 and Ki-67 with citrate buffer (Vector Laboratories Inc.) at 95°C for 15 minutes. For double immunofluorescence, secondary antibodies were Alexa Fluor 594 goat anti-rat or anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes Inc., Eugene, OR). After immunostaining of mouse sections, slides were coded, and six to eight images per sample were randomly selected and captured at ×200 or ×400 magnification for quantification of Ki-67- and CD31-expressing cells, respectively, by a blinded investigator (E.G.). The area between the epithelial basement membrane and the posterior border of the cartilage plates in the tracheal mucosa was measured with the NIS-elements BR2.30 software (Nikon, Tokyo, Japan), and the number of immunoreactive cells in these areas was quantified in a blinded fashion. Human endobronchial biopsy specimens were immunostained for FABP4, and the total number of FABP4-immunoreactive vessels in the subepithelial area that extended 100 μm beneath the epithelial basement membrane was similarly quantified and normalized to the total area.
Quantitative Real-Time PCR Analysis
Tracheas were homogenized, and total RNA was isolated with TRIZOL (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized with SuperScript First-Strand Synthesis System for Real-Time-PCR (Invitrogen) according to the manufacturer’s instructions. Real-time PCR reaction was performed in a 20-μL volume with SYBR Green (Bio-Rad, Hercules, CA) with the use of pooled cDNA samples. The PCR primers are listed in Table 1.
Table 1.
Real-Time PCR Primer Sequences
| Gene | Primer sequences |
|---|---|
| Cyclophilin A | |
| Forward | 5′-TCTGAGCACTGGAGAGAAAGGA-3′ |
| Reverse | 5′-TATGGCGTGTAAAGTCACCACC-3′ |
| eNOS | |
| Forward | 5′-ATGCCTACAGCATTGGTTGCAAGG-3′ |
| Reverse | 5′-AGCATATGAAGAGGGCAGCAGGAT-3′ |
| FABP4 | |
| Forward | 5′-TCACCATCCGGTCAGAGAGTA-3′ |
| Reverse | 5′-GCCATCTAGGGTTATGATGCTC-3′ |
| IL-β1 | |
| Forward | 5′-ACGGACCCCAAAAGATGAAG-3′ |
| Reverse | 5′-TTCTCCACAGCCACAATGAG-3′ |
| MCP1/CCL2 | |
| Forward | 5′-GTCCCTGTCATGCTTCTGG-3′ |
| Reverse | 5′-GCTCTCCAGCCTACTCATTG-3′ |
| SCF | |
| Forward | 5′-TCAAGAGGTGTAATTGTGGACG-3′ |
| Reverse | 5′-GGGTAGCAAGAACAGGTAAGG-3′ |
Bone Marrow Transplantation
VEGF-TG and VEGF-TG/FABP4−/− mice were irradiated with 12 Gy in two split doses given 4 hours apart.24 WT and FABP4−/− mice of matching age were sacrificed on the same day, and bone marrow was harvested from the right tibia and femur under sterile conditions. Two hours after irradiation, VEGF-TG and VEGF-TG/FABP4−/− mice were injected with 2 × 106 bone marrow cells in 150 μL of sterile saline from FABP4−/− and WT mice, respectively, via the tail vein. The mice were administered dox-water for 3 or 7 days and euthanized 8 to 9 weeks after bone marrow transplantation (BMT). Tracheas were harvested and processed as described in Animals and Human Specimens.
Statistical Analysis
Results are presented as means ± SEMs unless otherwise noted. Statistical significance was determined with Kruskal-Wallis and U-tests for ordinal and Fisher Exact test for nominal variables. P values <0.05 were considered significant.
Results
Endothelial Cell FABP4 Expression Is Induced by VEGF in the Mouse Airway Mucosa
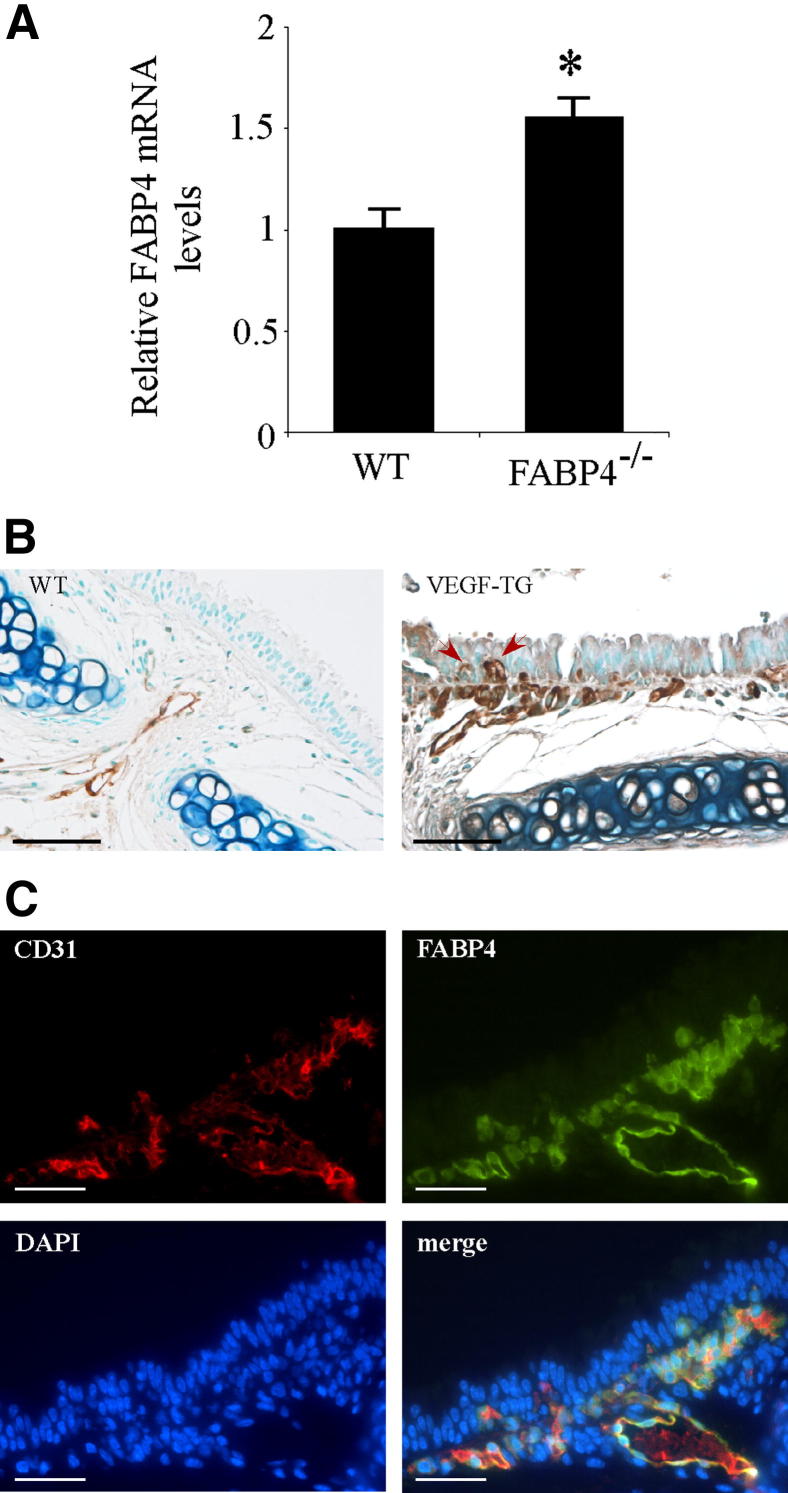
To characterize the relation between VEGF and FABP4 in vivo, WT and VEGF-TG mice were given dox-water for 2 weeks and sacrificed. Tracheas were harvested, and mRNA was isolated and analyzed by reverse transcription real-time PCR for FABP4 expression. Relative mRNA levels of FABP4 were significantly increased in VEGF-TG mice than in WT mice (P < 0.05) (Figure 1A). The localization of FABP4 on tracheal sections was determined by immunohistochemistry in mice given dox-water for 3 days (Figure 1B). In control WT mouse tracheas, FABP4 immunoreactivity was observed in endothelial cells of capillaries and small blood vessels in the lamina propria. In VEGF-TG mouse tracheas, FABP4-immunoreactive endothelial cells were detected in the same location but were increased in number. Furthermore, some FABP4+ capillaries had expanded to reach an intraepithelial location as previously reported.23 Double-immunofluorescence for CD31, a pan-endothelial cell marker, and FABP4 confirmed the endothelial cell localization of FABP4 in mouse tracheal sections (Figure 1C). FABP4 was colocalized with CD31 in most but not all of the CD31+ endothelial cells.
Figure 1.
Endothelial cell FABP4 expression is induced by VEGF in the airway mucosa. A: WT and VEGF-TG mice were given dox-water for 2 weeks (n = 6 per group). Tracheas were harvested and snap frozen. RNA was isolated and reverse transcribed to first-strand cDNA, and real-time PCR for FABP4 was performed. Bar graph represents means ± SEM values. *P < 0.05. B: VEGF-TG mice were given dox-water for 3 days (n = 6 per group). Tracheas were harvested, fixed in 10% formalin, embedded in paraffin, and immunostained for FABP4. Representative images are shown. Arrows indicate intraepithelial capillaries with FABP4+ endothelial cells. C: Representative images of double immunofluorescence analysis for FABP4 and CD31 on mouse tracheal sections after 3 days of dox-water treatment. Scale bars: 100 μm (B); 25 μm (C).
FABP4-Knockout Mice Exhibit Attenuated Airway Angiogenesis in Response to VEGF
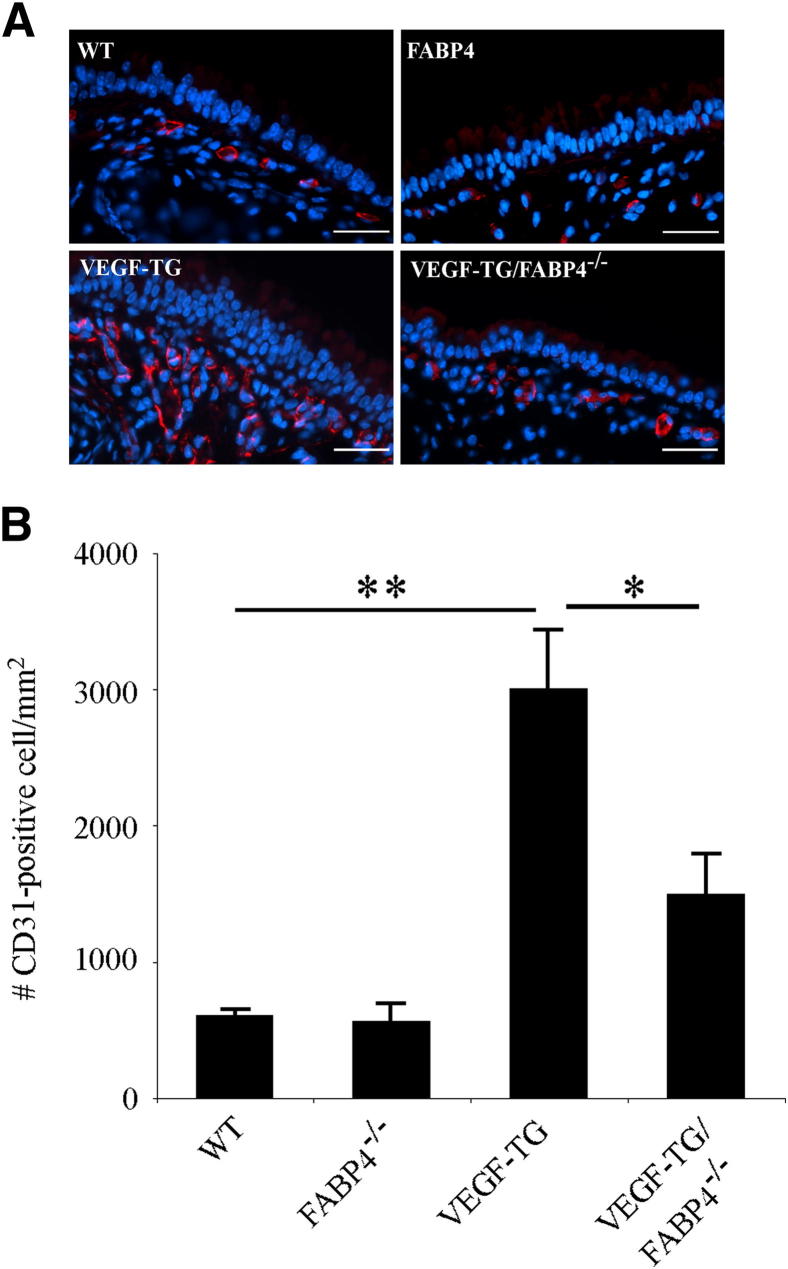
To determine whether FABP4 plays a role in VEGF-induced airway angiogenesis, VEGF-TG and FABP4−/− mouse lines were crossed. VEGF-TG and VEGF-TG/FABP4−/− mice and their WT and FABP4−/− littermates were given dox-water for 3 days, based on pilot experiments that showed an obvious increase in vascular density, which was most amenable to quantification, at this time point. Tracheas were harvested, and immunofluorescence analysis for CD31 was performed on formalin-fixed, paraffin-embedded sections (Figure 2A). Quantification of CD31+ cells did not show any differences between WT and FABP4−/− mice, whereas the number of CD31+cells was significantly higher in the VEGF-TG group than in the WT group as expected (P < 0.01) (Figure 2B). Although the number of CD31+ cells was higher in the VEGF-TG/FABP4−/− group than in the WT or FABP4−/− groups, they were approximately 50% lower than in the VEGF-TG mice (P < 0.05). Thus, FABP4 deficiency significantly attenuated VEGF-induced airway angiogenesis in mice.
Figure 2.
FABP4 deficiency attenuates VEGF-induced angiogenesis in mouse airways. A: Mice were given dox-water for 3 days. Tracheas were harvested, fixed in 10% formalin, and embedded in paraffin. Immunofluorescence analysis was performed for CD31. Representative images are shown. Scale bar = 25 μm. B: CD31+ endothelial cells localized between the airway lumen and posterior border of the cartilage plates were counted and normalized to the area described. Bar graph represents means ± SEM values from 5 to 7 mice per group. ∗P < 0.05, ∗∗P < 0.01.
FABP4-Knockout Mice Show Decreased Cell Proliferation in Response to VEGF
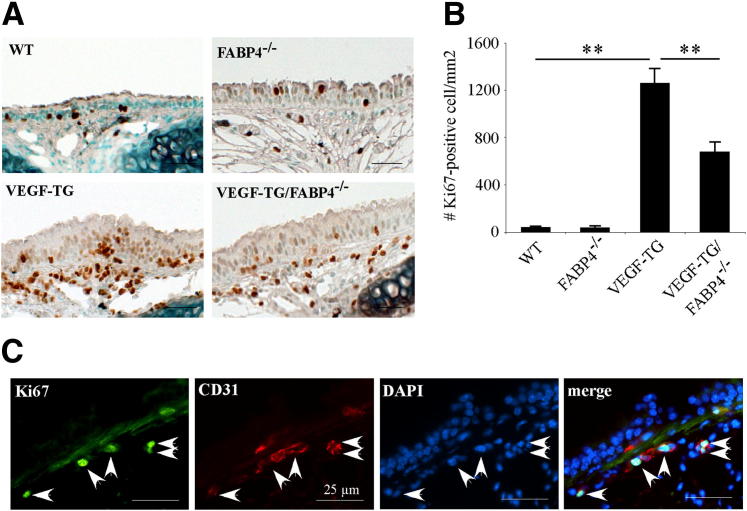
To determine whether VEGF-induced endothelial cell proliferation is regulated by FABP4, immunohistochemical analysis for the proliferation marker Ki-67 was performed on mouse tracheal sections (Figure 3A). The number of Ki-67+ cells was similar in WT and FABP4−/− groups and, as expected, was significantly higher in the VEGF-TG mice than in these two groups (P < 0.01) (Figure 3B). In accordance with the CD31 data, a significantly lower number of Ki-67+ cells was detected in the VEGF-TG/FABP4−/− mice than in the VEGF-TG mice (P < 0.01). Double immunofluorescence for Ki-67 and CD31 showed that most Ki-67+ cells were colocalized with CD31 and, thus, were endothelial cells (Figure 3C).
Figure 3.
FABP4 deficiency impairs cell proliferation in VEGF-TG mouse airways. A: WT, FABP4−/−, VEGF-TG, and VEGF-TG/FABP4−/− mice were given dox-water for 3 days. Tracheas were harvested, fixed in 10% formalin, embedded in paraffin, and immunostained for the proliferation marker Ki-67. Representative images are shown. B: Ki-67+ cell nuclei localized between the airway lumen and posterior border of the cartilage plates were counted and normalized to the area described. Bar graph represents means ± SEM values from 5 to 6 mice per group. ∗∗P < 0.01. C: Representative images of double immunofluorescence for CD31 and Ki-67 are shown. Arrows indicate colocalization of CD31 and Ki-67 in endothelial cells. Scale bars: 25 μm (B and C).
FABP4-Knockout Mice Exhibit Attenuated Airway Inflammation in Response to VEGF
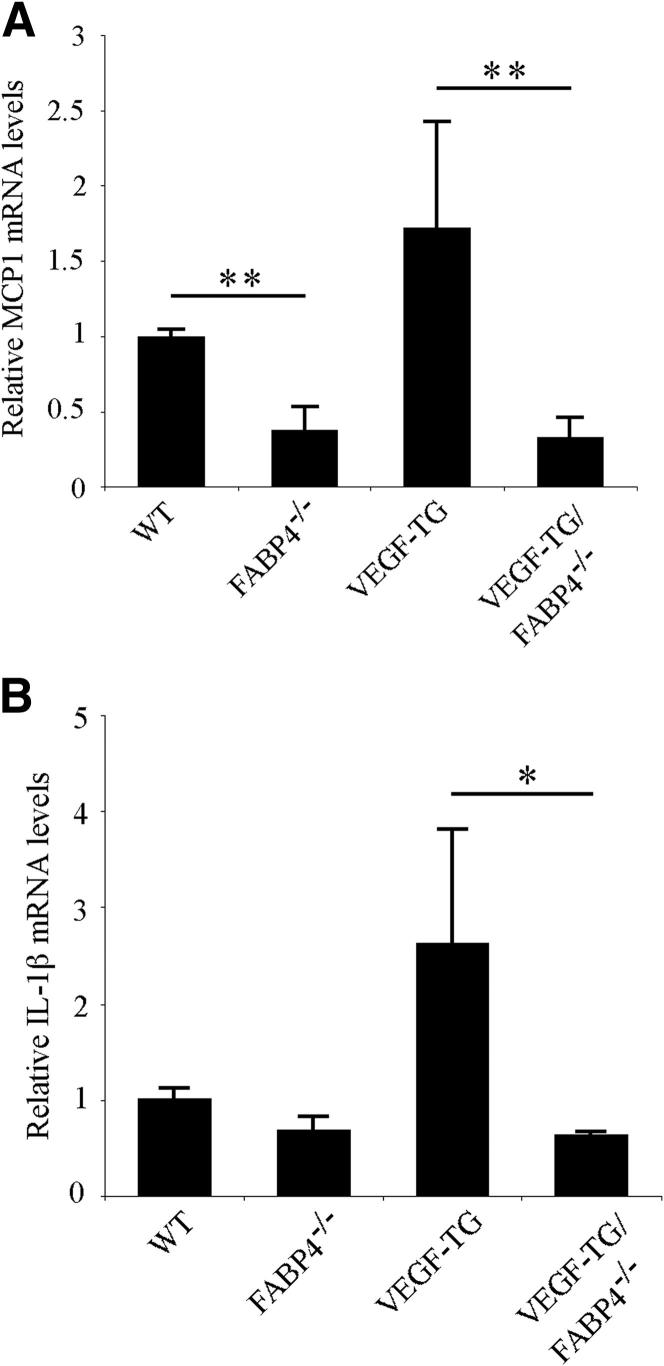
To determine whether FABP4 had an effect on VEGF-induced airway inflammation, we analyzed the mRNA expression levels of two key proinflammatory mediators, monocyte chemeotactic protein (MCP)-1 and IL-1β, in the trachea by real-time PCR (Figure 4). Relative MCP-1 mRNA level was significantly reduced in FABP4−/− mouse tracheas than in WT samples (P < 0.01) (Figure 4A). MCP-1 mRNA levels was also significantly decreased in the VEGF-TG/FABP4−/− mice than in the VEGF-TG samples (P < 0.01). Similarly, the mRNA levels of IL-1β, another key proinflammatory mediator released by activated macrophages, was significantly reduced in the VEGF-TG/FABP4−/− tracheas than in the VEGF-TG samples (Figure 4B).
Figure 4.
FABP4 deficiency ameliorates VEGF-induced airway inflammation. WT, FABP4−/−, VEGF-TG, and VEGF-TG/FABP4−/− mice were given dox-water for 14 days (n = 6 per group). Total RNA was isolated, and real-time PCR was performed to determine the relative steady-state mRNA expression levels of the proinflammatory cytokines MCP1 (A) and IL-1β (B). Bar graphs represent means ± SEM values. ∗P < 0.05, ∗∗P < 0.01.
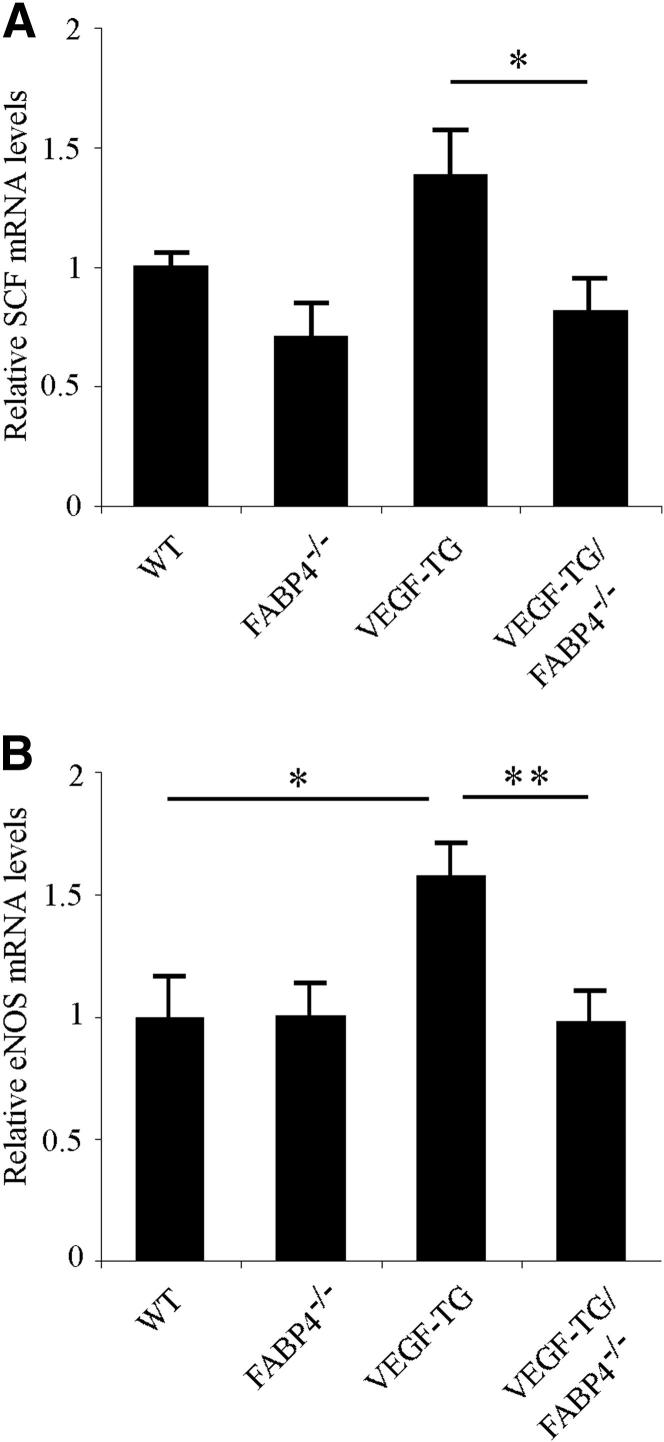
FABP4 Deficiency Is Associated With Decreased Tissue mRNA Levels of SCF and eNOS
In previous in vitro studies, we have identified stem cell factor (SCF) as a key mediator of decreased angiogenic function in cultured FABP4-deficient endothelial cells and FABP4−/− aortic explants.21 To determine whether SCF played a role in the attenuated angiogenic response in VEGF-TG/FABP4−/− mice, we analyzed SCF mRNA levels in mouse tracheas after 14 days of dox-water administration (Figure 5A). Consistent with our in vitro data, SCF mRNA levels were decreased by approximately 50% in VEGF-TG/FABP4−/− tracheas than in VEGF-TG samples (P < 0.05). Similar to our findings in human umbilical vein endothelial cells, VEGF induced SCF expression in vivo, but this difference did not reach a statistical significance. We have previously reported that the expression of eNOS, which is a critical mediator of VEGF-induced pulmonary responses, including angiogenesis, is also regulated by FABP4 in human umbilical vein endothelial cells.21,25,26 To determine whether eNOS was regulated by FABP4 in vivo, we examined eNOS mRNA levels in VEGF-TG mouse tracheas after 14 days of dox-water treatment. As previously reported, eNOS levels were significantly induced in VEGF-TG mice than in WT mice.25 No differences were observed in eNOS mRNA levels between WT and FABP4−/− mice. VEGF induced eNOS mRNA levels as expected (P < 0.05), and VEGF-TG/FABP4−/− mice showed significantly decreased levels of eNOS than did VEGF-TG mice (P < 0.01) (Figure 5B). Thus, these in vivo data support our previous in vitro findings and suggest that FABP4 modulates VEGF-induced responses in murine airways, at least in part, via regulation of SCF and eNOS pathways.
Figure 5.
FABP4 regulates SCF and eNOS expression in mouse airways. WT, FABP4−/−, VEGF-TG, and VEGF-TG/FABP4−/− mice were given dox-water for 14 days (n = 6 per group). Tracheas were harvested, and total RNA was isolated. Real-time PCR was performed to determine the relative steady-state mRNA expression levels of SCF and eNOS. Bar graphs represent means ± SEM values. ∗P < 0.05, ∗∗P < 0.01.
Endothelial Cell FABP4 Regulates VEGF-Mediated Airway Angiogenesis
In addition to endothelial cells, FABP4 is expressed in macrophages,27,28 which have the capacity to modulate angiogenic responses.29,30 To determine the contribution of macrophages or other bone marrow-derived cells to the attenuated neovascularization and airway inflammation in FABP4−/− mice, chimeric mice were generated by BMTs. VEGF-TG and VEGF-TG/FABP4−/− mice were lethally irradiated and reconstituted with FABP4−/− or WT bone marrow, respectively. The mice were allowed to recover for 8 weeks and then were given dox-water to induce VEGF expression. The absolute numbers of CD31− and Ki-67+ cells in the chimeric mice were lower than in the mice that did not receive BMT, likely because of their older age at the time of VEGF induction. However, similar differences to those observed in the VEGF-TG versus VEGF-TG/FABP4−/− mice persisted in the chimeric mice. Thus, VEGF-TG mice reconstituted with FABP4−/− hematopoietic cells (VEGF-TGch) had significantly higher number of CD31+ endothelial cells (P < 0.05) and Ki-67+ cells (P < 0.01) than VEGF-TG/FABP4−/− mice reconstituted with WT hematopoietic cells (VEGF-TG/FABP4−/−ch) (Figure 6, A and B, and Supplemental Figure S1). These results indicate that lack of FABP4 in resident endothelial cells is responsible for the attenuated neovascular responses to VEGF in FABP4−/− mice.
Figure 6.
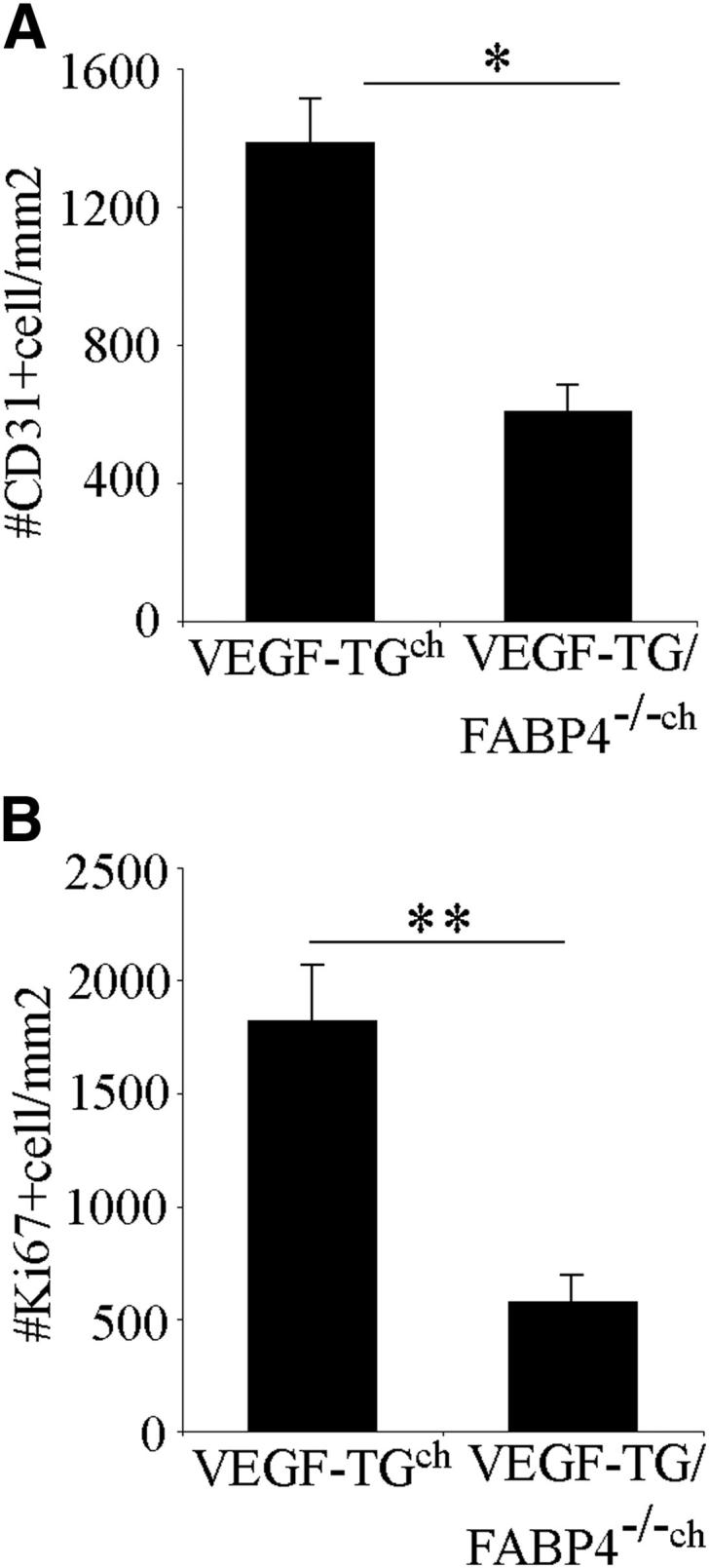
Endothelial-cell FABP4 regulates VEGF-induced airway angiogenesis. VEGF-TG and VEGF-TG/FABP4−/− mice were lethally irradiated and reconstituted with FABP4−/− (VEGF-TGch) or WT bone marrow (VEGF-TG/FABP4−/−ch), respectively. The mice were allowed to recover for 8 weeks and then were given dox-water for 3 days. The number of CD31+ (A) and Ki-67+ (B) cells were quantified. Bar graphs represent means ± SEM values from 6 mice per group. ∗P < 0.05, ∗∗P < 0.01.
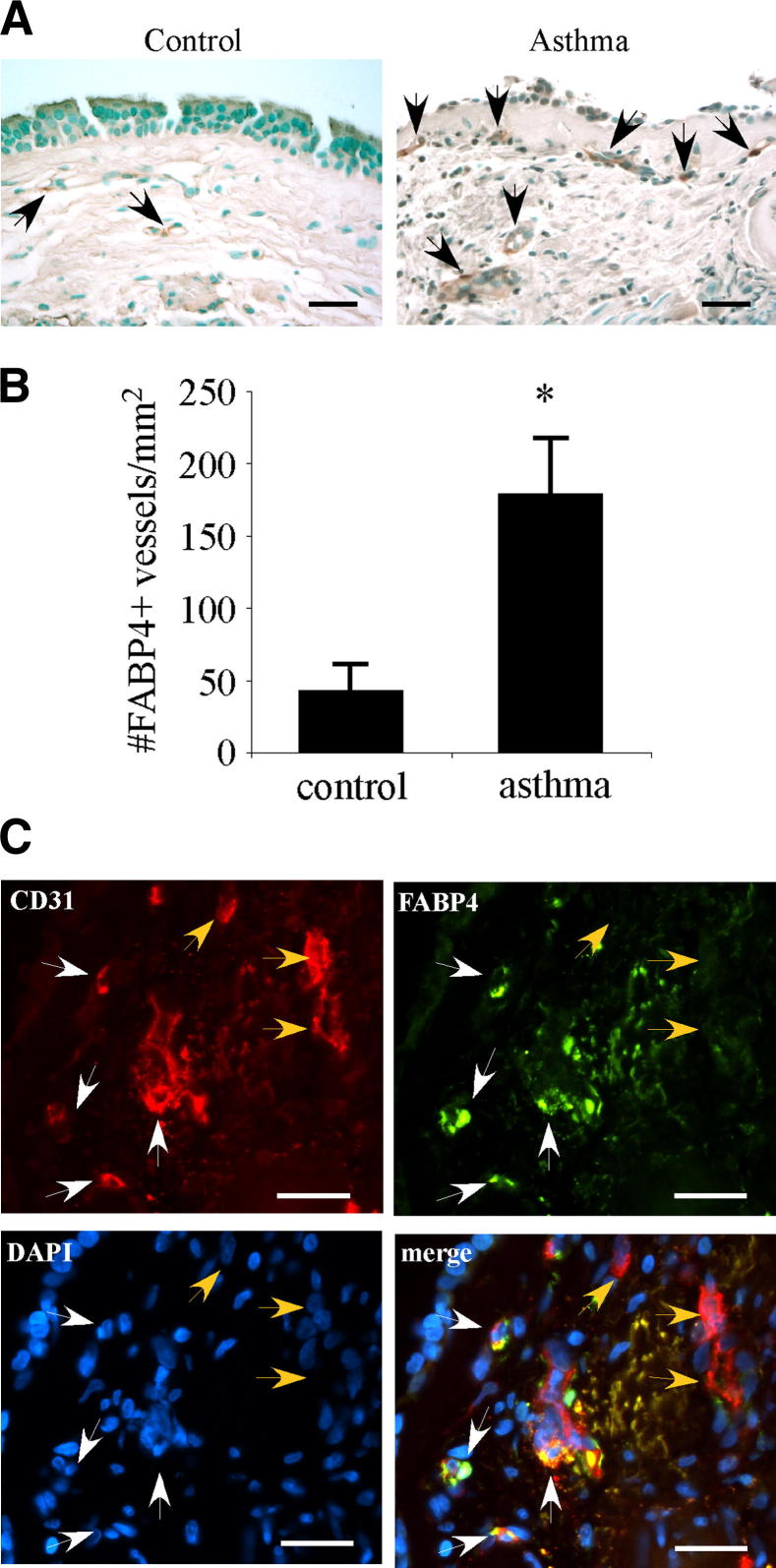
FABP4+ Vessel Density Is Increased in the Asthmatic Airways
We analyzed FABP4 expression in endobronchial biopsy samples obtained from patients with asthma and control subjects. Patient characteristics are shown in Table 2. In control specimens, FABP4 immunoreactivity was detected in rare vascular endothelial cells in the lamina propria (Figure 7A). In asthma samples, several blood vessels in the lamina propria harbored FABP4+endothelial cells. Although most airway epithelial cells were denuded in asthma samples, some were noted to have faint diffuse FABP4 immunoreactivity. Quantification of FABP4+ blood vessels showed that they were significantly increased in asthma samples compared with controls (P < 0.05) (Figure 7B). Double immunofluorescence for FABP4 and CD31 in asthma samples showed colocalization of FABP4 and CD31 in some vascular endothelial cells, most of which were in closer proximity to the airway epithelium than the ones that only expressed CD31 (Figure 7C).
Table 2.
Patient Characteristics
| Patient characteristics | Control (n = 5) | Asthma (n = 6) | P value |
|---|---|---|---|
| Age, means ± SD (years) | 33.6 ± 6.4 | 37.4 ± 10.7 | |
| Sex, N/n | 3/2 | 2/4 | |
| FEV1, means ± SD (%) | 94.3 ± 10.8 | 76.3 ± 17.4 | 0.05 |
| FVC, means ± SD (%) | 100.1 ± 11.2 | 93.0 ± 18.1 | |
| FEV1/FEC, means ± SD | 0.80 ± 0.06 | 0.68 ± 0.06 | <0.05 |
| Exhaled NO, means ± SD (ppb) | 17.6 ± 7.0 | 39.7 ± 32.1 | 0.05 |
FEV1, forced expiratory volume 1 second; FVC, forced vital capacity; NO, nitric oxide.
Figure 7.
FABP4+ vessel number is increased in human asthmatic airways. A: FABP4 immunohistochemistry was performed on endobronchial biopsy specimens from healthy control subjects and patients with asthma. A representative case from each group is shown. Black arrows indicate FABP4+vessels. B: The total number of FABP4+vessels in the subepithelial area extending 100 μm beneath the epithelial basement membrane was quantified and normalized to the total area (n = 5 to 6 per group). Bar graph represents means ± SEM values. ∗P < 0.05. C: Representative images of double immunofluorescence analysis for FABP4 and CD31 on an endobronchial biopsy specimen from a patient with asthma. White arrows indicate endothelial cells, where FABP4 is coexpressed with CD31, whereas orange arrows mark endothelial cells that only express CD31. Scale bars: 25 μm (A and C).
Discussion
The findings of this study demonstrate that FABP4 deficiency significantly attenuates VEGF-induced airway angiogenesis and inflammation in mice. Through generation of chimeric mouse models, we showed that endothelial cell FABP4 is responsible for modulating VEGF-induced responses in the murine trachea. Furthermore, we found an increased density of FABP4-immunoreactive blood vessels in the asthmatic airways, thus providing evidence for the clinical relevance and translational potential of our findings.
In a recent study, FABP4−/− mice were found to have attenuated airway inflammation with decreased eosinophils in the ovalbumin-induced model of asthma.18 In that study, generation of chimeric mice confirmed that nonhematopoietic cells, most likely airway epithelial cells, regulated allergic lung inflammation in FABP4−/− mice. The ovalbumin-induced murine model of asthma was not suitable for the purposes of our study because ovalbumin administration does not induce airway angiogenesis in mice (E.G. and S.C., unpublished observations). Therefore, we used the VEGF-TG mouse model, which exhibits both airway angiogenesis and type 2 helper T cell type inflammation. Because mouse lungs do not have a functional bronchial circulation below the level of the main stem bronchus31,32 and FABP4 is primarily expressed in the bronchial/systemic vasculature-derived endothelial cells,22 we focused our histologic analysis to the level of the trachea, where angiogenesis was previously characterized in detail in this model.23 Our findings showed that the lack of FABP4 confers protection against VEGF-induced pathologic angiogenesis as well as airway inflammation. Previous studies have shown decreased inflammatory activity in association with decreased NF-κB and enhanced peroxisome proliferator-activated receptor-γ activity in FABP4-deficient macrophages.33 Because inflammation and angiogenesis are closely linked processes34 and macrophages can contribute to neovascularization,30 we examined the possibility that lack of macrophage FABP4 could have contributed to the observed phenotype in the VEGF-TG/FABP4−/− mice. Interestingly, our findings in chimeric mice show that macrophage FABP4 does not play a role in regulation of VEGF-induced airway angiogenesis. In previous studies, we have found decreased expression of endothelial cell activation markers, such as E-selectin and intercellular adhesion molecule 1, in FABP4-deficient endothelial cells.21 Taken together, our findings suggest that endothelial cell FABP4 may have a novel role in coordinated regulation of endothelial cell activation and angiogenesis. Regulation of eNOS by FABP4 appears to be a potential mechanism that could account for some of these effects because VEGF-induced neovascularization as well as airway inflammation are, at least in part, eNOS dependent.25 Another potential mechanism for the attenuated airway inflammation in VEGF-TG/FABP4−/− mice may be a decrease in trafficking of inflammatory cells and mediators because of decreased number of neovessels.
Although our findings are most relevant for asthma-related vascular remodeling in the airways, they also provide some general insights into the potential role of FABP4 in expansion of bronchial circulation in other diseases, such as bronchopulmonary dysplasia22 and idiopathic pulmonary hypertension.35 The lung has a dual circulation through pulmonary and bronchial vasculatures.36 Bronchial vasculature, which arises from the systemic circulation, provides blood supply to the areas around large airways, visceral pleura, and vasa vasorum. Several lines of evidence from animal studies as well as humans have suggested that the bronchial vasculature has a higher angiogenic capacity than the pulmonary vasculature.32,37 However, the underlying causes for the differential angiogenic responses of these two vascular beds remains largely unknown. The findings of our study strongly suggest that FABP4 may be a key mediator that renders a pro-angiogenic and pro-inflammatory phenotype to the bronchial/systemic endothelium.
FABP4−/− mice are viable, fertile, and do not show any developmental phenotypes.38 Thus, FABP4 does not appear to interfere with VEGF-mediated vasculogenesis or angiogenesis in the embryo, but rather to selectively regulate postnatal angiogenesis, a phenomenon that has been noted also for other effector molecules, such as Notch 1.39 However, we cannot exclude the possibility of compensation by other members of the FABP family, particularly endothelial FABP5, in FABP4−/− embryos.
In endobronchial biopsy specimens from patients with asthma, we observed several vessels with CD31+endothelial cells that lacked FABP4 expression. Furthermore, FABP4+ vessels were noted to have a closer location to the bronchial epithelium than the FABP4− vessels. These observations support the notion that endothelial cell FABP4 is primarily expressed in vessels undergoing remodeling and/or angiogenesis and may serve as a more reliable marker of airway neovascularization than a pan-endothelial cell marker, such as CD31.
In conclusion, our findings establish a novel role for endothelial cell FABP4 in promotion of VEGF-induced airway angiogenesis and inflammation in vivo and, in conjunction with previous studies,18 suggest that FABP4 inhibition should be explored further as a potential therapeutic strategy in asthma because it appears to target at least two diverse pathologic processes that involve airway epithelial and endothelial cells in this disease.
Acknowledgment
We thank Dr. Alan Fine (Boston University, Boston, MA) for helpful discussions.
Footnotes
Supported by grants from the American Heart Association (11GRNT4900002 to S.C.) and BWH Biomedical Research Institute (S.C.) and in part by NIH grant DK064360 (G.S.H.).
Current address of H.E., Division of Hematology/Oncology, University of Virginia School of Medicine, Charlottesville, Virginia; of M.T., Department of Pharmacology, Columbia University, College of Physicians and Surgeons, New York, New York.
Supplemental Data
Endothelial-cell FABP4 regulates VEGF-induced airway angiogenesis. VEGF-TG and VEGF-TG/FABP4−/− mice were lethally irradiated and reconstituted with FABP4−/− (VEGF-TGch) or WT bone marrow (VEGF-TG/FABP4−/−ch), respectively. The mice were allowed to recover for 8 weeks and were then given dox-water for 3 days (n = 6 per group). Immunohistochemistry for CD31 (A) and Ki-67 (B) was performed on paraffin-embedded, formalin-fixed tracheal sections. Representative images are shown. Scale bars: 25 μm (A and B).
References
- 1.Al-Muhsen S., Johnson J.R., Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Hoshino M., Nakamura Y., Hamid Q.A. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 3.Hoshino M., Takahashi M., Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 4.Simcock D.E., Kanabar V., Clarke G.W., O’Connor B.J., Lee T.H., Hirst S.J. Proangiogenic activity in bronchoalveolar lavage fluid from patients with asthma. Am J Respir Crit Care Med. 2007;176:146–153. doi: 10.1164/rccm.200701-042OC. [DOI] [PubMed] [Google Scholar]
- 5.Feltis B.N., Wignarajah D., Zheng L., Ward C., Reid D., Harding R., Walters E.H. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006;173:1201–1207. doi: 10.1164/rccm.200507-1105OC. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Wilson J.W. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 7.Kuwano K., Bosken C.H., Pare P.D., Bai T.R., Wiggs B.R., Hogg J.C. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Resp Dis. 1993;148:1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 8.Barbato A., Turato G., Baraldo S., Bazzan E., Calabrese F., Panizzolo C., Zanin M.E., Zuin R., Maestrelli P., Fabbri L.M., Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 9.Wilson J. The bronchial microcirculation in asthma. Clin Exp Allergy. 2000;30(Suppl 1):51–53. doi: 10.1046/j.1365-2222.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 10.Detoraki A., Granata F., Staibano S., Rossi F.W., Marone G., Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65:946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonald D.M. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.G., Link H., Baluk P., Homer R.J., Chapoval S., Bhandari V., Kang M.J., Cohn L., Kim Y.K., McDonald D.M., Elias J.A. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guntur V.P., Reinero C.R. The potential use of tyrosine kinase inhibitors in severe asthma. Current opinion in allergy and clinical immunology. 2012;12:68–75. doi: 10.1097/ACI.0b013e32834ecb4f. [DOI] [PubMed] [Google Scholar]
- 14.Gerber H.P., Dixit V., Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 15.Siner J.M., Jiang G., Cohen Z.I., Shan P., Zhang X., Lee C.G., Elias J.A., Lee P.J. VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 2007;21:1422–1432. doi: 10.1096/fj.06-6661com. [DOI] [PubMed] [Google Scholar]
- 16.Hertzel A.V., Bernlohr D.A. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 17.Makowski L., Hotamisligil G.S. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shum B.O., Mackay C.R., Gorgun C.Z., Frost M.J., Kumar R.K., Hotamisligil G.S., Rolph M.S. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmasri H., Karaaslan C., Teper Y., Ghelfi E., Weng M., Ince T.A., Kozakewich H., Bischoff J., Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cataltepe O., Arikan M.C., Ghelfi E., Karaaslan C., Ozsurekci Y., Dresser K., Li Y., Smith T.W., Cataltepe S. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. 2012;38:400–410. doi: 10.1111/j.1365-2990.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 21.Elmasri H., Ghelfi E., Yu C.W., Traphagen S., Cernadas M., Cao H., Shi G.P., Plutzky J., Sahin M., Hotamisligil G., Cataltepe S. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15:457–468. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghelfi E., Karaaslan C., Berkelhamer S., Akar S., Kozakewich H., Cataltepe S. Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2011;45:550–556. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baluk P., Lee C.G., Link H., Ator E., Haskell A., Elias J.A., McDonald D.M. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuhashi M., Fucho R., Gorgun C.Z., Tuncman G., Cao H., Hotamisligil G.S. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandari V., Choo-Wing R., Chapoval S.P., Lee C.G., Tang C., Kim Y.K., Ma B., Baluk P., Lin M.I., McDonald D.M., Homer R.J., Sessa W.C., Elias J.A. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhandari V., Choo-Wing R., Lee C.G., Yusuf K., Nedrelow J.H., Ambalavanan N., Malkus H., Homer R.J., Elias J.A. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol. 2008;39:420–430. doi: 10.1165/rcmb.2007-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makowski L., Boord J.B., Maeda K., Babaev V.R., Uysal K.T., Morgan M.A., Parker R.A., Suttles J., Fazio S., Hotamisligil G.S., Linton M.F. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erbay E., Babaev V.R., Mayers J.R., Makowski L., Charles K.N., Snitow M.E., Fazio S., Wiest M.M., Watkins S.M., Linton M.F., Hotamisligil G.S. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuno Y., Nakamura-Ishizu A., Kishi K., Suda T., Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264–5272. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nucera S., Biziato D., De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Develop Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 31.Mitzner W., Lee W., Georgakopoulos D., Wagner E. Angiogenesis in the mouse lung. Am J Pathol. 2000;157:93–101. doi: 10.1016/S0002-9440(10)64521-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitzner W., Wagner E.M. Vascular remodeling in the circulations of the lung. J Appl Physiol. 2004;97:1999–2004. doi: 10.1152/japplphysiol.00473.2004. [DOI] [PubMed] [Google Scholar]
- 33.Makowski L., Brittingham K.C., Reynolds J.M., Suttles J., Hotamisligil G.S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arroyo A.G., Iruela-Arispe M.L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montani D., Perros F., Gambaryan N., Girerd B., Dorfmuller P., Price L.C., Huertas A., Hammad H., Lambrecht B., Simonneau G., Launay J.M., Cohen-Kaminsky S., Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:116–123. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 36.Aird W.C. Phenotypic heterogeneity of the endothelium, II: representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 37.Moldobaeva A., Wagner E.M. Difference in proangiogenic potential of systemic and pulmonary endothelium: role of CXCR2. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1117–L1123. doi: 10.1152/ajplung.00370.2004. [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil G.S., Johnson R.S., Distel R.J., Ellis R., Papaioannou V.E., Spiegelman B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 39.Takeshita K., Satoh M., Ii M., Silver M., Limbourg F.P., Mukai Y., Rikitake Y., Radtke F., Gridley T., Losordo D.W., Liao J.K. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endothelial-cell FABP4 regulates VEGF-induced airway angiogenesis. VEGF-TG and VEGF-TG/FABP4−/− mice were lethally irradiated and reconstituted with FABP4−/− (VEGF-TGch) or WT bone marrow (VEGF-TG/FABP4−/−ch), respectively. The mice were allowed to recover for 8 weeks and were then given dox-water for 3 days (n = 6 per group). Immunohistochemistry for CD31 (A) and Ki-67 (B) was performed on paraffin-embedded, formalin-fixed tracheal sections. Representative images are shown. Scale bars: 25 μm (A and B).