Abstract
A dualistic pathway model of ovarian carcinoma (OvCA) pathogenesis has been proposed: type I OvCAs are low grade, genetically stable, and relatively more indolent than type II OvCAs, most of which are high-grade serous carcinomas. Endometrioid OvCA (EOC) is a prototypical type I tumor, often harboring mutations that affect the Wnt and phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling pathways. Molecular and histopathologic analyses indicate type I and II OvCAs share overlapping features, and a subset of EOCs may undergo type I→type II progression accompanied by acquisition of somatic TP53 or PIK3CA mutations. We used a murine model of EOC initiated by conditional inactivation of the Apc and Pten tumor suppressor genes to investigate mutant Trp53 or Pik3ca alleles as key drivers of type I→type II OvCA progression. In the mouse EOC model, the presence of somatic Trp53 or Pik3ca mutations resulted in shortened survival and more widespread metastasis. Activation of mutant Pik3ca alone had no demonstrable effect on the ovarian surface epithelium but resulted in papillary hyperplasia when coupled with Pten inactivation. Our findings indicate that the adverse prognosis associated with TP53 and PIK3CA mutations in human cancers can be functionally replicated in mouse models of type I→type II OvCA progression. Moreover, the models should represent a robust platform for assessment of the contributions of Trp53 or Pik3ca defects in the response of EOCs to conventional and targeted drugs.
Surgical pathologists continue to use traditional morphology-based schemes for classifying ovarian carcinomas (OvCAs). The schemes are based largely on the degree of resemblance of the OvCAs to non-neoplastic epithelia in the female genital tract. However, mounting clinicopathologic and molecular data have led Kurman and Shih1 to propose a “dualistic” model of OvCA pathogenesis in which OvCAs are divided into two main categories, type I and type II.1–3 Type I OvCAs are suggested to be low-grade, relatively indolent and genetically stable tumors that typically arise from recognizable precursor lesions such as endometriosis or so-called borderline (low malignant potential) tumors. Type I OvCAs frequently harbor somatic mutations (eg, KRAS, BRAF, CTNNB1, PTEN) that dysregulate specific cell signaling pathway factors or certain chromatin remodeling complexes (eg, ARID1A). Type I OvCAs include most endometrioid, clear cell, and mucinous carcinomas and low-grade serous carcinomas. In contrast, type II OvCAs are proposed to be high-grade, biologically aggressive tumors from their outset, with a propensity for metastasis from small-volume primary lesions. Most type II OvCAs are high-grade serous carcinomas, virtually all of which harbor mutant TP53 alleles.4 These tumors have a high level of chromosomal instability.5 Although varied gene defects besides TP53 mutation have been identified in type II tumors, with the exception of frequent genetic/epigenetic inactivation of BRCA1/2 and NF1, RB1, and CDK12 mutations, each of the somatic gene defects is found in a small fraction of tumors.6–8 Notably, recent data suggest that many (and perhaps most) high-grade serous carcinomas arise from epithelium in the fallopian tube, rather than the ovarian surface epithelium (OSE).9–11 The identification of precursor lesions with p53 protein overexpression and clonal TP53 mutations in the fallopian tube epithelium (including “p53 signature lesions” and tubal intraepithelial carcinomas) suggests TP53 mutation is an early event in the pathogenesis of most type II OvCAs.12
The dualistic pathway model represents an advance in conceptualizing OvCA pathogenesis, but the model is likely an oversimplified view of a complex group of cancers. For example, there are uncertainties about classification of clear cell carcinomas as type I versus type II, because their molecular features are more in keeping with type I tumors, but cytologic grade and clinical behavior are often more like type II tumors.3 As another example, there is significant overlap in the morphologic and molecular features of high-grade serous and high-grade endometrioid OvCAs (hereafter referred to as EOCs) such that some pathologists now default the majority of gland-forming or near-solid cytologically high-grade OvCAs to the serous category and consider “true” high-grade EOCs to be rare or nonexistent.13 In this scenario, all low-grade EOCs would be classified as type I, and the category of high-grade EOCs would be eliminated.
In a prior analysis of a substantial number of primary human EOCs, we found that mutations predicted to activate the canonical Wnt and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathways frequently co-occur in low-grade EOC, a prototypical type I OvCA.14 Activation of Wnt signaling typically occurs as a consequence of oncogenic CTNNB1 mutations, or rarely bi-allelic APC inactivation, whereas PI3K/AKT/mTOR signaling is usually activated because of PTEN inactivation and/or oncogenic PIK3CA mutations. Notably, we identified 4 of 21 tumors with gene expression and/or mutational profiles like type I EOCs, but the tumors had also acquired TP53 mutations, suggesting type I→type II progression may occur in a sizeable subset (nearly 20%) of EOCs.14 Similarly, we identified four EOCs with mutations in both PTEN and PIK3CA, rather than a single mutation dysregulating PI3K/AKT/mTOR signaling. Importantly, TP53 mutation has been associated with adverse outcome in women with endometrioid carcinomas of the endometrium and ovary,15–18 and PIK3CA mutation has been linked to poor outcome in patients with several types of cancer, including carcinomas of the endometrium, breast, and colon.19–22 The effect of PIK3CA mutations on the prognosis of patients with OvCA is unclear, because some studies have shown an adverse effect on outcome, whereas others showed no or even a favorable effect.23–25 The notion that type II tumors can progress from type I tumors is not unique to EOCs. Indeed, type I→type II progression in serous carcinomas has also been described and is often associated with acquisition of TP53 mutation.26–29
We previously described a murine model for type I human EOC in which concurrent dysregulation of Wnt and PI3K/AKT/mTOR signaling is achieved through conditional inactivation of the Apc and Pten tumor suppressor genes in the OSE.14 In this model, ovarian bursal injection of recombinant adenovirus expressing Cre recombinase (AdCre) in Apcflox/flox;Ptenfloxf/flox mice results in the development of EOC-like tumors with complete penetrance. The purpose of the present study was to determine the effect of mutant Trp53 and Pik3ca on the Apc−/−;Pten−/− murine EOC tumor phenotype as models of type I→type II progression.
Materials and Methods
Strains of Transgenic Mice
In Apcflox/flox;Ptenflox/flox mice, conditional inactivation of the Apc and Pten tumor suppressor genes in the mouse OSE (MOSE) results in ovarian tumor development with 100% penetrance.14 Trp53 LSL-R172H/+ (01XM2), Trp53R270H/+ (01XM1), and Trp53flox/flox (01XC2) mice were purchased from the National Cancer Institute (Bethesda, MD) mouse repository and cross-bred with Apcflox/flox;Ptenflox/flox mice to generate the respective triple transgenic strains. Pik3caLSL-E545K/+ mice were developed in which a conditional mutant (LSL-E545K) allele is knocked into one of the endogenous Pik3ca loci.30 Cre-mediated deletion of a lox-STOP-lox cassette inserted upstream of the first coding exon activates expression of Pik3caE545K from the endogenous locus. The mice were crossed with Apcflox/flox;Ptenflox/flox mice to generate triple transgenic Apcflox/flox;Ptenfloxflox;Pik3ca LSL-E545K/+mice. All strains were maintained on a mixed C57BL/6;FVB;129 background.
Induction of Murine Ovarian Tumors
For tumor induction, 5 × 107 plaque-forming units of replication-incompetent AdCre (purchased from the University of Michigan’s Vector Core) with 0.1% Evans blue (Sigma-Aldrich, Indianoplis, IN) were injected into the right ovarian bursal cavities of 6- to 10-week-old female mice as previously described.14,31 In each mouse, the left ovarian bursa was not injected and served as control.
Mouse Histopathology and Immunohistochemistry
All tumor-bearing mice were euthanized per the Committee on Use and Care of Animals guidelines at the University of Michigan for end-stage illness and humane endpoints and then examined at necropsy. The genital tract and other major organs were collected from each mouse, fixed in 10% (v/v) buffered formalin, and embedded in paraffin. H&E-stained tissue sections were evaluated by a board-certified surgical pathologist with expertise in gynecologic cancer diagnosis (K.R.C). Immunohistochemical staining was performed with standard methods; antigen–antibody complexes were detected with the avidin-biotin peroxidase method with the use of 3,3′-diaminobenzidine as the chromogenic substrate. Antibodies used in this study include rat anti-cytokeratin 8 (CK8, #TROMA 1; Developmental Studies Hybridoma Bank, University of Iowa); rabbit anti-p53 (Vector Laboratories Inc., Burlingame, CA), rabbit anti–phospho-AKT (Ser473; #4060; Cell Signaling Technology, Inc., Danvers, MA), rabbit anti-PTEN (clone 138G6, #9559; Cell Signaling Technology, Inc.), mouse anti–β-catenin (Transduction Laboratories, Lexington, KY), goat anti–E-cadherin (R&D Systems, Minneapolis, MN), rabbit anti–WT-1 (sc-192; Santa Cruz Biotechnology, Inc., Dallas, TX), and rabbit anti-PAX8 (NBP1-74734; Novus Biologicals, Littleton, CO).
Genotyping and Recombination Analysis of Mouse Tumors
Genomic DNA was isolated from tail snips, ovarian tumors, or other organs, and PCR was performed with primers that allowed distinction between endogenous, genetically modified, and recombined alleles (primer sequences are available on request). Representative data are shown in Supplemental Figure S1.
Statistical Analysis
Graphs were constructed and statistical functions were analyzed with GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA). Kaplan-Meier survival curves were compared separately for each experimental pair by log-rank (Mantel-Cox) tests; P < 0.05 was considered statistically significant. Metastasis between groups was compared with a χ2 contingency table test for independence.
Results
More Aggressive Phenotype of Murine Apc−/−;Pten−/− Ovarian Tumors with the Addition of Somatic Missense Trp53 Mutations
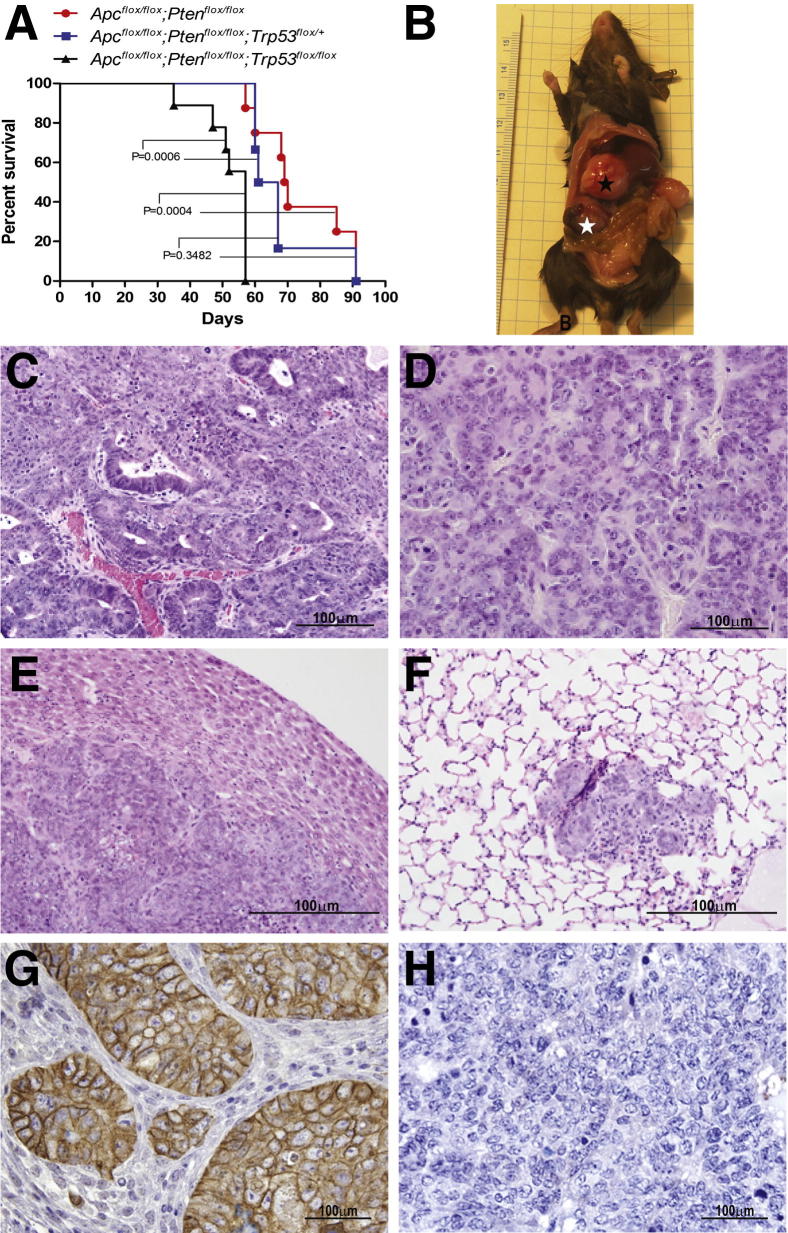
Somatic missense substitutions in the TP53 gene which result in the R175H and R273H alleles are among the most frequently identified p53 mutations in human cancers; both the R175H and R273H TP53 alleles have been studied for their loss-of-function effects on the transcription activity of p53, as well as for potential gain-of-function effects. In OvCAs, R273H, R248W, and R175H are the most common missense TP53 mutations (Catalogue of Somatic Mutations in Cancer; http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=gene&ln=TP53). As a means of modeling type I to type II progression of EOCs, we introduced the murine equivalents of the R273H (R270H) and R175H (R172H) mutations into Apcflox/flox;Ptenfloxflox mice. Specifically, Apcflox/flox;Ptenfloxflox mice were crossbred with mice that carried a conditional mutant Trp53LSL-R172H allele32 or constitutive mutant Trp53R270H allele33 to yield Apcflox/flox;Ptenfloxflox;Trp53LSL-R172H/+ and Apcflox/flox;Ptenfloxflox;Trp53R270H/+ mice. Ovarian tumors were induced by injection of AdCre into the right ovarian bursa, and tumor-bearing mice were monitored for several weeks thereafter. Mice with Apc−/−;Pten−/− ovarian tumors also expressing R172H or R270H mutant p53 had significantly shorter survival than littermate mice with Apc−/−;Pten−/− tumors that had wild-type Trp53 alleles (P = 0.0001 and P = 0.0227, respectively) (Figure 1, A and B). Similar to the Apc−/−;Pten−/− ovarian tumors we previously described, tumors expressing mutant p53 showed areas with glandular, overtly epithelial differentiation admixed with more poorly differentiated spindle-cell areas (Figure 1, C and D). Metastatic carcinoma, represented by small tumor implants on the surface of abdominal organs, including liver and kidney (Figure 1, E and F), was identified more frequently in mice expressing R172H mutant p53 than Apcflox/flox;Ptenfloxflox;Trp53+/+ tumor-bearing littermates (P = 0.025). Microscopic lung metastases were observed only in mice whose tumors expressed mutant p53, albeit in a small fraction. Immunohistochemical staining showed nuclear accumulation of p53 in the tumor cells expressing either the R172H or R270H mutant (Figure 1, G and H); in some cases p53 overexpression was focal and in others more diffuse. Data collected from ovarian tumor-bearing Apcflox/flox;Ptenfloxflox;Trp53LSL-R172H/+ and Apcflox/flox;Ptenfloxflox;Trp53R270H/+ mice are summarized in Table 1.
Figure 1.
The phenotype of murine Apc−/−;Pten−/− ovarian tumors is more aggressive with the addition of missense Trp53 mutations. Kaplan-Meier survival curves of Apcflox/flox;Ptenfloxflox;Trp53LSL-R172H/+ mice (n = 11) and Apcflox/flox;Ptenfloxflox littermate controls (n = 9) (A) and Apcflox/flox;Ptenfloxflox;Trp53R270H/+ mice (n = 11) and Apcflox/flox;Ptenfloxflox littermate controls (n = 10) (B) after ovarian bursal AdCre injection. H&E-stained sections of Apc−/−;Pten−/−;Trp53R172H/+ ovarian tumors showing glandular epithelial differentiation admixed (C) with more poorly differentiated spindle-cell areas (D). Representative metastases on the surface of the liver (E) and kidney (F; aggregate of metastatic tumor cells indicated by an arrow) are shown. Immunohistochemical staining of p53 in representative ovarian tumors arising in Apcflox/flox;Ptenfloxflox;Trp53LSL-R172H/+ (G) and Apcflox/flox;Ptenfloxflox;Trp53R270H/+ (H) mice. Scale bars: 100 μm (C–H).
Table 1.
Effects of Missense and Null Trp53 Mutations on the Apc−/−;Pten−/− Tumor Phenotype
| Trp53 genotype | Tumor size (mm3) | Metastasis (n/N) | P∗ | Lung metastasis (n/N) | Median survival (days) | P† |
|---|---|---|---|---|---|---|
| LSL-R172H/+ | 4752.7 ± 1767.8 | 8/11 | 1/11 | 57 | ||
| 0.025 | 0.0001 | |||||
| +/+ | 4632.8 ± 2178.6 | 2/9 | 0/9 | 76 | ||
| R270H/+ | 4331.2 ± 1383.3 | 4/11 | 1/11 | 71 | ||
| 0.757 | 0.0227 | |||||
| +/+ | 4838.2 ± 1868.1 | 3/10 | 0/10 | 75 | ||
| flox/flox | 3068.7 ± 877.0 | 9/9 | 1/9 | 57 | flox/flox vs flox/+, 0.0006 | |
| flox/+ | 3137.6 ± 1536.9 | 6/8 | 0.016 | 0/8 | 64 | flox/flox vs +/+, 0.0004 |
| +/+ | 2422.8 ± 1351.7 | 2/6 | 0/6 | 69.5 | flox/+ vs +/+, 0.3482 |
flox, flox (del ex2-10).
Determined by χ2 test.
Determined by log-rank test for median survival.
Mice Bearing Apc−/−;Pten−/−;Trp53−/− Tumors Have the Shortest Survival and Frequently Develop Bulky Metastatic Disease
Because null and missense Trp53 mutations have been shown to have different effects on the tumor phenotype in several mouse model systems34 and roughly 10% of TP53 mutations in human EOCs result in loss of p53 protein expression (ie, nonsense, insertion, deletion; http://www.sanger.ac.uk/search/s), we also wished to test the effects of Trp53 deletion in the Apc−/−;Pten−/− model of EOC. Notably, in humans, distant metastases are nearly eightfold more common in patients with OvCAs that carry TP53 null mutations compared with those with missense mutations.35 Apcflox/flox;Ptenfloxflox mice were crossed with mice harboring conditional knockout (Trp53flox) alleles, allowing Cre-mediated deletion of Trp53 exons 2 through 10.36 Ovarian tumors were induced in Apcflox/flox;Ptenfloxflox;Trp53flox/flox as well as Apcflox/flox;Ptenfloxflox;Trp53flox/+ and Apcflox/flox;Ptenfloxflox;Trp53+/+ littermates with bursal AdCre injection. Mice with Apc−/−;Pten−/−;Trp53−/− ovarian tumors had significantly reduced survival than mice with Apc−/−;Pten−/− tumors in which one or both Trp53 alleles were intact (P = 0.0006 and P = 0.0004, respectively). No significant difference in survival was observed between mice whose tumors had Cre-mediated deletion of one Trp53 allele compared with mice with two intact alleles (P = 0.3482) (Figure 2A and Table 1). At necropsy, all of the tumor-bearing Apcflox/flox;Ptenfloxflox;Trp53flox/flox mice had grossly visible abdominal metastases (Figure 2B). Representative photomicrographs of tumors arising in Apcflox/flox;Ptenfloxflox;Trp53flox/flox and Apcflox/flox;Ptenfloxflox;Trp53flox/+ mice are shown in Figure 2, C and D. The distribution of tumor growth outside of the ovary was similar to that seen in patients with OvCA stage IV disease, because some mice showed deep extension of tumor into the parenchyma of liver (Figure 2E) and/or kidney, and one mouse had metastasis to the lung (Figure 2F). Metastatic carcinoma was observed significantly less frequently in tumor-bearing Apcflox/flox;Ptenfloxflox;Trp53+/+ littermates (P = 0.016). Apc−/−;Pten−/−;p53−/− ovarian tumors and their metastases were poorly differentiated, but the epithelial components showed strong expression of E-cadherin (Figure 2G) and cytokeratin 8 (not shown). As expected, the tumor cells showed complete absence of p53 expression (Figure 2H).
Figure 2.
Mice bearing Apc−/−;Pten−/−;p53−/− tumors have shortest survival and develop bulky metastatic disease. A: Kaplan-Meier survival curves of Apcflox/flox;Ptenfloxflox;Trp53flox/flox (n = 9), Apcflox/flox;Ptenfloxflox;Trp53flox/+ (n = 8), and Apcflox/flox;Ptenfloxflox;Trp53+/+ (n = 6) mice after ovarian bursal injection of AdCre. B: Image of representative tumor-bearing Apcflox/flox;Ptenfloxflox;Trp53flox/flox mouse showing grossly visible ovarian tumor (white star) and abdominal metastasis (black star). Representative photomicrographs of H&E-stained sections showing primary ovarian tumor from Apcflox/flox;Ptenfloxflox;Trp53flox/flox mouse (C), primary ovarian tumor from Apcflox/flox;Ptenfloxflox;Trp53flox/+ mouse (D), parenchymal liver metastasis (E), and lung metastasis (F) in tumor-bearing Apcflox/flox;Ptenfloxflox;Trp53flox/flox mice. Immunohistochemical stains showing strong expression of E-cadherin (G) and absence of p53 expression (H) in sections from a representative Apc−/−;Pten−/−;p53−/− ovarian tumor. Scale bars: 100 μm (C–H).
EOC Development in Mice Requires Combined Defects in Canonical Wnt and PI3K/AKT/mTOR Signaling, Even in the Presence of Mutant p53
Studies of human high-grade serous OvCAs and precursor lesions in the fallopian tube suggest that TP53 mutations occur early and may be required for their development. In contrast, only a subset (≈20%) of type I EOCs with canonical Wnt and PI3K/AKT/mTOR signaling pathway defects also have TP53 mutations, suggesting that mutant p53 is not required for EOC development but may be associated with type I to type II progression. In previous studies, we showed that EOCs fail to develop unless both Wnt and PI3K/AKT/mTOR signaling pathways are concomitantly dysregulated. Specifically, no tumors formed after ovarian bursal AdCre injection in any of 61 Apcflox/flox or 63 Ptenflox/flox mice.14 Here, we wished to test whether Trp53 point mutations (R172H or R270H) could cooperate with dysregulation of either canonical Wnt or PI3K/AKT/mTOR signaling in initiating EOCs in our mouse model. Ovarian bursal AdCre injection was performed in Apcflox/flox;Ptenflox/+;Trp53LSL-R172H/+ (n = 2) and Apcflox/+;Ptenflox/flox;Trp53R270H/+ (n = 2) mice, and animals were monitored for up to 9 months. Although only a small number of mice with each genotype were tested, none of the mice developed ovarian tumor, further supporting the conclusion that dysregulation of both Wnt and PI3K/AKT/mTOR signaling is required for murine EOC development, even in the presence of mutant p53.
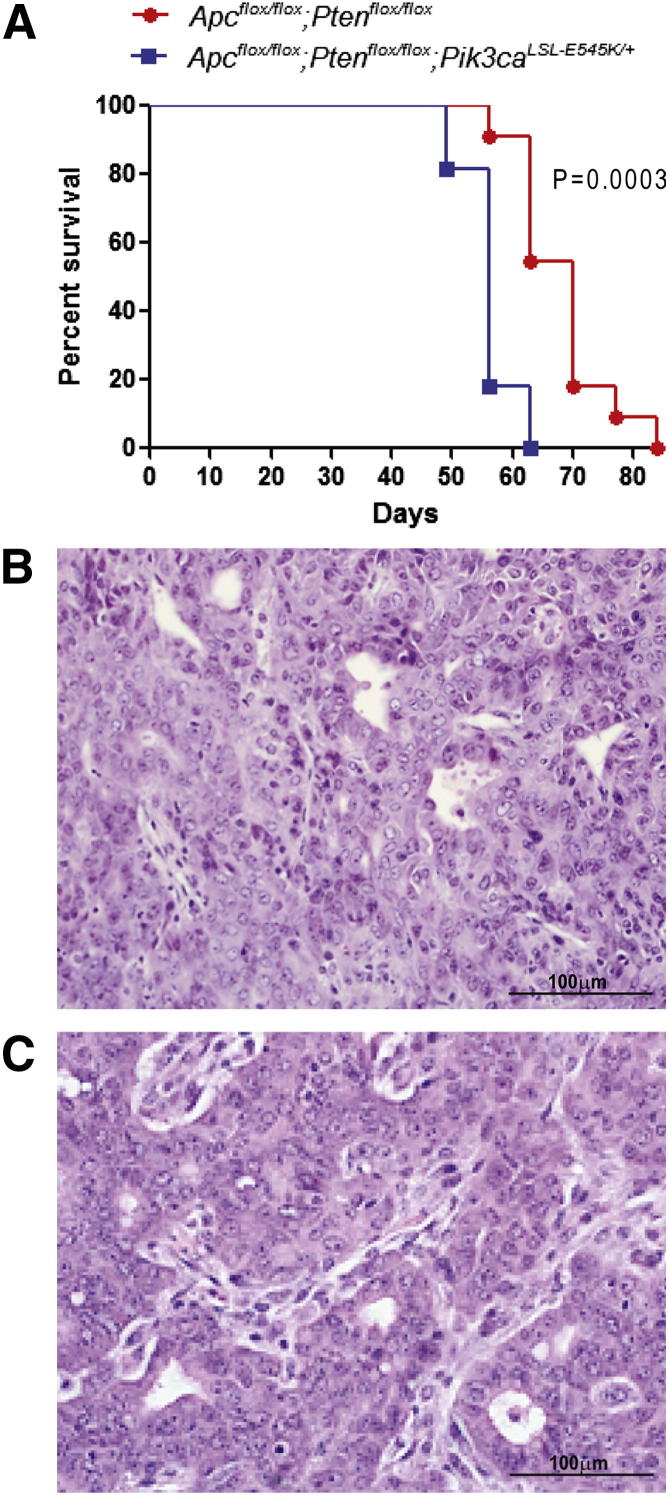
Mice with Apc−/−;Pten−/−;Pik3caE545K/+ EOCs Have Shortened Survival Compared with Mice with EOCs Lacking Mutant Pik3ca
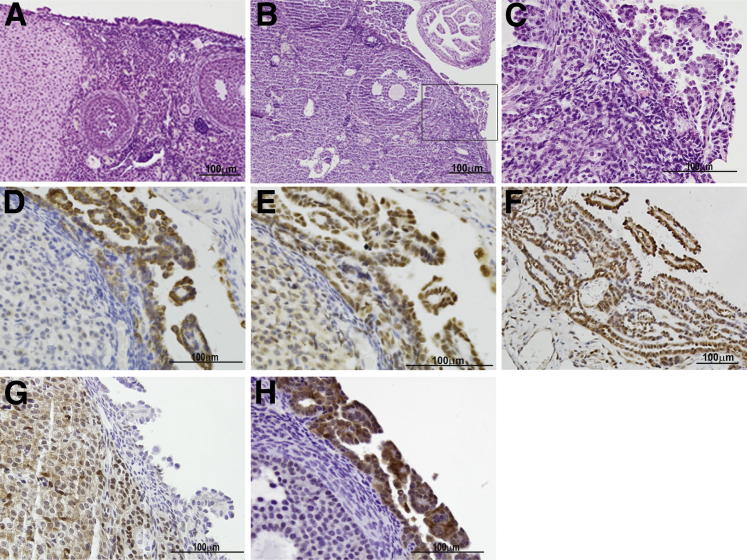
In our previous analysis of 21 human EOCs with type I OvCA gene expression and mutational profiles, 7 had mutations predicted to dysregulate PI3K/AKT/mTOR signaling, including 3 EOCs that had inactivating PTEN mutations in addition to activating PIK3CA mutations in exons 9 or 20.14,37 Because frequent mutations in PIK3CA exons 1 to 7 have been reported in endometrial adenocarcinomas,38 we subsequently evaluated these exons in our set of EOCs. One additional tumor (which also had mutant PTEN) was found to harbor two mutations in PIK3CA exon 1 (R88Q and K111N; Cho laboratory). Both of these have previously been reported as gain-of-function mutations.39–41 To determine effects of mutant Pik3ca on our murine EOC tumor phenotype, Apcflox/flox;Ptenfloxflox mice were crossed with mice in which a conditional mutant (LSL-E545K) allele is knocked into one of the endogenous Pik3ca loci. Ovarian bursal AdCre injection was used to induce tumors in Apcflox/flox;Ptenfloxflox;Pik3caLSL-E545K/+ and Apcflox/flox;Ptenfloxflox;Pik3ca+/+ littermates. Mice with Apc−/−;Pten−/−;Pik3caE545K/+ tumors (n = 11) had significantly shorter survival (P = 0.0003) and more frequent ascites and metastasis than littermate controls (n = 11), with Apc−/−;Pten−/− tumors lacking mutant Pik3ca (Figure 3A and Table 2). The addition of mutant Pik3ca had no appreciable effect on tumor morphology (Figure 3, B and C). As observed by Liang et al42 and Kinross et al,43 activation of mutant Pik3ca alone (n = 4) was insufficient to initiate tumors in the MOSE. However, in contrast to these other studies, we did not observe significant epithelial hyperplasia in mice expressing only mutant Pik3ca in the MOSE (Figure 4A). Ovarian bursal injection of AdCre in Ptenflox/flox;Pik3caLSL-E545K/+ mice resulted in the development of nonepithelial hamartoma-like tumor masses (to be reported separately) in 9 of 11 mice similar to those reported in the soft tissue of humans with PTEN hamartoma tumor syndromes.44 In addition, all 11 Ptenflox/flox;Pik3caLSL-E545K/+ mice displayed micropapillary proliferation of the MOSE that resembled low-grade (type I) serous carcinoma between 9 and 30 weeks after AdCre administration (Figure 4, B and C). The hyperplastic epithelium showed strong expression of CK8, WT1, and PAX8, consistent with a Mullerian epithelial rather than mesothelial proliferation (Figure 4, D, E, and F). In addition, as expected, the hyperplastic epithelial cells were negative for Pten (Figure 4G) but showed elevated expression of p-AKT (Figure 4H). The changes in our Ptenflox/flox;Pik3caLSL-E545K/+ mice are similar to the hyperproliferative surface epithelium observed by Kinross et al43 after expression of Pik3caH1047R in the MOSE.
Figure 3.
Mice with Apc−/−;Pten−/−;Pik3caE545K EOCs have shortened survival. A: Kaplan-Meier survival curve of tumor-bearing Apcflox/flox;Ptenfloxflox;Pik3caE545K/+ (n = 11) and Apcflox/flox;Ptenfloxflox (n = 11) mice. Photomicrographs of representative primary OvCAs from Apcflox/flox;Ptenfloxflox (B) and Apcflox/flox;Ptenfloxflox;Pik3caE545K/+ (C) mice. Scale bars: 100 μm (B and C).
Table 2.
Effects of Mutant Pik3ca on the Phenotype of Ovarian Tumors with Mutant Pten, Apc, and/or Trp53 Alleles
| Genotype | No. | Normal | MOSE proliferation | Carcinoma | Peritoneal metastasis | Lung metastasis |
|---|---|---|---|---|---|---|
| Pten+/+;Pik3caLSL-E545K/+ | 4 | 4 | ||||
| Ptenflox/+;Pik3caLSL-E545K/+ | 3 | 3 | ||||
| Pik3caLSL-E545K/+;Trp53R270H/+ | 4 | 4 | ||||
| Ptenflox/flox;Pik3caLSL-E545K/+ | 11 | 11 | ||||
| Ptenflox/flox;Pik3caLSL-E545K/+;Trp53LSI-R172H/+ | 5 | 5 | ||||
| Apcflox/+;Ptenflox/flox;Pik3caLSL-E545K/+ | 5 | 3 | 2 | |||
| Apcflox/flox;Ptenflox/+;Pik3caLSL-E545K/+ | 6 | 2 | 4 | |||
| Apcflox/flox;Ptenflox/flox;Pik3caLSL-E545K/+ | 11 | 11 | 7 | 1 | ||
| Apcflox/flox;Ptenflox/flox | 11 | 11 | 4 |
Figure 4.
Pik3caE545K induces epithelial hyperplasia in the context of Pten loss and in combination with other genes. A: Normal-appearing ovary from Pik3caLSL-E545K mouse 40 weeks after AdCre injection. B: Ovary from Ptenflox/flox;Pik3caLSL-E545K mouse 9 weeks after AdCre injection showing focal papillary proliferation of surface epithelium (H&E). C: Higher magnification photomicrograph of boxed area in B. Immunohistochemical stains showing expression of CK8 (D), WT1 (E), and PAX8 (F) in the hyperplastic epithelial cells. Additional immunohistochemical stains confirming loss of Pten (G) and increased pAkt expression (H) in the hyperplastic epithelium. Scale bars: 100 μm (A–H).
Mice with several other combinations of mutant Apc, Pten, and Pik3ca alleles were also tested for their ability to form ovarian tumors after bursal AdCre injection (Table 2). In previous work, we showed that none of the 20 Apcflox/flox;Ptenflox/+ mice and only 1 of the 20 Apcflox/+;Ptenflox/flox mice developed OvCA after AdCre injection.14 In this study, we found that AdCre induced OvCAs in two of five Apcflox/+;Ptenfloxflox;Pik3caLSL-E545K/+ mice, whereas the other three mice displayed hamartoma-like masses and micropapillary proliferation of the MOSE similar to that observed in Ptenfloxflox;Pik3caLSL-E545K/+ mice. The carcinomas were not accompanied by hamartomatous lesions, presumably because the carcinomas progressed rapidly and may have overgrown early hamartomatous lesions. Similarly, four of six Apcfloxflox;Ptenflox/+;Pik3caE545K/+ mice developed OvCAs after AdCre injection. None of the Ptenflox/+;Pik3caLSL-E545K (n = 3) or Trp53R270/+;Pik3caLSL-E545K (n = 4) mice developed ovarian surface epithelial alterations or tumors after AdCre injection within the 40-week surveillance period. Hence, tumor formation is most efficient when both alleles of Apc and Pten are inactivated, but addition of mutant Pik3ca increases the frequency of tumor formation when one copy of either Apc or Pten is intact.
Discussion
Understanding differences in the biology and genetics of type I versus type II OvCAs is considered critical for identifying new therapeutic strategies that will improve outcome for patients with OvCA. Type I OvCAs are characterized as low-grade, slow-growing tumors that are more resistant to conventional chemotherapy but more likely to be responsive to hormonal therapy than their high-grade (type II) counterparts.45 The type I tumors have a more favorable prognosis, largely because many are diagnosed when the tumors are confined to the ovary and curable with surgical removal. It is important to emphasize that women with advanced-stage type I tumors have a poor prognosis. In a recent study of >600 consecutive OvCAs, no significant difference in progression-free or overall survival was observed between women with advanced-stage type I versus type II tumors.46 Because many women with type I tumors will die of their disease, understanding the molecular events underlying type I tumor progression and metastasis and how type I and type II tumors differ are important goals. The dualistic model originally argued for the existence of two distinct pathogenetic pathways for OvCA development, but molecular and histopathologic analyses suggest that the two pathways are not mutually exclusive and that a sizeable subset of endometrioid (and smaller subset of serous) carcinomas may undergo type I→type II progression, perhaps associated with acquisition of TP53 and/or PIK3CA mutations. The murine model systems described herein provide support for the adverse prognostic effects of TP53 and PIK3CA mutations observed in human endometrial, ovarian, and other types of carcinomas.
The murine EOCs that develop in our model system show morphologic features similar to their human counterparts, including distinct gland formation and occasional foci of squamous differentiation. Unlike typical primary human EOCs, the murine tumors also show areas of less-differentiated cells with spindle-cell morphology, possibly representing epithelial-mesenchymal transition.14 It is difficult to compare survival of tumor-bearing mice with that of patients with EOC, in part because women with EOC are usually treated with surgical resection (with or without chemotherapy), and this intervention makes it nearly impossible to determine the natural history of untreated EOC in humans. Nonetheless, it is important to note that tumors arising in our model are usually quite large before mice require euthanasia, and we have performed studies showing that mice can be “cured” with surgical resection several weeks after tumor initiation with AdCre injection (Cho laboratory, unpublished data), suggesting that metastasis occurs relatively late in the biological progression of disease. This is similar to the behavior of type I human tumors, which often become large enough for clinical detection before metastasis has occurred. In contrast, type II tumors are believed to acquire metastatic potential much earlier, often when the primary tumor is small and clinically undetectable. Given these features, we believe the murine tumors recapitulate many, but by no means all, features of human EOCs. Importantly, the model system is also valuable for studying the relative contribution of specific genetic alterations to tumor progression. The work presented here is illustrative of this latter point, particularly given our specific focus on the same gene/pathway defects that are present in human EOCs.
We note with interest that different groups have observed variable effects of expressing mutant Pik3ca in the MOSE. Although Kinross et al43 and Liang et al42 found that expression of activated Pik3ca alone resulted in MOSE hyperplasia, expression of Pik3caE545K did not cause striking epithelial proliferation in our model system when Pten function remained intact. The phenotypic differences among the three studies could reflect different genetic backgrounds of the mice, differences in the methods with which Cre-mediated recombination is induced in the MOSE, or the specific mutant Pik3ca alleles evaluated. Although Kinross et al43 also used bursal injection of AdCre to induce Cre-mediated activation of mutant Pik3ca in the MOSE, they used a mutation (H1047R) that affects the kinase domain of the p110α protein. Liang et al42 generated mice in which the MISIIR promoter was used to drive expression of Pik3ca with the avian src myristoylation sequence added to the N-terminus, resulting in constitutive expression of activated p110α.30 Mice in both of these studies developed epithelial hyperplasia but no carcinomas within the 18-month monitoring period. The E545K mutation that we used is in the helical domain of p110α. Helical domain and kinase domain mutations of PIK3CA induce gain of function by different mechanisms.47 The functional consequences of helical domain mutations do not depend on binding to p85 but require interaction with RAS-GTP, whereas function of the kinase domain mutants depend on the interaction with p85, but not RAS-GTP binding. Recently, Ross et al48 showed that the effects of mutant PIK3CA are both cell type and mutation specific. Specifically, they found that in urothelial cells, both helical and kinase domain mutants induced increased urothelial cell motility and migration toward a chemoattractant. In these cells, the E545K mutant was more potent than the H1047R mutant in inducing signaling downstream of AKT. In NIH3T3 cells, the kinase domain mutant H1047R induced higher levels of AKT activation than the helical domain mutants, and the latter were less able to confer anchorage-independent growth. Collectively, the studies suggest that Pik3ca mutations affecting the kinase versus helical domains also have different functional consequences in the MOSE, with the kinase domain mutants being more potent in stimulating epithelial hyperplasia. Clearly, further studies are needed to better understand how different PIK3CA mutations might contribute to both human and murine OvCA development and/or progression. The hyperplastic OSE observed in Ptenflox/flox;Pik3caLSL-E545K/+ mice is positive for CK8, WT1, and PAX8. Interestingly, we also observed CK8, WT1, and patchy PAX8 expression in normal MOSE. Notably, although some studies have shown that human OSE is usually negative for PAX8,49,50 others have shown that PAX8 is often expressed in the OSE.51 Some investigators have suggested that the presence of PAX8+ cells on the ovarian surface may be due to transfer of tubal epithelium to the ovarian surface,52 whereas others suggest that the genes expressed by the OSE may reflect a dynamic balance between mesothelial and Mullerian differentiation.53 At this point, we are unable to distinguish between these two possibilities.
In summary, we have used a murine model of type I OvCA to examine in depth the functional effects of mutant Trp53 or Pik3ca alleles on type I to type II progression, given the evidence that TP53 or PIK3CA mutations may be linked to progression and poor outcome in some women with type I OvCA. In the mouse, Apc−/−;Pten−/− tumors displayed more aggressive behavior when the tumors also harbored mutant Trp53 or Pik3ca alleles, with shorter overall survival and more widespread metastases. In their recent review, Romero and Bast45 suggested that animal models that more closely recapitulate type I and type II OvCAs are needed to facilitate the identification of novel therapeutic targets and to predict response to combinations of new agents. Because the number of women with advanced-stage type I tumors is relatively modest, only a limited number of clinical trials that test targeted agents can be performed, and careful selection of the appropriate patients for trial enrollment will be critical. It is easier and less expensive to test different drug combinations and dosing schedules in animal models than in humans. We have recently shown that Apc−/−;Pten−/− murine model of EOC has utility for preclinical testing of PI3K/AKT/mTOR signaling inhibitors.31,54 Tumor growth in this model system is inhibited by conventional chemotherapeutic agents (cisplatin and paclitaxel), two different AKT inhibitors, and the mTOR inhibitor rapamycin. We are now well positioned to test how mutations of Trp53 or Pik3ca alter tumor response and acquisition of resistance to conventional and molecularly targeted drugs.
Footnotes
Supported by National Cancer Institute grants RO1CA94172 (K.R.C.) and RO1CA135554 (S.J.B.) and Department of Defense grant W81XWH-10-2-0013 (K.R.C. and E.R.F.).
Supplemental Data
PCR-based analyses of floxed and recombined transgenes with the use of genomic DNA isolated from primary ovarian tumors and tails. Representative data for Apc-conditional null allele (A), Pten-conditional null allele (B), Trp53-R270H constitutional mutant allele (C), Trp53-conditional null allele (D), and Pik3ca-E545K conditional mutant allele (E) are shown.
References
- 1.Kurman R.J., Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–160. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih IeM., Kurman R.J. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidman J.D., Cho K.R., Ronnett B.M., Kurman R.J. Surface epithelial tumors of the ovary. Blaustein’s pathology of the female genital tract, ed 6. In: Kurman R.J., Ellenson L.H., Ronnett R.M., editors. Springer; New York: 2011. pp. 679–784. [Google Scholar]
- 4.Ahmed A.A., Etemadmoghadam D., Temple J., Lynch A.G., Riad M., Sharma R., Stewart C., Fereday S., Caldas C., Defazio A., Bowtell D., Brenton J.D. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowtell D.D. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 6.Cho K.R., Shih I.M. Ovarian cancer. Annu Rev Pathol Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangha N., Wu R., Kuick R., Powers S., Mu D., Fiander D., Yuen K., Katabuchi H., Tashiro H., Fearon E.R., Cho K.R. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia. 2008;10:1362–1372. doi: 10.1593/neo.08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Network C.G.A.R. integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kindelberger D.W., Lee Y., Feltmate C., Callahan M., Garner E., Berkowitz R.S., Muto M.G., Crum C.P. Coexisting tubal intraepithelial carcinoma (TIC) and ovarian serous carcinoma: frequency and implications for the fallopian tube as a source of “ovarian” serous neoplasia. Lab Invest. 2006;86(Supplement):184A. [Google Scholar]
- 10.Kindelberger D.W., Lee Y., Miron A., Hirsch M.S., Feltmate C., Medeiros F., Callahan M.J., Garner E.O., Gordon R.W., Birch C., Berkowitz R.S., Muto M.G., Crum C.P. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 11.Crum C.P., Drapkin R., Miron A., Ince T.A., Muto M., Kindelberger D.W., Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 12.Jarboe E.A., Pizer E.S., Miron A., Monte N., Mutter G.L., Crum C.P. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol. 2009;22:345–350. doi: 10.1038/modpathol.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCluggage W.G. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61:152–163. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 14.Wu R., Hendrix-Lucas N., Kuick R., Zhai Y., Schwartz D.R., Akyol A., Hanash S., Misek D.M., Katabuchi H., Williams B.O., Fearon E.R., Cho K.R. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/B-catanin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Okuda T., Otsuka J., Sekizawa A., Saito H., Makino R., Kushima M., Farina A., Kuwano Y., Okai T. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003;88:318–325. doi: 10.1016/s0090-8258(02)00149-x. [DOI] [PubMed] [Google Scholar]
- 16.Leitao M.M., Soslow R.A., Baergen R.N., Olvera N., Arroyo C., Boyd J. Mutation and expression of the TP53 gene in early stage epithelial ovarian carcinoma. Gynecol Oncol. 2004;93:301–306. doi: 10.1016/j.ygyno.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Willner J., Wurz K., Allison K.H., Galic V., Garcia R.L., Goff B.A., Swisher E.M. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–613. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Strang P., Nordstom B., Nilsson S., Bergstrom R., Tribukait B. Mutant p53 protein as a predictor of survival in endometrial carcinoma. Eur J Cancer. 1996;32A:598–602. doi: 10.1016/0959-8049(95)00636-2. [DOI] [PubMed] [Google Scholar]
- 19.Ogino S., Nosho K., Kirkner G.J., Shima K., Irahara N., Kure S., Chan A.T., Engelman J.A., Kraft P., Cantley L.C., Giovannucci E.L., Fuchs C.S. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477–1484. doi: 10.1200/JCO.2008.18.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S.Y., Rong M., Grieu F., Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 21.Liao X., Morikawa T., Lochhead P., Imamura Y., Kuchiba A., Yamauchi M., Nosho K., Qian Z.R., Nishihara R., Meyerhardt J.A., Fuchs C.S., Ogino S. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catasus L., Gallardo A., Cuatrecasas M., Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–139. doi: 10.1038/modpathol.3800992. [DOI] [PubMed] [Google Scholar]
- 23.Woenckhaus J., Steger K., Sturm K., Munstedt K., Franke F.E., Fenic I. Prognostic value of PIK3CA and phosphorylated AKT expression in ovarian cancer. Virchows Arch. 2007;450:387–395. doi: 10.1007/s00428-006-0358-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Helland A., Holm R., Kristensen G.B., Borresen-Dale A.L. PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat. 2005;25:322. doi: 10.1002/humu.9316. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M., Nakayama K., Rahman M.T., Nakayama N., Ishikawa M., Katagiri A., Iida K., Nakayama S., Otsuki Y., Shih I.M., Miyazaki K. Clinicopathologic and biological analysis of PIK3CA mutation in ovarian clear cell carcinoma. Hum Pathol. 2012;43:2197–2206. doi: 10.1016/j.humpath.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Quddus M.R., Rashid L.B., Hansen K., Sung C.J., Lawrence W.D. High-grade serous carcinoma arising in a low-grade serous carcinoma and micropapillary serous borderline tumour of the ovary in a 23-year-old woman. Histopathology. 2009;54:771–773. doi: 10.1111/j.1365-2559.2009.03283.x. [DOI] [PubMed] [Google Scholar]
- 27.Dehari R., Kurman R.J., Logani S., Shih Ie M. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31:1007–1012. doi: 10.1097/PAS.0b013e31802cbbe9. [DOI] [PubMed] [Google Scholar]
- 28.Boyd C., McCluggage W.G. Low-grade ovarian serous neoplasms (low-grade serous carcinoma and serous borderline tumor) associated with high-grade serous carcinoma or undifferentiated carcinoma: report of a series of cases of an unusual phenomenon. Am J Surg Pathol. 2011;36:368–375. doi: 10.1097/PAS.0b013e31823732a9. [DOI] [PubMed] [Google Scholar]
- 29.Parker R.L., Clement P.B., Chercover D.J., Sornarajah T., Gilks C.B. Early recurrence of ovarian serous borderline tumor as high-grade carcinoma: a report of two cases. Int J Gynecol Pathol. 2004;23:265–272. doi: 10.1097/01.pgp.0000130049.19643.f6. [DOI] [PubMed] [Google Scholar]
- 30.Robinson G., Parker M., Kranenburg T.A., Lu C., Chen X., Ding L. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu R., Hu T., Rehemtulla A., Fearon E.R., Cho K.R. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clin Cancer Res. 2011;17:7359–7372. doi: 10.1158/1078-0432.CCR-11-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olive K.P., Tuveson D.A., Ruhe Z.C., Yin B., Willis N.A., Bronson R.T., Crowley D., Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 33.de Vries A., Flores E.R., Miranda B., Hsieh H.M., van Oostrom C.T., Sage J., Jacks T. Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc Natl Acad Sci U S A. 2002;99:2948–2953. doi: 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lozano G. The oncogenic roles of p53 mutants in mouse models. Curr Opin Genet Dev. 2007;17:66–70. doi: 10.1016/j.gde.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Sood A.K., Sorosky J.I., Dolan M., Anderson B., Buller R.E. Distant metastases in ovarian cancer: association with p53 mutations. Clin Cancer Res. 1999;5:2485–2490. [PubMed] [Google Scholar]
- 36.Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 37.Cho K.R. Ovarian cancer update: lessons from morphology, molecules, and mice. Arch Pathol Lab Med. 2009;133:1775–1781. doi: 10.5858/133.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd M.L., Price J.C., Fogoros S., Godwin A.K., Sgroi D.C., Merino M.J., Bell D.W. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollestelle A., Elstrodt F., Nagel J.H., Kallemeijn W.W., Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 40.Gymnopoulos M., Elsliger M.A., Vogt P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A. 2007;104:5569–5574. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogt P.K., Hart J.R., Gymnopoulos M., Jiang H., Kang S., Bader A.G., Zhao L., Denley A. Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol. 2010;347:79–104. doi: 10.1007/82_2010_80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang S., Yang N., Pan Y., Deng S., Lin X., Yang X., Katsaros D., Roby K.F., Hamilton T.C., Connolly D.C., Coukos G., Zhang L. Expression of activated PIK3CA in ovarian surface epithelium results in hyperplasia but not tumor formation. PLoS One. 2009;4:e4295. doi: 10.1371/journal.pone.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinross K.M., Montgomery K.G., Kleinschmidt M., Waring P., Ivetac I., Tikoo A., Saad M., Hare L., Roh V., Mantamadiotis T., Sheppard K.E., Ryland G.L., Campbell I.G., Gorringe K.L., Christensen J.G., Cullinane C., Hicks R.J., Pearson R.B., Johnstone R.W., McArthur G.A., Phillips W.A. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest. 2012;122:553–557. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurek K.C., Howard E., Tennant L.B., Upton J., Alomari A.I., Burrows P.E., Chalache K., Harris D.J., Trenor C.C., III, Eng C., Fishman S.J., Mulliken J.B., Perez-Atayde A.R., Kozakewich H.P. PTEN hamartoma of soft tissue: a distinctive lesion in PTEN syndromes. Am J Surg Pathol. 2012;36:671–687. doi: 10.1097/PAS.0b013e31824dd86c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero I., Bast R.C., Jr. Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–1602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braicu E.I., Sehouli J., Richter R., Pietzner K., Denkert C., Fotopoulou C. Role of histological type on surgical outcome and survival following radical primary tumour debulking of epithelial ovarian, fallopian tube and peritoneal cancers. Br J Cancer. 2011;105:1818–1824. doi: 10.1038/bjc.2011.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L., Vogt P.K. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross R.L., Askham J.M., Knowles M.A. PIK3CA mutation spectrum in urothelial carcinoma reflects cell context-dependent signaling and phenotypic outputs. Oncogene. 2013;32:768–776. doi: 10.1038/onc.2012.87. [DOI] [PubMed] [Google Scholar]
- 49.Bowen N.J., Logani S., Dickerson E.B., Kapa L.B., Akhtar M., Benigno B.B., McDonald J.F. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 50.Li J., Abushahin N., Pang S., Xiang L., Chambers S.K., Fadare O., Kong B., Zheng W. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 51.Ozcan A., Shen S.S., Hamilton C., Anjana K., Coffey D., Krishnan B., Truong L.D. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–764. doi: 10.1038/modpathol.2011.3. [DOI] [PubMed] [Google Scholar]
- 52.Tong G.X., Hamele-Bena D. The differential expression of PAX2 and PAX8 in the ovarian surface epithelium and fallopian tubal epithelium is an important issue. Am J Surg Pathol. 2012;36:1099–1100. doi: 10.1097/PAS.0b013e3182500c1b. [DOI] [PubMed] [Google Scholar]
- 53.Ozcan A., Truong L.D. PAX2 and PAX8 expression in the ovarian surface epithelium and inclusion cysts. Am J Surg Pathol. 2012;36:1100–1102. [Google Scholar]
- 54.Wang H., Galban S., Wu R., Bowman B., Witte A., Vetter K., Galban CJ., Ross BD., Cho KR., Rehemtulla A. Molecular imaging reveals a role for AKT in resistance to cisplatin for ovarian endometrioid adenocarcinoma. Clin Cancer Res. 2013;19:158–169. doi: 10.1158/1078-0432.CCR-12-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR-based analyses of floxed and recombined transgenes with the use of genomic DNA isolated from primary ovarian tumors and tails. Representative data for Apc-conditional null allele (A), Pten-conditional null allele (B), Trp53-R270H constitutional mutant allele (C), Trp53-conditional null allele (D), and Pik3ca-E545K conditional mutant allele (E) are shown.