Abstract
Invasive ductal carcinoma (IDC) not otherwise specified (NOS), the most common type of breast cancer, demonstrates great intratumoral morphological heterogeneity, which encompasses the presence of different types of morphological structures—tubular, trabecular, solid, and alveolar structures and discrete groups of tumor cells, the origins of which remain unclear at present. In this study of 162 IDC NOS patients, we investigated whether the distribution of different types of morphological structures is related to the basic clinicopathological parameters of IDC NOS. Our results showed that in patients with only one type of tumor structure, the presence of any one of the five types was equally probable; however, cases with two types of structures were more likely to contain trabecular structures than the other four types. The development of intratumoral morphological heterogeneity was not associated with menopausal status, tumor size, histological grade, hematogenic metastasis, or recurrence. However, the number of different types of morphological structures was significantly higher in luminal tumors than in triple-negative tumors. An increase in the frequency of lymph node metastasis correlated with the increased number of different types of structures in breast tumors; however, in contrast to premenopausal patients, this association was explained by the presence of alveolar structures in postmenopausal women. In addition, we showed a significant decrease in the numbers of positive lymph nodes in tumors with high numbers of morphological variants. The frequency of lymph node metastases and the number of positive nodes were generally independent features and formed by different mechanisms. Based on the evidence, the term “phenotypic drift” has been designated as the basis for the development of intratumoral morphological heterogeneity of IDC NOS.
Key words: breast cancer, lymph node metastasis, menopause, phenotypic drift, tumor heterogeneity
Introduction
Invasive ductal carcinoma (IDC) not otherwise specified (NOS) or no special type is the most common histological type of breast cancer (up to 75% of all invasive lesions). It is described as a heterogeneous group of tumors with extremely diverse morphological “portraits”1,2 that make it difficult to predict the individual prognoses of IDC NOS patients. IDC NOS tumors often contain minor components with a special type of histology, including architectural features of the invasive process, which may vary widely both within a single tumor and from case to case.3 Previously, we described five different morphological types of invasive components or morphological structures in IDC NOS tumors—tubular, trabecular, solid, and alveolar structures and discrete groups of tumor cells. We also reported an association between the presence of alveolar structures and an increased risk of lymph node metastasis, which was more significant in postmenopausal patients than in premenopausal patients.4 However, it is not clear so far whether such intratumoral morphological heterogeneity is an initial characteristic of an IDC NOS tumor or a later phenomenon formed during tumor development. In this context, there is great interest in elucidating a potential link between the clinicopathological parameters, which reflect tumor progression potential, and the development of intratumoral morphological heterogeneity. In a study by Wilson and Evans,5 phenotypic drift was described as the phenomenon of dedifferentiation of tubular cancer, a prognostically favorable type of breast cancer, into high-grade tumors of mixed tubular and ductal nonspecial type morphologies with elevated life-threatening potential. In the present study, the term “phenotypic drift” was defined as the change in the morphological phenotype of an IDC NOS tumor. In other words, any alteration in the distribution of various morphological structures, namely their numbers, during tumor development was attributed to phenotypic drift.
Based on the definition for phenotypic drift, we aimed to investigate the patterns of development of intratumoral morphological heterogeneity in IDC NOS, particularly focusing on whether the distribution of different types of morphological structures is dependent on the basic clinicopathological parameters of breast tumors.
Materials and Methods
In total, 162 patients (mean age of 43.3±6.7 years for premenopausal women and 61.5±8.0 years for postmenopausal women) with IDC NOS diagnosed in the Cancer Research Institute (Tomsk, Russia) between 1999 and 2006 and not treated by neoadjuvant chemotherapies were included (Table 1). The distinction of IDC NOS from other histological types was performed according to the World Health Organization Classification of Tumors.6
Table 1.
Characteristics of Study Group of Breast Cancer Patients
| |
n (%) |
|
|
|---|---|---|---|
| Tumor characteristic | Premenopausal patients N = 53 | Postmenopausal patients N = 109 | p-Value |
| Tumor size | |||
| <20 mm | 30 (56.7) | 37 (33.8) | 0.006 |
| 20–50 mm | 21 (39.6) | 66 (60.5) | 0.01 |
| >50 mm | 2 (3.7) | 6 (5.7) | 0.72 |
| Grade | |||
| Low | 4 (7.5) | 7 (6.4) | 1.00 |
| Medium | 49 (92.5) | 97 (89.0) | 0.58 |
| High | 0 (0.0) | 5 (4.6) | — |
| ER status | |||
| Positive | 31 (68.9) | 59 (67.0) | 0.82 |
| Negative | 14 (31.1) | 29 (33.0) | |
| Unknown | 8 | 21 | |
| PR status | |||
| Positive | 33 (73.3) | 48 (55.2) | 0.04 |
| Negative | 12 (26.7) | 39 (44.8) | |
| Unknown | 8 | 22 | |
| HER2/neu status | |||
| Negative | 31 (81.6) | 61 (81.3) | 0.82 |
| Positive | 7 (18.4) | 14 (18.7) | |
| Unknown | 15 | 34 | |
| Molecular subtypes | |||
| Luminal | 30 (81.1) | 51 (68.9) | 0.26 |
| HER2-enriched | 3 (8.1) | 7 (9.5) | 1.00 |
| Triple-negative | 4 (10.8) | 16 (21.6) | 0.19 |
| Unknown | 16 | 35 | |
| Lymph node metastases | |||
| − | 36 (67.9) | 66 (60.6) | 0.36 |
| + | 17 (32.1) | 43 (39.4) | |
| Hematogenic metastases | |||
| − | 41 (77.4) | 96 (88.1) | 0.08 |
| + | 12 (22.6) | 13 (11.9) | |
| Recurrence | |||
| − | 46 (86.8) | 102 (93.6) | 0.23 |
| + | 7 (13.2) | 7 (6.4) | |
n, number of patients presenting with a specific tumor characteristic; N, total number of patients in group of pre- or postmenopausal patients; ER, estrogen receptor; PR, progesterone receptor; −, absent; +, present.
Tumors were staged according to the tumor-node-metastasis (TNM) classification of malignant tumors (T1–3 N0–3 M0). Fresh tumor tissues were fixed in 10% neutral formalin (Karbolit) for 24 h, rinsed with a mixture of isopropanol (Biovitrum), and embedded in paraffin (Biovitrum). Morphological and immunohistochemical analyses were performed by light microscope (Jenamed, Carl Zeiss). All tumor slides (five from each sample) were reviewed by three experienced pathologists. Intra-observer variability was 5%. In case of discrepancy, results were discussed between the pathologists, and a consensus was reached.
Morphological analysis included determination of histological grade and calculation of different types of morphological structures in breast tumors. Sections of 5–6 μm thickness were stained by hematoxylin (Dako) and eosin (Dako). Histological grade was scored according to the modified Scarff-Bloom-Richardson grading system,7 taking into account the number of tubular and ductal structures, mitoses, and nuclear atypia/pleomorphism. The presence of different types of morphological structures was evaluated in tumor slides stained by hematoxylin and eosin. Tubular structures used in tumor grading were presented by rows of tiny tube-shaped cell aggregations. Trabecular structures were formed by two or more rows of cells located within the stroma. Solid structures represented groups of hundreds of cells with different sizes and shapes. Discrete groups of tumor cells were detected as single cells or as groups of up to five cells. Alveolar structures with rounded shapes contained 5–25 cells.4 It is important to note that tumors from different patients could have different types of morphological structures.
Immunohistochemical analysis was performed using antibodies directed against estrogen receptors (ER, ID5 clone, ready-to-use, nuclear expression, Dako), progesterone receptors (PR, PgR636 clone, ready-to-use, nuclear expression, Dako), and c-erbB2 (also known as HER2/neu, polyclonal antibody, 1:500 dilution, membrane expression, Dako). Five- to six-micrometer-thick sections were deparaffinized in three changes of 96% ethanol, rinsed with distilled water for 5 min, placed in plastic racks and subjected to high temperature antigen unmasking technique in 0.01 M citrate buffer, pH 6.0 (Dako). Antigen retrieval was carried out in three steps: heating for 7 min at 60 W, cooling-down for 1 min, and heating 7 min at 40 W. After cooling the sections to room temperature and rinsing in two changes of phosphate buffer (Biolot), incubation was performed with peroxidase blocking reagent (Dako) for 10 min. Further, the sections were rinsed with distilled water for 5 min and phosphate buffer for 5 min, and incubated with antibodies for 60 min at 25°C. After washing, the sections were treated with biotinylated antibodies (ready-to-use, Dako) for 10 min. Following another buffer wash, the sections were labeled with streptavidin-biotin complex (Dako) for 10 min. To visualize the immunoreactions, the sections were incubated with diaminobenzidine (Dako) for 10 min. The sections were counterstained with hematoxylin (Dako) and mounted in balsam (Panreac, Spain). Immunohistochemical visualization was performed with LSAB2 system-HRP (Dako).
Molecular classification of breast cancer cases was performed based on the ER, PR, and HER2/neu status. In this study, we focused luminal subtype, including A and B (ER, PR positive, absence or overexpression of HER2/neu), HER2-enriched (ER, PR negative, overexpression of HER2/neu), and triple-negative (ER, PR negative, absence of HER2/neu).8 Long-term results, namely the presence of hematogenic metastases and recurrences, analyzed throughout 5 years by patient records. This study was approved by the institutional review board, and all patients signed an informed consent for voluntary participation.
Statistical processing was performed by Pearson's chi-squared test and regression analysis. Yates' corrected chi-square test (when expected frequencies <10 were found)9 and Fisher's exact test (when expected frequencies <6 were found)10 were also utilized. All analyses were performed using STATISTICA 8.0 software (StatSoft) and 2x2 Contingency Table (http://vassarstats.net/odds2x2.html). Results were considered statistically significant at p<0.05, and p-values were two-sided.
Results
This study was conducted to reveal whether the distribution of different types of morphological structures is related to basic clinicopathological parameters of breast cancer, including menopausal status, tumor size, histological grade, molecular subtypes, lymph node involvement, hematogenic metastasis, and recurrence. We did not find any significant association between the distribution of different structural types and menopausal status, tumor size, histological grade, hematogenic metastasis, or recurrence. However, intratumoral morphological heterogeneity of IDC NOS was strongly associated with molecular subtypes and lymph node involvement, including the number of positive nodes.
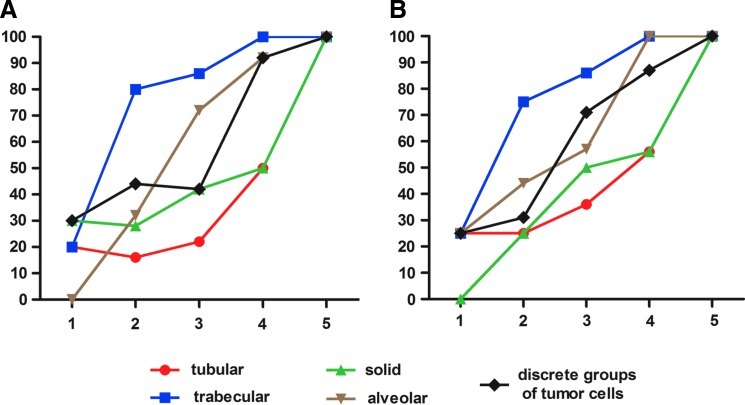
To understand which types of morphological structures originate first and which ones occur later, we performed comparisons of the frequencies of their combinations in the study group (Fig. 1). Regardless of menopausal status, there was a nearly equal distribution of each structural variant in tumors with only one type of structure. Nonetheless, in individuals with any two types of structures, trabecular structures were detected in a majority (75%–80%) of cases.
FIG. 1.
Distribution of different types of morphological structures in (A) pre- and (B) postmenopausal patients. The x-axis designates the number of different types of morphological structures. The y-axis designates the percentage of cases with a particular variant of structures. Regardless of menopausal status, there was a nearly equal distribution of each structural variant in tumors with only one type of structure. Nonetheless, in individuals with any two types of structures, trabecular structures were detected in a majority (75%–80%) of cases. In patients with two to four types of structural variants, there were insignificant differences between the numbers of tubular, solid, and alveolar structures and of discrete groups of tumor cells. Cases with five types logically contain all variants of morphological structures.
With regard to the dependence on molecular classification, tumors of postmenopausal patients with any single type of morphological structure were more frequently found in the triple-negative subtype than in the luminal breast cancers (18.7% vs. 3.9%; p=0.04; Table 2). In contrast, tumors with all variants of structures more frequently occurred in the luminal cancers than in the triple-negative ones (23.5% vs. 6.3%; p=0.04; Table 2). Similar tendencies were also observed in premenopausal women; however, the study group was too small to reach statistical significance. The disparity between the number of ER, PR positive (Table 1), and luminal (Table 2) patients, as well as between the number of HER2/neu positive and HER2-enriched patients, may be explained by the following reasons. First, in ER and PR expression, some of the HER2/neu positive tumors were considered as luminal B. Second, the simultaneous assessment of the ER, PR, and HER2/neu status that was not included in this study would be needed to define molecular subtype of breast cancer.
Table 2.
Number of Different Types of Morphological Structures in the Different Molecular Subtypes of Breast Tumors
| |
Premenopausal, n (%) |
Postmenopausal, n (%) |
||||
|---|---|---|---|---|---|---|
| No. of different types of morphological structures | Luminal | HER2-enriched | Triple-negative | Luminal | HER2-enriched | Triple-negative |
| 1 | 1 (3.3) | 1 (33.3) | 1 (25.0) | 2 (3.9) | 0 (0.0) | 3 (18.7)a |
| 2 | 9 (30.0) | 0 (0.0) | 1 (25.0) | 9 (17.6) | 3 (42.9) | 3 (18.7) |
| 3 | 11 (36.7) | 0 (0.0) | 0 (0.0) | 16 (31.5) | 3 (42.9) | 5 (31.3) |
| 4 | 7 (23.3) | 2 (66.7) | 2 (50.0) | 12 (23.5) | 1 (14.2) | 4 (25.0) |
| 5 | 2 (6.7) | 0 (0.0) | 0 (0.0) | 12 (23.5)a | 0 (0.0) | 1 (6.3) |
| Total | 30 | 3 | 4 | 51 | 7 | 16 |
p=0.04 (Fisher's exact test, differences between patients with luminal and triple-negative subtype).
n, number of patients with molecular subtype.
Previously, we reported the association between the presence of alveolar structures in breast tumors and an increased risk of lymph node metastasis, which was more significant in postmenopausal than in premenopausal women.4 Based on the present results, it is interesting to note that the percentage of premenopausal lymph node positive subjects, who had alveolar structures in tumors, increased with an increasing number of structural variants (β=0.97; p=0.02; Table 3). In contrast, only the presence of alveolar structures, but not the number of structural variants, was associated with a high likelihood of lymph node involvement in postmenopausal patients (β=0.69; p=0.30; Table 3).
Table 3.
Percentage of Lymph Node Positive Patients with Presence and Absence of Alveolar Structures in Breast Tumor
| |
Lymph node positive patients with alveolar and other morphological structures, n/N (%) |
|||
|---|---|---|---|---|
| |
Premenopausal |
Postmenopausal |
||
| No. of different types of morphological structures | Alveolar | Other | Alveolar | Other |
| 1 | 1/1 (100.0) | 0/3 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| 2 | 1/7 (14.2) | 2/9 (22.2) | 4/8 (50.0)a | 1/17 (5.9) |
| 3 | 3/8 (37.5) | 1/6 (16.7) | 15/26 (58.0)a | 1/10 (10.0) |
| 4 | 7/16 (43.7) | 0/0 (0.0) | 13/22 (59.0) | 0/2 (0.0) |
| 5 | 2/3 (66.7) | 0/0 (0.0) | 8/14 (57.1) | 0/0 (0.0) |
| Regression coefficient (β) | 0.97 | 0.69 | ||
| p-Value | 0.02 | 0.30 | ||
Regression coefficient and p-value are based on four observations: one, two, three, and four types.
p=0.02 (Fisher's exact test, differences between postmenopausal patients with alveolar and other structures).
n, number of patients with alveolar/other structures and lymph node metastases; N, number of patients in sample.
Next, we studied how the number of different types of morphological structures in breast tumors is related to the frequency of lymph node metastasis and the number of involved lymph nodes. In both pre- and postmenopausal patients, the frequency of lymph node involvement was elevated with an increasing number of structural variants; however, the differences reached statistical significance only in postmenopausal patients (0.02<p<0.06; Table 4). In addition, in postmenopausal women, the number of affected lymph nodes decreased with an increasing number of structural variants (0.04<p<0.07; Table 4).
Table 4.
Association Between Parameters of Lymph Node Metastasis and the Number of Different Types of Morphological Structures in Breast Tumors
| |
Premenopausal patients |
Postmenopausal patients |
||
|---|---|---|---|---|
| No. of different types of morphological structures | Frequency of lymph node metastasis, n/N (%) | Number of lymph nodes with metastases, mean±SD (n) | Frequency of lymph node metastasis, n/N (%) | Number of lymph nodes with metastases, mean±SD (n) |
| 1 | 1/4 (25.0) | 5.0 (1) | 1/10 (10.0) | 3.0 (1) |
| 2 | 3/16 (18.7) | 4.0±2.8 (3) | 5/25 (20.0) | 10.5±8.8 (5) |
| 3 | 4/14 (28.5) | 5.5±8.3 (4) | 16/36 (44.4)* | 5.0±4.4 (16)† |
| 4 | 7/16 (43.7) | 4.8±4.8 (7) | 13/24 (54.2)** | 4.2±3.3 (13)†† |
| 5 | 2/3 (66.7) | 1.0±0.0 (3) | 8/14 (57.1)*** | 3.5±3.1 (8)††† |
p=0.06 (differences between patients with three types and one or two types of morphological structures).
p=0.02 (differences between patients with four types and one or two types of morphological structures).
p=0.03 (differences between patients with five types and one or two types of morphological structures).
p=0.071 (differences between patients with three types and two types of morphological structures).
p=0.036 (differences between patients with four types and two types of morphological structures).
p=0.059 (differences between patients with five types and two types of morphological structures).
n, number of patients with lymph node metastases; N, number of patients in sample.
Discussion
Breast cancer shows significant heterogeneity at both inter- and intratumoral levels.11–13 Intratumoral heterogeneity, which refers to the presence of different clones of tumor cells with different malignancy potential and responsiveness to treatment, is a primary obstacle in oncology, confounding the molecular classification, disease prognostication, and therapeutic intervention of breast cancers.14–18 Several hypotheses have been advanced to explain the origin of intratumoral heterogeneity in breast cancer, beginning with the cancer stem cell and clonal evolution models and ending with the mutator phenotype model.14,19–21 Mathematical approaches have been applied to study the evolutionary dynamics of intratumoral heterogeneity. Calculations have shown that old but not young tumors, and the accumulation of neutral but not advantageous mutations, are associated with high levels of intratumoral diversity.22,23 In other words, tumors take a long time to become phenotypically diverse, while advantageous variations should probably be absent because their presence would eventually result in the formation of dominant clones. Therefore, the development of intratumoral heterogeneity is a time-dependent and mutation effect–controlled process.
In this study, we evaluated whether intratumoral morphological heterogeneity in invasive ductal carcinoma, the most common histological type of breast cancer, is dependent on clinicopathological parameters of the disease. Specifically, five different types of morphological structures were examined: tubular, trabecular, solid, and alveolar structures and discrete groups of tumor cells. In patients with only one type of tumor structure, the presence of any one of the five types was equally probable; however, cases with two types of structures were more likely to contain trabecular structures than the other four types. The mechanisms of origin and evolution of different types of morphological structures in breast tumors remain unclear. However, using laser capture microdissection (PALM, Carl Zeiss) our preliminary data demonstrate that there are differences in genotypes of some cancer genes24 and in expression of multidrug resistance genes25 between different morphological variants.
From the clinicopathological parameters we studied, only molecular subtypes and lymph node metastasis were linked with the development of intratumoral morphological heterogeneity. In particular, tumors with all of the structural variants were often detected in the luminal subtype, whereas cases with any single type of structure were mostly triple negative. There are two possible reasons for this observation: the expression of hormone receptors and the effect of tumor progression time. Regarding the first reason, previous studies have demonstrated an association between estrogen receptor expression and increased tumor differentiation, mediated by the ER-linked up-regulation of adherens junction proteins.26,27 Such data suggest that estrogen receptor expression should be linked to low intratumoral heterogeneity, which conflicts with our data showing high intratumoral diversity in estrogen receptor positive (luminal) breast cancer. The second reason suggests that due to a favorable prognosis of luminal breast cancer, there is more time to allow the formation of all morphological variants in this type of tumor than in the triple-negative cases.
Despite comparable distributions of different types of structures in breast tumors of pre- and postmenopausal women, there are observations to suggest that menopausal status influences the contribution of intratumoral morphological heterogeneity to lymph node metastasis.4 From this study, the percentage of premenopausal lymph node positive subjects, who had alveolar structures in tumors, increased with an increasing number of different types of morphological structures (Table 3). However, in postmenopausal group (Table 3), the high risk of lymphogenic metastasis was linked to the presence of alveolar structures, as was previously suggested.4 In premenopausal patients, the nature of the association between a high frequency of lymph node involvement and a large number of morphological variants could be related to the occurrence of diverse subpopulations of tumor cells with various biological characteristics, including the ability to form lymph node metastases. In contrast, the significance of alveolar structures in lymph node involvement in postmenopausal patients could be linked to menopause-specific factors28–30 that provoke tumor cells within these structures to metastasize. However, further studies are needed to understand the mechanisms of these associations.
An important observation in this study was an inversely proportional correlation between the number of lymph nodes with metastases and the number of different types of morphological structures in postmenopausal breast tumors; however, it is interesting that the frequency of lymph node involvement increased with increasing intratumoral diversity (Table 4). At first sight, it would appear that our data are contradictory. However, the frequency of lymph node metastasis and the number of positive nodes are generally independent features formed by different mechanisms. Otherwise, the values of these two prognostic factors would be equal. The number of positive lymph nodes has a greater prognostic significance for assessment of risk of hematogenic metastasis. Meanwhile, metastasis involving four or more lymph nodes denotes a high probability of development of hematogenic metastases. In addition, high variability in the numbers of positive nodes (from 1 to 20 and more) in the origins of lymph node metastases indicates that the two features are independent from each other.31
The importance of our data is highlighted by the fact that there are no known factors that can determine the number of involved lymph nodes. Based on the numbers of morphological structure variants in breast tumors, it is possible to estimate the numbers of lymph nodes, which would facilitate the diagnosis and treatment of tumors with high metastatic potential.
To designate changes in the distribution of various types of morphological structures, namely in their numbers, during tumor development, the term “phenotypic drift” was applied. Previously, phenotypic drift32 was proposed to explain rapid shifts in the metastatic behaviors of tumor cells and spontaneous and reproducible “waves” in their sensitivities to chemotherapeutical drugs.33,34 Later, Wilson and Evans5 used the term to describe dedifferentiation of tubular cancers into high-grade tumors of mixed tubular and ductal nonspecial type morphologies. Recently, phenotypic drift was illustrated as the natural evolution of the more aggressive cells in a heterogeneous tumor to become the dominant histological type.35 In any case, phenotypic drift or “dynamic heterogeneity,” as it is called by Hill and colleagues,36 reflects specific fluctuations in the functional status of tumor cells, owing to the time-dependent continuous generation of diverse cell subpopulations and maintained by different genetic alterations and environmental factors.32,37,38 In the present study, and in the context of breast cancer development dynamics, phenotypic drift could be considered as a shift of the morphological phenotype of IDC NOS or change of the distribution of different types of morphological structures containing tumor cells with specific biological features. Collectively, data from previous reports and the current study confirm that phenotypic drift is an original source of intratumoral heterogeneity, as has been suggested by Nicolson.34 However, we demonstrated that intratumoral morphological heterogeneity of IDC NOS has functional manifestations in terms of its association with lymph node metastasis and chemotherapy efficiency, as has been found earlier.39 Accordingly, in this study phenotypic drift was observed for not only morphological features, but also for functional features of tumor cells during breast cancer development. Interestingly, the drift of morphological phenotype of IDC NOS appears to be nonrandom, because it is strongly associated with tumor behavior.
It is interesting to note that phenotypic drift was also placed in lymph node involvement of IDC NOS. With an increase in the degree of replacement of lymphoid tissue by cancer cells, the morphological diversity of metastases grew. In up to 10% of replacement of lymphoid tissue, the number of different types of morphological structures was 1.8±0.9 (mean±standard deviation), from 10% to 50% (2.7±0.9), from 50% to 75% (3.2±1.0), and more than 75% (2.9±1.0; data not shown).
Therefore, our data provide evidence that phenotypic drift is a cause for the development of intratumoral morphological heterogeneity in invasive ductal carcinoma NOS, represented by different types of morphological structures within tumors. In particular, intratumoral diversity was found to be dependent on the molecular subtype of breast cancer and associated with the frequency of lymph node metastasis and the number of positive nodes. Furthermore, the association between alveolar structures and lymph node metastasis was exclusively found in postmenopausal patients.
Acknowledgments
This work was supported by the Ministry of Education and Science of the Russian Federation (projects 8291 and 8595), the Russian Federation President Grant (16.120.11.1259-ML), and a grant from the OPTEK company (1/11KTS).
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- 1.Breast Pathology. In: O'Malley FP, editor; Pinder SE, editor; Mulligan AM, editor. 2nd. Elsevier; Philadelphia, PA: 2011. [Google Scholar]
- 2.Early Diagnosis and Treatment of Cancer: Breast Cancer. In: Jacobs L, editor; Finlayson CA, editor. Elsevier; Philadelphia, PA: 2011. [Google Scholar]
- 3.Goldschmidt RA. Histopathology of malignant breast disease. In: Winchester DJ, editor; Winchester DP, editor. Atlas of Clinical Oncology. Breast Cancer. B.C. Decker Inc.; Hamilton, Canada: 2000. pp. 89–98. [Google Scholar]
- 4.Zavyalova MV. Perelmuter VM. Vtorushin SV, et al. The presence of alveolar structures in invasive ductal NOS breast carcinoma is associated with lymph node metastasis. Diagn Cytopathol. 2011 doi: 10.1002/dc.21852. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ARM. Evans AJ. E3. Over-diagnosis and breast cancer screening. Eur J Cancer Suppl. 2006;4:6–9. [Google Scholar]
- 6.World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. In: Tavassoli FA, editor; Devilee P, editor. IARC Press; Lyon, France; 2003. [Google Scholar]
- 7.Elston CW. Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Gruver AM. Portier BP. Tubbs RR. Molecular pathology of breast cancer: the journey from traditional practice toward embracing the complexity of a molecular classification. Arch Pathol Lab Med. 2011;135:544–557. doi: 10.5858/2010-0734-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 9.Brown JD. Yates correction factor. Shiken: JALT Testing &. Evaluation SIG Newsletter. 2004;8:22–27. [Google Scholar]
- 10.Motulsky H, editor. Oxford University Press; New York: 1995. Intuitive Biostatistics. [Google Scholar]
- 11.Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121:3786–3788. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertos NR. Park M. Breast cancer—one term, many entities? J Clin Invest. 2011;121:3789–3796. doi: 10.1172/JCI57100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marusyk A. Almendro V. Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 14.Denison TA. Bae YH. Tumor heterogeneity and its implication for drug delivery. J Control Release. 2012;164:187–191. doi: 10.1016/j.jconrel.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders NA. Simpson F. Thompson EW, et al. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO Mol Med. 2012;4:675–684. doi: 10.1002/emmm.201101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlo LM. Maley CC. The role of genetic diversity in cancer. J Clin Invest. 2010;120:401–403. doi: 10.1172/JCI42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner NC. Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 18.Michor F. Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila). 2010;3:1361–1364. doi: 10.1158/1940-6207.CAPR-10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russnes HG. Navin N. Hicks J, et al. Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest. 2011;121:3810–3818. doi: 10.1172/JCI57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marusyk A. Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata D. Cancer. Heterogeneity and tumor history. Science. 2012;336:304–305. doi: 10.1126/science.1222361. [DOI] [PubMed] [Google Scholar]
- 22.Durrett R. Foo J. Leder K, et al. Intratumor heterogeneity in evolutionary models of tumor progression. Genetics. 2011;188:461. doi: 10.1534/genetics.110.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasa Y. Michor F. Evolutionary dynamics of intratumor heterogeneity. PLoS One. 2011;6:e17866. doi: 10.1371/journal.pone.0017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denisov EV. Tashireva LA. Dultseva TS, et al. Molecular genetic characterization of intratumoral heterogeneity in invasive ductal NOS breast carcinoma. European Human Genetics Conference, Nurnberg, Germany. Eur J Hum Genet. 2012;20(Suppl 1):388. [Google Scholar]
- 25.Denisov E. Tsyganov M. Tashireva L, et al. Intratumoral heterogeneity in expression of chemotherapy response markers in invasive ductal breast carcinoma NOS. 4th WIN Symposium: Efficacy of Biomarkers and Personalized Cancer Therapeutics, Paris, France. Ann Oncol. 2012;23:v21–v22. [Google Scholar]
- 26.Deroo BJ. Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maynadier M. Chambon M. Basile I, et al. Estrogens promote cell-cell adhesion of normal and malignant mammary cells through increased desmosome formation. Mol Cell Endocrinol. 2012;364:126–133. doi: 10.1016/j.mce.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Perel'muter VM. Zav'ialova MV. Vtorushin SV, et al. Genetic and clinical and pathological characteristics of breast cancer in premenopausal and postmenopausal women. Adv Gerontol (Russian). 2008;21:643–653. [PubMed] [Google Scholar]
- 29.Anders CK. Acharya CR. Hsu DS, et al. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS One. 2008;3:e1373. doi: 10.1371/journal.pone.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol. 2008;66:65–74. doi: 10.1016/j.critrevonc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter CL. Allen C. Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Neri A. Nicolson GL. Phenotypic drift of metastatic and cell-surface properties of mammary adenocarcinoma cell clones during growth in vitro. Int J Cancer. 1981;28:731–738. doi: 10.1002/ijc.2910280612. [DOI] [PubMed] [Google Scholar]
- 33.Welch DR. Nicolson GL. Phenotypic drift and heterogeneity in response of metastatic mammary adenocarcinoma cell clones to adriamycin, 5-fluoro-2′-deoxyuridine and methotrexate treatment in vitro. Clin Exp Metastasis. 1983;1:317–325. doi: 10.1007/BF00121194. [DOI] [PubMed] [Google Scholar]
- 34.Nicolson GL. In: Encyclopedia of Cancer. Autocrine and paracrine growth mechanisms in cancer progression and metastasis. In: Bertino JR, editor. 2nd. Elsevier Science Ltd.; San Diego, CA: 2002. pp. 165–177. edition. [Google Scholar]
- 35.Cink TM. Breast cancer screening update and evaluation of the high-risk patient. S D Med. 2010:23–30. Spec No: [PubMed] [Google Scholar]
- 36.Hill RP. Chambers AF. Ling V, et al. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science. 1984;224:998–1001. doi: 10.1126/science.6719130. [DOI] [PubMed] [Google Scholar]
- 37.Nenci I. Expression and modulation of estrogen receptors in human breast cancer. J Steroid Biochem. 1985;23:1093–1096. doi: 10.1016/0022-4731(85)90025-1. [DOI] [PubMed] [Google Scholar]
- 38.Sheahan K. Shuja S. Murnane MJ. Cysteine protease activities and tumor development in human colorectal carcinoma. Cancer Res. 1989;49:3809–3814. [PubMed] [Google Scholar]
- 39.Zavyalova MV. Litvyakov NV. Garbukov EY, et al. Relationship between tumor sensitivity to neoadjuvant chemotherapy and histologic pattern of primary tumor in unicentric infiltrating ductal breast carcinoma. Sib J Oncol (Russ). 2008;6:30–34. [Google Scholar]