Abstract
Glutaraldehyde (GA) is largely used in the cross-linking of collagen matrices to improve their mechanical and biological properties for applications in cardiovascular surgery. However, GA has major drawbacks, including graft degeneration, calcification, and durability. The aim of this study was to test the hypothesis that filling the interstitial space in the bovine pericardium with various space fillers could prevent tissue calcification. GA, genipin, and 1-ethyl-3(-3 dimethyl aminopropyl) carbodiimide hydrochloride fixation with spacefiller treatment have been studied in order to improve the properties of heart valve xenografts. Crosslinking efficiency of GA treated group was better than genipin or 1-(3-dimethyl aminopropyl)-3-ethyl carbodiimide/N-hydroxysuccinimide treated group in vitro mechanical, enzymatic degradation resistance tests. Space-filling samples have shown significantly reduced calcification in the rabbit intramuscular implantation model. Regardless of the filling effect, the level of calcification and the cytotoxicity was low in a genipin-treated group compared to levels in the GA-treated group. The results indicated that GA and genipin fixation with space-filler treatment were effective in anticalcification for biological tissue preservation.
Key words: tissue engineering
Introduction
Heart valve diseases have significant mortality. Heart valve replacement is the most common treatment for heart valve disease worldwide. Recently, bioprosthetic valves have been used extensively in cardiac surgery.1 Although these valves are in widespread use to treat valve diseases, durability problems remain unresolved.2 The common way to prepare xenogenic valves is treatment of the animal pericardium with glutaraldehyde (GA; JUNSEI), a chemical cross-linking reagent, to improve biostability and structural integrity of the collagen material. GA generates a cross-link between lysine and hydroxylysine residues of the tissue proteins, and it can be used as an intramolecular or intermolecular bridge.3 However, GA treatment does not completely eliminate the immune response and calcification. Although the exact mechanism for calcification is unclear, the process has been attributed to several factors: (1) the degree of cross-linking,4,5 (2) the presence of free aldehyde groups,6 and (3) localized areas of stress and cellular debris.7 It is assumed that antigenicity is associated predominantly with the cellular component of the tissue, and that removal of the native cells will remove antigenicity.8–11 The decellularization may attenuate tissue calcification itself and in synergy with other anticalcification treatment.
Carbodiimide directly cross-links polypeptide chains. Cross-linking with carbodiimides involves the activation of the carboxylic acid groups of glutamic or aspartic acid residues by carbodiimides to form O-acylisourea groups. Cross-links are formed after nucleophilic attack by free amino groups of lysine or hydroxyline residues.12 Genipin (Wako, Osaka, Japan), which is less cytotoxic than GA, can be obtained from its parent compound, geniposide, which is isolated from the fruits of Gardenia jasminoides Ellis.13,14
In addition to simply using a different cross-linking reagent, several procedures have been introduced in an effort to reduce calcification in GA-treated tissue. These include treatment with ethanol, α-amino-oleic acid (AOA), and glutamic-acid amino compounds.15–18 A small number of researchers have investigated filling interstitial void spaces in GA-pretreated tissue with a macromolecular substance to prevent calcification. They hypothesized that reaction of macromolecules with free aldehyde groups of GA can inactivate them or mask platelet receptor sites on collagen with macromolecules to prevent aggregation of platelets.19–22 Polyethylene glycol (PEG) is a versatile polymer having mostly hydrophilic and hydrophobic functionalities and is capable of stabilizing the biological properties of protein from proteolytic degradation.23 Although monomeric acrylamide is neurotoxic, polyacrylamide (pAAm) hydrogel is a nontoxic, stable, and highly biocompatible material.24 pAAm appears to not cause degradation, granulomas, inflammation, or fibrosis.25
Materials and Methods
Decellularized bovine pericardia were cross-linked with GA, 1-(3-dimethyl aminopropyl)-3-ethyl carbodiimide/N-hydroxysuccinimide (EDC/NHS) or genipin after treatment with various space fillers (Jeffamine, pAAm, or PEG), and then the pericardia were examined for tensile strength, thermal stability, in vitro enzymatic degradation resistance, and cytotoxicity. Histologic and calcium analysis were done after 4 or 8 weeks of rabbit intramuscular implantation.
Cell isolation and culture
To obtain aortic smooth muscle cells, segments of aorta taken from pigs were used. We stripped off the adventitia physically. After one end of aorta was ligatured with suture thread, the lumen was filled with Hank's balanced salt solution (HBSS) containing 0.02% collagenase type II (Sigma). The other end was ligatured, and then the aorta was incubated at 37°C for 15 min. After incubation, endothelial cells and cell sheets from the digest were washed twice. After predigestion for 1 h in HBSS containing 0.02% collagenase type II, the remaining media was digested in a new aliquot of the same solution. The digest was filtered though a mesh to remove debris and centrifuged for 10 min at 3200 g. The cell pellet was suspended in Dulbecco's modified Eagle's medium (WelGENE, Korea) containing 10% fetal bovine serum (Gibco), 1% antibiotics (Gibco), plated on a 1.5% gelatin-coated culture dish (BD Falcon™), and incubated in 5% CO2/95% O2 at 37°C for approximately 1–2 weeks until confluent. The medium changes were performed twice a week. Cells of 4–10 passages (split ratio 1:4) were used for the assays.
Tissue decellularization and cross-linking
Fresh bovine pericardia were obtained from the local slaughter house and transported immediately in 4°C normal saline bags to our facility. The tissue was processed aseptically in a sterile clean room where excess fat and damaged tissue was removed from the pericardial sacks. All tissues were disinfected in 0.1% (v/v) peracetic acid with 4% ethanol in distilled water for 2 h, then washed vigorously for 1 h with distilled water. To decellularize the pericardium, cell extraction was performed with a hypotonic solution (1 L of distilled water, 10 mM Tris, pH 8) with 0.25% sodium dodecyl sulfate for 24 h, followed by the hypotonic solution with 0.5% Triton X-100 for 24 h, and then washed with distilled water for 12 h at 4°C. Finally, the tissues were washed with phosphate-buffered solution (PBS, 0.01 M, pH 7.4) for 1 h at 4°C and stored for use. After decellularization, the pericardia were cross-linked by the method described in Table 1.
Table 1.
Tissue Cross-Linking Process
| 1. | 30 mM EDC and 6 mM NHS in 0.05 M MES (pH 5.5) | for 3 days at RT | → | |
| EDC/NHS+75% ethanol+5% octanol | for 2 days at RT | → | 0.1 M glycine for 1 day at 37°C | |
| 2. | 0.06 M poly(propylene glycol) bis 2-(aminopropyl) ether (Jeffamine) in 0.25 M MES (pH 5) | for 1 day at RT | → | EDC and NHS (group 1) |
| 3. | 30 g acrylamide/bisacrylamide, 37:1 ratio and 0.4 % (w/w) DMPA in 0.01 M PBS (pH 7.4) | for 1 days at RT | → | EDC and NHS (group 1) |
| 4. | 50% PEG (1000 MW) in 0.01 M PBS (pH 7.4) | for 1 day at RT | → | EDC and NHS (group 1) |
| 5. | 0.25% GA in 0.05 M HEPES (pH 7.4) | for 3 days at RT | → | |
| 0.25% GA + 75% ethanol + 5% octanol | for 2 days at RT | → | ||
| 0.25% GA | for 1 week at RT | → | 0.1 M glycine for 1 day at 37°C | |
| 6. | 0.06 M Jeffamine in 0.25 M MES (pH 5) | for 1 day at RT | → | GA fixation (group 5) |
| 7. | 30 g acrylamide/bisacrylamide, 37:1 ratio and 0.4 % (w/w) DMPA in 0.01 M PBS (pH 7.4) | for 1 day at RT | → | GA fixation (group 5) |
| 8. | 50% PEG (1000 MW) in 0.01 M PBS (pH 7.4) | for 1 day at RT | → | GA fixation (group 5) |
| 9. | 0.3% genipin in 0.01 M PBS (pH 7.4) | for 3 days at RT | → | |
| 0.3% genipin + 75% ethanol + 5% octanol | for 2 days at RT | → | 0.1 M glycine for 1 day at 37°C | |
| 10. | 0.06 M Jeffamine in 0.25 M MES (pH 5) | for 1 days at RT | → | genipin fixation (group 9) |
| 11. | 30 g acrylamide/bisacrylamide, 37:1 ratio and 0.4 mass% DMPA in 0.01 M PBS (pH 7.4) | for 1 day at RT | → | genipin fixation (group 9) |
| 12. | 50% PEG (1000 MW) in 0.01 M PBS (pH 7.4) | for 1 day at RT | → | genipin fixation (group 9) |
| 13. | Control |
EDC, 1-(3-dimethyl aminopropyl)-3-ethyl carbodiimide; NHS, N-hydroxysuccinimide; MES, MES hydrate; RT, room temperature; DMPA, 2,2-bis(hydroxymethyl)propionic acid; PBS, phosphate-buffered saline; PEG, polyethylene glycol; GA, glutaraldehyde.
Tensile strength test and elasticity test
We measured the tensile strength using 0.5 cm×1 cm samples of bovine pericardium, which were measured at an angle of 30° because of their irregular collagen fibrous arrangement. We used a tensile testing machine (K-ML-1000N; M-TEC) equipped with a digital force gauge (DS2-200N; IMADA). Each bovine pericardium sample was loaded at a speed of 100 mm/min and measured in units of MPa (=N/mm2). We also used a Mitutoyo thickness gauge (Quick-Mini 700-117) to test the relationship between width and strength of the pericardium samples by repeated measurements.
Thermal stability test
To assess the degree of fixation of bovine pericardium, we measured the tissue shrinkage temperature by a hydrothermal method. Tissue strips (8 mm×30 mm) were loaded to 95 g, held at constant extension along the long axis, and placed in a water bath. The temperature of water bath was increased ∼2.5°C–5°C/min and the width of the strip was measured using a microscope. We used a waterproof digital thermometer (−50°C–200°C [−58°F–392°F]) to measure the shrinkage temperature. The sharp deflection point at which shrinkage occurred was identified as the shrinkage temperature.
Resistance towards protease digestion
We measured the dry weight of about ten 10-mm bovine pericardium samples to establish a baseline value. Then the tissues were incubated with shaking (22 g) at 50°C for 24 h in a protease solution prepare by dissolving 0.5 mg/mL Pronase® in HEPES 0.01 M (pH 7.4), glycine 0.1 M, and CaCl2 0.01 M. The sample remains were removed from protease solution, washed with deionized water, dried, and weighed. The actual weight loss was calculated as the post-degradation dry mass (using before/after mass ratio).
Contact cytotoxicity test
The porcine smooth muscle cells were seeded into each well at a density that would achieved confluence on attachment to a six-well culture plate. Ten-millimeter disks of tissue samples were place on the center of a well in a six-well culture plate using sterile fixing supports. A fixing support only, cells alone, and dimethyl sulfoxide (DMSO)-treated cells were used as controls. Plates were then incubated at 37°C in 5% (v/v) CO2 in air for 48 h. The confluence and morphology of the cells were then examined by light microscopy. Cells were washed twice with cold PBS and then resuspended in 1×binding buffer. We transferred 100 μL of the solution to 5-mL culture tube, added 5 μL of fluorescein isothiocyanate (FITC) Annexin V and 5 μL of propidium iodide (PI; FITC Annexin V apoptosis detection kit 1, BD Pharmingen), and then gently vortexed the cells and incubated them for 15 min at room temperature (25°C) in the dark. Next, 400 μL of 1× binding buffer was added to each tube and analyzed by flow cytometry (FACS Caliber, Becton-Dickinson) within 1 h.
Rabbit intramuscular implantation
This study was approved by Institutional Animal Care and Use Committee of the Clinical Research Institute, Seoul National University Hospital (IACUC No. 10-0091). This facility was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care international (AAALAC). Male New Zealand white rabbits (6 weeks old, 1.5 kg) were used. All surgery was performed using aseptic techniques. Anesthesia used was zoletil (15 mg/kg IM) and xylazine hydrochloride (5 mg/kg IM). Each rabbit's hind leg was shaved, an incision was made through the skin and into the femoral muscle, and the tissue sample was inserted between the muscle layers. Each rabbit hind leg received six 10-mm samples. The tissue samples were harvested from the intramuscular region along the spine at 4 or 8 weeks, examined visually for infection and calcium, and then placed in normal saline.
Calcium analysis
Harvested tissue samples (12 samples for each group; six samples at 4 weeks and six samples at 8 weeks) were washed with normal saline, dried at 90°C for 24 h, and weighed. Samples were then hydrolyzed with 5.0 normality (N) HCl solution. Sample were redried and hydrolyzed. Calcium combines with Ortho Cresol Phthalein Complexone method to form a reddish-purple complex. We measured the absorbance of the reddish-purple complex to obtain the amount of calcium. Inorganic phosphate is combined with ammonium molybdate as the phosphomolybdate is reduced by lactate p-methylaminophenol molybdenum blue. Thus, we determined the amount of inorganic phosphate by measuring the absorbance of the molybdenum blue (HITACHI 7070, Hitachi High-Technologies Corporation).
Statistics
Data were expressed as mean±standard deviation. We used the one-way ANOVA and Mann-Whitney tests as a comparison method. We also used the Bonferroni and Tamhene method as post hoc methods. All statistical analyses were performed by SPSS (SPSS 12.0 for Windows, SPSS Inc., Chicago, IL). Statistical significance was defined as p<0.05.
Results
Decellularization and cross-linking
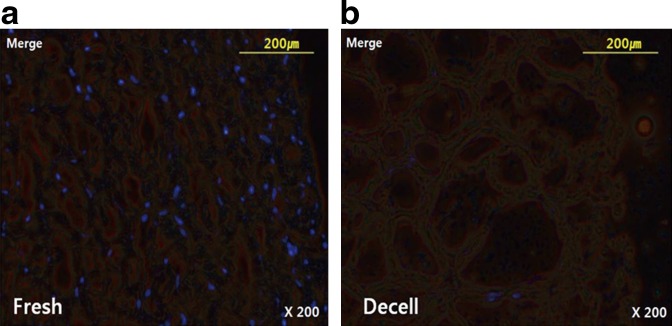
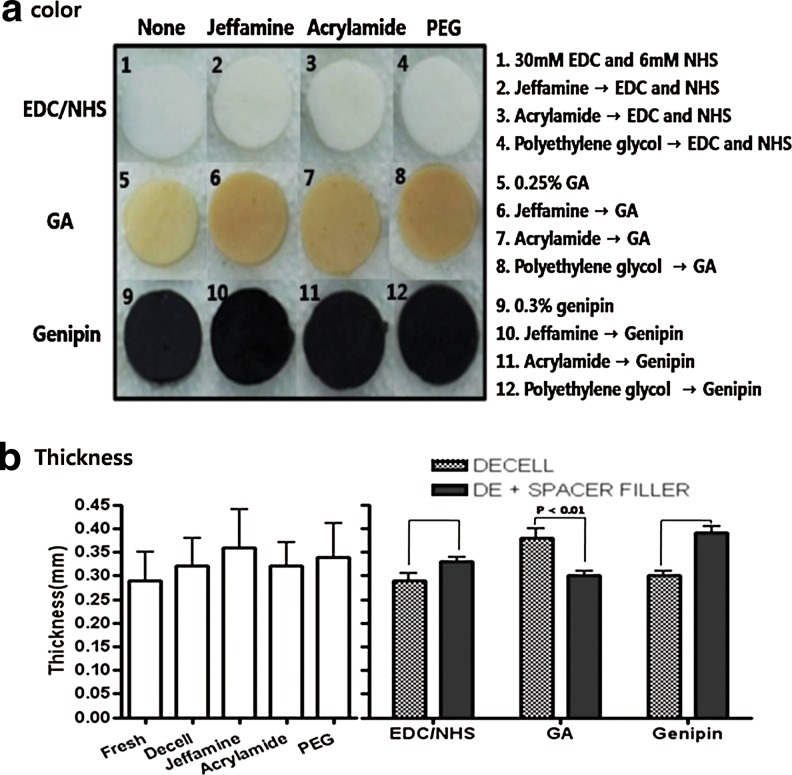
In Figure 1, 4′,6-diamidino-2-phenylindole, dihydrochloride staining demonstrated a significant reduction of DNA in decellularized bovine pericardium (Fig. 1b) compared to fresh control (Fig. 1a). After decellularization, we confirmed that the bovine pericardium was completely decellularized. Decellularized pericardia were filled with space fillers (Jeffamine, acrylamide, PEG), then cross- linked with EDC/NHS, GA, or genipin. After cross-linking, the color changed; dark yellow after GA treatment and blue-black after genipin treatment (Fig. 2a). If we used space filler, the overall color was more vivid and even. Color was whitish after EDC/NHS treatment. In Figure 2b, after space filler processing, most tissues became thicker (each group, n=10). In contrast, the GA group (decellularized only; 0.38±0.06 mm) was thinner after space filler treatment (Jeffamine, 0.34±0.03 mm; acryl, 0.27±0.05 mm; PEG, 0.29±0.03 mm). The graph shows the average value of Jeffamine, acrylamide, PEG.
FIG. 1.
4′,6-Diamidino-2-phenylindole, dihydrochloride (DAPI) staining of decellularized pericardium. DAPI staining demonstrated a significant reduction of DNA in decellularized bovine pericardium. (a) Fresh bovine pericardium; (b) decellularized (decell) bovine pericardium (fluorescence microscopy, original magnification ×200).
FIG. 2.
Changes of pericardia after completing all of the processing (1–12). (a) The color of the pericardium changed to white (EDC/NHS group), ivory-yellow (GA group), or blue-black (genipin groups). (b) After space filler groups (Jeffamine, 0.34±0.03; acryl, 0.27±0.05; PEG, 0.29±0.03) processing most tissues become thicker than the previous (each group, n=10), excepting the GA groups (decellularized only, 0.38±0.06). All data represent mean±SEM.
Tensile strength
Tensile strength and strain values are shown (Table 2). These data indicate that GA, EDC/NHS, and genipin are all effective cross-linking reagents. Additionally, it was found that the maximum loads of the GA-, EDC/NHS-, and genipin-fixed tissues were greater than the fresh tissue. This suggests that that the cross-linking of free amino groups within biological tissue may increase the mechanical strength of the tissue.
Table 2.
Tensile Strength of Bovine Pericardium
| |
|
Tensile strength |
||
|---|---|---|---|---|
| Group (bovine pericardium) | Sample | Thickness (mm) | Ultimate strength (MPa) | Strain at fracture (%) |
| Fresh bovine pericardium | n=50 | 0.29±0.06 | 17.53±4.58 | 42.47±7.55 |
| DE-EDC/NHS | n=10 | 0.29±0.05 | 13.89±9.24 | 39.33±12.35 |
| DE-Jeffamine-EDC/NHS | n=10 | 0.31±0.06 | 11.82±3.56 | 47.33±8.29 |
| DE-acryl-EDC/NHS | n=10 | 0.36±0.04 | 18.24±6.06 | 39.67±4.57 |
| DE-PEG-EDC/NHS | n=10 | 0.32±0.05 | 17.84±6.52 | 45.67±6.68 |
| DE-GA | n=10 | 0.38±0.06 | 16.63±4.91 | 56.67±24.09 |
| DE-Jeffamine-GA | n=10 | 0.34±0.03 | 15.61±4.34 | 38.33±5.03 |
| DE-acryl-GA | n=10 | 0.27±0.05 | 13.02±3.37 | 35.33±8.04 |
| DE-PEG-GA | n=10 | 0.29±0.03 | 15.43±3.70 | 47.33±4.39 |
| DE-genipin | n=10 | 0.30±0.03 | 13.49±3.53 | 45.00±7.41 |
| DE-Jeffamine-genipin | n=10 | 0.45±0.06 | 8.31±3.95 | 57.67±24.70 |
| DE-acryl-genipin | n=10 | 0.32±0.03 | 15.47±8.04 | 41.33±9.71 |
| DE-PEG-genipin | n=10 | 0.41±0.08 | 16.37±6.13 | 55.33±9.05 |
DE, decellularization.
Cross-linking efficiency
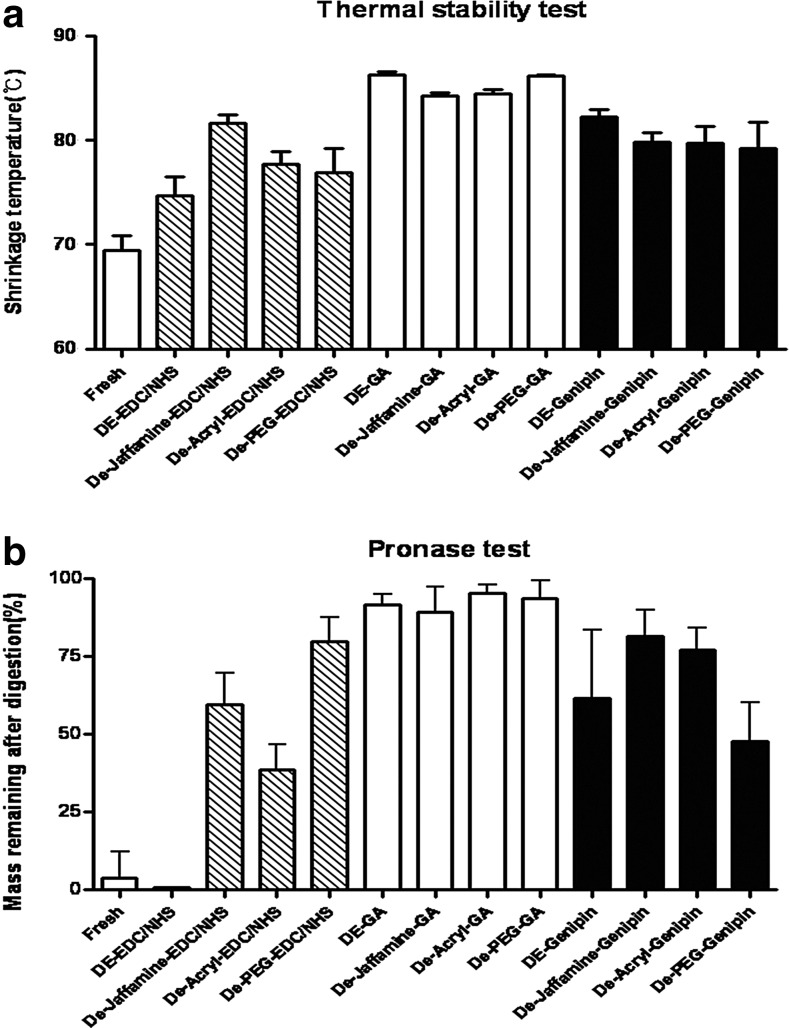
In Figure 3A, shrinkage temperatures of all cross-linked groups were significantly higher than that of fresh pericardium (69.42°C; p<0.05). GA-treated groups (86.28°C, 84.23°C, 84.44°C, 86.14°C) showed the highest shrinkage temperature. Genipin-treated groups (82.28°C, 79.82°C, 79.70°C, 79.20°C) were slightly higher than EDC/NHS-treated groups (74.72°C, 81.68°C, 77.74°C, 76.87°C). Except in the EDC/NHS-treated groups, the spacer filler did not affect at the thermal stability significantly. In EDC/NHS groups, thermal stability was reached in the Jeffamine group at 81.68°C. The resistance to degradation by pronase was studied (Fig. 3b). This study showed a similar pattern to the thermal stability. After enzymatic degradation in vitro, the relative weight loss in the GA- and genipin-treated groups was lower than fresh bovine pericardium. Genipin treatment was significantly less effective than GA. Protease resistance was very weak in the groups treated with EDC/NHS (without spacer filler); more than 90% of the pericardium was degraded. Only when we treated with a combination of EDC/NHS and space filler was the resistance was markedly increased. After enzymatic degradation, 89.3%–95.4% of GA samples remained. Independent of filler effect, GA was the most effective cross-linking reagent.
FIG. 3.
In vitro cross-linking efficiency tests. (a) Thermal stability test. Shrinkage temperatures of all cross-linked groups were significantly higher than that of fresh pericardium (69.42°C; p<0.05). (b) Pronase test is shown in a similar pattern to the thermal stability. All data represent mean±SD.
Contact cytotoxicity
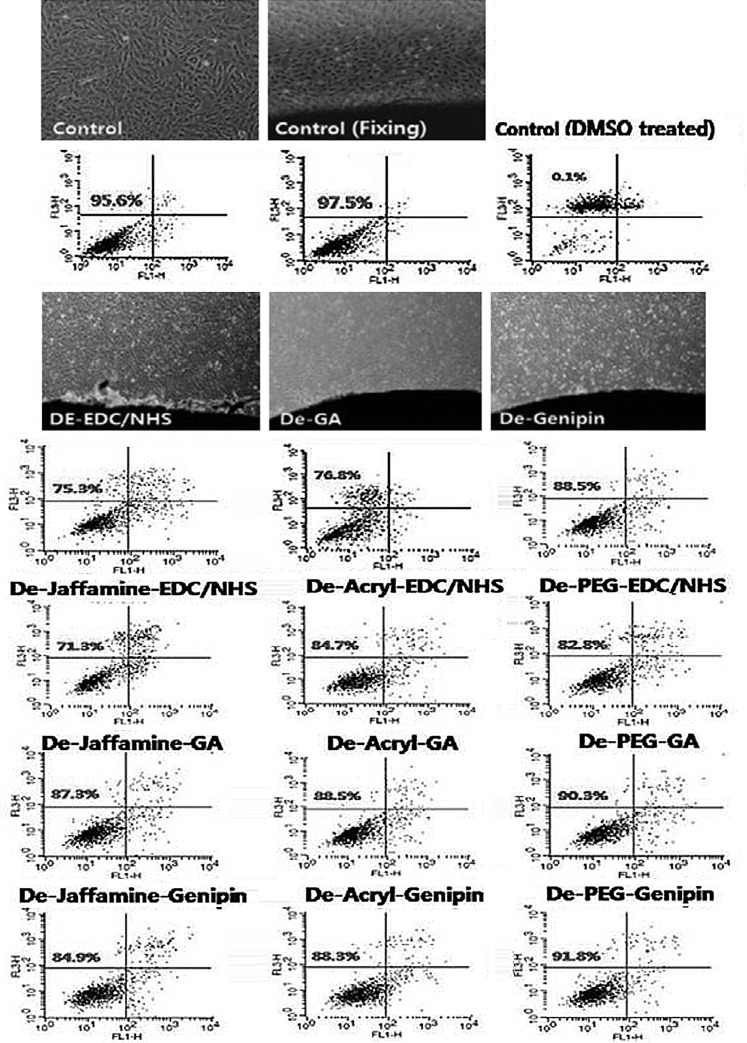
We confirmed cross-linking efficiency in vitro. Before rabbit intramuscular implantation, we checked the contact cytotoxicity of samples (Fig. 4). There was no change in morphology of the cells compare to the positive control. In contrast, the DMSO (negative control) caused cell death. For quantitative analysis, PI and Annexin V stain cell apoptosis was analyzed by flow cytometry. Around 95% of cells were alive in the positive control and only 0.1% of cells were alive in negative control. In the EDC/NHS-treated group, 75.3% (none), 71.3% (Jeffamine), 84.7% (acryl), and 82.8% (PEG) cells lived. This fixation method was the most toxic. In the GA-treated group, 76.8% (none), 87.3% (Jeffamine), 88.5% (acryl), and 90.3% (PEG) cells lived. These data indicated that space filler treatment elevated cell viability about 10%. In the genipin-treated group, 88.8% (none), 84.9% (Jeffamine), 88.3% (Acryl), and 91.8% (PEG) cells lived. We confirmed that regardless of filler treatment genipin groups were not toxic. We compared this result with the degree of calcification (Fig. 5), fewer live cells increased the degree of calcification. After space filler treatment, cell activity has increased over the decellularized only group. This means, except for a few cases, space filler treatment showed less cytotoxicity.
FIG. 4.
Contact cytotoxicity of bovine pericardium with porcine aortic smooth muscle cells. We confirmed cross-linking efficiency in vitro. Phase-contrast microscopy image of contact cultured cells with pericardium (original magnification ×40). Cells were labeled with Annexin V fluorescein isothiocyanate (FL1) and propidium iodide (FL2), then identified using flow cytometry.
FIG. 5.
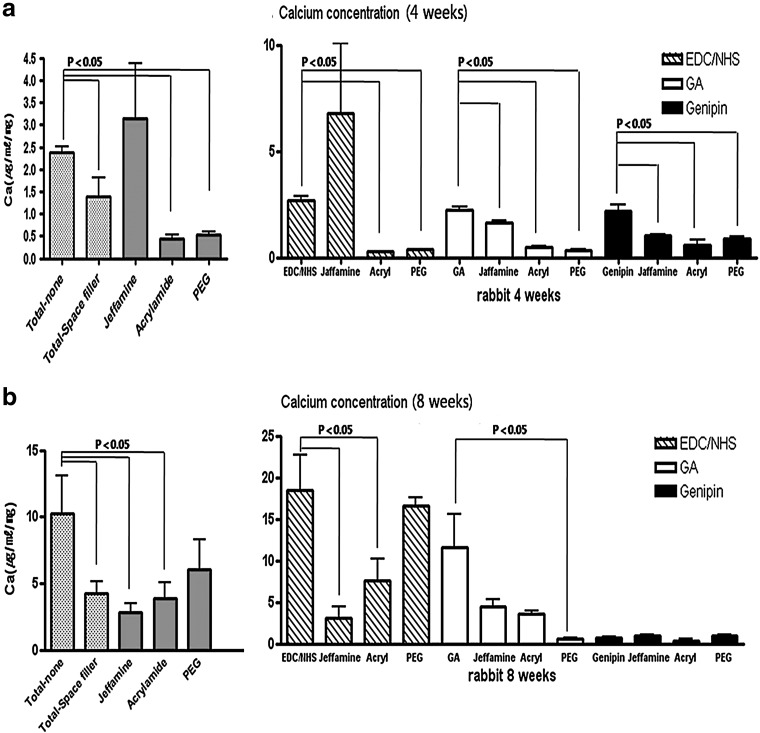
Calcium concentration of explanted pericardium. Samples were implanted into rabbit intramuscular space for 4 or 8 weeks. We confirm that regardless of filler treatment, genipin groups were not toxic. We compared this result with the degree of calcification and found that the lower the population of living cells, the higher the degree of calcification. All data represent mean±SEM (μg/mL/mg, dry weight, n=5).
Calcium analysis
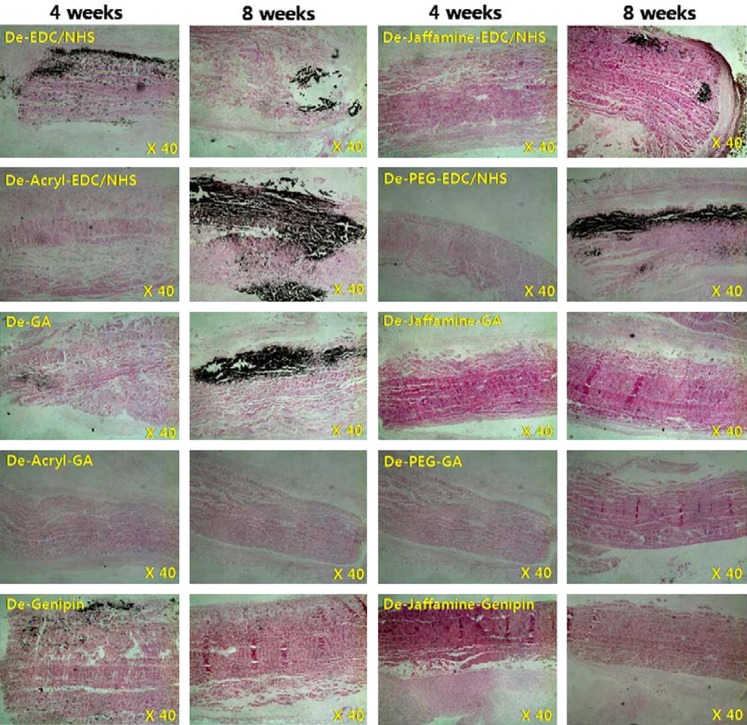
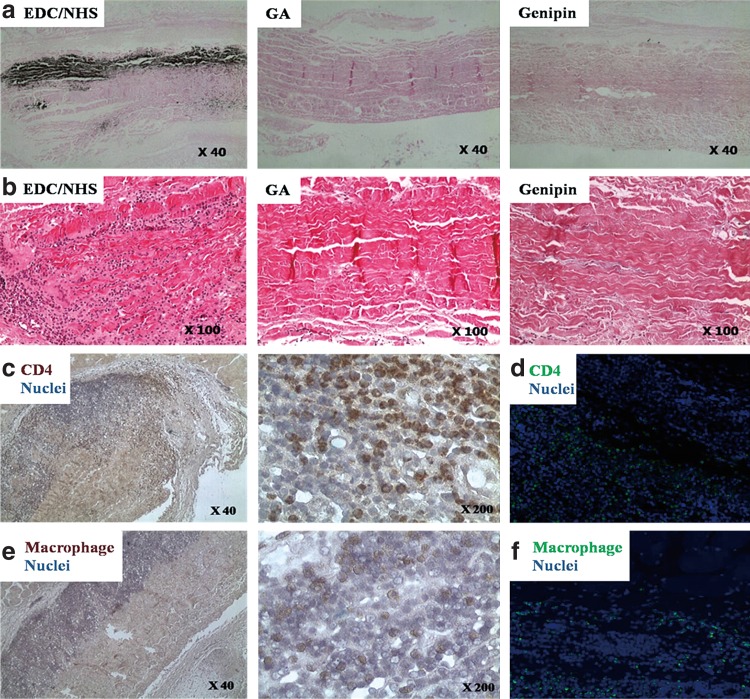
To analyze calcification, we performed rabbit intramuscular implantation for 4 and 8 weeks. As Figure 5 shows, 4 weeks is insufficient for calcification. In Figure 5b, in the genipin-treated group, calcification barely occurred (less than 1 μg/mL/mg). In terms of EDC/NHS or GA treatment groups, the space filler treatment reduced calcification (p<0.05). This pattern was similar to that seen in contact cytotoxicity assays. The GA-PEG group especially showed a very low degree of calcification (0.62±0.1 μg/mL/mg). Von Kossa staining showed similar results (Fig. 6). When more calcification occurs, more recipient cells deeply infiltrated from the tissue border line to the center of the tissue (Fig. 7, hematoxylin and eosin stain). These cells were identified as CD4 antibody positive (abcam, ab61131; Sigma-Aldrich, A0545) and macrophages (Fig. 7; immunohistochemistry and immunofluorescence). According to these results, death of donor cells occurred due to tissue contact cytotoxicity, which then recruited immune cells to the transplanted tissue. These immune cells penetrated into the center of the tissue, leading to tissue calcification.
FIG. 6.
Explanted pericardium with von Kossa staining. Samples were implanted into rabbit intramuscle for 4 or 8 weeks. In particular the GA-PEG group showed a very low degree of calcification (0.62±0.1 μg/mL/mg). These results can be observed with the von Kossa stain. Light microscopic image of bovine pericardium (original magnification ×40).
FIG. 7.
Von Kossa staining (a, c, e), hematoxylin and eosin staining (b), and immunofluorescence (d, f) of PEG-treated pericardium. Samples were implanted into rabbit intramuscle for 4 or 8 weeks. These cells were identified as CD4 (c, d) and macrophage (e, f) [immunohistochemistry (c, e) and immunofluorescence (d, f)]. According to the results, dead donor cells due to tissue contact cytotoxicity recruited immune cells from tissue surrounding the transplanted tissue. These immune cells penetrated into the center of the transplanted tissue and then led to tissue calcification. (a–c, e) Light microscopic images of bovine pericardium (original magnification ×40, ×100, ×200). (d, f) Fluorescence microscopy images of bovine pericardium (original magnification ×100).
Discussion
In this study, we investigated the ideal cross-linking method for low toxicity, minimal calcification, and enhanced stability. To achieve this goal, we devised a three-step process: (1) use decellularization for removal of cells from a bovine pericardium; (2) fill the interstitial space with a macromolecular substance (Jeffamine, pAAm, or PEG) to prevent calcification; and (3) cross-link with GA, EDC/NHS, or genipin to improve mechanical and immunogenic properties. Among 12 groups, the decellularization–PEG–GA method was the most effective way to improve the mechanical and chemical tissue properties and prevent tissue calcification. Xenogenic biomaterials require a chemical stabilization process that improves the biochemical and mechanical properties before their use in cardiovascular surgery. The processes developed to date are based on cross-linking agents, with GA being the most widely used. GA generates a cross-link between lysine and hydroxylysine residues of the tissue proteins, and it can be used as an intramolecular or intermolecular bridge.3 However, GA is cytotoxic and has been implicated in calcification of the tissue. Alternative tissue fixation methods have been developed, and several cross-linking methods have been reported.
In this study, we demonstrated the anticalcification efficacy of a three-step process with bovine pericardium in the rabbit intramuscular implantation model. Residual free aldehyde groups are cytotoxic and are known to result in tissue calcification.26,27 Step 1, decellularization, removes cells that can cause an immune response to graft, but space is formed and fixing with GA does not completely remove it. Further, there is the possibility of calcium being deposited. These problems can be solved by treating the filler (step 2) before fixing. Oosthuysen et al.28 reported that GA cross-links probably restricted acrylamide penetration into tissue by rendering a more rigid and dense tissue structure. This research group treated with acrylamide to remove void space caused by fixed GA (no decellularization). But this two-step process is insufficient to completely eliminate calcification. Our three-step process includes ethanol, octanol, and glycine treatment at final step of cross-linking.15,18,29,30 Ethanol, long chain alcohol, ether, or surfactant did mitigate calcification. Amino-reagent treatment required a warm temperature (37°C), but was able to detoxify tissue satisfactorily.
In EDC/NHS treatment groups, Jeffamine treatment reduced calcification. Everaerts et al.31 reported a new carbodiimide-based cross-linking method, blocking of free amine groups with butanal, followed by carbodiimide activated cross-linking of carboxyl groups with amine-terminated polypropylene glycols. We found, however, Jeffamine treatment was able to effectively reduce calcification even without blocking processes. Also Zilla et al.32 proposed that both the zero-length cross-links between adjacent NH2 and COOH groups initiated by storage in EDC or the combined creation of zero-length cross-links as well as Jeffamine-based cross-links between carboxyl groups in the EDC/Jeffamine treatment did not seem to have any effect on calcification; it left the reaction products of AOA as the crucial elements responsible for the near abolition of calcification in the surface two thirds of the tissue. In the entire group treated with glycine instead of AOA, according to our results, EDC treatment did not seem to have any effect on calcification, but EDC/Jeffamine combination treatment seem to affect calcification. We have the same opinion about the effect of EDC; the difference in the effects of Jeffamine was caused by the difference in processing time. Zilla et al.32 treated with Jeffamine for only 30 min, but we treated for 24 h. The processing time as well as pretreatment before the fixing in order to effectively penetrate the filler are important.
In the GA treatment groups, all three kinds of fillers were effective to prevent calcification. Oosthuysen et al.28 reported that calcium content of 0.2% GA-fixed tissue was 97.4 μg/mg and calcium content of acrylamide-filled fresh tissue was 13.7 μg/mg after 8 weeks in a rat subcutaneous implantation model. Because we did cell-free treatment and anticalcification treatment, calcium content was only 11.6 μg/mg after 8 weeks in a rabbit subcutaneous implantation model. Calcium content was further lowered after the filler treatment (4.5, 3.6, and 0.6 μg/mg). After PEG treatment, calcification rarely occurred.
In the genipin treatment groups, there was little toxicity, and space filler did not affect the calcification. Genipin can be obtained from its parent compound, geniposide, which can be isolated from gardenia fruits.33 Genipin can react spontaneously with amino acids or proteins to form dark blue pigments,34 which is a disadvantage. With regard to thermal stability and resistance towards protease digestion, the genipin-treated group was lower than the GA-treated groups. Considering the complex mechanism of calcification of GA-fixed tissue, combining anticalcification treatments targeting different calcification processes are needed. In conclusion, we developed an ideal cross-linking method that improved mechanical properties and had low toxicity and calcification characteristics. In this study, we confirmed that our three-step process effectively reduced calcification and did not affect the mechanical properties. The tissue treated with our decellularization process had low immunogenicity. This method might reduce the risk of unknown viral infection and acute rejection in xenotransplantation.
This study has some limitations. The number of samples in each cross-linking experiment is not enough, so it is difficult to conclude which space filler is better. Further studies must be done using a large animal long-term circulatory model to verify our results.
This study shows that the decellularization–PEG–GA method was the most effective way to improve the mechanical and chemical tissue properties and prevent tissue calcification among the preservation methods tested. Our results suggest that the interstitial space filler treatment might be a useful approach for preparation of bioprosthetic heart valves.
Acknowledgment
This work was supported by grants from the Korea Health 21 Research & Development Project, Korean Ministry for Health, Welfare & Family Affairs (A04004-006), Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Neuenschwander S. Hoerstrup SP. Heart valve tissue engineering. Transplant Immunol. 2004;12:359–365. doi: 10.1016/j.trim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt CE. Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 3.Jayakrishnan A. Jameela SR. Glutaraldehyde as a fixation in bioprostheses and drug delivery matrices. Biomaterials. 1996;17:471–484. doi: 10.1016/0142-9612(96)82721-9. [DOI] [PubMed] [Google Scholar]
- 4.Golomb G. Schoen FJ. Smith MS, et al. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol. 1987;127:122–130. [PMC free article] [PubMed] [Google Scholar]
- 5.Nimni ME. Cheung D. Strates B, et al. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987;21:741–771. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 6.Gong G. Ling Z. Seifter E, et al. Aldehyde tanning: the villain in bioprosthetic calcification. Eur J Cardiothorac Surg. 1991;5:288–289. doi: 10.1016/1010-7940(91)90037-k. [DOI] [PubMed] [Google Scholar]
- 7.Thubrikar MJ. Deck JD. Aouad J. Nolan SP. Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg. 1983;86:115–125. [PubMed] [Google Scholar]
- 8.Zhai W. Chang J. Lin K, et al. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials. 2006;27:3684. doi: 10.1016/j.biomaterials.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Girardot JM. Girardot MN. Amide cross-linking: an alternative to glutaraldehyde fixation. J Heart Valve Dis. 1996;5:518–525. [PubMed] [Google Scholar]
- 10.Huang LL. Sung HW. Tsai CC. Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res. 1998;42:568–576. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Jorge-Herrero E. Fernández P. Turnay J, et al. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials. 1999;20:539–545. doi: 10.1016/s0142-9612(98)90205-8. [DOI] [PubMed] [Google Scholar]
- 12.Timkovich R. Detection of the stable addition of carbodiimide to proteins. Anal Biochem. 1977;79:135–143. doi: 10.1016/0003-2697(77)90387-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsai TH. Westly J. Lee TF. Chen CF. Identification and determination of geniposide, genipin, gardenoside, and geniposidic acid from herbs by HPLC/photodiode-array detection. J Liq Chromatography. 1994;17:2199–2205. [Google Scholar]
- 14.Fujikawa S. Yokota T. Koga K. Kumada J. The continuous hydrolysis of geniposide to genipin using immobilized-glucosidase on calcium alginate gel. J Biotechnol Lett. 1987;9:697. [Google Scholar]
- 15.Shen M. Kara-Mostefa A. Chen L, et al. Effect of ethanol and ether in the prevention of calcification of bioprostheses. Ann Thorac Surg. 2001;71(Suppl. 5):S413–416. doi: 10.1016/s0003-4975(01)02521-8. [DOI] [PubMed] [Google Scholar]
- 16.Gott J. Pan C. Dorsey L, et al. Calcification of porcine valves: a successful new method of antimineralization. Ann Thorac Surg. 1992;53:207–215. doi: 10.1016/0003-4975(92)91321-y. [DOI] [PubMed] [Google Scholar]
- 17.Grimm M. Grabenwoger M. Eybl E, et al. Improved biocompatibility of bioprosthetic heart valves by L-glutamic acid treatment. J Cardiovasc Surg. 1992;7:58–64. doi: 10.1111/j.1540-8191.1992.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 18.Zilla P. Fullard L. Trescony P, et al. Glutaraldehyde detoxification of aortic wall tissue: a promising perspective for emerging bioprosthetic valve concepts. J Heart Valve Dis. 1997;6:510–520. [PubMed] [Google Scholar]
- 19.Chanda J. Anticalcification treatment of pericardial prostheses. Biomaterials. 1994;15:465–469. doi: 10.1016/0142-9612(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 20.Chanda J. Kuribayashi R. Abe T. Use of the glutaraldehyde-chitosan-treated porcine pericardium as a pericardial substitute. Biomaterials. 1996;17:1087–1091. doi: 10.1016/0142-9612(96)85909-6. [DOI] [PubMed] [Google Scholar]
- 21.Vasudev S. Chandy T. Sharma C. The antithrombotic versus calcium antagonistic effects of polyethylene glycol grafted bovine pericardium. J Biomater Appl. 1999;14:48–66. doi: 10.1177/088532829901400103. [DOI] [PubMed] [Google Scholar]
- 22.Oosthuysen A. Zilla PP. Human PA, et al. Bioprosthetic tissue preservation by filling with a poly(acrylamide) hydrogel. Biomaterials. 2006;27:2123–2130. doi: 10.1016/j.biomaterials.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Han DK. Jeong SY. Kim YH. Evaluation of blood compatibility of PEO grafted heparin immobilized polyurethanes. J Biomed Mater Res Appl Biomater. 1989;23:211–228. [PubMed] [Google Scholar]
- 24.Carpentier A. Nashef A. Carpentier S, et al. Techniques for prevention of calcification of valvular bioprostheses. Circulation. 1984;70(3 Pt 2):I165–168. [PubMed] [Google Scholar]
- 25.Christensen L. Breiting V. Aasted A, et al. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003;111:1883–1890. doi: 10.1097/01.PRS.0000056873.87165.5A. [DOI] [PubMed] [Google Scholar]
- 26.Stacchino C. Bona G. Bonetti F, et al. Detoxification process for glutaraldehyde-treated bovine pericardium: biological, chemical and mechanical characterization. J Heart Valve Dis. 1998;7:190–194. [PubMed] [Google Scholar]
- 27.Valente M. Pettenazzo E. Thiene G, et al. Detoxified glutaraldehyde cross-linked pericardium: tissue preservation and mineralization mitigation in a subcutaneous rat model. J Heart Valve Dis. 1998;7:283–291. [PubMed] [Google Scholar]
- 28.Oosthuysen A. Zilla PP. Human PA, et al. Bioprosthetic tissue reservation by filling with a poly (acrylamide) hydrogel. Biomaterials. 2006;27:2123–2130. doi: 10.1016/j.biomaterials.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Pathak CP. Adams AK. Simpson T, et al. Treatment of bioprosthetic heart valve tissue with long chain alcohol solution to lower calcification potential. J Biomed Mater Res A. 2004;69:140–144. doi: 10.1002/jbm.a.20129. [DOI] [PubMed] [Google Scholar]
- 30.Lee C. Kim SH. Choi SY. Kim YJ. High-concentration glutaraldehyde fixation of bovine pericardium in organic solvent and post-fixation glycine treatment: in vitro material assessment and in vivo anticalcification effect. Eur J Cardiothorac Surg. 2011;39:381–387. doi: 10.1016/j.ejcts.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Everaerts F. Torrianni M. van Luyn M, et al. Reduced calcification of bioprostheses, cross-linked via an improved carbodiimide based method. Biomaterials. 2004;25:5523–5530. doi: 10.1016/j.biomaterials.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 32.Zilla P. Bezuidenhout D. Human P. Carbodiimide treatment dramatically potentiates the anticalcific effect of alpha-amino oleic acid on glutaraldehyde-fixed aortic wall tissue. Ann Thorac Surg. 2005;79:905–910. doi: 10.1016/j.athoracsur.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Sung HW. Huang RN. Huang LL, et al. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Touyama R. Takeda Y. Inoue K, et al. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem Pharm Bull. 1994;42:668–673. [Google Scholar]