Abstract
The goal of our study was to evaluate whether drugs currently used for smoking cessation (i.e., nicotine replacement therapy, varenicline [a partial agonist at nicotinic acetylcholine receptors (nAChR)] and bupropion [which acts in part as a nAChR antagonist]) can affect beta cell function and determine the mechanism(s) of this effect. INS-1E cells, a rat beta cell line, were treated with nicotine, varenicline and bupropion to determine their effects on beta cell function, mitochondrial electron transport chain enzyme activity and cellular/oxidative stress. Treatment of INS-1E cells with equimolar concentrations (1μM) of three test compounds resulted in an ablation of normal glucose-stimulated insulin secretion by the cells. This disruption of normal beta cell function was associated with mitochondrial dysfunction since all three compounds tested significantly decreased the activity of mitochondrial electron transport chain enzyme activity. These results raise the possibility that the currently available smoking cessation pharmacotherapies may also have adverse effects on beta cell function and thus glycemic control in vivo. Therefore whether or not the use of nicotine replacement therapy, varenicline and bupropion can cause endocrine changes which are consistent with impaired pancreatic function warrants further investigation.
Keywords: bupropion, diabetes, INS-1E cells, mitochondrial dysfunction, nicotine, varenicline
Introduction
Although the adverse health effects associated with cigarette smoking have been well documented, cigarette smoking remains one of the leading causes of global burden of disease and premature death (Ezzati and Lopez, 2003). Although many smokers report a desire to quit, nicotine dependence is a significant obstacle to smoking cessation (Benowitz, 2010). Indeed smoking cessation is associated with nicotine cravings and symptoms of nicotine withdrawal including anger, anxiety, depression, difficulty concentrating, impatience, insomnia and restlessness (Hughes, 2007). Consequently, most smokers who attempt to quit without pharmacologic smoking cessation aids are unsuccessful; only 3–5% achieve long-term (6–12 months) abstinence (Benowitz, 2010). Therefore, unless contraindicated, smoking cessation pharmacotherapies are recommended for all smokers trying to quit (US Public Health Services, 2000).
Currently, there are three first line smoking cessation pharmacotherapies that have been approved for use in North America: nicotine replacement therapy (NRT), varenicline (Champix®), and sustained-release bupropion (Zyban®); all of which have been shown to be more effective than placebo for smoking cessation (McNeil et al., 2010). Nicotine is a full agonist of nicotinic acetylcholine receptors (nAChR) whereas varenicline is an α4β2 nAChR partial agonist with a similar structure to nicotine (Potts and Garwood, 2007). Bupropion is an atypical antidepressant that acts as a norepinephrine and dopamine reuptake inhibitor, and an nAChR antagonist (Slemmer et al., 2000). Of these, NRT is the oldest and most commonly used smoking cessation pharmacotherapy (reviewed in McNeil et al., 2010).
Although smoking cessation is associated with numerous health benefits, there have been few studies which have looked at whether smoking cessation improves or worsens glycemic control, despite the fact that smoking is associated with an increased risk of type 2 diabetes in a dose related manner (Willi et al., 2007) and weight gain, a common outcome following smoking cessation (Inoue et al., 2011), is a risk factor for the development of diabetes. However, there have been reports suggesting that at least one of the smoking cessation pharmacotherapies, namely nicotine, can impair pancreatic function. Indeed, long-term NRT use has been reported to be associated with abnormal endogenous insulin secretion in humans (Eliasson et al., 1996) and results from animal studies and in vitro experiments have demonstrated that nicotine exposure results in beta cell loss and impaired beta cell function (Bruin et al., 2008a; Bruin et al., 2010; Yoshikawa et al., 2005). To date, there have been no studies that have investigated the effect of varenicline on beta cell function but cytisine, another α4β2 nAChR agonist, was reported to inhibit tolbutamide-stimulated insulin secretion (Yoshikawa et al., 2005). Similarly, the effects of bupropion on beta cell function have received little attention but there is one study which reported increased insulin secretion from isolated pancreatic islets treated with bupropion (el-Dakhakhny et al., 1996). Taken together these data suggest that all three of the smoking cessation pharmacoptherapies currently approved for use in North America may have the potential to affect glycemic control, however a direct comparison of these drugs on beta cell function has not been reported. Therefore, the goals of this study were 1) to examine the effects of nicotine, varenicline and bupropion on beta cell function and 2) examine mechanistic pathways by which these drugs could affect beta cell function.
Materials and Methods
Cell Culture Maintenance
INS-1E cells were generously provided by Dr. Claes Wollheim (University of Geneva, Geneva, Switzerland). Cells were cultured in a humidified atmosphere of 95% O2 and 5% CO2 in RPMI-1640 medium (Sigma Aldrich, Oakville, ON), supplemented with 10mM HEPES, 1mM L-glutamine, 1mM Na+-pyruvate, 50μM 2-mercaptoethanol, 1U/mL penicillin, 1μg/mL streptomycin (Sigma Aldrich) and 5% heat-inactivated fetal bovine serum (Hyclone, Logan, UT).
Insulin secretion
Acute exposure: To determine the effects of acute exposure to nicotine, varenicline, and bupropion on insulin secretion (i.e. beta cell function), INS-1E cells were seeded in six-well plates (2.5 × 105 cells/well). After 72 hours, cells were washed twice with PBS and then incubated for 1 hour in Krebs-Ringer bicarbonate HEPES (KRBH) buffer (135mM NaCl, 3.6mM KCl, 5mM NaHCO3, 0.5mM H2PO4, 0.5mM MgCl2, 1.5mM CaCl2, 10mM HEPES, 5mM glucose and 0.1% bovine serum albumin) containing vehicle, 1nM or 1μM of the three test compounds: nicotine bitartrate (Sigma Aldrich), varenicline (generously provided by Pfizer Inc., Groton, CT) or bupropion hydrochloride (Sigma Aldrich) (n=5 per treatment condition). The doses of nicotine were chosen to cover the maximum (Cmax:1.8–112 nM) and average concentrations of nicotine (AUC0-∞:17.8 nM•h - 2.3 μM•h) which have been reported in pharmacokinetic studies of nicotine replacement therapies including gum, lozenges, mouth sprays, transdermal patches and electronic nicotine delivery devices (Bullen et al., 2010; DeVeaugh-Geiss et al., 2010; Kraiczi et al., 2011). Equivalent concentrations of varenicline and bupropion were tested to allow direct comparisons between the three test compounds. Following the final incubation period, media was collected, centrifuged and frozen at −20°C until analysis. Insulin content in the media was determined using a commercially available rat insulin radioimmunoassay (LINCO, St. Charles, MO) according to the manufacturer’s instructions. Cells were lysed with 0.4M NaOH to determine protein content (BCA Protein Assay, Thermo Scientific, Rockford, IL).
Glucose-stimulated insulin secretion
Based on the observation that acute insulin secretion was only inhibited in the presence of 1μM nicotine, we chose to determine whether or not chronic exposure to nicotine at the same dose would affect glucose-stimulated insulin secretion. INS-1E cells were seeded in 6 well plates at a density of 2.5 × 105 cells/well for 24 hours. Cells were washed twice with PBS and then incubated with RPMI-1640 medium (as above) containing vehicle, nicotine, varenicline or bupropion hydrochloride for 48 hours (n=5 per treatment condition). This incubation time has been shown to be sufficient to observe nicotine-induced impairments in glucose-stimulated insulin secretion in rat and human pancreatic islets (Yoshikawa et al., 2005). To directly compare the effects of nicotine, bupropion and varenicline on our outcome measures, cells were treated with equimolar concentrations (i.e., 1μM) of all three test compounds. Following the 48 hour incubation, cells were washed twice with Krebs-Ringer bicarbonate HEPES (KRBH) buffer (135mM NaCl, 3.6mM KCl, 5mM NaHCO3, 0.5mM H2PO4, 0.5mM MgCl2, 1.5mM CaCl2, 10mM HEPES, 2.8mM glucose, pH 7.4) supplemented with 0.1% bovine serum albumin (all reagents from Sigma Aldrich) and incubated for 1 hour. Cells were then washed twice and incubated for 45 minutes in KRBH buffer containing either 3.3 or 16.7 mM glucose. Following the final incubation period, media was collected, centrifuged and frozen at −20°C until analysis. Insulin content in the media and cellular protein were determined as described above. Glucose-stimulated insulin release was determined by comparing the amount of insulin released at 16.7 mM glucose relative to 3.3 mM glucose.
Mitochondrial function
Normal mitochondrial function is critical for the maintenance of beta cell function (i.e., insulin secretion) (Patti and Corvera, 2010) and nicotine has been shown to cause mitochondrial dysfunction in a pancreatic beta cell line (Bruin et al., 2010). Since treatment of INS-1E cells with 1μM of nicotine, buproprion and varenicline adversely affected glucose-stimulated insulin secretion, therefore we examined the effects of all three drugs at the same concentration (i.e., 1 μM) on mitochondrial electron transport chain enzyme activity.
INS-1E cells were seeded in 10 cm dishes in culture medium (described above) for 3–4 days until approximately 80% confluent. Cells were then washed twice with PBS and then incubated with RPMI culture medium containing vehicle, nicotine, varenicline, or bupropion (1μM) for 48 hours. Treatments were done in triplicate in minimum of 3 independent experiments. Following the incubation period, cells were washed in Dulbeccos Modified PBS (DPBS; HyClone, Logan, UT), removed from the plate using cell scrapers, pelleted by centrifugation (1000 × g for 10 min at 4 C) and resuspended in homogenization buffer (5 mM HEPES, 100 mM KCl, 70 mM sucrose, 220 mM mannitol, 1 mM EGTA, pH 7.4) supplemented with Complete EDTA-free protease inhibitor (Roche Applied Science, Laval, PQ) and fatty acid free BSA (2mg/ml; Roche Applied Science). The cell suspension was sonicated (Microsonix 200) at a low power setting (7Hz) for 3 10 sec bursts. The mitochondrial fraction was prepared by differential centrifugation. The sonicate was centrifuged at 1000 × g for 10 min at 4°C. The supernatant was centrifuged for 16000 × g for 20 min at 4°C and the pellet was re-suspended in BSA-free Hepes buffer for enzyme activity assays or RIPA lysis buffer (15mM Tris-HCl, 1% (v/v) Triton X-100, 0.1% (w/v) SDS, 167mM NaCl, 0.5% (w/v) sodium deoxycholatic acid) containing protease inhibitor (Roche Applied Science) for western blot analysis (see below). The mitochondrial suspensions were flash frozen in liquid nitrogen and stored at −80°C until analysis. Citrate synthase activity, an indicator of total mitochondrial mass (Figueiredo et al., 2008) was measured using the thiol reagent 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB, Sigma Aldrich). Complex IV (cytochrome c oxidase) activity was assessed by measuring the rate of cytochrome c (from equine heart; Sigma Aldrich) oxidation. Both activity assays were performed using UV-spectrophotometry (Varian Inc., CA) as previously described (Parise et al., 2005). Data are expressed as the mean enzyme activity relative to the protein content in the sample.
Cellular and oxidative stress
There is considerable evidence both in vivo and in vitro showing that exposure to nicotine alone results in increased oxidative stress (Zhao and Reece, 2005; Crowley-Weber et al., 2003; Wetscher et al., 1995; Halima et al., 2010; Gallo et al., 2010). Moreover, pancreatic beta cells are known to be particularly sensitive to oxidative damage due to their relative low expression of antioxidant enzymes compared to other cell types (Lenzen et al., 1996). To determine whether bupropion and varenicline can also cause oxidative stress and/or oxidative damage to pancreatic beta cells, we determined the expression of heat shock protein 70 [Hsp70; protects pancreatic islets from the cytoxicity of reactive oxygen intermediates (Bellman et al., 1995)], 4-hydroxy-2-nonenal [4-HNE; a marker of oxidative damage of lipids], copper/zinc superoxide dismutase [CuZnSOD; an antioxidant enzyme] and manganese superoxide dismutase [MnSOD; an antioxidant enzyme] by western blotting and determined the levels of protein carbonyl formation [a marker of oxidative damage of proteins] by ELISA.
To determine the effect of the three smoking cessation drugs on Hsp70, CuZnSOD, MnSOD and 4-HNE, expression, cells were treated with vehicle, nicotine, varenicline and bupropion (1μM) for 48 hours (N=3 independent experiments); a time and dose which adversely affected beta cell function. Following treatment with the test compounds, cells were washed with Ca2+, Mg2+-free DPBS (HyClone) and collected in DPBS containing a protease inhibitor (Roche Applied Science). Cells were pelleted by centrifugation (2000 rpm for 5 minutes), re-suspended in RIPA lysis buffer containing protease inhibitor (Roche Applied Science) and centrifuged at 2000 rpm for 5 minutes. Protein content in the supernatants was determined using a BCA protein assay kit according to the manufacturer’s instructions (Thermo Scientific Fisher Inc., Rockford, IL) and samples were stored at −80°C. Protein (4-HNE 50μg; Hsp70 30μg; CuZnSOD and MnSOD; 10μg) was subjected to SDS-PAGE and then electrotransferred to polyvinylidene difluoride blotting membrane. As mitochondria are particularly vulnerable to oxidative damage, we also examined 4-HNE levels in the mitochondrial fraction (10 μg). Membranes were blocked overnight with 5% (wt/vol) skim milk in TTBS (Tris-buffered saline [TBS], 0.5% [vol/vol] Tween 20) at 4°C on a rocking platform and then incubated for 1 hour at room temperature in primary antibody. immunoblots were normalized using β-actin (1:5000; AbCam, Cambridge, MA). The following antibodies were used for this study (all rabbit polyclonal except 4-HNE): HSP70 (1:2000, 72 kDa, Cell Signaling Technology, Danvers, MA); MnSOD (1:10000, 25kDa, Santa Cruz Biotechnology, CA); CuZnSOD (1:70000, 23kDA, Santa Cruz Biotechnology, CA); and 4-HNE (mouse polyclonal, 1:500, Santa Cruz, CA). After washing with TTBS, blots were incubated with peroxidase-conjugated secondary anti-rabbit (1:2000; Santa Cruz) or anti-mouse (1:2000 Amersham Biosciences, NJ) for 1 h at room temperature on a rocking platform. Blots were developed and quantified as previously described (Bruin et al., 2008b).
Protein carbonyl levels were determined in whole cell protein homogenates from INS-1E cells treated for 48 hours with nicotine, varenicline, or bupropion (N=5 independent experiments per treatment group) using the commercially available OxiSelect Protein Carbonyl ELISA kit (Cell Biolabs, San Diego, CA) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using SigmaStat (v.3.1, SPSS, Chicago, IL, USA). The results are expressed as mean ± SEM. Data were tested for normality as well as equal variance, and when normality or variance tests failed, data were analyzed using appropriate non-parametric tests. To determine whether the test compounds resulted in altered insulin secretion following acute exposure, insulin release for each drug was compared to control values by one-Way ANOVA followed by appropriate post-hoc tests were performed when significance was indicated (p<0.05). Glucose-stimulated insulin release for each test compound was determined by comparing the amount of insulin released at 16.7 mM glucose relative to 3.3 mM glucose using Student’s t-test (α=0.05). All other outcome measures were compared between control and treatment groups by one-way ANOVA followed by appropriate post-hoc tests were performed when significance was indicated (p<0.05).
RESULTS
Insulin secretion
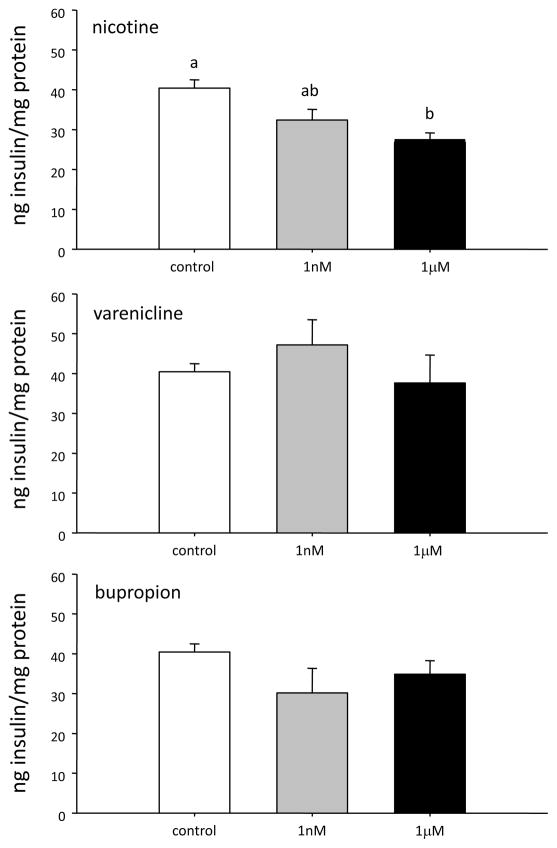
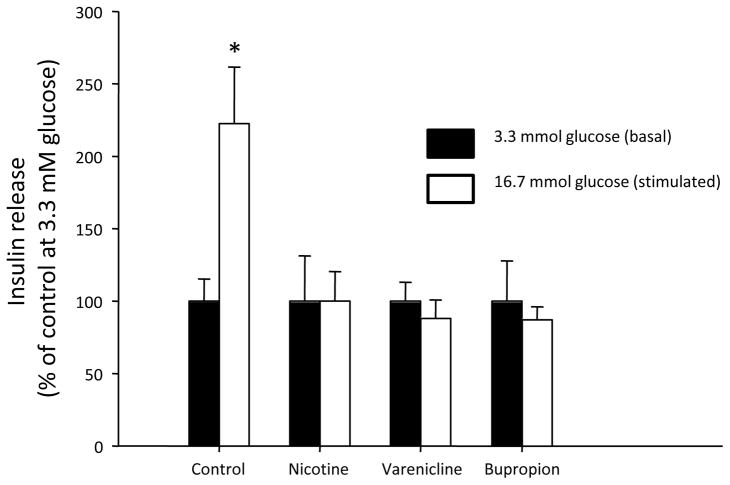
Acute exposure to nicotine inhibited insulin secretion from INS-1E cells; an effect which was only significant at the highest dose tested (i.e., 1μM) (Figure 1). Neither varenicline nor bupropion affected insulin secretion at either dose tested (all p>0.1). However, treatment of INS-1E cells for 48 hours with 1μM concentration of all three drugs, nicotine, bupropion and varenicline caused an ablation of GSIS (Figure 2).
Figure 1.
Insulin secretion following a 1 hour exposure to A) nicotine; B) varenicline and C) bupropion. Data are presented as mean ± SE (N=4–6). Within each treatment group values with different superscripts are significantly (p<0.05) different from each other.
Figure 2.
Glucose-stimulated insulin secretion in INS-1E cells following a 48 hour exposure to nicotine, varenicline, and bupropion. Data are presented as mean ± SE (N=5). Values with an asterisk are significantly different from basal (i.e., at 3.3 mM glucose) insulin release (p<0.05).
Mitochondrial function
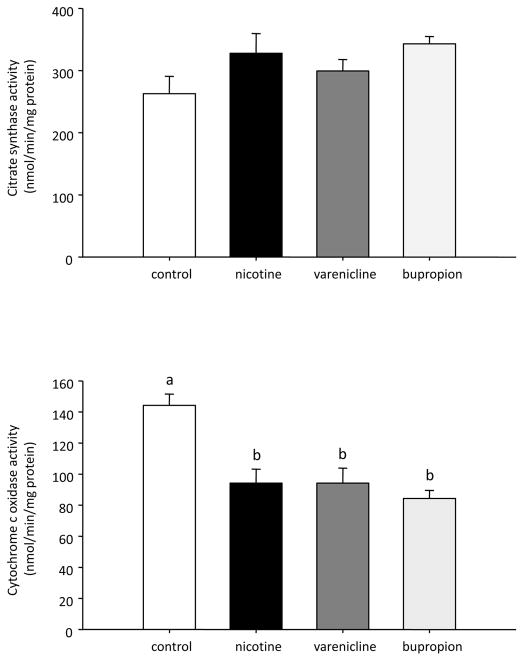
Treatment of INS-1E cells with nicotine, bupropion and varenicline (1 μM) for 48 hours significantly decreased mitochondrial complex IV activity (Figure 3). There was no difference in citrate synthase activity (an indicator of mitochondrial mass) in any treatment group.
Figure 3.
Citrate synthase (A) and cytochrome c oxidase (B) activity in INS-1E cells treated for 48 hours with nicotine, varenicline, and bupropion. Data are presented as mean ± SE (N=3). Values with different superscripts are significantly (p<0.05) different from each other.
Cellular and oxidative stress
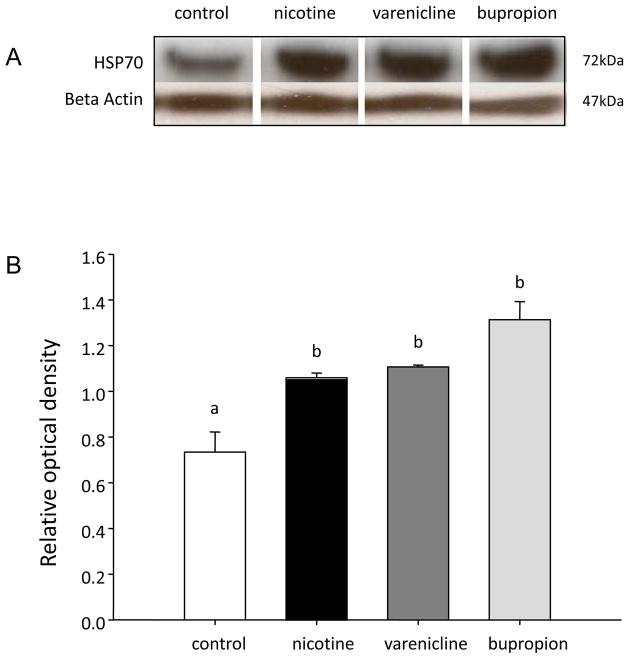
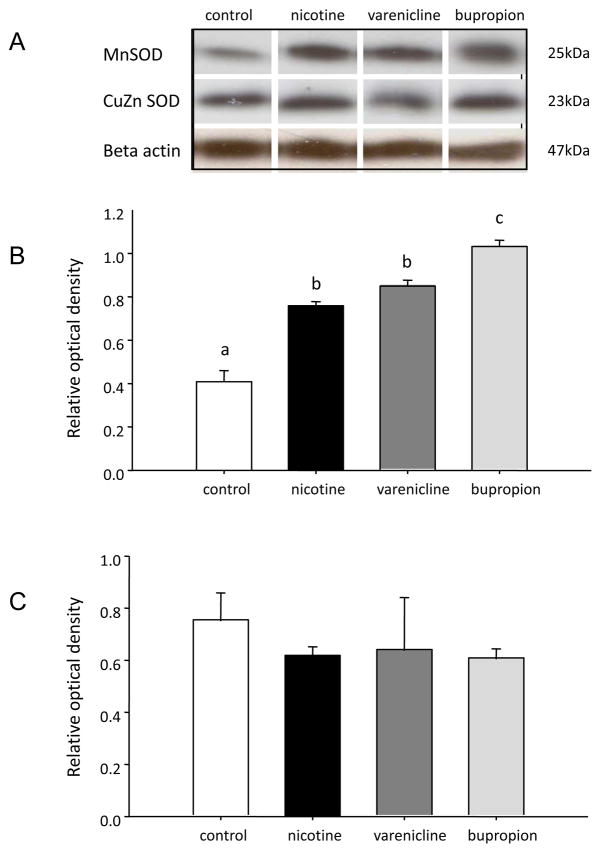
INS-1E cells treated with 1 μM nicotine, bupropion and varenicline had significantly (P<0.05) elevated levels of Hsp70 relative to vehicle-treated (control) cells; there was no difference in Hsp70 expression between treatment groups (Figure 4). There was no effect of nicotine, bupropion or varenicline (1 μM) on the expression of CuZnSOD in INS-1E cells (p>0.05) whereas MnSOD expression was significantly elevated by all three drugs relative to vehicle-treated (i.e., control) cells. Moreover, treatment of INS-1E cells with 1μM bupropion resulted in higher MnSOD expression relative to that seen in cells treated with an equimolar concentration of either nicotine or varenicline (Figure 5).
Figure 4.
Quantification of Hsp70 in INS-1E cells treated with nicotine, varenicline or bupropion. A) Representative Western blot for each protein B) Quantification of protein expression of Hsp70. All protein expression was quantified relative to a β-actin loading control. Data are presented as mean ± SE (N=3). Values with different superscripts are significantly (p<0.05) different from each other.
Figure 5.
Quantification of MnSOD and CuZn SOD in INS-1E cells treated with nicotine, varenicline or bupropion. A) Representative Western blot for each protein B) Quantification of protein expression of MnSOD. C) Quantification of protein expression of CuZnSOD. All protein expression was quantified relative to a β-actin loading control. Data are presented as mean ± SE (N=3). Values with different superscripts are significantly (p<0.05) different from each other.
Oxidative damage
Treatment of INS1E cells with 1μM of nicotine, bupropion or varenicline did not affect lipid peroxidation (i.e., 4-HNE expression) in either the whole cell lysates or mitochondrial fraction (data not shown). Similarly, there was no change in protein carbonyl formation (data not shown) suggesting that under these treatment conditions none of these drugs caused increased oxidative damage in INS-1E cells.
Discussion
Cigarette smoking is associated with numerous adverse health consequences, including an increased risk of T2DM (Willi et al., 2007). Although cigarette smoke contains over 4000 chemicals (Rustemeier et al., 2002), clinical evidence suggests that nicotine, the addictive component in cigarettes, may play a role in the development of T2DM. Indeed, a number of clinical studies report that nicotine is associated with decreased insulin sensitivity, which results in increased insulin resistance and uncontrolled hepatic glucose production; key factors in the development of T2DM (Assali et al., 1999; Eliasson et al., 1996; Epifano et al., 1992). Furthermore, nicotine has been shown to directly impair beta cell function (i.e., insulin secretion) (Yoshikawa et al., 2005; this study). Functional responses of beta cells to nicotine treatment are thought to be mediated via the activation of nicotinic acetylcholine receptors (nAChR) present in pancreatic beta cells (Yoshikawa et al., 2005; Ohtani et al., 2006). The other two main smoking cessation pharmacotherapies, varenicline (Champix®) and bupropion (Zyban®) do not involve the direct administration of nicotine, but rather exert their actions by reducing or blocking activation of nAChRs (Slemmer et al., 2000; Potts and Garwood, 2007). Therefore we hypothesized that varenicline and/or bupropion would have less impact on beta cell function than nicotine. Indeed, as predicted, a 60 minute acute exposure to varenicline and bupropion at either 1nM or 1μM did not significantly affect insulin secretion. However, a 48 hour exposure to all three drugs resulted in the ablation of glucose-stimulated insulin secretion. Importantly, this effect of bupropion and varenicline to affect GSIS was seen at a concentration (i.e., 1μM) which is lower than average plasma levels reported in pharmacokinetic studies of bupropion (AUC0-infinity: 1164 ng • h/ml (i.e., 4.2μM•h) (Hsyu et al., 1997) and within the reported range of average plasma varenicline concentrations (AUC0-infinity 91.4 ng • h/ml (i.e., 0.4 μM•h; 1mg single dose) and 226 ng • h/ml (i.e., 1.1 μM•h; 2mg single dose) (Faessel et al., 2010). Since varenicline is a partial agonist at the nAChR it was not surprising that GSIS was reduced in both the nicotine-and varenicline-treated cells. However, the finding that GSIS was also significantly reduced in the bupropion-treated cells was unexpected, as bupropion acts as a non-competitive antagonist of nAChR (Slemmer et al., 2000). Moreover, the effect of bupropion is more likely due to its action as a dopamine-norepinephrine reuptake inhibitor rather than a non-specific response to nAChR antagonism as treatment of cells for 48 hours with 100μM mecamylamine, a nonselective nAChR antagonist, had no effect on GSIS (data not shown).
In beta cells, the function of the mitochondrial electron transport chain (ETC) is critical for glucose stimulated insulin secretion (Maechler and de Andrade, 2006). Since there is considerable evidence both in vivo and in vitro which demonstrates that nicotine exposure has adverse effects on mitochondrial structure and function (Bruin et al., 2010; Crowley-Weber et al., 2003; Wang et al., 2009), we wondered whether or not the ability of the test compounds to inhibit GSIS was mediated via reduced mitochondrial ETC activity. Indeed, treatment of beta cells with 1μM of nicotine, bupropion and varenicline for 48 hours significantly decreased mitochondrial ETC activity. Since it is well established that that exposure to nicotine results in increased ROS production and oxidative stress in a number of tissues including the pancreas (Zhao and Reece, 2005; Crowley-Weber et al., 2003; Wetscher et al., 1995; Halima et al., 2010; Gallo et al., 2010; Bruin et al 2008b) and mitochondrial function is particularly sensitive to the effects of pro-oxidants and oxidative stress (Drews et al., 2010), we investigated the hypothesis that the unifying mechanism by which these 3 compounds could cause mitochondrial dysfunction was via increased oxidative stress.
A basal level of reactive oxygen species (ROS) production is required for normal cell function, but if the level of ROS exceeds the antioxidant capacity of the cell, oxidative stress will ensue (Kaneto et al., 2005). The consequences of oxidative stress include damage to mitochondrial and non-mitochondrial proteins, lipids and nucleic acids. Of these, the mitochondria are particularly sensitive to ROS-induced damage (Wallace, 2005). Nicotine is reported to be a pro-oxidant (Zhao and Reece, 2005; Crowley-Weber et al., 2003; Wetscher et al., 1995; Halima et al., 2010; Gallo et al., 2010; Bruin et al 2008b) and although not proven experimentally, Kovavic (2007) propose that based on a structural analysis, varenicline treatment may also produce ROS and oxidative stress. Furthermore, it has been suggested that bupropion treatment, through its effects on the regulation of the dopaminergic system may also increase oxidative stress in vivo, although bupropion treatment of a human neuroblastoma cell line, SH-SY5Y cells, did not result in increased ROS production (Jang et al., 2011). In the current study we did not find unequivocal evidence of oxidative stress and/or oxidative damage with any of the drugs tested. Oxidative damage would be expected only if treatment with these drugs increased the formation of ROS beyond the ability of the cell to mitigate their actions. However, our observed increase in MnSOD suggests that nicotine, bupropion and varenicline evoke a compensatory increase in this enzyme, and that this increase is sufficient to prevent oxidative damage. Moreover, the fact that we detected an increase in MnSOD protein expression, but not CuZnSOD protein expression, suggests that the antioxidant response, and therefore the oxidative insult, may be localized within the mitochondria and not in the cytosol. This finding is consistent with the reduced mitochondrial ETC activity detected in all treatment groups since mitochondrial function is particularly sensitive to the effects of pro-oxidants and oxidative stress and altered mitochondrial function and subsequently impaired insulin secretion may be evident at low levels of oxidative stress prior to overt cellular oxidative damage (Drews et al., 2010).
In summary, results of this study have demonstrated that all three of the currently available smoking cessation pharmacotherapies, nicotine, varenicline and bupropion, have the potential to disrupt normal beta cell function; an effect which appears to be mediated via mitochondrial dysfunction. Although smoking cessation is associated with numerous health benefits, the fact that nicotine, varenicline and bupropion have the potential to disrupt beta cell function in vitro raises the possibility that these drugs may also adversely affect insulin secretion and therefore glycemic control in vivo. Therefore, the inclusion of endocrine and metabolic outcomes in clinical trials evaluating smoking cessation drugs warrants consideration.
Highlights.
Nicotine, varenicline and buproprion all disrupt beta cell function
All three drugs caused an antioxidant response which prevented oxidative damage
The impaired beta cell function appears to be a result of mitochondrial dysfunction
These drugs cause impaired beta cell function consistent with type 2 diabetes
Acknowledgments
Varenicline was generously provided by Pfizer Inc. (Groton, CT)
Funding
This work was supported by an Operating grant from the Canadian Institutes of Health Research [MOP 86474 to A.C.H.]. A.K.W and C.J.N were recipients of the Ashley Studentship for Research in Tobacco Control.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Assali AR, Beigel Y, Schreibman R, Shafer Z, Fainaru M. Weight gain and insulin resistance during nicotine replacement therapy. Clin Cardiol. 1999;25:357–360. doi: 10.1002/clc.4960220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995;95:2840–5. doi: 10.1172/JCI117989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Morrison KM, Holloway AC. Increased pancreatic beta-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicol Sci. 2008a;103:362–370. doi: 10.1093/toxsci/kfn012. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Petre MA, Lehman MA, Raha S, Gerstein HC, Morrison KM, Holloway AC. Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic Biol Med. 2008b;44:919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Petrik JJ, Hyslop JR, Raha S, Tarnopolsky MA, Gerstein HC, Holloway AC. Rosiglitizone improves pancreatic mitochondrial function in an animal model of dysglycemia: the role of the insulin-like growth factor axis. Endocrine. 2010;37:303–311. doi: 10.1007/s12020-009-9294-8. [DOI] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preference and nicotine deliver: randomized cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Crowley-Weber CL, Dvorakova K, Crowley C, Bernstein H, Bernstein C, Garewal H, Payne CM. Nicotine increases oxidative stress, activates NF-kappaB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: relevance to colon carcinogenesis. Chem Biol Interact. 2003;145:53–66. doi: 10.1016/s0009-2797(02)00162-x. [DOI] [PubMed] [Google Scholar]
- DeVeaugh-Geiss AM, Chen LH, Kotler ML, Ramsay LR, Durcan MJ. Pharmacokinetic comparison of two nicotine transdermal systems, a 21-mg/24-hour patch and a 25-mg/16-hour patch: a randomized, open-label, single dose, two-way crossover study in adult smokers. Clin Ther. 2010;32:1140–1148. doi: 10.1016/j.clinthera.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Drews G, Krippeit-Drews P, Dufer M. Oxidative stress and beta-cell dysfunction. Eur J Physiol. 2010;460:703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- El-Dakhakhny M, Abdel el-Latif HA, Ammon HP. Different effects of the antidepressant drugs imipramine, maprotiline and bupropion on insulin secretion from mouse pancreatic islets. Arzneimittelforschung. 1996;46:667–669. [PubMed] [Google Scholar]
- Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94:878–881. doi: 10.1161/01.cir.94.5.878. [DOI] [PubMed] [Google Scholar]
- Epifano L, Di VA, Fanelli C, Porcellati F, Perriello G, De FP, Motolese M, Brunetti P, Bolli GB. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus. Eur J Clin Pharmacol. 1992;43:257–263. doi: 10.1007/BF02333019. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Figueiredo PA, Ferreira RM, Appell HJ, Duarte JA. Age-induced morphological, biochemical, and functional alterations in isolated mitochondria from murine skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:350–9. doi: 10.1093/gerona/63.4.350. [DOI] [PubMed] [Google Scholar]
- Gallo C, Renzi P, Loizzo S, Loizzo A, Piacente S, Festa M, Caputo M, Tecce MF, Capasso A. Potential therapeutic effects of vitamin e and C on placental oxidative stress induced by nicotine: an in vitro evidence. Open Biochem J. 2010;4:77–82. doi: 10.2174/1874091X01004010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halima BA, Sarra K, Kais R, Salwa E, Najoua G. Indicators of oxidative stress in weanling and pubertal rats following exposure to nicotine via milk. Hum Exp Toxicol. 2010;29:489–96. doi: 10.1177/0960327109354440. [DOI] [PubMed] [Google Scholar]
- Hsyu PH, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol. 1997;37:737–43. doi: 10.1002/j.1552-4604.1997.tb04361.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takeshima F, Kadota K, Yoda A, Tatsuta Y, Nagaura Y, Yoshioka S, Nakamichi S, Nakao K, Ozono Y. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance. Intern Med. 2011;50:707–712. doi: 10.2169/internalmedicine.50.4600. [DOI] [PubMed] [Google Scholar]
- Jang EH, Park CS, Kang JH. Bupropion, an atypical antidepressant, induces endoplasmic reticulum stress and caspase-dependent cytotoxicity in SH-SY5Y cells. Toxicology. 2011;285:1–7. doi: 10.1016/j.tox.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kawamori D, Matsuoka TA, Kajimoto Y, Yamasaki Y. Oxidative stress and pancreatic beta-cell dysfunction. Am J Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- Kovacic P. Mechanism of varenicline (clinical ramifications): electron transfer and oxidative stress. Med Hypotheses. 2007;68:1184–5. doi: 10.1016/j.mehy.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kraiczi H, Hansson A, Perfekt R. Single-dose pharmacokinetics of nicotine when given with a novel mouth spray for nicotine replacement therapy. Nicotine Tob Res. 2011;13:1178–1182. doi: 10.1093/ntr/ntr139. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–6. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Maechler P, de Andrade PB. Mitochondrial damages and the regulation of insulin secretion. Biochem Soc Trans. 2006;34:824–827. doi: 10.1042/BST0340824. [DOI] [PubMed] [Google Scholar]
- McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation—recent advances. Cardiovasc Drugs Ther. 2010;24:359–367. doi: 10.1007/s10557-010-6246-8. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Oka T, Badyuk M, Xiao Y, Kellar KJ, Daly JW. Mouse beta-TC6 insulinoma cells: high expression of functional alpha3beta4 nicotinic receptors mediating membrane potential, intracellular calcium, and insulin release. Mol Pharmacol. 2006;69:899–907. doi: 10.1124/mol.105.014902. [DOI] [PubMed] [Google Scholar]
- Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med. 2005;39:289–295. doi: 10.1016/j.freeradbiomed.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts LA, Garwood CL. Varenicline: the newest agent for smoking cessation. Am J Health-Syst Pharm. 2007;64:1381–1384. doi: 10.2146/ajhp060428. [DOI] [PubMed] [Google Scholar]
- Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol. 2002;40:93–104. doi: 10.1016/s0278-6915(01)00085-0. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damai MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- US Public Health Services. A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kim JM, Donovan DM, Becker KG, Li MD. Significant modulation of mitochondrial electron transport system by nicotine in various rat brain regions. Mitochondrion. 2009;9:186–195. doi: 10.1016/j.mito.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, Hinder RA. Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med. 1992;18:877–82. doi: 10.1016/0891-5849(94)00221-5. [DOI] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Hellstrom-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism. 2005;54:247–254. doi: 10.1016/j.metabol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Reece EA. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Res B Dev Reprod Toxicol. 2005;74:383–91. doi: 10.1002/bdrb.20052. [DOI] [PubMed] [Google Scholar]