Abstract
Reactive oxygen or nitrogen species play an integral role in both myocardial injury and repair. This dichotomy is differentiated at the level of species type, amount, duration of free radical generated. Homeostatic mechanisms designed to prevent free radical generation in the first instance, scavenge, or enzymatically convert them to less toxic forms and water, play crucial roles in maintenance of cellular structure and function. The outcome between functional recovery and dysfunction is dependent upon the inherent ability of these homeostatic antioxidant defenses to withstand acute free radical generation, in the order of seconds to minutes. Alternatively, pre-existent antioxidant capacity (from intracellular and extracellular sources) may regulate the degree of free radical generation. This converts reactive oxygen and nitrogen species to the role of second messenger involved in cell signalling. The adaptive capacity of the cell is altered by the balance between death or survival signal converging at the level of the mitochondria, with distinct pathophysiologic consequences that extends the period of injury from hours to days and weeks. Hyperglycemia, hyperlipidemia, and insulin resistance enhance oxidative stress in diabetic myocardium that cannot adapt to ischemia reperfusion. Altered glucose flux, mitochondrial derangements and nitric oxide synthase uncoupling in the presence of decreased antioxidant defense and impaired prosurvival cell signalling may render the diabetic myocardium more vulnerable to injury, remodelling and heart failure.
Keywords: cell signalling, diabetes mellitus, glycobiology, ischemia and reperfusion, mitochondrial permeability transition, myocardial injury, oxidative stress
Introduction
Coronary artery atherosclerosis is the leading cause of death in the western world [1] and its prevalence is increasing worldwide on an annual basis. It is estimated that more than one million people will experience or be at risk for life threatening myocardial infarction annually. Diabetes mellitus increases the risk of developing cardiovascular disease nearly five fold [2]. Nearly 25 million adult United States citizens have been diagnosed with concomitant diabetes and coronary artery atherosclerosis [3]. The risks of heart failure and death after myocardial infarction (MI) are increased 2 to 4 fold in patients with diabetes [4–6].
Effective re-establishment of coronary perfusion to preserve the myocardium is a mainstay of therapy for patients with coronary artery atherosclerosis. Unfortunately, diabetes is associated with increased cardiac morbidity and mortality following surgical or nonsurgical (angioplasty and vascular stenting) revascularization techniques [7–11]. Ischemia-reperfusion injury following myocardial revascularization is a major risk factor in the development of adverse outcomes in this patient population.
The principle mediator of myocardial injury secondary to ischemia and reperfusion in type 2 diabetes is oxidative stress [12]. Oxidative stress mediated myocardial injury is the consequence of an imbalance between the free radical generation and elimination due to increased reactive oxygen and nitrogen species (RONS) generation and/or inadequate antioxidant defense [13]. Oxidative stress arises directly or indirectly from hyperglycemia, hyperlipidemia, hyperinsulinemia, and insulin resistance that characterize type 2 diabetes. These perturbations, either alone or in combination, are thought to contribute to altered cellular structure and function of diabetic myocardium. The cellular and molecular mechanisms of diabetic cardiomyopathy in the absence of coronary artery atherosclerosis have been considered in detail elsewhere [14, 15]. This review will focus on the role of free radical biology in the pathogenesis of injury in diabetic heart with concomitant coronary artery atherosclerosis.
Oxidative stress and myocardial ischemia-reperfusion injury
The most common forms of free radicals identified in the human heart include the superoxide anion (O2•)−, the hydroxyl radical (OH•), hydrogen peroxide (H2O2), singlet oxygen, carbon-centered radical, peroxynitrite (ONOO−), nitric oxide (NO•), and nitrogen dioxide radical [16]. The O2•− serves as the precursor to the formation of most of the other free radicals [13].
The generation of free radicals in the heart is low under basal conditions. It is normally the results of electron leakage from the mitochondrial electron transport chains (ETC) under physiologic conditions. The cardiomyocyte response to low-level O2•− generation includes its conversion to less cytotoxic H2O2 by the action of enzyme superoxide dismutase (SOD) in cytoplasm or mitochondria. Hydrogen peroxide is subsequently converted to water by either catalase (CAT) or by the glutathione peroxidase (GPx) system [13].
In contrast, during ischemia and reperfusion, O2•− generation is markedly increased and originates from multiple cellular sources. These include the mitochondrial electron transport damage and uncoupling, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, uncoupled nitric oxide synthase (NOS), xanthine oxidase, cytochrome P450 monooxygenase, and cyclooxygenase. In addition, the cellular antioxidant defense system is depleted during ischemia and reperfusion with lower activities of endogenous free radical scavenging enzymes such as SOD, GPx and CAT. The severity of cardiomyocyte damage during ischemia and reperfusion ranges from reversible to fatal, proportionally to the magnitude of RONS reactions within the cells. We have recently published a detailed review of the topic and this is not considered at length in this article [13].
The OH•, which is thought to be the most damaging radical species in the cell, is extensively generated during ischemia and reperfusion [17]. Ischemia and early reperfusion result in low pH and hypoxic conditions that favor the release of iron cations (Fe2+) from metalloproteinases. In the presence of iron cations, H2O2 from SOD scavenge of O2•− will more likely be converted via the Fenton reaction to the OH• (instead of being scavenged by the CAT or GPx) [18]. Nitric oxide, whose myocardial production is also greatly increased during ischemia-reperfusion, reacts with increased O2•− to yield ONOO−. With increased intracellular acidification, ONOO− becomes more protonated to form peroxynitrous acid (ONOOH). ONOON rapidly forms nitrogen dioxide and OH•. The ONOO−/ONOOH degradation contributes in parallel with the Fenton reaction to OH• generation during ischemia and reperfusion [13].
The conditions of early reperfusion may be the primary determinant of tissue injury [19]. Reperfusion stimulates NADPH oxidase, cytochrome P450 and cyclooxygenase activity to increase and accelerate free radical production. Paradoxically, re-oxygenation of tissues with accumulated oxidative substrates uncouples the ETC, leading to a large-scale generation of RONS. Large-scale RONS generation [20, 21] and depleted energy stores [22, 23], induce a process called mitochondrial permeability transition (mPT) [24, 25]. mPT results in pore (mPTP) formation and opening on the inner mitochondrial membrane that permits the passage of molecules below 1.5 kDa in size [26, 27]. mPTP dissipates the mitochondrial membrane potential (ΔΨm) by providing protons an alternate route to the matrix. Cellular adenosine triphosphate (ATP) stores are further depleted. Molecules greater than 1.5 kDa exert a colloid osmotic pressure that causes the mitochondrial matrix to swell. The integrity of the outer membrane is compromised, cytochrome c is released to the cytoplasm to initiate pro-apoptotic signals [28]. The severity and the duration of mPT directly affect maintenance of ATP stores and cellular integrity. If mPT is transient, the cell can recover [29]. If mPT is prolonged and ATP depletion is severe, necrosis occurs [25]. To this end, the number of mitochondria undergoing mPT correlates with the likelihood of cardiomyocyte loss [30]. The severity of mPT is proportional to the functional recovery in an isolated heart model [31]. In general, acidic condition during ischemia inhibits mPT. As pH normalizes during early reperfusion, mPT is enhanced [32–34].
Myocardial injury during ischemia and reperfusion in diabetes
Although there is conflicting evidence from experimental studies on whether diabetic myocardium is more or less vulnerable to ischemia and reperfusion injury compared to normal heart, clinical data strongly supports an increased susceptibility to myocardial ischemia-reperfusion injury in patients with diabetes mellitus [35–38]. Clinically, diabetes is considered as an independent risk factor for myocardial injury during cardiac surgery [38]. The risk of post-myocardial infarction death is increased 2 to 4 fold in diabetic patients compared to those without diabetes [4, 6]. The pathophysiology of myocardial ischemia and reperfusion injury involves multifactorial mechanisms. The underlying mechanisms responsible for the increased injury in diabetic myocardium are not fully understood. The enhanced oxidative stress (excessive free radical generation and/or depleted endogenous antioxidant defense system) and impaired cellular cardioprotective prosurvival signal pathways have been implicated.
Enhanced oxidative stress in diabetic myocardium
It is well known that oxidative stress is involved in the development and progression of diabetes mellitus and diabetic complications [39, 40]. Yokota et al reported the increased O2•− production, lipid peroxidation, and NADPH oxidase activity in the plasma and tissue of type 2 diabetic mice [41]. The oxidative protein products and lipid peroxidation are elevated in patients with type 2 diabetes [42, 43]. Lack of functional GPx-1 was detected in diabetic apolipoprotein E-deficient mice [44]. In patients with type 2 diabetes, both the levels and the activities of enzymatic (GPx, SOD, and CAT) and non-enzymatic (β-carotene, retinol, vitamin C and E, and uric acid) antioxidants in erythrocytes were decreased compared to normal subjects [42]. Antioxidants therapy can normalize the increased ROS production in diabetic animals [39]. Although the beneficial effects of antioxidants on diabetes and its complications are still controversial, diabetic patients with antioxidants administration show a significant suppression of plasma markers for lipid oxidation [45]. These findings provide the confirmatory evidence of enhanced oxidative stress in diabetes.
In diabetic myocardium there are three major sources of free radicals: mitochondria, NADPH oxidase and/or NOS. Diabetes is associated with impaired mitochondrial morphology and function [46]. Myocardial mitochondrial perturbations were identified in both type 2 diabetic mice [47] and human diabetes [15]. Increased leakage of electron and the generation of O2•− from the ETC results from mitochondrial dysfunction. In contrast to normal conditions involving physiologic generation of O2•− by ETC Complex I and III, in diabetes, myocardial O2•− generation is enhanced by stimulation of ETC Complex II [48, 49]. Elevated NADPH oxidase activity was also observed in myocardium and vascular tissues from animal models with type 2 diabetes [50, 51]. In diabetic patients, both the activity of NADPH oxidase system and the levels of NADPH oxidase protein subunits were significantly increased [52, 53]. NADPH oxidase inhibitors were demonstrated to significantly inhibit the increased free radical formation in diabetic animals. In addition, reduced availability of the cofactor tetrahydrobiopterin (BH4) was identified in diabetic animal vessels and endothelial cells. The depletion of BH4 functionally “uncouples” endothelial NOS (eNOS) to generate more O2•− and less nitric oxide [54]. The accompanying increase in NADPH oxidase 2 and 4 in diabetes [55] further predisposes the heart to NOS uncoupling and ONOO− generation. Moreover, inducible NOS (iNOS) is activated in diabetes by inflammatory mediators, which makes iNOS uncoupling a predominant contributor for oxidative/nitrosative stress in diabetic myocardium. Reversal of iNOS uncoupling by BH4 treatment is shown to increase myocardial tolerance to ischemia and reperfusion injury by increasing the bioavailability of iNOS-derived nitric oxide and eliminating oxidative stress in the diabetic rat heart [56].
The mechanisms underlying mitochondrial dysfunction, high NADPH oxidase activation and NOS uncoupling in diabetic myocardium are still unclear. However, various abnormalities in type 2 diabetes, including hyperglycemia, hyperlipidemia, hyperinsulinemia, and insulin resistance have all been implicated [57].
Hyperglycemia induces O2•− generation in the heart primarily by disruption of the ETC, activation of NADPH oxidase, and uncoupling of NOS [49, 58]. High glucose can impair the activities of ETC complex enzymes, and contribute to mitochondrial O2•− overproduction. Chronic elevated oxidative stress in type 2 diabetes may in turn impair mitochondrial energy metabolism, lead to further mitochondrial dysfunction [15] and form a vicious metabolic cycle of irreversible cell and tissue damage. High glucose has been demonstrated to activate NADPH oxidase and increased NADPH oxidase-derived O2•− formation in vitro. Hyperglycemia is also shown to increase NOS-dependent O2•− production in human endothelial cells. Alternative pathways of glucose metabolism and the formation and activation of advanced glycation endproducts (AGEs) may also play a major role in enhancing oxidative stress [59–61]. In addition, glycation can inactivate antioxidant enzymes like SOD to impair the myocardial antioxidant defense [62, 63]. Hyperglycemia has been shown to decrease total antioxidant capacity, erythrocyte glutathione (GSH) content and SOD activity in patients with impaired glucose regulation [64].
Free fatty acids (FFAs) levels are elevated in diabetes [47, 65]. FFAs stimulate NADPH oxidase and the ETC to generate ROS [66]. Elevated levels of FFAs also decrease intracellular GSH [67] to additionally impair the endogenous defense against free radical mediated injury.
Hyperinsulinemia and insulin resistance are usually linked to type 2 diabetes. Insulin resistance is related to oxidative stress in obese children [68]. Insulin administration increases NADPH oxidase activity to produce free radicals in cultured cells. By using a cardiomyocyte insulin receptor deletion mice model, Boudina et al reported impaired myocardial insulin signalling induced mitochondrial uncoupling, and promoted H2O2 production and oxidative stress [69].
Impaired endogenous cardioprotective signalling pathways in diabetic heart
Yue et al reported that cardiomyocyte necrosis is significantly increased in ventricular myocardial biopsies of diabetic patients, confirming a primary impairment in myocardial tolerance to ischemia and reperfusion injury [70]. It is well recognized that effective intraoperative myocardial protection is crucial to preserve high-risk myocardium and improve patient post-operative outcomes. To date, extensive research has focused on increasing the myocardial tolerance to ischemia using conditioning strategies.
Myocardial ischemia and/or pharmacological pre- and/or post-conditioning are known to reduce the severity of ischemia-reperfusion injury by activating cell survival pathways and altering free radical reactions in the myocardium. Unfortunately, the diabetic myocardium is resistant to physical or pharmacologic pre- and post-conditioning stimulus. This has been explained experimentally on the basis of prosurvival signal transduction impairment and enhanced mPT. This could explain enhanced susceptibility to injury in ischemia-reperfused diabetic myocardium.
Conditioning in general involves diverse cell signalling mechanisms. It appears to be predicated on the direct and/or indirect activation of several key cellular pro-survival pathways including the phosphoinositide-3 kinase (PI3K)-AKT and janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) pathways, which in turn converge on key-end-effectors such as mPTP.
The PI3K/AKT prosurvival pathway is central to physical and pharmacologic pre- and postconditioning and salvaging the ischemia-reperfused myocardium. The activation of AKT induces a cytoprotective effect via the actions of its putative downstream effectors, such as eNOS and anti-apoptotic B-cell lymphoma-2 (BCL-2), to prevent mitochondrial-directed cell death [71, 72]. In diabetes, myocardium is resistant to preconditioning stimulus [73, 74]. The threshold stimulus for myocardial preconditioning is raised and subject to a critical level of AKT activation, to mediate a cardioprotective effect [73, 74]. The extent of AKT activation has been found to be significantly decreased in a Goto-Kakizaki rat type 2 diabetic model [74]. Impaired AKT activation has been characterized in patients with diabetes in clinical studies [73, 75]. It has been postulated that the principle negative regulator of the PI3K/AKT pathway is phosphatase and tensin homologue on chromosome 10 (PTEN) [76–78]. PTEN is constitutively expressed and subject to regulation by free radical biology. In part, hyperglycemia induced RONS generation affects PTEN antagonism of AKT activation [79, 80]. The level of PTEN has been found to be increased in Goto-Kakizaki Rat myocardium [77]. Our group recently found a positive correlation between blood glucose level, oxidative stress and PTEN level detected in human diabetic myocardium [43]. The extent of AKT phosphorylation negatively correlated with increased levels of myocardial PTEN in patients with diabetes. These findings suggest increased PTEN levels negatively regulate AKT prosurvival signalling in human diabetic myocardium [43]. Further study is required. In addition, high levels of circulating FFAs impair insulin-stimulated activation of PI3K, AKT, and eNOS [65, 81, 82]. FFAs are shown to up-regulate PTEN expression in vitro [81]. FFA treatment of cardiomyocytes has been shown to result in decreased insulin-stimulated eNOS activation [81]. These findings confirmed the impaired prosurvival AKT signalling in diabetic heart, which may render the heart resistant to cardioprotective preconditioning interventions.
The activation of transcription factor STAT3 is another cardioprotection obtained independently of AKT [83]. STAT3 is activated during ischemia and reperfusion by the JAKs [84]. STAT3 is phosphorylated by activated JAK at tyrosine 705. Activated STAT3 then translocates to the nucleus, regulating gene transcription [85]. In the heart, STAT3 positively regulates the expression of several anti-apoptotic genes such as B-cell lymphoma-extra large (BCL-XL) and BCL-2 [86]. In addition to its role as a transcriptional factor, STAT3 can also act as a signalling molecule by direct phosphorylation of various cytoplasmic components [85–87]. Mitochondrial energetics are preserved via direct action of STAT3 on the ETC [88] or prevention of mPT via the actions of AKT and BCL-2, two of STAT3’s potential downstream effectors [89, 90]. Experimentally, decreased STAT3 activity and phosphorylation, observed in aged mice [91] or with pharmacological inhibition, [92] is associated with a loss of the cardioprotection normally achieved by pre- and post-conditioning. STAT3-deficient mice are more susceptible to myocardial ischemia-reperfusion injury and MI, show increased cardiac apoptosis and infarct size, and have reduced cardiac function and survival [93]. In contrast, transgenic mice that over-express STAT3 have decreased infarct size subsequent to ischemia and reperfusion compared to wild type mice [94]. Although there is no direct evidence on STAT3 expression and activation in human diabetes, the rat modals of diabetes demonstrated significant decrease in myocardial STAT3 expression and activation, which was associated with a resistance to conditioning strategies [95].
Susceptibility to oxidant-mediated ischemia and reperfusion injury in the diabetic heart
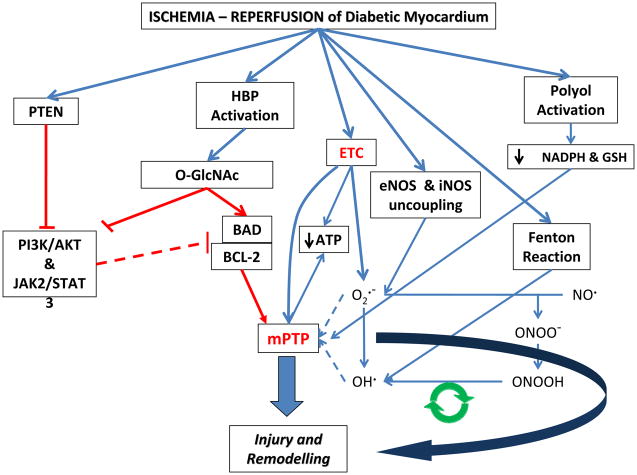
The diabetic myocardium and vasculature appear more vulnerable to ischemia and reperfusion injury. This includes oxidant mediated structural alternations to cardiomyocyte sarcomeres, mitochondria and accompanying interstitial, and microvascular ultrastructure [96]. The underlying mechanisms responsible for exacerbation of oxidant-mediated injury in the ischemic reperfused diabetic heart are not clearly known. In addition to the general increased basal oxidative state and impaired cellular prosurvival signallings already described in previous sections, the primary etiology also relates to the impact of glucose flux, impaired cardiac stress or adaptive responses, and NOS uncoupling in the ischemic reperfused diabetic heart, to confer further injury (See Figure 1).
Figure 1.
Ischemia-reperfusion injury in the diabetic myocardium is multi-factorial and complex. a) Overexpression of phosphatase and tensin homologue on chromosome 10 (PTEN) in diabetes results in an inability to activate cellular protective pathways such as, PI3K/AKT and JAK2/STAT3 to prevent ischemia and reperfusion injury. b) Hexose biosynthase pathway (HBP) activation due to hyperglycemia results in glycosylation of proteins. Glycosylation of BAD, for example, leads to higher binding to and inactivation of anti-apoptotic BCL-2. This renders the cardiomyocyte more susceptible to mPTP. c) The multitude of effects of hyperglycemia and diabetes lead to uncoupling of the mitochondrial electron transport chains (ETC) and have a net effect of ROS overproduction and lower ATP production that renders the myocardium unable to ward off injury. d) Uncoupling of NOS enzymes and the resultant overproduction of peroxynitrite is a cyclical mode of cellular injury. This cycle is fed by overproduction of superoxide, which itself is formed from multiple sources during diabetes and ischemia and reperfusion. e) Release of divalent cations from metalloproteinases during ischemia and reperfusion promote production of OH• radical from H2O2 through the fenton reaction. f) Activation of aldose reductase during ischemia and reperfusion depletes NADPH, the cofactor required for glutathione reductase (GR) activity. Decreased GR activity leads to lower levels of anti-oxidant glutathione (GSH), which also contributes to lower glutathione peroxidase (GPx) activity. Lower GPx activity decreases the amount of H2O2 neutralized and therefore contributes to higher oxidative stress and susceptibility to mPTP and resultant cardiac injury and remodeling.
In diabetic heart, the flux of glucose also activates aldose reductase, sorbital dehydrogenase, and the conversion of glucose to fructose to promote oxidant stress through the polyol pathway. Ischemia-reperfusion activates aldose reductase induced depletion of intracellular NADPH, the cofactor needed for the activity of glutathione reductase (GR) in the heart. The level of antioxidant GSH is depleted, rendering the heart vulnerable to oxidant mediated injury. It is reported that aldose reductase mediated oxidative stress enhances mPTP openings [97], which contributes to myocardial contractile dysfunction and tissue damage in ischemia-reperfused rat hearts [98, 99]. Indeed, the pharmacological inhibition of aldose reductase may represent a novel adjunctive approach for protecting the diabetic ischemic hearts.
In addition to high glucose, hyperlipidemia and RONS generation during ischemia and reperfusion create greater flux through the hexose biosynthase pathway (HBP). This may increase protein O-GlcNAc modification at regulatory serine/threonine phosphorylation sites. O-GlcNAc modification impairs prosurvival PI3K-AKT-eNOS signalling in diabetes [100, 101]. Increased O-GlcNAcylation of BCL-2-associated death promoter (BAD) will lead to an increase in BAD-BCL-2 (or BCL-XL) dimerization and the subsequent decrease in free BCL-2 (or BCL-XL) [102, 103]. The impact of blood glucose level on the downregulation of BCL-2 expression in skin biopsy from patients with diabetes has been previous described [104]. Similarly our group recently found an inverse correlation between blood glucose and BCL-2 level in human diabetic myocardium [43]. Decreased free BCL-2 (or BCL-XL) expression will predispose to mPT and increased cardiomyocyte death to extend injury. Targeting glycobiology of the diabetic heart may promote cell protection rather than corrupt important cellular prosurvival signalings responsible for metabolic homeostasis of the heart.
Compensatory mechanisms to protect the heart against oxidant-mediated injury include upregulation of metallothionein1 and 2 (MT1 and 2), redox regulators of free radicals scavenger GR [105]. MT expression increases in response to the oxidant stress in the diabetic heart. MT1 and 2 are also targets genes involved in STAT3-mediated cardioprotection [106]. However the response may be inadequate in diabetes. Decreased STAT3 expression in the diabetic heart may sensitize myocardium to the effects of RONS production during ischemia-reperfusion to exacerbate this form of injury [107, 108] via the insufficient regulation on MT1/2 expression.
Additionally, heme oxygensae (HO)-1 is the stress response protein responsible for oxidant heme degradation, or the generation of antioxidant bilirubin or carbon monoxide. Its absence has been associated with increased mortality following MI. Thus, the inability to elaborate HO-1 may also exacerbate ischemia-reperfusion injury in the diabetic heart [109].
Alternatively, thioredoxin (TRX)-1, a key intracellular antioxidant that regulates cell survival pathways [110], is also modified in diabetes. ONOO− generation secondary to eNOS uncoupling may be responsible for nitration of TRX-1 [111]. Glycation of TRX-1 may also occur in hyperglycemia and diabetes [112]. The subsequent inactivation of TRX-1 due to nitration or glycation may represent a major mechanism responsible for ischemia and reperfusion in diabetes [110].
Progressive myocardial dysfunction and degeneration after MI
Oxidative stress mediated mechanisms of injury are also the leading cause of myocardial repair and remodeling after MI [113, 114]. This is especially the case with the diabetic heart [115], where oxidative stress is extensively enhanced. Smith et al demonstrated elevated oxidative stress in the diabetic MI animals as indicated by increased levels of myocardial 8-isoprostane (a marker of oxidative stress), oxidized glutathione (GSSG), SOD and CAT protein expression. This was associated with depressed indices of cardiac function at 4 weeks post-MI [116]. Aragno et al found that oxidative stress induced by experimental chronic hyperglycemia promoted profibrogenic gene expression and extracellular matrix deposition, which led to cardiac fibrosis and dysfunction [117]. These findings provide evidence to support oxidative stress as a key factor in the pathogeneses of myocardial dysfunction and degeneration after MI in diabetes.
Oxidative stress-induced cardiac remodeling involves multiple mechanisms [114, 118], including the effects of RONS on degeneration of lipid, protein and nuclei acid, RONS-promoted cardiomyocytes apoptosis, direct damage of RONS on cardiac contractile function, RONS-activated cardiac inflammatory response, regulation of RONS on extracellular matrix remodeling, and activation of a series of protein kinase which response for hypertrophic response by RONS stimulation. Study in diabetes demonstrated that the loss of myocardial insulin signalling led to accelerated post-MI left ventricular dysfunction [119], which may partially due to a reduction of substrate utilization and availability in the diabetic myocardium induced by oxidative stress related mitochondrial dysfunction (identified by a combination of declined mitochondrial fatty acid oxidative capacity and limited glucose transport capacity). In addition, mitochondria in human diabetic myocardium have decreased tolerance to calcium and increased propensity towards calcium induced mPTP opening, which is associated with increased levels of caspase-9, the mitochondrial cell death protease [120]. Oxidant induced alterations in key mPTP components including the adenine nucleotide transporter and glutathione depletion may play a role in mitochondrial calcium sensitivity of the mPTP. Finally, our group recently described the association between myocardial 15-F2t-isoprostane (a marker for oxidative stress) generation, increased expression of PTEN, and decreased levels of key mitochondrial regulator BCL-2 in human diabetic myocardium [43], which may, in part, explain this effect in diabetic myocardium.
Taken together, the prolonged impact of metabolic perturbations, mitochondrial dysfunction in association with insulin resistance and oxidative stress in the diabetic state, has profound impact on the myocardium’s tolerance to ischemia reperfusion injury. Oxidative stress subsequently plays a role in the pathogenesis and increased frequency of heart failure following MI associated with diabetes.
Conclusions
Oxidative stress, an imbalance between the free radial generation and elimination or detoxification, is a principle mediator of myocardial injury during ischemia and reperfusion. The magnitude of oxidative stress is proportional to the functional recovery of the heart. Among the multiple cellular sources of free radicals during tissue ischemia and reperfusion, mitochondria-derived RONS is extremely important in the ischemic and reperfused myocardium.
In diabetes, enhanced oxidative stress arises directly or indirectly from hyperglycemia, hyperlipidemia, hyperinsulinemia, and insulin resistance. These perturbations, either alone or in combination, contribute to mitochondrial dysfunction, high NADPH oxidase activation and NOS uncoupling, which lead to excessive free radical generation in diabetic myocardium that cannot readily adapt to ischemia reperfusion. Altered glucose flux, mitochondrial derangements and NOS uncoupling in the presence of decreased antioxidant defense and impaired prosurvival cell signalling may render the diabetic myocardium more vulnerable to injury, remodelling and heart failure.
No therapeutic strategy has yet been demonstrated clinically effective against cardiac injury in diabetic population. The complex nature of the free radical biology in myocardial homeostasis, injury and repair strengthens the understanding of the underlying biologic mechanisms and the challenges to prevent cardiac injury in patients with diabetes. Simultaneously targeting perioperative glucose control, inhibition of oxidative stress in cardiac tissues, and activation of cellular prosurvival signalling may prove to be an effective treatment alternative for the cardioprotection of surgical patients with type 2 diabetes. This approach is the subject of ongoing research in laboratory models and clinical studies.
Acknowledgments
This work is supported by Canadian Institutes of Health Research Operating Grant #82757. The authors would like to thank Jayant Shravah, MSc candidate for his helpful suggestions in the writing of this article and the accompanying figure design. Jayant Shravah is supported by Canada Graduate Scholarships from the Canadian Institutes of Health Research.
Footnotes
Conflict of interest: There is no conflict of interest in this paper.
Author contributions
Both authors wrote this manuscript.
References
- 1.Roger VL, Roger VL, Go AS, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:1–195. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, Brown JR, Edwards FH, et al. The diabetic disadvantage: historical outcomes measures in diabetic patients undergoing cardiac surgery -- the pre-intravenous insulin era. Seminars in thoracic and cardiovascular surgery. 2006;18:281–288. doi: 10.1053/j.semtcvs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Miketic JK, Miketic JK, Hravnak M, et al. Factors influencing the outcomes of patients with both coronary artery disease and diabetes enrolled in standard cardiac rehabilitation programs: a literature review. The Journal of cardiovascular nursing. 2011;26:210–217. doi: 10.1097/JCN.0b013e31820017dc. [DOI] [PubMed] [Google Scholar]
- 4.Fisher BM, Fisher BM. Heart abnormalities in IDDM. Diabetologia. 1997;40 (Suppl 2):S127–129. doi: 10.1007/s001250051427. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty JD, Flaherty JD, Davidson CJ, et al. Diabetes and coronary revascularization. JAMA : the journal of the American Medical Association. 2005;293:1501–1508. doi: 10.1001/jama.293.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Katayama T, Katayama T, Nakashima H, et al. Clinical outcomes and left ventricular function in diabetic patients with acute myocardial infarction treated by primary coronary angioplasty. International heart journal. 2005;46:607–618. doi: 10.1536/ihj.46.607. [DOI] [PubMed] [Google Scholar]
- 7.Brener SJ, Mehran R, Dressler O, et al. Diabetes mellitus, myocardial reperfusion, and outcome in patients with acute ST-elevation myocardial infarction treated with primary angioplasty (from HORIZONS AMI) Am J Cardiol. 2012;109:1111–1116. doi: 10.1016/j.amjcard.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, Carson JL, Scholz PM, et al. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. Journal of the American College of Cardiology. 2002;40:418–423. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 9.Cohen Y, Cohen Y, Raz I, et al. Comparison of factors associated with 30-day mortality after coronary artery bypass grafting in patients with versus without diabetes mellitus. Israeli Coronary Artery Bypass (ISCAB) Study Consortium. The American journal of cardiology. 1998;81:7–11. doi: 10.1016/s0002-9149(97)00797-2. [DOI] [PubMed] [Google Scholar]
- 10.Luciani N, Luciani N, Nasso G, et al. Coronary artery bypass grafting in type II diabetic patients: a comparison between insulin-dependent and non-insulin-dependent patients at short- and mid-term follow-up. The Annals of thoracic surgery. 2003;76:1149–1154. doi: 10.1016/s0003-4975(03)00838-5. [DOI] [PubMed] [Google Scholar]
- 11.Szabó Z, Szabó Z, Håkanson E, et al. Early postoperative outcome and medium-term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting. The Annals of thoracic surgery. 2002;74:712–719. doi: 10.1016/s0003-4975(02)03778-5. [DOI] [PubMed] [Google Scholar]
- 12.Anderson EJ, Anderson EJ, Kypson AP, et al. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. Journal of the American College of Cardiology. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raedschelders K, Ansley DM, Chen DDY. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Boudina S, Boudina S, Abel ED, et al. Diabetic cardiomyopathy, causes and effects. Reviews in endocrine & metabolic disorders. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88:229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen AF, Chen D-D, Daiber A, et al. Free radical biology of the cardiovascular system. Clin Sci. 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 17.Khalid MA, Ashraf M. Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Is the hydroxyl radical really the most damaging radical species? Circ Res. 1993;72:725–736. doi: 10.1161/01.res.72.4.725. [DOI] [PubMed] [Google Scholar]
- 18.Tanguy S, de Leiris J, Besse S, et al. Ageing exacerbates the cardiotoxicity of hydrogen peroxide through the Fenton reaction in rats. Mech Ageing Dev. 2003;124:229–235. doi: 10.1016/s0047-6374(02)00185-9. [DOI] [PubMed] [Google Scholar]
- 19.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 20.Zorov DB, Zorov DB, Filburn CR, et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. The Journal of experimental medicine. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorov DB, Zorov DB, Juhaszova M, et al. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et biophysica acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Lisa F, Lisa F, Menabò R, et al. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. The Journal of biological chemistry. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 23.Vercesi AE, Vercesi AE, Kowaltowski AJ, et al. The role of reactive oxygen species in mitochondrial permeability transition. Bioscience reports. 1997;17:43–52. doi: 10.1023/a:1027335217774. [DOI] [PubMed] [Google Scholar]
- 24.Halestrap AP, Halestrap AP, Clarke SJ, et al. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular Research. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Kim J, He L, et al. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochemical and biophysical research communications. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, Halestrap AP, Clarke SJ, et al. The role of mitochondria in protection of the heart by preconditioning. Biochimica et biophysica acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda HM, Honda HM, Ping P, et al. Mitochondrial permeability transition in cardiac cell injury and death. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 2006;20:425–432. doi: 10.1007/s10557-006-0642-0. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson AB, Gustafsson AB, Gottlieb RA, et al. Heart mitochondria: gates of life and death. Cardiovascular Research. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 29.Hausenloy D, Hausenloy D, Wynne A, et al. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 30.Juhaszova M, Juhaszova M, Zorov DB, et al. Role of glycogen synthase kinase-3beta in cardioprotection. Circulation research. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr PM, Kerr PM, Suleiman MS, et al. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. The American journal of physiology. 1999;276:H496–502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- 32.Bernardi P, Bernardi P, Vassanelli S, et al. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations The Journal of biological chemistry. 1992;267:2934–2939. [PubMed] [Google Scholar]
- 33.Qian T, Qian T, Nieminen AL, et al. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. The American journal of physiology. 1997;273:C1783–1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 34.Selivanov VA, Selivanov VA, Zeak JA, et al. The role of external and matrix pH in mitochondrial reactive oxygen species generation. The Journal of biological chemistry. 2008;283:29292–29300. doi: 10.1074/jbc.M801019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scrutinio D, Scrutinio D, Giannuzzi P, et al. Comorbidity in patients undergoing coronary artery bypass graft surgery: impact on outcome and implications for cardiac rehabilitation. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2008;15:379–385. doi: 10.1097/HJR.0b013e3282fd5c6f. [DOI] [PubMed] [Google Scholar]
- 36.Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34:104–112. doi: 10.1016/s0008-6363(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 37.Whittington HJ, Babu GG, Mocanu MM, et al. The diabetic heart: too sweet for its own good? Cardiology Research and Practice. 2012;2012:845698. doi: 10.1155/2012/845698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balakumar P, Sharma NK. Healing the diabetic heart: Does myocardial preconditioning work? Cellular Signalling. 2011;24:53–59. doi: 10.1016/j.cellsig.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 40.Folli F, Corradi D, Fanti P, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 41.Yokota T, Kinugawa S, Hirabayashi K, et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;297:H1069–1077. doi: 10.1152/ajpheart.00267.2009. [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008;45:41–46. doi: 10.1007/s00592-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Wang B, Raedschelders K, et al. Differences in myocardial PTEN expression and Akt signalling in type 2 diabetic and nondiabetic patients undergoing coronary bypass surgery. Clinical endocrinology. 2011;74:705–713. doi: 10.1111/j.1365-2265.2011.03979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis P, Stefanovic N, Pete J, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 45.Fardoun RZ. The use of vitamin E in type 2 diabetes mellitus. Clin Exp Hypertens. 2007;29:135–148. doi: 10.1080/10641960701361601. [DOI] [PubMed] [Google Scholar]
- 46.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Sack MN. Type 2 diabetes, mitochondrial biology and the heart. Journal of Molecular and Cellular Cardiology. 2009;46:842–849. doi: 10.1016/j.yjmcc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishikawa T, Nishikawa T, Edelstein D, et al. The missing link: a single unifying mechanism for diabetic complications. Kidney international Supplement. 2000;77:S26–30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa T, Nishikawa T, Edelstein D, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 50.Matsushima S, Kinugawa S, Yokota T, et al. Increased myocardial NAD(P)H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H409–416. doi: 10.1152/ajpheart.01332.2008. [DOI] [PubMed] [Google Scholar]
- 51.Inoguchi T, Sonta T, Tsubouchi H, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 52.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 53.Takayanagi R, Inoguchi T, Ohnaka K. Clinical and experimental evidence for oxidative stress as an exacerbating factor of diabetes mellitus. J Clin Biochem Nutr. 2011;48:72–77. doi: 10.3164/jcbn.11-014FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okazaki T, Otani H, Shimazu T, et al. Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radical Research. 2011;45:1173–1183. doi: 10.3109/10715762.2011.605361. [DOI] [PubMed] [Google Scholar]
- 55.Maalouf RM, Eid AA, Gorin YC, et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597–604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okazaki T, Otani H, Shimazu T, et al. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. Journal of Molecular and Cellular Cardiology. 2011;50:534–544. doi: 10.1016/j.yjmcc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010;88:241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- 58.Wolff SP, Wolff SP, Dean RT, et al. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. The Biochemical journal. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy AL, Kennedy AL, Lyons TJ, et al. Glycation, oxidation, and lipoxidation in the development of diabetic complications. Metabolism: clinical and experimental. 1997;46:14–21. doi: 10.1016/s0026-0495(97)90311-5. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt AM, Schmidt AM, Hori O, et al. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arteriosclerosis and thrombosis : a journal of vascular biology/American Heart Association. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 61.Yim MB, Yim MB, Yim HS, et al. Protein glycation: creation of catalytic sites for free radical generation. Annals of the New York Academy of Sciences. 2001;928:48–53. [PubMed] [Google Scholar]
- 62.Kawamura N, Kawamura N, Ookawara T, et al. Increased glycated Cu,Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetis mellitus. The Journal of clinical endocrinology and metabolism. 1992;74:1352–1354. doi: 10.1210/jcem.74.6.1592880. [DOI] [PubMed] [Google Scholar]
- 63.Morgan PE, Morgan PE, Dean RT, et al. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Archives of biochemistry and biophysics. 2002;403:259–269. doi: 10.1016/s0003-9861(02)00222-9. [DOI] [PubMed] [Google Scholar]
- 64.Song F, Jia W, Yao Y, et al. Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed Type 2 diabetes. Clin Sci. 2007;112:599–606. doi: 10.1042/CS20060323. [DOI] [PubMed] [Google Scholar]
- 65.Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians. 1999;111:241–248. doi: 10.1046/j.1525-1381.1999.99220.x. [DOI] [PubMed] [Google Scholar]
- 66.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 67.Sheikh-Ali M, Chehade JM, Mooradian AD. The antioxidant paradox in diabetes mellitus. Am J Ther. 2011;18:266–278. doi: 10.1097/MJT.0b013e3181b7badf. [DOI] [PubMed] [Google Scholar]
- 68.Ozgen IT, Tascilar ME, Bilir P, et al. Oxidative stress in obese children and its relation with insulin resistance. J Pediatr Endocrinol Metab. 2012;25:261–266. doi: 10.1515/jpem-2011-0397. [DOI] [PubMed] [Google Scholar]
- 69.Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yue T-L, Bao W, Gu J-L, et al. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554–562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]
- 71.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 72.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J of Investigative Surgery. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 73.Sivaraman V, Hausenloy DJ, Wynne AM, et al. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–1746. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsang A, Hausenloy DJ, Mocanu MM, et al. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 75.Sasso FC, Torella D, Carbonara O, et al. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Kang KH, Lemke G, Kim JW. The PI3K-PTEN tug-of-war, oxidative stress and retinal degeneration. Trends in Molecular Medicine. 2009;15:191–198. doi: 10.1016/j.molmed.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mocanu MM, Field DC, Yellon DM. A potential role for PTEN in the diabetic heart. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 2006;20:319–321. doi: 10.1007/s10557-006-8876-4. [DOI] [PubMed] [Google Scholar]
- 78.Ruan H, Li J, Ren S, et al. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 79.Seo JH, Ahn Y, Lee S-R, et al. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song P, Wu Y, Xu J, et al. Reactive Nitrogen Species Induced by Hyperglycemia Suppresses Akt Signaling and Triggers Apoptosis by Upregulating Phosphatase PTEN (Phosphatase and Tensin Homologue Deleted on Chromosome 10) in an LKB1- Dependent Manner. Circulation. 2007;116:1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- 81.Wang XL, Wang XL, Zhang L, et al. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 82.Xueliang, Xueliang, Edelstein D, et al. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. The Journal of clinical investigation. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lecour S. Multiple protective pathways against reperfusion injury: a SAFE path without Aktion? Journal of Molecular and Cellular Cardiology. 2009;46:607–609. doi: 10.1016/j.yjmcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Reich NC. STAT3 revs up the powerhouse. Science Signaling. 2009;2:pe61. doi: 10.1126/scisignal.290pe61. [DOI] [PubMed] [Google Scholar]
- 85.Boengler K, Hilfiker-Kleiner D, Drexler H, et al. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? Journal of Molecular and Cellular Cardiology. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Goodman MD, Koch SE, Fuller-Bicer GA, et al. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wegrzyn J, Potla R, Chwae Y-J, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boengler K, Hilfiker-Kleiner D, Heusch G, et al. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith CCT, Dixon RA, Wynne AM, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–1270. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circulation Research. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 92.Lacerda L, Somers S, Opie L, et al. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 93.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circulation Research. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 94.Haghikia A, Stapel B, Hoch M, et al. STAT3 and cardiac remodeling. Heart Fail Rev. 2011;16:35–47. doi: 10.1007/s10741-010-9170-x. [DOI] [PubMed] [Google Scholar]
- 95.Drenger B, Ostrovsky IA, Barak M, et al. Diabetes Blockade of Sevoflurane Postconditioning Is Not Restored by Insulin in the Rat Heart: Phosphorylated Signal Transducer and Activator of Transcription 3- and Phosphatidylinositol 3-Kinase-Mediated Inhibition. Anesthesiology. 2011;114:1364–1372. doi: 10.1097/ALN.0b013e31820efafd. [DOI] [PubMed] [Google Scholar]
- 96.Schneider R, Welt K, Aust W, et al. Cardiac ischemia and reperfusion in spontaneously diabetic rats with and without application of EGb 761: I. cardiomyocytes. Histol Histopathol. 2008;23:807–817. doi: 10.14670/HH-23.807. [DOI] [PubMed] [Google Scholar]
- 97.Ananthakrishnan R, Ananthakrishnan R, Kaneko M, et al. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. American journal of physiology Heart and circulatory physiology. 2009;296:H333–341. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang WH, Tang WH, Kravtsov GM, et al. Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins. Journal of molecular and cellular cardiology. 2010;49:58–69. doi: 10.1016/j.yjmcc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang WH, Tang WH, Wu S, et al. Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free radical biology & medicine. 2008;45:602–610. doi: 10.1016/j.freeradbiomed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 100.LX, LX, Edelstein D, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. The Journal of clinical investigation. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Federici M, Menghini R, Mauriello A, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 102.Marsh SA, Dell’Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajamani U, Joseph D, Roux S, et al. The hexosamine biosynthetic pathway can mediate myocardial apoptosis in a rat model of diet-induced insulin resistance. Acta Physiol (Oxf) 2011;202:151–157. doi: 10.1111/j.1748-1716.2011.02275.x. [DOI] [PubMed] [Google Scholar]
- 104.Hasnan J, Yusof MI, Damitri TD, et al. Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singapore Med J. 2010;51:50–55. [PubMed] [Google Scholar]
- 105.Song Y, Wang J, Li Y, et al. Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol. 2005;167:17–26. doi: 10.1016/S0002-9440(10)62949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oshima Y, Fujio Y, Nakanishi T, et al. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res. 2005;65:428–435. doi: 10.1016/j.cardiores.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 107.Barry SP, Townsend PA, Knight RA, et al. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol. 2010;91:506–514. doi: 10.1111/j.1365-2613.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barry SP, Townsend PA, McCormick J, et al. STAT3 deletion sensitizes cells to oxidative stress. Biochem Biophys Res Commun. 2009;385:324–329. doi: 10.1016/j.bbrc.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X, Wei J, Peng DH, et al. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 110.Luan R, Liu S, Yin T, et al. High glucose sensitizes adult cardiomyocytes to ischaemia/reperfusion injury through nitrative thioredoxin inactivation. Cardiovasc Res. 2009;83:294–302. doi: 10.1093/cvr/cvp085. [DOI] [PubMed] [Google Scholar]
- 111.Yin T, Hou R, Liu S, et al. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–361. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 112.Wang XL, Lau WB, Yuan YX, et al. Methylglyoxal increases cardiomyocyte ischemia-reperfusion injury via glycative inhibition of thioredoxin activity. Am J Physiol Endocrinol Metab. 2010;299:E207–214. doi: 10.1152/ajpendo.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007;334:197–205. doi: 10.1097/MAJ.0b013e318157388f. [DOI] [PubMed] [Google Scholar]
- 114.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eguchi M, Eguchi M, Kim YH, et al. Ischemia-reperfusion injury leads to distinct temporal cardiac remodeling in normal versus diabetic mice. PloS one. 2012;7:1–10. doi: 10.1371/journal.pone.0030450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith HM, Smith HM, Hamblin M, et al. Greater propensity of diabetic myocardium for oxidative stress after myocardial infarction is associated with the development of heart failure. Journal of molecular and cellular cardiology. 2005;39:657–665. doi: 10.1016/j.yjmcc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Aragno M, Mastrocola R, Alloatti G, et al. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- 118.Gurusamy N, Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–1988. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sena S, Sena S, Hu P, et al. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. Journal of molecular and cellular cardiology. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anderson EJ, Anderson EJ, Rodriguez E, et al. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. American journal of physiology Heart and circulatory physiology. 2011;300:H118–124. doi: 10.1152/ajpheart.00932.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]