Abstract
β-thalassemia is a disease associated with decreased β-globin production leading to anemia, ineffective erythropoiesis, and iron overload. New mechanisms associated with modulation of erythropoiesis and iron metabolism have recently been discovered in thalassemic mice, improving our understanding of the pathophysiology of this disease. These discoveries have the potential to be translated into clinically-relevant therapeutic options to reduce ineffective erythropoiesis and iron overload. A new generation of therapies based on limiting ineffective erythropoiesis, iron absorption, and the correction of iron maldistribution could be on the way, possibly complementing and improving the current standard of patient care.
Keywords: β-thalassemia, ineffective erythropoiesis, Jak2, iron overload, hepcidin, transferrin
Introduction
β-thalassemia is a disease characterized by reduced or absent production of β-globin chains. The phenotype is associated with development of anemia, production of abnormal red blood cells (RBC), ineffective erythropoiesis (IE), and iron overload. More than 200 mutations in the β-globin gene or its promoter have been associated with this disease, contributing to a diverse phenotypic manifestation and adding an additional degree of complexity to the understanding and management of this disease. The most severe form, β-thalassemia major or Cooley’s anemia, is caused by mutations that completely abrogate synthesis of β-globin protein. Patients affected by this form of the disease develop a severe anemia requiring a regular blood transfusion regimen to sustain life. On the other hand, mutations that allow for production of some β-globin protein lead to milder forms of the disease, β-thalassemia intermedia. Although these patients are usually transfusion independent, iron overload develops due to increased iron uptake in the duodenum, leading to organ damage.1 In this paper, we will focus on novel experimental models modulating IE and iron overload in β-thalassemic mice to showcase their potential utility as new therapies for patients affected by this devastating disease.
Ineffective erythropoiesis in β-thalassemia
IE is defined as the failure of erythroid progenitors to differentiate either as a consequence of cell death or an intrinsic differentiation defect, leading to abnormal production of mature RBCs. The premature death or hemolysis of circulating RBCs can also contribute to this process. Feedback from anemia triggers an increase in erythropoietic activity and results in erythroid expansion in the bone marrow as well as de novo proliferation in the spleen and liver, a process known as extra medullary hematopoiesis (EMH). As a consequence, patients often develop hepatosplenomegaly, a common finding in β-thalassemic patients. In normal individuals, the main function of the spleen is to clear senescent RBCs from circulation. For this reason it is believed that the splenomegaly observed in β-thalassemia increases RBC sequestration, worsening the anemia and leading to an escalation of transfusion requirements. Patients often undergo splenectomy to reduce their transfusion requirement and limit iron overload.
Ferrokinetic studies conducted 50 years ago have served as the basis of our understanding of IE in β-thalassemia.2,3 In these studies it was observed that β-thalassemic patients had 10 times the plasma iron turnover of normal individuals, while their output of RBCs into the peripheral blood was markedly reduced. This suggested that the percentage of thalassemic erythroid precursors undergoing apoptosis was dramatically increased compared to that of normal individuals. Subsequent reports confirmed the increased apoptosis of erythroid progenitors in bone marrow and other sites of extramedullary erythropoiesis in β-thalassemic patients.4,5 Taken together, these studies confirmed that the majority of erythroid progenitors from β-thalassemic subjects die during the maturation process. Apoptosis is associated with accumulation of α-globin chains resulting from the reduction in β-globin synthesis. In normal erythropoiesis, erythroid progenitors possess mechanism to control iron uptake and coordinate the production of all hemoglobin components. Iron was shown to modulate heme biosynthesis, and heme bioavailability to control globin production via the heme-regulated inhibitor kinase system.6,7 Under the pathological conditions of β-thalassemia excesses of these elements leads to increased oxidative stress and accumulation of hemichromes. This is hypothesized to cause alterations in the cytoskeletal membrane proteins band-3 and spectrin, and to induce membrane lipid peroxidation,8,9 leading to exposure of the anionic phospholipids phosphatidylethanolamine and phosphatidylserine.10 All of these alterations in thalassemic erythroid cells undoubtedly contribute to the increased apoptosis observed. In agreement with the hypothesis that β-globin accumulation contributes to the development of IE, Kong and colleagues showed that lack of α-hemoglobin stabilizing protein (AHSP) impairs erythropoiesis and worsens the β-thalassemia phenotype, with increased apoptosis of erythroid precursors.11 In summary, markedly increased apoptosis of erythroid precursors and the decreased life span of β-thalassemic RBC in the peripheral blood seem to be major factors contributing to anemia and driving IE.
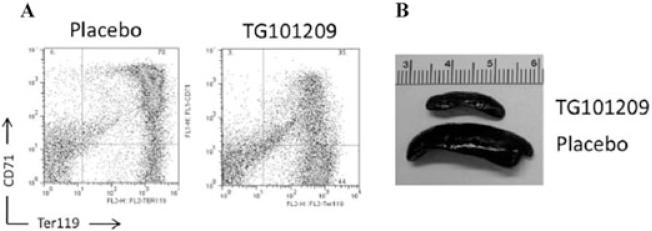
Although there is unequivocal evidence of increased apoptosis in β-thalassemic erythroid precursors the data suggests that the percentage of apoptotic cells is much lower than originally suggested by the ferrokinetic studies.2-5,12 The original studies predicted between 60% and 80% mortality of erythroid precursors but these researchers did not have the benefit of taking into consideration the role of hepcidin in increased iron absorption or the fact that a significant fraction of this iron would be stored in the liver.13,14 We recently reported that erythroid marrow expansion and EMH are also associated with increased proliferation and decreased differentiation of erythroid progenitor cells, in addition to increased apoptosis.12,15 Our data, obtained in mouse models of β-thalassemia intermedia (th3/+) and major (th3/th3), indicated that cell-cycle promoting genes were upregulated in β-thalassemic erythroid precursors limiting their differentiation, and likely worsening IE.12 Interestingly, use of a Jak2 inhibitor, to block the erythropoietin (Epo) signaling pathway, was effective in reducing the relative and absolute number of undifferentiated erythroid cells and had a profound beneficial effect on splenomegaly (Fig. 1).12 These observations corroborated our hypothesis that hyper proliferation of erythroid precursors with limited cell differentiation is an intrinsic feature of IE in β-thalassemia. In our original studies, use of the Jak2 inhibitor was associated with mild worsening of the anemia. Our new data in th3/+ mice indicate that a tailored dose of the Jak2 inhibitor leads to improvement of IE without worsening of anemia.15 Moreover, in th3/th3 animals, we determined that if the inhibitor was administered together with blood transfusions, the splenomegaly was ameliorated and the effectiveness of the transfusions was improved.15 Higher levels of hemoglobin were maintained over time compared to the situation in untreated transfused th3/th3 animals15 (and Melchiori 2010, unpublished data). Overall, these preclinical results support the use of Jak2 inhibitors to prevent splenectomy, especially under conditions of increased thrombotic risk.
Figure 1.
Effect of Jak2 on ineffective erythropoiesis in β-thalassemia. (A) Erythroid populations in splenic cell suspensions measured by classical CD71 and ter119 co-staining showing a decreased percentage of double positive cells in the animals treated with Jak2 inhibitor (right panel) compared to those of placebo treated mice (left panel). (B) Decrease in spleen size caused by treatment with the Jak2 inhibitor.
In conclusion, apoptosis driven by accumulation of α-globin aggregates plays a major role in IE. However, our data suggest that additional mechanisms may exacerbate IE. Epo signals erythroid cells to expand, promoting their proliferation and leading to erythroid expansion. On the other hand, it is unlikely that the mechanisms leading to decreased cell differentiation depend on Epo production alone. We postulate that other factors contribute for this process including activation of unknown targets by iron, ROS, heme, and globin chain imbalance, possibly through heme-regulated inhibitor kinase.16 This is an area of active research, especially because new tools have been identified to dissect erythropoiesis in normal and disease conditions.17
Preventing the need for splenectomy in β-thalassemia might also limit thrombosis in this disorder. It has been suggested that abnormal erythrocytes might predispose one to thrombotic events and that removal of the spleen might exacerbate this process.18 Several observations suggest that a hypercoagulable state is present in β-thalassemia.19,20 A number of abnormalities affecting coagulation have been seen in β-thalassemia, including activation of platelets, endothelium, and monocytes and altered levels of both coagulation factors and inhibitors.19-21 Moreover, abnormal RBCs, through their increased cohesiveness, contribute to the higher risk from thrombotic complications. In addition, the asymmetry of thalassemic RBC membranes contributes to activation of important coagulation factors, such as thrombin enhancing the hypercoagulable state.19-21
Thus, use of Jak2 inhibitors may lead to new treatments for and better management of IE, EMH, splenomegaly and, potentially, thrombosis in this disease.
Hepcidin in β-thalassemia
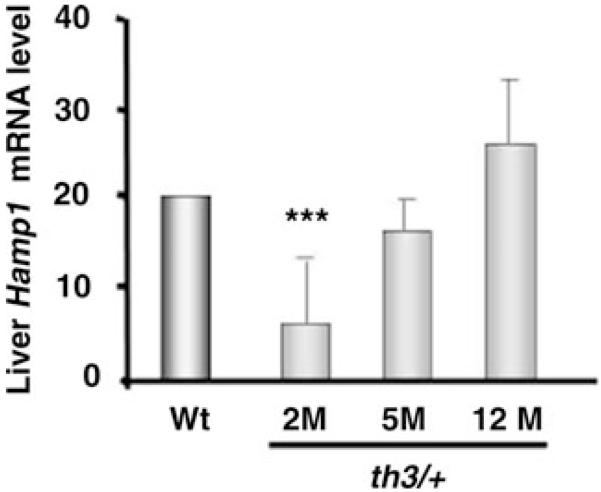
Several studies, reviewed in,16,22,23 dissected the role of hepcidin in β-thalassemia thereby adding to our understanding of the mechanisms affecting iron accumulation in this disorder. In particular, our study14 revealed interesting differences that helped to further characterize the regulation of iron metabolism in β-thalassemia: (1) despite a greater degree of iron overload, animals affected by β-thalassemia major (th3/th3) had the lowest hepcidin expression, indicating that these animals are relatively insensitive to their iron burden under conditions of extreme IE; (2) mimicking the situation in human patients,24 transfusion lead to increased hepcidin expression, hypothetically associated with decreased erythropoietic activity; (3) in mice affected by β-thalassemia intermedia (th3/+) hepcidin expression was disproportionally low compared to their iron load (Fig. 2).14 However, hepcidin expression increased as the animals aged, with elevated iron intake from the duodenum seemingly associated with increased ferroportin expression in this organ. Low hepcidin expression has also been reported in patients affected by β-thalassemia.13,25 These and other studies suggest that the expression of hepcidin is low due to the high erythropoietic activity.26 A corollary of this scenario is that erythroid cells secrete a protein, called the erythroid factor, which suppresses hepcidin expression. Pak and colleagues corroborated this model showing that blockage of erythropoietic activity in vivo was sufficient to abolish the downregulation of hepcidin mediated by anemia, even in the presence of high levels of Epo.26 Therefore, if an erythroid factor is secreted by erythroid progenitors, an increase in their number would augment the total amount of secreted erythroid factor, and result in a stronger downregulation of hepcidin. Sera from β-thalassemic patients suppressed hepcidin expression in a human hepatoma cell line, HepG2, further corroborating the existence of the erythroid factor. This effect was not observed using sera from normal individuals or from patients affected by hemochromatosis.27 Based on all of these observations, and because we expect that β-thalassemia major is associated with a greater degree of IE, we hypothesized that the erythroid factor would be at its highest concentration exerting the greatest degree of hepcidin repression. We demonstrated that hepcidin expression in th3/th3 mice is lower, despite a highest iron concentration in the liver, compared to th3/+ and normal mice.14 However, under conditions of limited anemia and IE as in th3/+ animals, iron overload is able to exert an effect on increasing hepcidin expression. In fact, although the level of hepcidin was disproportionally low in these animals, hepcidin expression increased as the iron burden worsened.14,16 This further supports the concept of independent pathways regulating hepcidin expression.
Figure 2.
Hepcidin mRNA expression in liver samples from thalassemic mice. Hepcidin mRNA expression in the liver of th3/+ mice at different ages as measured by qPCR, showing very low levels of hepcidin expression in the early stages of the disease (2m) and an increase of hepcidin levels at later stages (1 year). The abbreviations 2m, 5m, and 12m indicate 2-month-old, 5-month-old, and 12-month-old mice.14
Recent studies introduced two members of the TGF-β superfamily, growth differentiation factor 15 (GDF15) and twisted gastrulation protein-1 (TWSG1), as potential candidates for the erythroid factor.28,29 The corresponding genes were shown to be upregulated in undifferentiated erythroid cells and analysis of serum from β-thalassemic patients confirmed that their concentrations were elevated. Hepcidin expression in primary hepatocytes was shown to respond to both administration and blockage of these proteins in culture, suggesting that these erythroid derived factors are involved in the regulation of hepcidin in β-thalassemia. However, although they seem to contribute to hepcidin regulation in β-thalassemia, it is still not clear if they are involved in other conditions of anemia.
Modulation of iron metabolism and ineffective erythropoiesis in β-thalassemia
Iron overload is one of the major complications of β-thalassemia and new therapies to complement iron chelation are of great interest. This disease is characterized by an excess of iron over that needed to form hemoglobin, which accumulates progressively in the liver. Therefore, untransfused β-thalassemic patients and animals absorb more iron than they need to fulfill their requirements for erythropoiesis. Therapies that decrease iron uptake from the diet could prove efficient in reducing iron overload with minimal effect on anemia. This hypothesis was investigated by generating thalassemic animals overexpressing hepcidin. This resulted in decreased iron absorption and amelioration of anemia (Gardhengi 2010, unpublished data). Recently, using the th1/th1 mouse model of β-thalassemia intermedia,30 Dr. Ginzburg’s group also suggested that iron maldistribution in β-thalassemia might contribute to IE.31 In this study, iron delivery to erythroid cells in β-thalassemic mice was modulated by administration of apo-transferrin, showing a remarkable improvement of their phenotypic manifestations, including reduction of splenomegally and IE, improvement in hemoglobin concentration, increased hepcidin expression and reduced iron accumulation. Treatment with transferrin resulted in an increased number of RBCs, although the hemoglobin per cell (MCH) was decreased. These data suggest that decreasing iron availability, by decreasing either iron absorption or transferrin saturation, is beneficial to abnormal RBCs. Decreased iron availability likely results in more effective erythropoiesis as iron use within erythroid cells is limited to that needed for hemoglobin synthesis and less excess iron is available to generate free heme or α-globin precipitates, associated with shortened RBC survival.
Recently, a new line of investigation in our laboratory is also showing promising results. Although intrinsic properties of RBCs seem to drive IE and expansion of erythroid precursors increases iron absorption, we postulate that the microenvironment in the bone marrow affects erythroid maturation and differentiation under conditions of IE. Erythoid precursors mature in specialized structures called erythroblastic islands in which they associate with a central macrophage.32 Moreover, macrophages within the reticuloendothelial system are also of crucial importance in the recycling of iron from senescent RBCs and play an important role in normal iron homeostasis. Finally, it has been proposed that macrophages participate in the regulation of hepcidin by a yet unknown mechanism.33,34 Because of their multiple functions in erythropoiesis and iron homeostasis, we hypothesized that macrophages might modulate the thalassemic phenotype. A suicide technique to eliminate macrophages has been developed using liposome-mediated intracellular delivery of clodronate, a simple bisphosphonate (dichloromethylene diphosphonate, Cl2MDP).35 Clodronate-containing liposomes are quickly ingested by macrophages via endocytosis, the liposome bilayers disrupted by lysosomal phospholipases, and the clodronate released into the cytoplasm. Subsequently, the clodronate can be metabolized to a nonhydrolysable analog of ATP, which causes collapse of the mitochondrial membrane potential, leading to apoptosis. Original studies have been performed to assess the effect of reducing or eliminating macrophages and macrophage-like cells in normal mice using clodronate. Splenic macrophages and hepatic Kupffer cells were targeted and eliminated by a single dose of intravenously injected clodronate. The effect is reversible because macrophage repopulation starts after about 1 week. Prolonged treatment with clodronate caused an almost complete depletion of macrophages from the spleen and a 58% reduction of those in the BM. These alterations were accompanied by an increase in both the life span and age-related changes of their RBCs, as well as a mild anemia associated with a reduced reticulocyte count.36 Therefore, use of clodronate is an extremely efficient way to systematically reduce the number of macrophages.35
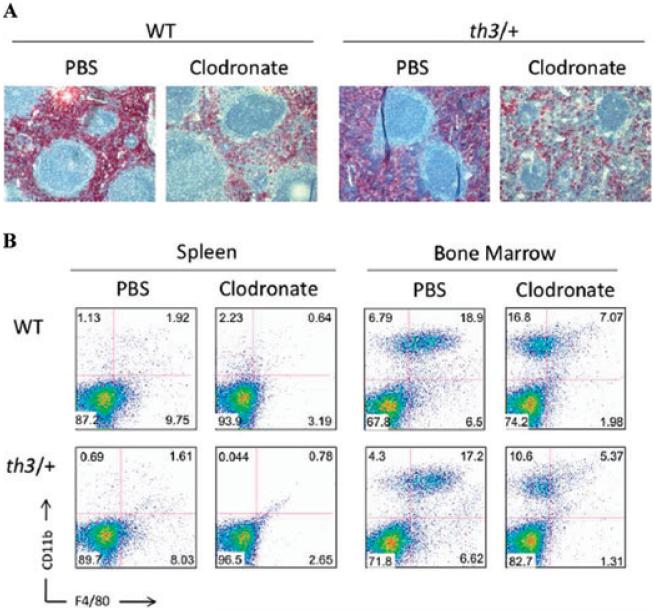
We performed some macrophage depletion studies using clodronate-containing liposomes. Using this approach, we confirmed by both flow cytometry and immunohistochemistry that macrophages in the spleen and bone marrow were markedly reduced (Fig. 3).37 Preliminary studies indicate that while this treatment leads to reduced RBC synthesis in normal mice, elimination of macrophages positively affects iron metabolism and IE in mouse models of β-thalassemia. Further experiments are in progress to corroborate these observations and to more fully understand the role of macrophages in this disorder (Ramos 2010, unpublished data).
Figure 3.
Depletion of macrophages using clodronate-containing liposomes. (A)Immunohistochemical staining of splenic sections from animals treated with PBS or clodronate-containing liposomes using the F4/80 antibody. (B) FACS analysis of cells from BM and spleen showing a reduction of F4/80 positive cells 48 hours after treatment with clodronate-containing liposomes.37
Future prospects
β-Thalassemia is a disease characterized by ineffective erythropoiesis, anemia, and iron overload. Recent studies shed light on the pathophysiology of β-thalassemia, including mechanisms driving ineffective erythropoiesis, with increased proliferation and decreased differentiation of erythroid progenitors in association with erythroid apoptosis, and increased iron absorption associated with inappropriately low hepcidin expression. In addition, studies in mice suggest that iron maldistribution may enhance IE and anemia in this disorder. Results obtained in mouse models of β-thalassemia suggest that use of Jak2 inhibitors, hepcidin, and transferrin might prove effective in limiting IE, iron absorption, or both. Additional studies related to the erythropoietic niche in the bone marrow, under conditions of chronic stress erythropoiesis and iron overload such as in β-thalassemia, will benefit our insight into the pathophysiology of this disorder and identify novel therapeutic targets to clinical practice. Human studies will be needed to corroborate these data and validate these approaches.
In conclusion, although the current treatments for β-thalassemia, blood transfusions and iron chelation, have proven to be effective in the management of this disease, recent observations suggest alternative strategies that may further improve the treatment of β-thalassemic patients.
Acknowledgments
This work was supported by the Cooley’s Anemia Foundation-CAF, the Associazione Veneta Lotta alla Talassemia (AVLT) (S.G.), and by grants from the Carlo and Micol Schejola Foundation, the Children’s Cancer and Blood Foundation and NIH-R21DK065169 (S.R.), R01DK55463 (R.W.G.), the American Portuguese Biomedical Fund (APBRF, USA)/Inova grant (P.R.). S.G. is a fellow of the Cooley’s Anemia Foundation. P.R. is a fellow from Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/24813/2005).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bannerman RM, et al. Thalassemia intermedia, with iron overload, cardiac failure, diabetes mellitus, hypopituitarism and porphyrinuria. Am. J. Med. 1967;42:476–486. doi: 10.1016/0002-9343(67)90276-8. [DOI] [PubMed] [Google Scholar]
- 2.Finch CA, Sturgeon P. Erythrokinetics in Cooley’s anemia. Blood. 1957;12:64–73. [PubMed] [Google Scholar]
- 3.Finch CA, et al. Ferrokinetics in man. Med. (Baltimore) 1970;49:17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yuan J, et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe beta-thalassemia (Cooley’s anemia) Blood. 1993;82:374–377. [PubMed] [Google Scholar]
- 5.Mathias LA, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp. Hematol. 2000;28:1343–1353. doi: 10.1016/s0301-472x(00)00555-5. [DOI] [PubMed] [Google Scholar]
- 6.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 7.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinar E, et al. Erythrocyte membrane skeleton abnormalities in severe beta-thalassemia. Blood. 1987;70:158–164. [PubMed] [Google Scholar]
- 9.Cappellini MD, et al. Metabolic indicators of oxidative stress correlate with haemichrome attachment to membrane, band 3 aggregation and erythrophagocytosis in beta-thalassaemia intermedia. Br. J. Haematol. 1999;104:504–512. doi: 10.1046/j.1365-2141.1999.01217.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuypers FA, et al. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91:3044–3051. [PubMed] [Google Scholar]
- 11.Kong Y, et al. Loss of alpha-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia. J. Clin. Invest. 2004;114:1457–1466. doi: 10.1172/JCI21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libani IV, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112:875–885. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Origa R, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 14.Gardenghi S, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melchiori L, et al. Use of Jak2 Inhibitors to limit ineffective erythropoiesis and iron absorption in Mice affected by β-thalassemia and other disorders of red cell production. Blood. 2009;114:2020. [Google Scholar]
- 16.Rivella S. Ineffective erythropoiesis and thalassemias. Curr. Opin. Hematol. 2009;16:187–194. doi: 10.1097/MOH.0b013e32832990a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappellini MD, et al. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br. J. Haematol. 2000;111:467–473. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- 19.Ataga KI, Cappellini MD, Rachmilewitz EA. Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br. J. Haematol. 2007;139:3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 20.Taher AT, et al. Thalassemia and hypercoagulability. Blood Rev. 2008;22:283–292. doi: 10.1016/j.blre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Rechavi G, Rivella S. Regulation of iron absorption in hemoglobinopathies. Curr. Mol. Med. 2008;8:646–662. doi: 10.2174/156652408786241401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivella S, Rachmilewitz E. Future alternative therapies for β-thalassemia. Expert Rev. Hematol. 2009;2:685–697. doi: 10.1586/ehm.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins ZA, et al. Iron homeostasis during transfusional iron overload in beta-thalassemia and sickle cell disease: changes in iron regulatory protein, hepcidin, and ferritin expression. Pediatr. Hematol. Oncol. 2007;24:237–243. doi: 10.1080/08880010701360700. [DOI] [PubMed] [Google Scholar]
- 25.Kattamis A, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 26.Pak M, et al. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weizer-Stern O, et al. Downregulation of hepcidin and haemojuvelin expression in the hepatocyte cell-line HepG2 induced by thalassaemic sera. Br. J. Haematol. 2006;135:129–138. doi: 10.1111/j.1365-2141.2006.06258.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanno T, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat. Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 29.Tanno T, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skow LC, et al. A mouse model for beta-thalassemia. Cell. 1983;34:1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- 31.Li H, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat. Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 32.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theurl M, et al. Kupffer cells modulate iron homeostasis in mice via regulation of hepcidin expression. J. Mol. Med. 2008;86:825–835. doi: 10.1007/s00109-008-0346-y. [DOI] [PubMed] [Google Scholar]
- 34.Makui H, et al. Contribution of Hfe expression in macrophages to the regulation of hepatic hepcidin levels and iron loading. Blood. 2005;106:2189–2195. doi: 10.1182/blood-2005-02-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani AL, et al. Changes in murine bone marrow macrophages and erythroid burst-forming cells following the intravenous injection of liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP) Eur. J. Haematol. 2001;66:221–229. doi: 10.1034/j.1600-0609.2001.066004221.x. [DOI] [PubMed] [Google Scholar]
- 37.Ramos P, et al. Macrophage depletion leads to modification of thalassemic phenotype. Blood. 2009;114:2023. [Google Scholar]