Key Points
Natural killer (NK) cells can be swiftly mobilized by danger signals and are among the earliest arrivals in target organs of disease. However, the role of NK cells in regulating inflammatory responses is far from completely understood in different organs. It is often complex and sometimes paradoxical.
The phenotypes and functions of NK cells in the liver, mucosal tissues, uterus, pancreas, joints and brain are influenced by the unique cellular interactions and the local microenvironment within each organ.
Hepatic NK cells exhibit an activated phenotype with high levels of cytotoxic effector molecules. These cells have been implicated in promoting liver injury and inhibiting liver fibrosis and regeneration. The liver is also enriched in NK cells with memory-like adaptive immune features.
NK cells are detected in healthy lymphoid tissues of the lung, skin and gut, and are recruited to these tissues during infection or inflammation. In the gastrointestinal tract, classical NK cells and a variety of innate lymphoid cells, such as the family of lymphoid tissue-inducer (LTi) cells, are likely to have crucial roles in controlling inflammatory responses.
NK cells represent the major lymphocyte subset in the pregnant uterus, with a unique phenotype resembling an early developmental state. Emerging evidence indicates that these cells play a crucial part in mediating the uterine vascular adaptations to pregnancy and promoting the maintenance of healthy pregnancy.
In non-obese diabetic (NOD) mice, NK cells are recruited early to the pancreas, become locally activated and then adopt a hyporesponsive phenotype. Although NK cells have a pathogenic role in the natural progression of diabetes in NOD mice, they contribute to diabetes protection induced by complete Freund's adjuvant and to islet allograft tolerance induced by co-stimulatory blockade.
NK cells in the inflamed joint uniquely express receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), which promote osteoclast differentiation. Although NK cells have a pathogenic role in collagen-induced arthritis in mice, they are also crucial for protection against antibody-induced arthritis mediated by CpG oligonucleotides.
Studies in a mouse model of multiple sclerosis have shown that NK cells arrive in the central nervous system (CNS) before pathogenic T cells and have a protective role in the development of CNS inflammation, probably by killing CNS-resident microglia that prime effector T cells.
During evolution, different organs might have evolved distinct ways to recruit and influence the effector functions of NK cells. Once we understand these mechanisms, the next challenge will be to exploit this information for harnessing NK cells to develop prophylactic and therapeutic measures against infectious agents, tumours and inflammatory diseases.
Subject terms: T cells
Each tissue in our body contains a unique microenvironment that can differentially shape immune reactivity. In this Review article, Shiet al. describe how organ-specific factors influence natural killer cell homing and phenotype, and discuss the local molecular and cellular interactions that determine the protective or pathogenic functions of natural killer cells in the different tissues.
Abstract
Natural killer (NK) cells can be swiftly mobilized by danger signals and are among the earliest arrivals at target organs of disease. However, the role of NK cells in mounting inflammatory responses is often complex and sometimes paradoxical. Here, we examine the divergent phenotypic and functional features of NK cells, as deduced largely from experimental mouse models of pathophysiological responses in the liver, mucosal tissues, uterus, pancreas, joints and brain. Moreover, we discuss how organ-specific factors, the local microenvironment and unique cellular interactions may influence the organ-specific properties of NK cells.
Main
Infection and autoimmunity are two common pathological processes, during which inflammation might be elicited in an organ-specific manner. Microorganisms can infect specific organs and induce inflammatory responses. Frequently, the host immune response against the invading pathogens may also cause tissue damage owing to bystander effects. Moreover, inflammation can sometimes trigger organ-specific autoimmune diseases or even malignant transformation of the affected organ.
Among the many cell types of the immune system, natural killer cells (NK cells) are one of the earliest cell types to arrive at target organs of inflammation1. The role of NK cells in modulating inflammatory responses in different organs is often complex, not always completely understood and sometimes even paradoxical2,3,4. For example, some studies have shown that NK cells may promote insulitis, destroy insulin-producing islet cells and promote the development of type 1 diabetes (T1D)5,6,7,8. In sharp contrast, other studies have shown that NK cells may inhibit the intensity of inflammatory responses within the central nervous system (CNS)9,10,11,12. In rheumatoid arthritis, however, both inhibitory and promoting roles for NK cells have been reported13,14. Although different NK cell subsets or functions might be involved during different stages of the inflammatory process2,3, emerging evidence indicates that additional elements, such as organ-specific factors and cellular interactions, may also influence the divergent functions of NK cells in different organs and during different pathological conditions.
Bone marrow-derived NK cell precursors undergo a maturation process that leads to the acquisition of their effector functions, changes in the expression of chemotactic receptors and adhesion molecules, and their migration from the bone marrow through the blood to the spleen, liver, lung and many other organs. The distribution of NK cells is not static because these cells can recirculate between organs15. Owing to the expression of chemokine receptors, such as CC-chemokine receptor 2 (CCR2), CCR5, CXC-chemokine receptor 3 (CXCR3) and CX3C-chemokine receptor 1 (CX3CR1), NK cells can respond to a large array of chemokines16, and thus they can be recruited to distinct sites of inflammation17,18,19,20,21,22,23,24,25 and extravagate to the parenchyma or body cavities. In contrast to B cells, T cells and dendritic cells (DCs), the detailed trafficking patterns of NK cells are not very well characterized. Nevertheless, it appears that chemokines produced by cells that are unique to specific organs may have a role in orchestrating NK cell migration to each organ. For example, the CX3C-chemokine ligand 1 (CX3CL1; also known as fractalkine) produced by neurons is necessary and sufficient to guide CX3CR1-bearing NK cells to the inflamed brain10,26. Similarly, Kupffer cell-derived CC-chemokine ligand 2 (CCL2; also known as MCP1) attracts CCR2-expressing NK cells to the liver during murine cytomegalovirus (MCMV) infection27,28. The production of unique chemokines by organ-specific cell types for the recruitment of NK cells (Table 1) suggests that organ-intrinsic elements may be important in shaping NK cell homing during physiological and pathological conditions.
Table 1.
Organ-specific determinants that can influence NK cell function*
| Organ-specific determinants | Liver | Mucosal tissues and skin | Uterus | Pancreas | Joints | Brain |
|---|---|---|---|---|---|---|
| Chemokines | Kupffer cell-derived CCL2, CXCL4, CXCL9, CXCL11, CXCL6 and CCL3 (Hs/Mm)16 | Lung: CCR2, CXCR3 and CX3CR1 ligands (Mm)16 | CXCL12 (Hs/Mm)74,75,76, 77,78,79,80,81,82 | CXCL10 (Mm)86 | CCR2 and CCR5 ligands (Hs)93,94 | Neuron-derived CX3CL1 and CX3CR1 expression on microglia (Mm)10 |
| Skin: TIG2, CXCR3 ligands and CCR5 ligands (Hs)65 | ||||||
| Unique cellular components | Kupffer cells, hepatic stellate cells, hepatocytes and NKT cells (Hs/Mm)42,52 | Gut: DCs adjacent to crytopatches; close proximity to epithelial stem cells and Paneth cells (Hs/Mm)67,68 | Invading trophoblasts; decidual stromal cells (Hs/Mm)71,72 | β-cells expressing RAE1 (an NKG2D ligand) and ligands for PD1 (Mm)85,88 | Osteoclasts, macrophages and monocytes (Hs/Mm)13,97 | Microglia, astrocytes and neuronal cells (Hs/Mm)1,106,107 |
| Skin: keratinocytes (Hs)63 | ||||||
| Soluble components | Abundance of IL-12 and IL-18; temporal IL-10 increase; frequent exposure to LPS (Hs/Mm)42,53,54,55 | Gut: IL-1 and IL-23 derived from DCs (Hs/Mm)68 | TGFβ derived from stromal cells (Hs)81; IL-15 (Hs/Mm)74; stem cell factor (Mm)17 | Effector T cell-derived cytokines and TReg cell-derived cytokines (Mm)6,85 | M-CSF, RANKL, IL-12, IL-15 and IL-18 (Hs/Mm)13,95,97,98,99 | Neurotransmitters; arrays of cytokines; proteins released by CNS-resident cells (Hs/Mm)1 |
| Lung: IL-15 from lung macrophages (Mm)64 | ||||||
| Skin: CCL5 and CXCL10 from keratinocytes (Hs)68 | ||||||
| Anatomical constituents | Exposure to food antigens and microbial products; perfusion with blood from the circulation and intestine; thin-walled sinusoids (Hs/Mm)28,42,43 | Gut: exposure to commensal microflora (Hs/Mm)68 | Mesometrial lymphoid aggregate of pregnancy (Hs/Mm)71 | β-cells in the islets of Langerhans (Hs/Mm)127 | Relative immune-privilege in normal conditions (Hs/Mm)13 | Distinctive blood–brain barrier in healthy conditions; neuro–immune–endocrine axis (Hs/Mm)2,100,128 |
| CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CNS, central nervous system; CX3CL, CX3C-chemokine ligand; CX3CR, CX3C-chemokine receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; DC, dendritic cell; IL, interleukin; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; NK, natural killer; NKG2D, natural killer group 2, member D; NKT, natural killer T; PD1, programmed cell death protein 1; RAE1, retinoic acid early inducible 1; RANKL, receptor activator of NF-κB ligand; TGFβ, transforming growth factor-β; TIG2, tazarotene-induced gene 2 (also known as chemerin); TReg, regulatory T. | ||||||
| *'Hs' indicates evidence found in humans, 'Mm' indicates evidence found in mice, and 'Hs/Mm' indicates evidence found in both humans and mice. | ||||||
In this article, we discuss recent research on organ-specific NK cells and argue that organ-intrinsic factors shape the cellular, molecular and functional features of NK cells in organ-specific inflammation. The impact of the local environment in tumours on NK cells will not be discussed here because this topic has been extensively covered elsewhere29,30.
Systemic NK cells
NK cell subsets. NK cells can be divided into functionally distinct subsets, which differ between humans and mice. Human NK cells can be functionally classified based on the levels of CD56 and CD16 expression. Most circulating NK cells are CD56lowCD16+, and can kill target cells and produce cytokines following specific recognition of their targets31,32. By contrast, CD56hiCD16− NK cells, which are the predominant NK cell subset in peripheral lymphoid organs, produce large amounts of cytokines — including interferon-γ (IFNγ), tumour necrosis factor (TNF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) — following stimulation by pro-inflammatory cytokines and acquire cytotoxicity only after prolonged activation31,32. It has been shown that the CD56hiCD16− NK cells can differentiate into CD56lowCD16+ NK cells, and recent studies have revealed that CD56lowCD16+ NK cells can undergo further differentiation and functional modification with time33,34,35.
Mouse NK cells share many characteristics with human NK cells, but the lack of CD56 expression and other surface markers on mouse NK cells makes it difficult to identify functionally comparable NK cell populations in mice. Nevertheless, mouse NK cells can be classified based on expression of CD11b (also known as αM integrin), CD27, CD127 and B220 (Refs 36, 37, 38). There is evidence that CD11bhiCD27+ and CD11bhiCD27− NK cell subsets in mice differ in the expression of activating and inhibitory receptors and chemokine receptors, with the CD11bhiCD27+NK cell subset exhibiting the highest levels of both cytokine production and cytotoxicity39. Mouse NK cells that express the interleukin-7 (IL-7) receptor CD127 resemble human CD56hiCD16− NK cells in that they produce copious amounts of cytokines and only acquire cytotoxic activities after prolonged activation. Furthermore, B220+CD11c+ NK cells are enriched in secondary lymphoid tissues and secrete higher levels of IFNγ than other mouse NK cells36,37,38.
More recently, NKp46 (also known as NCR1) has been identified as a common marker for human and murine NK cells. However, some subsets of innate lymphocytes that are distinct from NK cells have also been found to express NKp46 (Box 1).
NK cell activation. NK cells are activated by target cells following the detection of missing or altered expression of self MHC class I molecules. NK cell activation can occur through three mechanisms: by engagement of the Fc portion of IgG antibodies that decorate cells; by the sensing of altered molecules on stressed cells; or by an inflammatory environment that is rich in cytokines, such as IL-12 or type I IFNs40,41. Cytotoxicity against infected or transformed cells and production of cytokines are the main effector functions of NK cells.
NK cells in the liver
Homing of NK cells to the liver. The liver represents a unique immunological environment, in which constant exposure to gut-derived antigens does not result in inflammation. In the liver, NK cells are preferentially located in the hepatic sinusoids, often adhering to the endothelial cells42. In mice, 5–10% of hepatic lymphocytes are NK cells, whereas, strikingly, up to 30–50% of all human hepatic lymphocytes are NK cells. Moreover, studies in mice have shown that the number of NK cells changes substantially in different experimental models of liver diseases. For example, mice infected with viral pathogens show profound accumulation of NK cells in the liver28,43. Likewise, administration of polyI:C (polyinosinic–polycytidylic acid; a synthetic version of double-stranded RNA) also induces NK cell accumulation in the liver. The chemokines CCL2, CCL3 (also known as MIP1α) and CXC-chemokine ligand 10 (CXCL10), which are produced by liver-resident cells, can recruit NK cells expressing CCR1, CCR2, CCR3, CCR5 and CXCR3 to the liver under various pathological conditions16.
Hepatic NK cell phenotype. Whereas CD11b+CD27− NK cells predominate in the peripheral blood of mice, CD11b−CD27+ NK cells are found at greater proportions in the liver44. Another hallmark of liver NK cells, compared with NK cells in peripheral blood, is the expression of the inhibitory receptor natural killer group 2, member A (NKG2A), which interacts with the MHC class I-related protein Qa-1 in mice (HLA-E in humans). Expression of NKG2A on NK cells may contribute to liver tolerance42.
The characteristics of NK cells in different organs may reflect their ability to adapt to different microenvironments. In support of this notion, splenic NK cells can migrate to the liver following adoptive transfer, where they adopt a phenotype and function that closely resembles that of liver-resident NK cells44. The recent discovery that NK cells are capable of antigen-specific immunological memory in humans and mice further suggests that NK cells are more versatile than previously anticipated45,46,47,48. This is well reflected by hepatic NK cells, as they can acquire and retain antigen-specific memory against haptens and virus-derived antigens even in the absence of antigen persistence49,50. Memory NK cells specific for such antigens are enriched in the liver (Fig. 1) and crucially depend on expression of CXCR6, a receptor for the chemokine CXCL16 (which is produced by the endothelium of liver sinusoids50). In addition to its role in NK cell-mediated memory, tonic engagement of CXCR6 protects the liver from NK cell-mediated hepatotoxicity by regulating the killing activity of NK cells50.
Figure 1. Unique effector properties of NK cells in the liver.
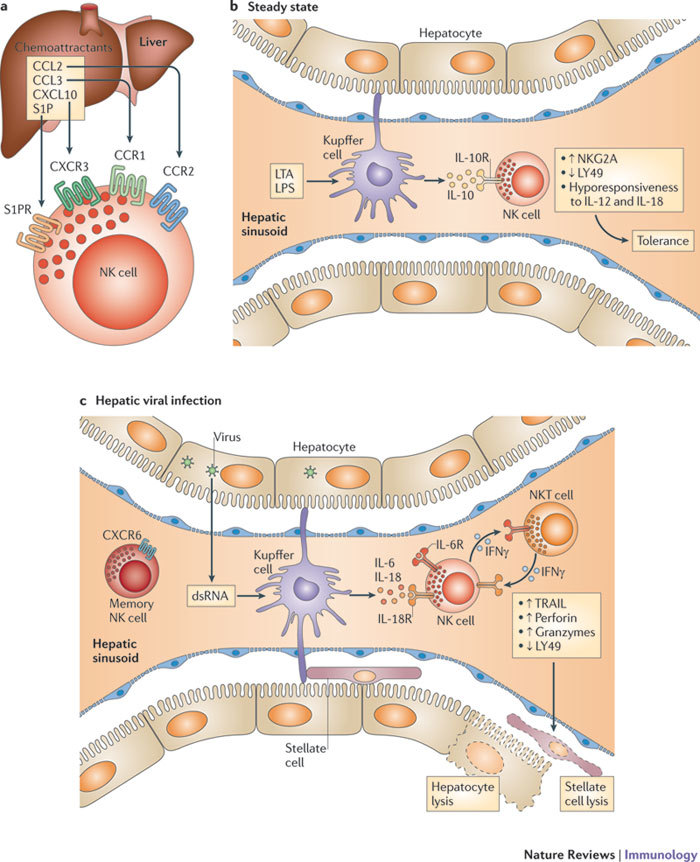
a | CC-chemokine ligand 2 (CCL2), CCL3, CXC-chemokine ligand 10 (CXCL10) and sphingosine-1-phosphate (S1P)125 derived from Kupffer cells and other hepatic cells attract natural killer (NK) cells to the inflamed liver. b | Hepatic NK cells reside in distinctive, thin-walled sinusoids, where Kupffer cells and other lymphocytes are also located. Hepatic NK cells face the challenging task of balancing immunity to multiple hepatotropic viruses and bacteria with tolerance to food and self antigens. In the steady state, commensal-derived lipoteichoic acid (LTA) and lipopolysaccharide (LPS) that arrive in the liver from the gut through the portal vein ligate Toll-like receptor 2 (TLR2) and TLR4, respectively, on Kupffer cells, triggering the release of interleukin-10 (IL-10)126. IL-10 in turn renders NK cells hyporesponsive to IL-12 and IL-18 and promotes tolerance in the liver. In addition, expression of the inhibitory receptor natural killer group 2, member A (NKG2A) is increased, whereas expression of the activating receptor LY49 is decreased on NK cells. c | During viral infection, double-stranded RNA (dsRNA) ligates TLR3 and triggers Kupffer cells to release IL-6 and IL-18, which in turn activate NK cells and boost their killing activity. This is reflected by the phenotype of hepatic NK cells, which is characterized by the expression of high levels of TNF-related apoptosis-inducing ligand (TRAIL), perforin and granzymes, and a lack of LY49. Activated hepatic NK cells can lyse hepatocytes or stellate cells via TRAIL-dependent pathways during hepatitis B virus infection or fibrosis, respectively. Thus, Kupffer cells appear to have a dual role in shaping hepatic NK cell phenotype and function. Hepatic NK cells are also subjected to the influence of interferon-γ (IFNγ) released by natural killer T (NKT) cells. Hepatic NK cells can acquire memory to antigens derived from influenza virus, vesicular stomatitis virus and HIV-1 (not shown). CCR, CC-chemokine receptor; CXCR, CXC-chemokine receptor; S1PR, S1P receptor.
Finally, the chronic stages of hepatic infections with hepatitis C virus (HCV) promote the appearance of NK cells with increased levels of TRAIL (TNF-related apoptosis-inducing ligand), NKp44 (also known as NCR2), NKG2C and CD122 (Refs 49, 50).
Crosstalk between NK cells and liver cells. NK cells may assist in maintaining liver tolerance through their interactions with various cell types. For example, NK cells co-cultured with hepatocytes have been described to promote the capacity of DCs to induce CD4+ regulatory T (TReg) cells51. The induction of TReg cells by DCs in the presence of NK cells is dependent on NKG2A engagement on NK cells during their co-culture with hepatocytes52.
Kupffer cells, which are mainly localized around the periportal regions in the liver, function as hepatic tissue macrophages and are crucial for endotoxin removal from the blood. Treatment of Kupffer cells with lipopolysaccharide (LPS) results in high levels of IL-10 production, which leads to decreased NK cell activation. It has been suggested that IL-10-mediated induction of NKG2A in liver NK cells might regulate liver NK cell function42 (Fig. 1). Although the timing and physiological circumstances under which IL-10 is released by Kupffer cells require further investigation, it is presumed that Kupffer cells produce IL-10 to downregulate the immune responses orchestrated by NK cells and other immune cells during late stages of inflammation to avoid bystander hepatic damage.
Soluble factors that shape NK cell phenotype in liver. The presence of IFNα, IFNγ, IL-2, IL-6, IL-12 and IL-18 in the inflamed liver largely contributes to an activated phenotype of NK cells, whereas IL-15 and IL-2 may sustain the survival and activation of these cells (Fig. 1). Among the liver-resident cells that can provide a source of these cytokines, hepatic natural killer T cells (NKT cells) can release large amounts of IFNγ, and Kupffer cells primarily release CCL2, IL-6 and other acute pro-inflammatory mediators (Fig. 1). As mentioned above, Kupffer cells can also release IL-10 (Ref. 53), which induces expression of NKG2A51. The liver encounters relatively high levels of LPS derived from the gut, which could be potentially harmful. Interestingly, it has been reported that crosstalk between Toll-like receptor (TLR) and complement receptor signalling pathways inhibits IL-12 production, and this may have a role in dampening excessive NK cell activation54,55.
Role of hepatic NK cells in disease. Chronic liver disease is often associated with the development of fibrosis, a wound-healing process. Fibrosis is characterized by the formation of new blood vessels, sinusoidal remodelling and pericyte (stellate cell) proliferation56. Hepatic stellate cells are activated following the phagocytosis of apoptotic bodies and cell debris, leading to their differentiation, proliferation and pro-fibrotic progression. NK cells have been suggested to be involved in hepatic stellate cell death, as depletion of NK cells leads to enhanced fibrosis in a mouse experimental model42. Interestingly, of the many pro-apoptotic molecules investigated in activated hepatic stellate cells, the upregulation of TRAIL receptors, which can bind NK cell-derived cytotoxic ligands may be crucial57.
The finding that NKG2A expression is increased on NK cells from patients chronically infected with HCV suggests a role for these cells in maintaining the persistent viral infection58,59, possibly by inhibiting antiviral T cell responses and facilitating pathogen persistence. Other studies have suggested that hepatic NK cells may contribute to innate resistance to HCV infection via increased expression of NKp30 (also known as NCR3)60. Finally, liver NK cells might contribute to the therapeutic activities of IFNα against hepatitis B virus (HBV) and HCV infection. In addition to its direct antiviral effects, IFNα treatment might affect NK cell activity61. Transcriptional profiling studies using gene arrays identified TRAIL as one of the most significantly upregulated genes in NK cells after IFNα stimulation. IFNα-stimulated NK cells killed HCV-infected hepatoma cells in a TRAIL-dependent manner. These findings suggest that IFNα-induced TRAIL expression on NK cells may be associated with control of HCV infection61.
Thus, steady-state hepatic NK cells promote liver tolerance and contain a population with memory-like features. Following viral infection, chemokines that are produced by liver-resident cells recruit more NK cells to the liver, and these cells acquire an activated phenotype to control viral infection. Crosstalk with liver-resident cells restrains NK cell-mediated cytotoxicity following viral clearance to avoid bystander tissue damage. However, during chronic liver infections, NK cells promote the persistence of the pathogen and liver injury while inhibiting liver fibrosis and tissue regeneration.
NK cells in mucosal tissues and the skin
NK cell homing to the lung, skin and gut. Interpretation of studies performed with NK cells in mucosal tissues is complicated by the fact that many studies often have not been able to distinguish bona fide NK cells from NKT cells and the growing population of innate lymphoid cells (ILCs) that are particularly prevalent in the gut-associated lymphoid tissue (GALT) (Box 1). Nevertheless, based on the available evidence, NK cells represent a major lymphocyte subset in the healthy lung (accounting for ∼10% of all lymphocytes in the lung in mice)16, and are also present in healthy skin in humans62 and in the GALT of humans and mice65.
In humans, infection with Staphylococcus aureus triggers a vigorous NK cell influx into the bronchoalveolar space64. Ligation of diverse chemokine receptors, including CCR2, CXCR3 and CX3CR1, with their corresponding ligands recruits NK cells to the lung during various pathogen encounters16.
In acute skin lesions, such as psoriatic plaques in human patients, 5–8% of the infiltrating cells are CD3−CD56+ NK cells, which are mostly localized in the mid and papillary dermis63. Skin NK cells express high levels of the chemokine receptors CXCR3 and CCR5, intermediate amounts of CXCR1, CCR6, chemokine-like receptor 1 (also known as CHEMR23) and CCR8, and low levels of CCR1, CCR2, CCR4, CCR7 and CX3CR1 (Ref. 65). Moreover, they home to the skin, directed by CXCL10 and CCL5 (Ref. 63) (Table 1).
In the intestine, NK cells are found predominantly within the lamina propria and are rare in lymphoid aggregates, although they can be found in the parafollicular region of caecal lymphoid patches, Peyer's patches and mesenteric lymph nodes65.
NK cell phenotype in mucosal tissues and the skin. NK cells generated in vitro from lung NK cell progenitors differ substantially from NK cells generated from bone marrow-derived NK cell progenitors with regard to expression of members of the LY49 family of NK cell receptors. Specifically, lung NK cells express multiple LY49 receptors, whereas most NK cells that develop from bone marrow-derived progenitors express CD94–NKG2 heterodimers but not LY49, suggesting a major impact of the microenvironment on NK cell development66. During S. aureus infection, lung NK cells produce increased amounts of TNF64.
In addition to their distinct patterns of chemokine receptor expression, NK cells in the skin of patients with psoriatic lesions exhibit a CD56hiCD16−CD158b− phenotype, fail to express the skin-homing cutaneous lymphocyte-associated antigen and release abundant IFNγ following stimulation63.
A major challenge in identifying gut NK cells lies in the difficulty in discriminating these cells from other gut ILCs. A subset of gut ILCs expresses NKp46, and among this subset one fraction lacks expression of retinoic acid receptor-related orphan receptor-γt (RORγt) and produces IFNγ67. Among gut ILCs, these NKp46+RORγt− cells resemble conventional NK cells in several respects, such as in the expression of several NK cell receptors and various molecules that are required for NK cell-dependent cytotoxicity63, and thus these cells are likely to be bona fide gut NK cells. However, adding to the complexity is the observation that, whereas RORγt expression is stable in 'non-NK' NKp46+RORγt+ cells in the small intestine, RORγt expression can be lost in these cells in the colon, leading to a population of IFNγ-polarized NKp46+RORγt− cells that are phenotypically indistinguishable from gut NK cells67.
Crosstalk between NK cells and mucosal tissue- or skin-resident cells. In the lung, alveolar macrophage-derived IL-15 supports NK cell proliferation and, conversely, lung macrophages show improved phagocytosis of S. aureus in the presence of NK cells64. Similarly, there appears to be a reciprocal relationship between skin-infiltrating NK cells and keratinocytes. Keratinocytes may provide a major source of CCL5 and CXCL10 to recruit NK cells, and skin-resident NK cells release large amounts of IFNγ and efficiently activate keratinocytes63. In the gut, signals emanating from DCs, epithelial cells and luminal contents constantly regulate GALT development and function, thereby contributing to the maintenance of a 'tolerogenic' status to innocuous antigens and commensal flora while initiating immune responses and tissue reparative mechanisms that restrain pathogen invasion67. For example, IL-23 enhances the production of IL-22 by NKp46+RORγt+ cells67,68, and this may function to preserve and restore epithelial barrier integrity67.
Role of mucosal NK cells in disease. NK cells, including those in the gut and other mucosal lymphoid organs, are thought to protect against infection by viruses and other intracellular pathogens. For example, within 24 hours of infection with orally administered Listeria monocytogenes, IFNγ production is induced by NK cells in the small intestine69. IFNγ production peaks at 48 hours post-inoculation in this model. Importantly, blocking IFNγ, but not IL-22, with neutralizing antibodies induces significant increases in bacterial loads in the GALT69. However, this does not exclude an important role for IL-22 in other bacterial infections or in other forms of tissue injury69,70.
In summary, NK cells are detected in healthy mucosal tissues and the skin. In response to infection, more NK cells are recruited to the lung, the gut and the skin, and these cells acquire an activated phenotype, which is essential for pathogen clearance. Moreover, in the gut, classical NK cells together with ILCs appear to control inflammatory responses.
NK cells in the uterus
NK cell homing to the uterus. NK cells are abundant in the decidua — the endometrium of the pregnant uterus that forms the maternal part of the placenta. There, uterine NK cells are thought to control endometrial tissue remodelling, vascular function and the formation of a placenta in the uterus71,72. Several studies have described the kinetics of uterine NK cell accumulation in pregnant mice72,73. Following fertilization and implantation, NK cells accumulate initially in the decidua basalis, which is subjacent to the trophoblast layer, and later in the mesometrial lymphoid aggregate of pregnancy, a lymphoid structure that transiently forms between the two layers of myometrial smooth muscle. Uterine NK cell numbers peak at mid-gestation (9–10 days) and decline in the last 5 days of pregnancy73. Uterine NK cells can either develop in situ or migrate from the peripheral blood through a CXCR4- and CXCL12-dependent mechanism (reviewed in Ref. 74) (Table 1).
Uterine NK cell phenotype. During pregnancy, the human uterine decidua is dominated by the presence of CD56hiCD16− NK cells75. In the mouse, two distinct subsets of CD3−CD122+ NK cells have been described in the uterus at mid-gestation76 (Table 2). The smaller subset expresses phenotypic markers of peripheral NK cells and might therefore represent peripheral NK cells that have recently been recruited into the uterus. The larger subset lacks NK1.1 expression and reacts with Dolichus biflorus agglutinin, which specifically identifies uterine NK cells in the mouse. Uterine NK cells express a unique repertoire of MHC class I-binding receptors, activation markers and adhesion molecules. The unique LY49 receptor repertoire of mouse uterine NK cells suggests that they develop within the uterus and that the local microenvironment may shape the LY49 receptor repertoire76. Similarly, NK cells in the human uterine mucosa have recently been characterized and found to resemble unique early developmental stages of human NK cell differentiation77.
Table 2.
Unique features of NK cells in distinct organs*
| Cell features and functions | Liver | Mucosal tissues and skin | Uterus | Pancreas | Joints | Brain |
|---|---|---|---|---|---|---|
| Receptor expression | NKG2A ↑, LY49 ↑, CD56low and CD16 ↓ (Hs/Mm)44,49,50 | Gut: RORγt+LIN−, RORγt+NKp46+NK1.1int, CD127+NKp46low, CD122intc-Kit+ and KIR− (Hs)65,67–69; LY49− (Mm)68 | CD16−CD56hi (Hs)75; NKp46+ (Hs/Mm)75; CD3−CD122+, LY49+, NK1.1− and DX5− (Mm)76,79 | KLRG1 ↑; NK cells from NOD mice express more NKC gene products than NK cells from B6.H-2g7 mice; CD25 and CD69 (Mm)6,85 | CD56hiCD16− ↑, CD94–NKG2A ↑ (Hs)87,92,95,96; RANKL+, chemokine receptors ↑ (Hs/Mm)13,99; M-CSFR+, adhesion molecules ↑ (Mm)13,97,98 | NKG2A ↑ (Mm)11,12; nicotinic acetylcholine receptors ↑ (Mm)‡ |
| Lung: LY49hi (Mm)64,66 | ||||||
| Skin: CD56hiCD16−CD158b−(Hs)63 | ||||||
| Effector molecule expression | NKp44+, NKG2C+, TRAIL ↑, perforin and granzymes ↑ (Mm)44,49,50 | Gut: IL-22+, IL-17+, IFNγ− and perforin ↓ (Hs/Mm)67 | CXCL8 and CXCL12 ↑ (Hs)82; IFNγ, VEGF and PLGF ↑ (Mm)74,78 | PD1+ and LAMP1+ (Mm)85,88 | Granzymes and IFNγ (Hs/Mm)13,87,92 | IFNγ, perforin and granzymes ↑ (Mm)11,12 |
| Lung: factors that enhance macrophage function (Mm)64 | ||||||
| Skin: IFNγ (Hs)63 | ||||||
| Cytotoxicity and proliferation | Increased early, decreased at later stages (Hs/Mm)42 | Gut: reduced or absent (Hs/Mm)67,68 | ADCC and cytotoxicity decreased (Hs)81 | Proliferation and degranulation increased early and decreased at later stages (Mm)85 | Increased (Mm)13 | Increased cytotoxicity against microglia (Mm)11,12 |
| Other functions | Crosstalk with Kupffer and NKT cells (Hs/Mm)42; promote TReg cells (Mm)51 | Control inflammation (Hs/Mm)67,84 | Regulate endometrial remodelling, angiogenesis and implantation (Hs/Mm)71; uterine NK cell-derived IFNγ modifies vascularity (Mm)78 | Reciprocal interactions with effector and TReg cells (Mm)6 | Promote formation of osteoclasts; colocalize with monocytes and promote their TNF production (Mm)13 | Condition the CNS; release cytokines; suppress myelin-specific TH17 cells (Mm)11,12 |
| Gut: TH17 cell-like functions; respond to DC-derived IL-23 and IL-1, but not IL-15; sustain lymphoid tissue integrity; promote tissue repair (Hs/Mm)67,68 | ||||||
| ADCC, antibody-dependent cell-mediated cytotoxicity; CNS, central nervous system; CXCL, CXC-chemokine ligand; DC, dendritic cell; IFNγ, interferon-γ; IL, interleukin; KLRG1, killer cell lectin-like receptor subfamily G member 1; LAMP1, lysosomal-associated membrane protein; LIN−, lineage negative; M-CSF, macrophage colony-stimulating factor; NK, natural killer; NKC, natural killer gene complex; NKG2, natural killer group 2; NKT, natural killer T; NOD, non-obese diabetic; PLGF, placental growth factor; RANKL, receptor activator of NF-κB ligand; TH17, T helper 17; TNF, tumour necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; TReg, regulatory T; VEGF, vascular endothelial growth factor. | ||||||
| *'Hs' indicates evidence found in humans, 'Mm' indicates evidence found in mice, and 'Hs/Mm' indicates evidence found in both humans and mice. | ||||||
| ‡Unpublished observations (F.-D.S.). | ||||||
Crosstalk between uterine NK cells and trophoblasts during pregnancy. The function of uterine NK cells is still not clear, but it appears that they interact with invading trophoblasts, and this interaction results in the release of factors that modify the uterine spiral arteries. This 'physiological' inflammatory process is important as it increases the blood supply to the mouse fetus71,78,79. Moreover, uterine NK cell-derived IFNγ is needed for vascular modifications to occur during pregnancy78. Although mice lacking uterine NK cells or components of the IFNγ pathway do not exhibit increased fetal loss, they show several abnormalities during pregnancy, including reduced decidual cellularity and inadequate modifications of the spiral arteries73.
In addition, the regulation of NK cell activation is crucial for normal placentation and hence a successful pregnancy. Interestingly, human immunogenetic studies have suggested that interactions between fetal HLA-C molecules and killer cell immunoglobulin-like receptors (KIRs) on uterine NK cells are important for reproductive success in humans, possibly through tuning the threshold for NK cell activation80.
Soluble factors that shape NK cell phenotype in the uterus. Transforming growth factor-β (TGFβ) produced by decidual stromal cells can reduce CD16 expression on NK cells, thereby impairing antibody-dependent cellular cytotoxicity (ADCC)81. CXCL12 is produced in the decidua, particularly by extravillous trophoblasts, and attracts peripheral NK cells towards the decidua82; on the other hand, the presence of IL-15 and stem cell factors may support NK cell development in situ74 (Table 1).
Role of uterine NK cells in pregnancy disorders. Uterine NK cell activation following interaction with invading trophoblasts has been associated with several pregnancy disorders. A seminal study described how a particular combination of fetal HLA-C molecules and maternal inhibitory NK cell receptors was associated with a predisposition for pre-eclampsia83. More recently, such studies have been extended to recurrent miscarriage and fetal growth restriction, revealing a common uterine NK cell-mediated mechanism underlying these pregnancy disorders84. Importantly, this study also showed that mothers with uterine NK cells that express activating and inhibitory NK cell receptors with similar specificities are less likely to suffer from unsuccessful pregnancy. Although data from human population genetics studies have provided interesting insights into the factors controlling interactions between uterine NK cells and trophoblasts of fetal origin, functional studies in humans are challenging. Thus, despite differences in placentation between humans and mice71, mice may provide an important model for studying the biology of uterine NK cells.
In summary, uterine NK cells can either develop in situ or migrate to the decidua after being attracted by locally produced chemokines. Their receptor repertoire suggests that their activation has to be tightly regulated to ensure normal pregnancy. Moreover, mouse and human studies suggest that crosstalk between uterine NK cells and trophoblasts contributes to vascular modifications during pregnancy.
NK cells in the pancreas
NK cell homing to the pancreas. NK cells are present not only in the pancreas of diabetes-prone non-obese diabetic (NOD) mice but also in the pancreas of mouse strains that are not susceptible to the development of diabetes85. This indicates that NK cells can be recruited to the pancreas in the absence of profound inflammation. Nevertheless, the severity of T1D in NOD mice correlates with increased numbers and early entry of NK cells into the pancreas5,6,85, suggesting a disease-promoting role for these infiltrating NK cells. Importantly, one study showed that NK cells accumulate before T cells in the islet infiltrates85. NK cells might be recruited to the pancreas by chemokines such as CXCL10 (a ligand for CXCR3 on NK cells) and CCL5 (a ligand for CCR5 on NK cells), which can be produced by multiple cell types, including pancreatic β-cells (Table 1), and their numbers are increased in the pancreas of NOD mice in an accelerated model of T1D86.
Pancreatic NK cell phenotype. Molecular, phenotypic and functional characteristics of NK cells in the pancreas and spleen have been compared between diabetes-prone NOD mice and diabetes-resistant mice5,85. Microarray studies revealed that the NK cell gene complex, which encodes a set of NK cell receptor genes, is expressed differently in diabetes-prone NOD mice versus diabetes-resistant B6.H-2g7 mice (which are congenic for the NOD MHC region). Immune phenotyping analyses further indicated that pancreatic NK cells from NOD mice share phenotypic characteristics with pancreatic NK cells from non-diabetic strains, but display increased levels of the activation markers CD69 and CD25, and of surface killer cell lectin-like receptor G1 (KLRG1), which is a marker for mature NK cells that have undergone proliferation85. Furthermore, a subset of pancreatic NK cells from young NOD mice produced IFNγ and expressed the lysosomal marker lysosome-associated membrane protein 1 (LAMP1; also known as CD107a)85, which is a marker for NK cell degranulation (Table 2).
However, proliferating and activated NK cells were detected only in the early pre-diabetic infiltrates85. During the later stages of diabetes development, most pancreatic NK cells of NOD animals became hyporesponsive, as reflected by diminished cytokine secretion and a lower capacity to degranulate in response to antibodies specific for activating receptors, and in response to activation by phorbol 12-myristate 13-acetate (PMA) and ionomycin. It was thus proposed that pancreatic NK cells from NOD mice initially mediate pro-inflammatory effector functions, but later become hyporesponsive owing to exhaustion or regulation85.
Crosstalk between NK cells and β-cells. Experiments in which splenic NK cells from NOD mice were transferred into recombination activating gene (RAG)- and common cytokine receptor γ-chain (γc)-deficient NOD mice (which lack B cells, T cells and NK cells) showed that NK cells migrate to the pancreas and upregulate CD69 and KLRG1 expression85. This suggests that the local microenvironment in the pancreas of NOD mice, in the absence of profound inflammation and adaptive immune cells, can recruit NK cells and regulate the phenotype of these cells. In addition, one study suggested that the NK cell activating receptor NKp46 can recognize ligands on β-cells that promote NK cell activation, which, in turn, might result in β-cell destruction8. Such activated NK cells might exhibit direct cytotoxicity against pancreatic β-cells and recruit other cell types, and thus have a pathogenic role during the early stages of diabetes development.
The hyporesponsive phenotype of NK cells that is observed during later stages of diabetes development might be partly caused by interactions between NK cell receptors and their ligands expressed by pancreatic β-cells. For example, it has been suggested that ligands for NKG2D — such as retinoic acid early inducible 1 (RAE1; also known as mRNA export factor) expressed by pancreatic β-cells — might downregulate the expression of NKG2D and 'turn off' NK cells to avoid excessive autoimmunity87. Furthermore, interaction of the inhibitory receptor programmed cell death protein 1 (PD1) on pancreatic NK cells with PD1 ligands on inflamed islets might dampen the response of pancreatic NK cells85,88.
Soluble factors that shape NK cell phenotype in the pancreas. The phenotype of NK cells in the pancreas might be influenced by cytokines produced by other cell types. For example, it has been suggested that cytokines derived from autoreactive T cells might contribute to the hyporesponsive phenotype of NK cells during the later stages of diabetes development in NOD mice87. There is also evidence that NK cell functions are kept in check by TReg cells, as ablation of CD4+FOXP3+ TReg cells in diabetes-prone BDC2.5 T cell receptor (TCR)-transgenic NOD mice results in rapid activation of NK cells within the insulitic lesions, before the activation of effector T cells6.
Role of pancreatic NK cells in disease. A pathogenic role of NK cells in T1D has been supported by evidence derived from several animal models5,6,7,8,86,89. Depletion of NK cells significantly inhibited diabetes development in three mouse models: BDC2.5 TCR-transgenic NOD mice treated with cytotoxic T lymphocyte antigen 4 (CTLA4)-specific antibodies5; transgenic NOD mice with pancreatic expression of suppressor of cytokine signalling 1 (SOCS1) that had been infected with coxsackievirus B4 (Ref. 89); and transgenic NOD mice with β-cell-specific expression of IFNβ86. Furthermore, blockade of the NK cell activating receptor NKG2D prevented disease in NOD mice7, although the contribution of other NKG2D-expressing cells (including CD8+ T cells) could not be ruled out in this model.
In addition to their pathogenic roles in autoimmune diabetes, NK cells can, paradoxically, exhibit protective functions in β-cell autoimmunity. NK cells have a crucial role in mediating the capacity of complete Freund's adjuvant (CFA) to protect NOD mice from diabetes90. NK cells also have an important role in islet allograft tolerance in C57BL/6 mice induced by blocking interactions between CD40 and CD154 or between lymphocyte function-associated antigen 1 (LFA1; also known as αLβ2 integrin) and intercellular adhesion molecule (ICAM)91. It is likely, however, that these ameliorating effects of NK cells on autoimmunity and allograft rejection are mediated by peripheral rather than pancreatic NK cells, possibly through them interacting directly with DCs or pathogenic T cells.
In summary, few NK cells home to the healthy pancreas, and the numbers of pancreatic NK cells are substantially increased in diabetes-prone and diabetic mice. The local microenvironment of the pre-diabetic pancreas seems to induce NK cell proliferation and activation. At later disease stages, crosstalk between pancreatic NK cells and β-cells or other immune cells leads to NK cell hyporesponsiveness. Despite their early pathogenic role in diabetes, NK cells have also been implicated in CFA-mediated diabetes protection and in islet allograft tolerance.
NK cells in the joints
NK cell homing to the joints. Whereas few NK cells are present in healthy joints, these cells are abundant in the inflamed joints of patients with rheumatoid arthritis92 and in mice with collagen-induced arthritis13. NK cells can be detected in the inflamed synovial tissue at any stage of the disease, and they constitute up to 20% of all lymphocytes in synovial fluid of patients with established rheumatoid arthritis92. These cells express increased levels of the chemokine receptor CCR5 and, to a lesser extent, CCR2 (Ref. 93). Chemokine ligands for these receptors have been detected in the synovial fluid of patients with rheumatoid arthritis94 (Table 1).
NK cell phenotype in the joints. Among synovial fluid NK cells in patients with rheumatoid arthritis, cells of the CD56hiCD16− phenotype predominate92,95,96, suggesting that this cell population is selectively recruited to the inflamed synovium and/or expands there. These cells also express high levels of the inhibitory receptor NKG2A, produce granzymes and exhibit enhanced cytotoxicity compared with systemic NK cells87,92 (Table 2). Uniquely, NK cells in the joints of mice with collagen-induced arthritis express receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)97,98, a feature that is shared with NK cells from patients with rheumatoid arthritis99.
Crosstalk between NK cells and other cells in inflamed joints. The molecular features of synovial fluid NK cells and their interaction with other cells in the inflamed joint have been investigated in animal models. NK cells in the inflamed joint that express RANKL and M-CSF are frequently juxtaposed with monocytes in the synovium of inflamed joints13 (Table 2). The reciprocal interactions between these two cell types can trigger the formation of osteoclasts in a process that is dependent on RANKL and M-CSF13.
Soluble factors that shape NK cell phenotype in joints. Cytokines, such as IL-12, IL-15 and IL-18, that are produced by myeloid cells in the inflamed joint can stimulate IFNγ production by NK cells95. These factors, in particular IL-15, might also contribute to the induction of RANKL and M-CSF in synovial NK cells13.
Role of joint NK cells in disease. Depletion of NK cells from mice before the induction of collagen-induced arthritis reduces the severity of subsequent arthritis and almost completely prevents bone erosion13. Paradoxically, however, NK cells were shown to have a crucial role in protection against inflammatory arthritis mediated by unmethylated CpG oligodeoxynucleotides following transfer of serum from K/BxN mice into wild-type mice14. This protection required interaction of NK cells with DCs in lymphoid organs, resulting in reduced neutrophil recruitment to the joints. These observations suggest opposite functions of NK cells in lymph nodes versus inflamed joints during rheumatoid arthritis. The current available information is not sufficient to provide a clear linkage between the divergent phenotypes and functions of NK cells in lymphoid organs and joints. Differences in the triggering antigens (that is, type II collagen versus glucose-6-phosphate isomerase) in different models of arthritis and their roles in inducing NK cells directly or indirectly invite further investigation.
Thus, NK cells are recruited to the inflamed joints, where they exhibit an activated phenotype and promote osteoclast differentiation and inflammation. By contrast, lymph node NK cells have been suggested to play a crucial part in CpG-induced protection against inflammatory arthritis in a mouse disease model.
NK cells in the CNS
Homing of NK cells to the CNS. In the CNS, the brain and spinal cord are shielded from the circulating blood by the blood–brain barrier and the epithelial blood–cerebrospinal fluid barrier, respectively, which are formed by the tight junctions of endothelial cells and prevent the influx of immune cells under normal physiological conditions. This view was recently challenged by the finding that activated T cells can breach the blood–brain barrier and the epithelial blood–cerebrospinal fluid barrier to perform immune surveillance functions in the CNS100. It is conceivable that peripherally activated lymphocytes, including NK cells, might also be able to penetrate the blood–brain barrier. However, data on the presence of NK cells in the human brain and spinal cord are sparse. Nevertheless, NK cells can be detected in mouse CNS tissues during a variety of infections, including infection by murine coronavirus101, Semliki Forest virus102 and L. monocytogenes103. NK cells are also recruited to the CNS in glioma-bearing mice; 1 week after tumour implantation, NK cells constitute approximately 50% of all leukocytes in the CNS, and after this peak their frequency gradually declines104. NK cells might be recruited to the CNS by chemokines such as CX3CL1 produced by neurons and CCL2 and CXCL10 produced by microglia, astrocytes or infiltrating inflammatory cells (Table 1).
NK cell phenotype in the CNS. The most intriguing data on the role for NK cells in CNS inflammation are derived from studies of experimental autoimmune encephalomyelitis (EAE). In this model, NK cells become fully activated and proliferate in the CNS even before the arrival of T cells105. Analysis of NK cells in the CNS of mice with EAE suggested that, compared with their peripheral counterparts, CNS-infiltrating NK cells upregulate the inhibitory receptor NKG2A and produce large amounts of CCL2, a chemoattractant for microglia11,12.
Crosstalk between NK cells and CNS-specific cell types. The CNS is distinguished from other organs with regard to the genesis and progression of inflammatory responses in that it harbours a unique spectrum of antigen-presenting cells (APCs; that is, astrocytes and microglia) and neuroantigens, and accommodates intimate interactions between the immune and nervous systems (Fig. 2). So, after homing to the inflamed CNS, NK cells become receptive to an array of cellular components that they have not encountered in the periphery. These include astrocytes, microglia and neurons106,107,108,109, which release numerous soluble factors with diversified and perhaps coordinated effects on NK cells (Fig. 2).
Figure 2. The brain alters NK cell phenotypes and functions.
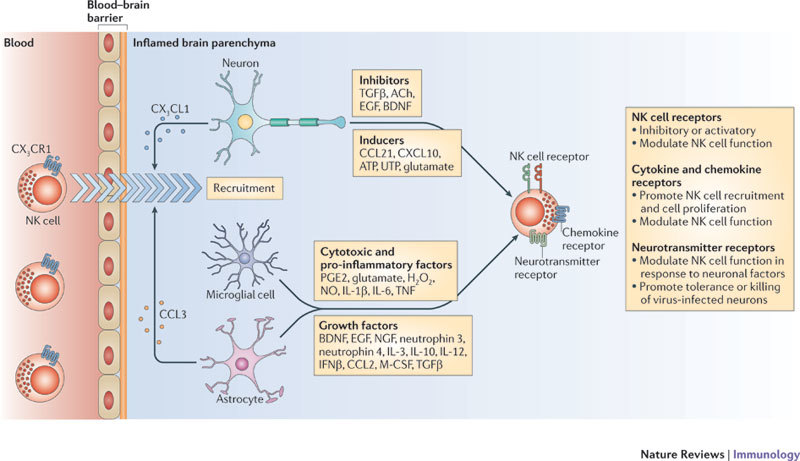
The entry of lymphocytes into the central nervous system (CNS) is normally shielded by the blood–brain barrier, which becomes compromised in several pathological circumstances. Natural killer (NK) cells are recruited to the inflamed brain by CX3C-chemokine ligand 1 (CX3CL1) produced by neurons. Brain-specific cell types and neurotransmitters alter the features of NK cells that migrate from the periphery. An array of cytokines, growth factors and neurotransmitters produced by microglial cells, astrocytes and neurons can influence NK cell activation and proliferation. The chemokine CC-chemokine ligand 3 (CCL3) attracts NK cells to the site of inflammatory foci. After acquiring new features, brain-resident NK cells lose tolerance to microglial cells, suppress pathogenic, myelin-reactive T helper cells and significantly attenuate the intensity of local inflammatory and autoimmune responses. NK cells also kill neurons infected by herpes simplex virus. In addition, mediators derived from neurons, microglial cells and astrocytes and multidirectional cellular interactions may influence NK cell tolerance and function. The combined effects of soluble factors and cellular interactions, as well as the timing of the immune response, may dictate NK cell activity in the CNS. ACh, acetylcholine; BDNF, brain-derived neurotrophic factor; CXCL10, CXC-chemokine ligand 10; EGF, epidermal growth factor; IFNβ, interferon-β; IL, interleukin; M-CSF, macrophage colony-stimulating factor; NGF, nerve growth factor; NO, nitric oxide; PGE2, prostaglandin E2; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor.
The finding that NK cells in the CNS control the magnitude of an inflammatory response in the EAE model11,12 suggests that, after homing to the CNS, NK cells exert specific functions that affect the outcome of inflammation. In particular, it has been suggested that NK cells in the CNS can directly lyse microglia11,12. Microglia are macrophage-like APCs that are capable of priming myelin-reactive T cells, and they exert direct effects on neuronal or glial cells depending on the timing of the inflammatory process and the activation status of these cells105,106,107,108,109,110,111. Microglia serve as sentinels for ongoing immune responses in the periphery that have the potential to spread to the CNS. In comparison with the behaviour of other types of APC in the CNS during brain inflammation, activated microglia appear to become targets of peripherally activated NK cells that enter the CNS11. Such interactions between NK cells and microglia are likely to be facilitated by the recruitment of microglia through CCL2 secretion by NK cells. As activated microglia have a crucial role in initiating and propagating pathogenic T cell responses during EAE, their elimination results in reduced numbers of myelin-reactive T helper 17 (TH17) cells in the CNS11. Although the exact mechanisms remain unclear, susceptibility of activated microglia to NK cell lysis may occur through the loss of MHC class I expression on these cells11,12 (Fig. 2). These findings in mice are in apparent contrast with a recent study suggesting that human NK cells can lyse resting microglia but spare activated microglia in an in vitro culture system105. However, it remains unclear which NK cell subset executed this killing activity and how microglia were maintained within a resting state during this experiment.
Soluble factors that shape the NK cell phenotype in the CNS. Under pathophysiological conditions, an array of soluble factors is produced by microglia, astrocytes and neuronal cells (Fig. 2). Based on their effect on NK cells, these mediators can be categorized as inhibitory (such as TGFβ, IL-10 and acetylcholine) or stimulatory (such as ATP, UTP, glutamate, prostaglandin E2, H2O2, nitric oxide, IL-1β, IL-6, TNF, epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3, neurotrophin 4, IL-3, IL-12 and M-CSF). So, the sequence of soluble signals received by NK cells, the physical location of the cellular source of these factors and the balance between inhibitory and stimulatory signals that NK cells receive may all influence the functional status of NK cells in the inflamed CNS.
Role of CNS-specific NK cells in disease. During EAE in C57BL/6 mice, depletion of NK cells exacerbates neurological deficits9,11. Similarly, CX3CR1-deficient mice, in which homing of NK cells to the brain is severely impaired, develop fatal inflammatory lesions following EAE induction10. As the numbers of peripheral NK cells in these mice are comparable with those of wild-type mice, these results imply that a CNS-specific deficit in NK cells leads to aggressive inflammation and autoimmunity in the CNS (Fig. 2). Thus, there is strong evidence that CNS-specific NK cells control CNS inflammation during EAE in mice.
These findings might be relevant to the mode of action of daclizumab, a humanized antibody specific for the IL-2 receptor α-chain (CD25). Daclizumab, alone or in combination with IFN preparations, consistently reduces CNS lesions in patients with multiple sclerosis112,113,114,115. One of the immunological effects of daclizumab is that it can expand CD56hi NK cell populations in treated patients107. In a human–mouse chimaera model, it has been demonstrated that EAE can be ameliorated by transfusion of human NK cells11. As administration of NK cells directly into the brain appeared to achieve enhanced clinical benefits compared with systemically administered cells11, these results suggest that NK cells within the brain may have an anti-inflammatory role. Furthermore, there is some evidence to suggest that other drugs that are currently used for the treatment of multiple sclerosis, such as IFN preparations and glatiramer acetate (a random polymer of the four amino acids found in myelin basic protein; also known as copolymer 1 or Copaxone (Teva Pharmaceuticals)), may achieve their clinical efficacy partially by inducing NK cells116,117 via a mechanism similar to that of daclizumab.
In addition to their protective role in autoimmune disease, NK cells control CNS infections. In detail, NK cells provide protection against encephalitis and demyelination induced by Theiler's murine encephalitis virus118, reduce viral replication and disease following intracerebral infection by murine coronavirus101, and exacerbate immunopathology and accelerate mortality during Semliki Forest virus infection102.
In summary, there are no data on the presence of NK cells in the steady-state CNS, but NK cells have been shown to migrate to the inflamed CNS. Although the exact cellular and molecular interactions that shape NK cell phenotype and function in the CNS still need to be determined, it appears that activated NK cells have a protective role in the brain under the circumstances of ongoing inflammation, as they eliminate viral infection and inhibit the activation of autoimmune T cells through the killing of activated microglia.
Linking organ-specific NK cell features and disease
The major functions of NK cells in the liver, mucosal tissues, skin, pancreas and perhaps the CNS are to amplify the local immune response and combat infectious agents. In doing so, NK cells may kill infected or transformed cells. When this process becomes dysregulated, damage to neighbouring cells and autoimmune disease might ensue. Determinants for these divergent roles of NK cells, as exemplified here for a number of different organs, include the timing of immune responses, the different pathogens targeted by the NK cells and the NK cell-orchestrated immune responses. Furthermore, the studies discussed here raise the possibility that organ-specific factors shape NK cell phenotype and contribute to the organ-specific functions of these cells. The organ-specific features of NK cells appear to be dictated by a number of factors that are intrinsic to a particular organ, including its anatomical structure, accessibility to peripheral immune cells, and the unique microenvironment of cells and soluble factors within the organ. Additional determinants include inciting pathogens or autoantigens that induce inflammation.
Concluding remarks
We have discussed here the organ-specific features of NK cells and given examples of how these cells may serve to maintain normal health and contribute to the development and control of inflammation in various organ-specific diseases. There are many outstanding questions and future challenges in this area (Box 2). Progress will be facilitated by novel approaches such as two-photon microscopy, which has been used to record the trafficking of several types of lymphocytes to reveal the cellular interactions within solid organs in physiological and pathological situations119,120,121,122. The application of this and other techniques to studies of NK cells in solid organs should reveal valuable information on the physical localization of NK cells within specific anatomical structures, as well as interactions with resident cellular components. Imaging studies in live animals may yield insight into the behaviour of NK cells during the course of organ-specific inflammation.
High-throughput DNA and protein arrays, aided by laser capture microscopy to sample highly purified NK cells from localized areas of pathology, may aid in defining molecular signatures of NK cells within the inflamed organs. Improved access to human organs will also be paramount. Furthermore, improved 'humanized' mouse models that are capable of maintaining transplanted, functional human immune cells, including NK cells, should aid our understanding of human NK cells in mouse organs. Many mouse cytokine, chemokine and cellular (for example, Notch-induced) signalling pathways can trigger the expression of human NK cell receptors123. Improving our understanding of organ-specific NK cell functions through the use of these techniques will allow us to take advantage of organ-intrinsic factors for boosting specific NK cell functions to control infection or tumours, or to silence excessive effector functions to protect against organ-specific autoimmunity.
During evolution, distinct organs might have evolved different ways to recruit and influence the effector functions of NK cells. Once we understand these mechanisms, the next challenge will be to exploit this information to harness NK cells for combating infectious agents and tumours and for avoiding autoimmune and inflammatory diseases.
Box 1 | Innate lymphoid cells.
Recent studies have identified several subsets of innate lymphoid cells (ILCs) that are developmentally related and evolutionarily conserved124. This family of cells includes natural killer (NK) cells, lymphoid tissue-inducer (LTi) cells (which produce interleukin-17 (IL-17) and/or IL-22), natural helper cells and nuocytes (which produce IL-5 and IL-13), and cells with characteristics of both NK cells and LTi cells. These latter cells, which have been referred to as NK-like cells or ILC22 cells, share with NK cells the expression of the natural cytotoxicity receptor NKp46 (but not NK1.1) and with LTi cells the expression of retinoic acid receptor-related orphan receptor-γt (RORγt). However, although NK cells generally produce interferon-γ and are cytotoxic, NKp46+RORγt+ ILCs produce IL-22 and are non-cytotoxic. Several subsets of ILCs are enriched in mucosal tissues, particularly in the gut.
NK cells have a crucial role in antiviral immunity; LTi cells are important for the development of lymph nodes during embryogenesis, tissue remodelling, homeostasis of epithelia and host defence; and natural helper cells and nuocytes have been implicated in immunity to extracellular parasites and in the development of asthma. However, the functions of NKp46+RORγt+ ILCs remain unclear.
Box 2 | Outstanding questions on the organ-specific features of NK cells.
What determines natural killer (NK) cell recruitment to different organs, and how does the phenotype of NK cells change following recruitment to specific organs under different experimental conditions?
Under what conditions do local NK cells become activated in different organs?
Are specific features of NK cells within individual organs the cause or the consequence of inflammatory responses within that organ?
How is the phenotype of NK cells linked to their particular functions within the inflamed organs?
How can organ-intrinsic factors that boost or silence certain NK cell functions be harnessed to control infection or reduce inflammation?
Do inflamed organs retain the recruited NK cells in an activated state, or do they allow for NK cell circulation back to other tissues and/or restoration of a resting NK cell phenotype?
How are the local effects of NK cells affected by tissue-specific MHC class I expression? In addition to altering effector responses, how do the different levels of tissue MHC class I expression affect the functionality of local NK cells through education or licensing processes? How are these processes affected by local inflammatory responses and the influx of additional inflammatory cells that may alter tissue MHC class I expression levels?
Acknowledgements
We thank present and past members of our laboratories for their contributions towards the understanding of NK cells in health and disease. We also thank Z. Tian, N. Björkström and Z. Tu for fruitful discussions and advice, and R. Liu, Y. Gan and S. Shi for assistance in preparing the manuscript. The authors' clinical and laboratory programmes are supported by the National Science Foundation of China (31170864 to F.-D.S.), the US Muscular Dystrophy Association (159281 to F.-D.S.), the Arizona Biomedical Research Commission (09-085 to F.-D.S.), the US National Institutes of Health (RO1AI083294 to F.-D.S.; RO1AR53295 to A.L.C.; and RO1AI070305, R21AI072417, RO1DK081536 and RO1HL089667 to L.V.K.), the Swedish Research Council (H.-G.L.), the Swedish Cancer Society (H.-G.L.), the Tobias Foundation (H.-G.L.), the Karolinska Institutet (H.-G.L.), and the Stockholm County Council (H.-G.L.). We apologize to those colleagues whose work has not been cited owing to space constraints.
Glossary
- Natural killer cells
(NK cells). Cytotoxic lymphocytes of the innate immune system. These cells have an important role in host defence against many intracellular pathogens and some tumours, regulate the development of adaptive immunity, and control the homeostasis of other immune cells. Human NK cells express the surface marker CD56, and mouse NK cells express the surface markers DX5 (CD49b) and, in some mouse strains (for example, C57BL/6), NK1.1 (CD161).
- Type 1 diabetes
A form of diabetes caused by autoimmune destruction of insulin-producing pancreatic β-cells. Autoimmune destruction is predominantly mediated by CD8+ T cells, but natural killer cells may affect disease outcome.
- Rheumatoid arthritis
A systemic inflammatory disorder that may affect many tissues and organs, but principally attacks synovial joints. The disease process produces an inflammatory response of the synovium (synovitis), secondary to hyperplasia of synovial cells, excess synovial fluid and the development of pannus in the synovium. The pathology of the disease process often leads to the destruction of articular cartilage and ankylosis of the joints.
- Kupffer cell
Also known as Browicz–Kupffer cells, Kupffer cells are specialized macrophages that line the walls of the liver sinusoids.
- Natural killer T cells
(NKT cells). A subset of T cells that are specific for lipid or glycolipid antigens bound to the MHC class I-related protein CD1d. Most NKT cells, which are referred to as type I or invariant NKT (iNKT) cells, express an invariant T cell receptor-α chain (Vα14–Jα18 in mice or Vα24–Jα18 in humans) together with various natural killer cell surface markers, such as NK1.1. NKT cells have a regulatory role during an immune response, bridging the innate and adaptive immune systems. iNKT cells can be most easily identified with multimeric CD1d molecules loaded with iNKT cell agonists, such as α-galactosylceramide.
- Trophoblast layer
A layer formed by trophoblast cells that arise from the trophectoderm that surrounds the blastocyst. The trophoblast layer attaches the embryo to the uterus and forms a part of the placenta.
- Collagen-induced arthritis
A model for human rheumatoid arthritis that is induced in susceptible strains of mice or rats by immunization with autologous or heterologous type II collagen in adjuvant.
- K/BxN mice
K/BxN mice express a transgene-encoded T cell receptor that is specific for the ubiquitously expressed enzyme glucose-6-phosphate isomerase, which is presented by the MHC class II molecule H2-Ag7. These mice spontaneously develop joint inflammation with features of human rheumatoid arthritis. Interestingly, transfer of serum from arthritic animals into healthy non-transgenic animals induces arthritis in a highly reproducible and controlled manner.
- Microglia
Non-neuronal cells of the central nervous system with phagocytic properties. They are haematopoietic cells and are involved in inflammatory and immune responses in the central nervous system.
- Experimental autoimmune encephalomyelitis
(EAE). An experimental model for human multiple sclerosis that can be induced in susceptible animals by immunization with neuroantigens and adjuvant.
- Multiple sclerosis
An inflammatory and demyelinating disease of the central nervous system that is primarily diagnosed in young adults. Clinical presentations are heterogeneous depending on the severity and location of central nervous system lesions.
Biographies
Fu-Dong Shi received his clinical training in neurology at Harbin Medical University and Peking Union Hospital in China and at the Barrow Neurological Institute (BNI), St. Joseph's Medical Center, Phoenix, Arizona, USA, and received his Ph.D. from the Karolinska Institutet, Stockholm, Sweden. He completed his postdoctoral research at The Scripps Research Institute, La Jolla, California, USA. In 2003, he joined the faculty of the Division of Neurology at the BNI, where he served as associate professor of neurology and professor of the BNI–ASU (Arizona State University) Neuroscience Program. His research focuses on natural killer (NK) cells, innate immunity and immune therapy in neurological disorders. As the recipient of a 'ChangJiang Scholar Award' and the '1000 Talent Plan' of China, he recently established a Center for Neuroinflammation in Tianjin, China.
Hans-Gustaf Ljunggren received his M.D. and Ph.D. from the Karolinska Institutet. He conducted postdoctoral research at the Massachusetts Institute of Technology, Cambridge, Massachusetts, USA, and has been a full professor at the Karolinska Institutet Department of Medicine since 2001. His research has focused on NK cells, where he has made original discoveries with regards to their molecular specificity. His research interests include studies of NK cell development and differentiation, and molecular specificity and recognition of virus-infected and transformed cells. He is also interested in using these cells in therapeutic settings.
Antonio La Cava received his M.D. and Ph.D. from the Federico II University of Naples, Italy, and received postdoctoral training at the University of California, San Diego, USA, and at The Scripps Research Institute. He is currently Professor of Medicine at the Department of Medicine of the University of California, Los Angeles (UCLA), USA, and Director of the FOCIS (Federation of Clinical Immunology Societies) Center of Excellence at UCLA. His research interests include the pathogenesis of autoimmune disease and the study of the mechanisms of immune peripheral tolerance.
Luc Van Kaer received a Ph.D. from Ghent University, Ghent, Belgium, for his work on bacteriophages. He carried out postdoctoral research with Susumu Tonegawa at the Massachusetts Institute of Technology, where he studied γδ T cells, non-classical MHC molecules and antigen presentation. In 1993, he joined the faculty of Vanderbilt University Medical Center, Nashville, Tennessee, USA, where he is currently Professor of Pathology, Microbiology and Immunology. His research programme is focused on the immunological functions of classical and non-classical MHC molecules, including the role of these molecules in regulating the functions of NK cells and NKT cells.
Related links
FURTHER INFORMATION
Competing interests
The authors declare no competing financial interests.
References
- 1.Shi F-D, Ransohoff RM. Natural Killer Cells. 2010. pp. 373–383. [Google Scholar]
- 2.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nature Rev. Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 3.French AR, Yokoyama WM. Natural killer cells and autoimmunity. Arthritis Res. Ther. 2004;6:8–14. doi: 10.1186/ar1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flodstrom-Tullberg M, Bryceson YT, Shi FD, Hoglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr. Opin. Immunol. 2009;21:634–640. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Poirot L, Benoist C, Mathis D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc. Natl Acad. Sci. USA. 2004;101:8102–8107. doi: 10.1073/pnas.0402065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogasawara K, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Gur C, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nature Immunol. 2010;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 11.Hao J, et al. Central nervous system (CNS)-resident natural killer cells suppresses Th17 responses and CNS autoimmune pathology. J. Exp. Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao J, et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 2011;69:721–734. doi: 10.1002/ana.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soderstrom K, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc. Natl Acad. Sci. USA. 2010;107:13028–13033. doi: 10.1073/pnas.1000546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HJ, et al. Inflammatory arthritis can be reined in by CpG-induced DC–NK cell cross talk. J. Exp. Med. 2007;204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nature Rev. Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire C, et al. The trafficking of natural killer cells. Immunol. Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thapa M, Kuziel WA, Carr DJ. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 2007;81:3704–3713. doi: 10.1128/JVI.02626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang D, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J. Clin. Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald O, et al. IFN-γ acts on T cells to induce NK cell mobilization and accumulation in target organs. J. Immunol. 2006;176:4716–4729. doi: 10.4049/jimmunol.176.8.4716. [DOI] [PubMed] [Google Scholar]
- 20.Widney DP, et al. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect. Immun. 2005;73:485–493. doi: 10.1128/IAI.73.1.485-493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-γ production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 22.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J. Clin. Invest. 2003;112:1862–1870. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiles LN, Hardison JL, Schaumburg CS, Whitman LM, Lane TE. T cell antiviral effector function is not dependent on CXCL10 following murine coronavirus infection. J. Immunol. 2006;177:8372–8380. doi: 10.4049/jimmunol.177.12.8372. [DOI] [PubMed] [Google Scholar]
- 25.Yu YR, et al. Defective antitumor responses in CX3CR1-deficient mice. Int. J. Cancer. 2007;121:316–322. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 26.Hamann I, et al. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011;133:62–73. doi: 10.1111/j.1365-2567.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-α/β-induced inflammatory responses and antiviral defense in liver. J. Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 28.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J. Exp. Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nature Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nature Rev. Immunol. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 32.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnani C, et al. CD56brightCD16− killer Ig-like receptor− NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 34.Chan A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J. Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Bjorkstrom NK, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 36.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J. Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferlazzo G, et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur. J. Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, et al. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J. Immunol. 2001;166:1590–1600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 39.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 40.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanier LL. Evolutionary struggles between NK cells and viruses. Nature Rev. Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krueger PD, Lassen MG, Qiao H, Hahn YS. Regulation of NK cell repertoire and function in the liver. Crit. Rev. Immunol. 2011;31:43–52. doi: 10.1615/CritRevImmunol.v31.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntyre KW, Welsh RM. Accumulation of natural killer and cytotoxic T large granular lymphocytes in the liver during virus infection. J. Exp. Med. 1986;164:1667–1681. doi: 10.1084/jem.164.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49− liver NK cells in a functionally hyporesponsive state. J. Immunol. 2010;184:2693–2701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc. Natl Acad. Sci. USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol. Rev. 2010;235:297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjorkstrom NK, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nature Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 50.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jinushi M, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang W, Sun R, Zhou R, Wei H, Tian Z. TLR-9 activation aggravates concanavalin A-induced hepatitis via promoting accumulation and activation of liver CD4+ NKT cells. J. Immunol. 2009;182:3768–3774. doi: 10.4049/jimmunol.0800973. [DOI] [PubMed] [Google Scholar]
- 53.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy S, et al. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 55.Knolle P, et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 56.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehal W, Imaeda A. Cell death and fibrogenesis. Semin. Liver Dis. 2010;30:226–231. doi: 10.1055/s-0030-1255352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jinushi M, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J. Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 59.Nattermann J, et al. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stegmann KA, et al. Interferon-α-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 62.Ebert LM, Meuter S, Moser B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J. Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 63.Ottaviani C, et al. CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur. J. Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 64.Small CL, et al. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 2008;180:5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 65.Reynders A, et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt− lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halim TYF, Fumio T. NK cell development from a novel progenitor found in the murine lung. J. Immunol. 2009;182:138.10. [Google Scholar]
- 67.Vonarbourg C, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 69.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary natural killer cells and the potential role of IL-22 during primary influenza virus infection. J. Virol. 2010;84:7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson MS, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nature Rev. Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 72.Colucci F, Boulenouar S, Kieckbusch J, Moffett A. How does variability of immune system genes affect placentation? Placenta. 2011;32:39–45. doi: 10.1016/j.placenta.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol. Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 74.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nature Rev. Immunol. 2007;9:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 75.Moffett-King A. Natural killer cells and pregnancy. Nature Rev. Immunol. 2002;9:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 76.Yadi H, et al. Unique receptor repertoire in mouse uterine NK cells. J. Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 77.Male V, et al. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 2010;185:3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashkar AA, Di Santo JP, Croy BA. Interferon γ contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nature Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 80.Sharkey AM, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- 81.Keskin DB, et al. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc. Natl Acad. Sci. USA. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanna J, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 83.Hiby SE, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hiby SE, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brauner H, et al. Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J. Immunol. 2010;184:2272–2280. doi: 10.4049/jimmunol.0804358. [DOI] [PubMed] [Google Scholar]
- 86.Alba A, et al. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-β. Clin. Exp. Immunol. 2008;151:467–475. doi: 10.1111/j.1365-2249.2007.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu R, et al. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J. Immunol. 2006;176:5247–5254. doi: 10.4049/jimmunol.176.9.5247. [DOI] [PubMed] [Google Scholar]
- 88.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flodstrom M, et al. Target cell defense prevents the development of diabetes after viral infection. Nature Immunol. 2002;3:373–382. doi: 10.1038/ni771. [DOI] [PubMed] [Google Scholar]
- 90.Lee IF, Qin H, Trudeau J, Dutz J, Tan R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J. Immunol. 2004;172:937–942. doi: 10.4049/jimmunol.172.2.937. [DOI] [PubMed] [Google Scholar]
- 91.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nature Med. 2005;11:1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 92.Tak PP, et al. Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994;37:1735–1743. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- 93.Mack M, et al. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 1999;42:981–988. doi: 10.1002/1529-0131(199905)42:5<981::AID-ANR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 94.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275:4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 95.Dalbeth N, et al. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J. Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 96.Zhang AL, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–2493. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/S8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 98.Lubberts E, et al. Increase in expression of receptor activator of nuclear factor κB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis. Arthritis Rheum. 2002;46:3055–3064. doi: 10.1002/art.10607. [DOI] [PubMed] [Google Scholar]
- 99.Geusens PP, et al. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006;54:1772–1777. doi: 10.1002/art.21896. [DOI] [PubMed] [Google Scholar]
- 100.Redzic Z. Molecular biology of the blood–brain and the blood–cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trifilo MJ, et al. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J. Virol. 2004;78:585–594. doi: 10.1128/JVI.78.2.585-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alsharifi M, et al. NK cell-mediated immunopathology during an acute viral infection of the CNS. Eur. J. Immunol. 2006;36:887–896. doi: 10.1002/eji.200535342. [DOI] [PubMed] [Google Scholar]
- 103.Hayashi T, et al. Critical roles of NK and CD8+ T cells in central nervous system listeriosis. J. Immunol. 2009;182:6360–6368. doi: 10.4049/jimmunol.0803798. [DOI] [PubMed] [Google Scholar]
- 104.Alizadeh D, et al. Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy. Clin. Cancer Res. 2010;16:3399–3408. doi: 10.1158/1078-0432.CCR-09-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lunemann A, et al. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J. Immunol. 2008;181:6170–6177. doi: 10.4049/jimmunol.181.9.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ponomarev ED, et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 107.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'on' and 'off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 109.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 111.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 112.Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann. Neurol. 2004;56:864–867. doi: 10.1002/ana.20287. [DOI] [PubMed] [Google Scholar]
- 113.Rose JW, et al. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- 114.Bielekova B, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bielekova B, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch. Neurol. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vandenbark AA, et al. Interferon-β-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 117.Sand KL, Knudsen E, Rolin J, Al-Falahi Y, Maghazachi AA. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell. Mol. Life Sci. 2009;66:1446–1456. doi: 10.1007/s00018-009-8726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paya CV, Patick AK, Leibson PJ, Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J. Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- 119.Bartholomaus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 120.Fan Z, et al. In vivo tracking of 'color-coded' effector, natural and induced regulatory T cells in the allograft response. Nature Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siffrin V, et al. In vivo imaging of partially reversible Th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 122.Bajenoff M, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage in humanized mice. Proc. Natl Acad. Sci. USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 125.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 126.Tu Z, et al. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]


