Abstract
Crystallography generally only provides static structural information. This can render it an ineffective technique for probing dynamic solution state processes. A crystal of HoDOTMA affords unique structures that effectively represent that of a lanthanide tetra-acetate chelate mid-way through the water exchange process.
Using crystallographic data to probe solution state structures can be frustrating. The investigator must always be on their guard: the static crystal phase frequently does not provide representation of the dynamic solution phase. Even in well-defined systems such as the chelates of DOTA‡ the risks of being misled by the crystal phase are ever present.1 But every once in a while a structure comes along that shines new light on a process occurring in the solution from which it grew. One such structure is that of HoDOTMA‡, presented herein.
Holmium occupies a special place in the lanthanide series. The coordination chemistry of DOTA is characterized by two geometries: the square antiprism (SAP) and the twisted square antiprism (TSAP).2, 3 In solution the two geometries interconvert by either rotation of the pendant arms (Δ ↔ Λ) or ring inversion (δδδδ ↔ λλλλ).2, 3 The earlier Ln3+ ions exhibit a marked preference for the TSAP geometry, which has a larger coordination cage that is better able to accommodate larger ions.4 As the Ln3+ ionic radius contracts across the series the more compact SAP isomer becomes increasingly favoured until at Ho3+ almost no TSAP isomer is observed. Curiously, as the Ln3+ ions contract further the TSAP isomer becomes increasingly more favoured again. The results of molecular modelling studies have shown that the energy of the SAP geometry continues to decrease all the way through to Lu3+.5 The observed reversal in the trend has generally been attributed to a change in the hydration state of the TSAP geometry:4 monohydrated before Ho3+ and dehydrated after. Recent results have suggested an alternative explanation for this observation and Ho3+, which lies at the cross over point, is ideally placed to provide insight.
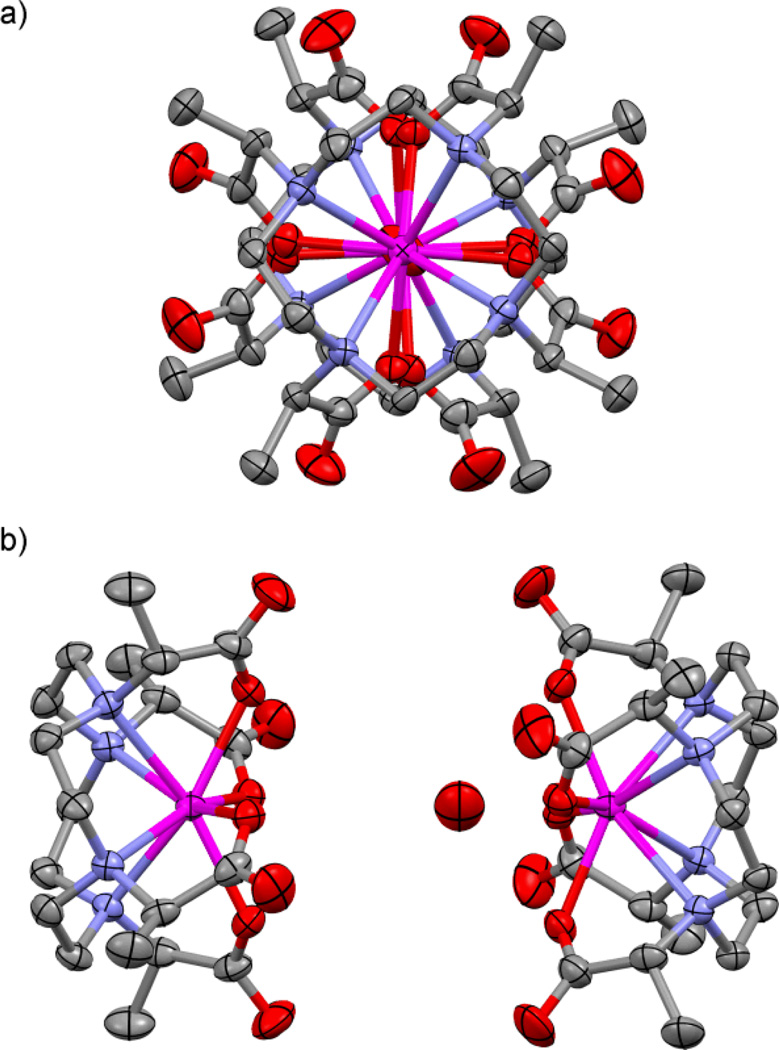
In the crystal HoDOTA adopts a SAP geometry6 and therefore provides no real insight into the chemistry of a TSAP chelate at this point in the series. In contrast, Ln3+ chelates of DOTMA exhibit a marked preference for the TSAP coordination geometry across the series, both in solution7 and in the crystal (Fig 1). HoDOTMA was prepared using an identical procedure to that used to prepare GdDOTMA.7 Large, pale pink X-ray quality crystals were obtained by slow diffusion of ethanol into a concentrated aqueous solution of NaHoDOTMA. In the unit cell of the crystal two HoDOTMA chelates are found in a ‘coordination dimer’ separated by a layer of disordered Na+ ions and water molecules. Each chelate adopts a TSAP Λ(λλλλ) coordination geometry. Of the two chelates, one is indisputably eight coordinate: without coordinated water. Although a water molecule lies above the apical coordination site in which water is often found, this water lies in excess of 4.7 Å from the metal ion. In solution this distance would put this water molecule in the second, rather than the inner, hydration sphere.8
Fig. 1.
50% Thermal ellipsoid plots, a) top view; b) side view; of the ‘coordination dimer’ in the unit cell of the crystal of (NaHoDOTMA)2·NaCl·(H2O)16. One HoDOTMA anion should be considered dehydrated and the other ‘semi’-hydrated. Hydrogen atoms, counter-ions and water molecules (except those close to apical positions) are omitted for clarity.
Hydration of the other chelate in the unit cell is somewhat more ambiguous; a water molecule is to be found closer to the apical coordination site but at a much greater distance (2.66 Å) than would normally be considered ‘bound’9 and is therefore not shown bonded (Fig 1). Although this water molecule is not formally bound to Ho3+, it is close enough that there can be no doubt that it interacts strongly with the metal ion. The hydration state (q) and metal-water distance (rHoO) are intrinsically linked parameters. Parker and co-workers realized that the value of q determined by Horrocks’ method depends upon the negative sixth power of the distance from the metal ion to the quenching OH oscillator.10 More distant water molecules have a weaker quenching effect and give rise to lower apparent q. On the basis of this relationship the value of q determined for these two chelates in a Horrocks type experiment would be in the region of 0.6.
Lukeš and Herman published an intriguing structure of an Y3+ chelate that crystalized as both mono- and dehydrated TSAP geometries.11 These structures led to the hypothesis that the metal ion moves down, towards the N4 coordination plane as the water molecule leaves and up towards the O4 plane as it returns. This leads to the ligand contracting and relaxing in a ‘breathing’ motion through the water exchange cycle. The dehydrated form of this chelate11 closely resembles the dehydrated chelate of HoDOTMA (Fig 1) in which the metal ion lies 0.59 of the way from the N4 to the O4 coordination planes. In contrast the position of the Ho3+ ion in the ‘semi’-hydrated chelate resembles neither that of the monohydrated form of the Y3+ chelate, nor the monohydrated GdDOTMA.7 The metal ion lies about 0.64 of the way from the N4 to the O4 coordination planes, whereas about 0.68 – 0.70 is usual for a monohydrated chelate. This, and the observed conformations of the ligands in these chelates, demonstrates that they are intermediate between mono- and de-hydrated states. This structure represents a chelate part way through a dissociative solvent exchange process. This exchange mechanism is of critical importance to the function of the analogous Gd3+ chelates as T1-shortening contrast agents in clinical MRI.
The structure of HoDOTMA is significant because it confirms the hypothesis of Hermann and Lukeš11, 12 that movement of the metal ion within the coordination sphere is linked to the water exchange process and not the observation of a ‘separate’ dehydrated form of the chelate. It also supports our own recent hypothesis that rHoO, which may alternatively be expressed as q, is intrinsically related to the rate of water exchange.13 Faster water exchange leads to longer time-averaged distances between metal and water, an effect apparently observed in other systems.14 No change in bond distance is proposed, just the average position of the water molecule.
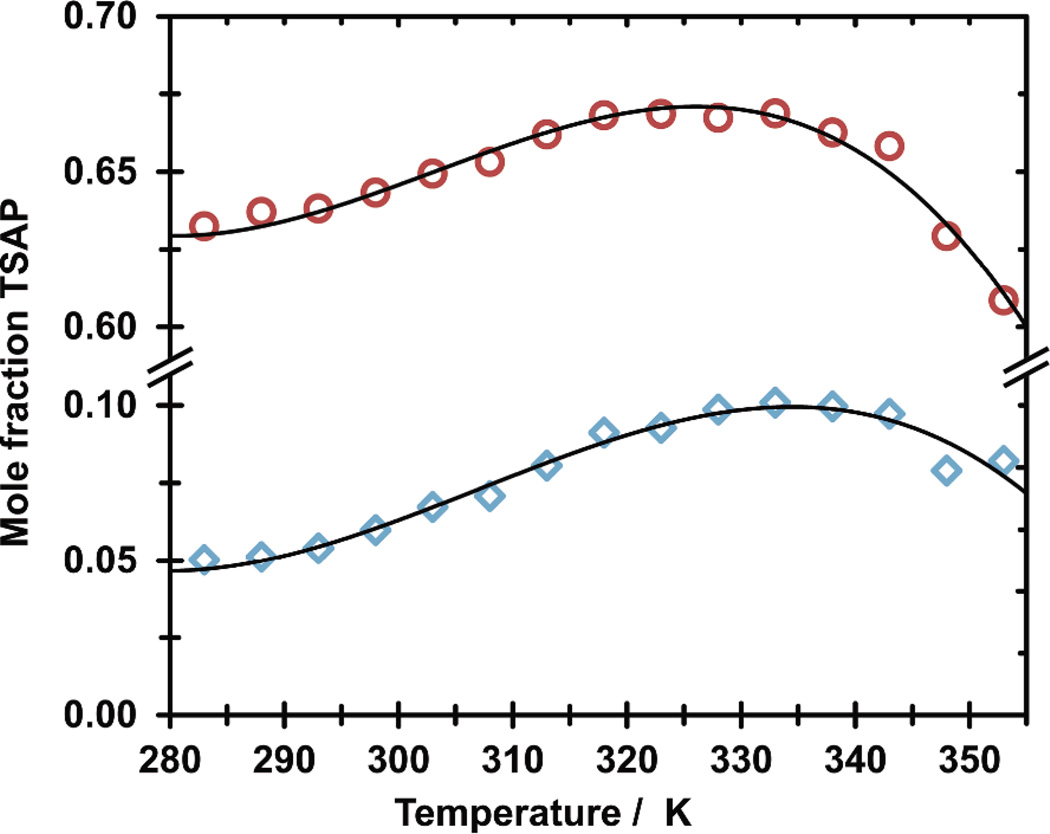
Realizing that these two parameters (one structural, the other dynamic) are related enables an unusual solution state observation to be explained. The temperature variation of the SAP/TSAP distribution for both HoDOTA and HoDOTMA is remarkable (Fig 2). Below room temperature about 95% of HoDOTA adopts the SAP geometry. As the temperature rises the proportion of the chelate in the TSAP geometry increases. On the surface, this can be attributed to the TSAP geometry being the higher energy (minor) structure. However, around 340K this trend abruptly reverses and the SAP geometry becomes more favoured with increasing temperature. The behaviour of HoDOTMA is more unusual still. At lower temperatures the TSAP geometry predominates about 2:1, but increasing the temperature actually increases the proportion TSAP geometry. Normally increasing temperatures would be expected to increase the proportion of the higher energy SAP geometry. As noted previously this unusual observation is often explained by invoking a change in the hydration state of the TSAP geometry with later Ln3+ ions. Typically a dehydrated TSAP isomer (denoted TSAP′) is invoked as an additional solution state structure and is often treated as if it were not participating in water exchange. Given the ligand conformations observed in crystallographic data of dehydrated TSAP chelates,13 such a structure must have quite different dipolar hyperfine shifts.15 The TSAP′ geometry would therefore be expected to have a different NMR spectrum from the TSAP. In the 1H NMR spectra of both HoDOTA and HoDOTMA (ESI) only a single species with a TSAP geometry is observed. Therefore, either there is only one TSAP geometry present or the TSAP and TSAP′ are in fast exchange, but this means they are both exchanging water.
Fig. 2.
Variation in the mole fraction of HoDOTA (blue diamonds) and HoDOTMA (red circles) in the TSAP geometry as a function of temperature determined by integration of the most shifted axS resonances in the 1H NMR spectrum in D2O (pD 5) at 400 MHz. Lines through the data are not fits and are to guide the eye only.
It seems clear from the structures in Fig 1 and previous molecular modelling data5 that water is able to access and interact with the Ho3+ ion in the TSAP geometry of DOTMA. However, from the distance between metal and water in Fig 1 it also seems reasonable to suppose that approaching the Ho3+ ion in this chelate is difficult for the water molecule. This presumably affords a solution state chelate with a long average rHoO (lower q value) and this must lead to fast water exchange kinetics.16, 17 As the temperature increases the exchange rate accelerates, presumably rapidly. This acceleration will cause the time-averaged rHoO to increase further decreasing q. The time-averaged structure of the chelate now more closely resembles the ‘semi’ hydrated chelates (Fig 1), than either a mono- or dehydrated structure. As the water molecule moves away from the Ho3+ ion in this way so the energy of the TSAP geometry falls. In the case of HoDOTMA it falls faster than the rate at which energy is introduced to the system, leading to an increase in the preponderance of the TSAP isomer. Eventually the water exchange rate is so fast and rHoO so long, that any further increase in temperature has no further effect on the energy of the TSAP geometry. Alone this would result in increasing SAP populations with further increases in temperature. But a similar effect is also taking place in the SAP isomer. Although water exchange is slower and the Ho3+ centre more readily accessed in the SAP geometry there comes a point at which the elongation of rHoO causes the energy of this structure to fall also. This would contribute to and accentuate the reversal in the trend observed for HoDOTMA around 340 K. It would also account for the reversal in the trend around the same temperature observed for HoDOTA. None of this should suggest to the reader that an increase in population of TSAP′ is not responsible for these observations. Applying the ergodic principle the effect of increasing the water exchange rate can be considered in terms of its effect on the ensemble of chelates. Faster exchange means more chelates in a dehydrated (or at least not fully hydrated) state; in other words over the ensemble more TSAP′ chelates are present when exchange is fast. Critically however, these TSAP′ chelates are in exchange with the bulk solvent.
The crystal structure of HoDOTMA (Fig 1) demonstrates the significance of the insights provided by both Parker,10 and Lukeš and Hermann.11 It strongly supports the hypothesis that Ln3+ ions coordinated by DOTA-type ligands move up and down in the coordination cage as the ‘bound’ water comes and goes through the water exchange process. It also reveals that water is able to access the central Ho3+ ion of a TSAP coordination isomer. In the context of the observed coordination chemistry of HoDOTA and HoDOTMA (Fig 2) this is particularly significant. It strongly suggests that a dehydrated TSAP′ species exists only transiently, as part of the exchange process, and does not exist as a discrete species. Thus, the change in hydration state often cited for the later Ln3+ ions is in fact a result of increased steric crowding around the water coordination site, leading to longer rLnO distances. But perhaps most interestingly, the value of rHoO seems to increase as water exchange rate accelerates, which is manifest in the unusual temperature dependence of the coordination chemistry.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health (EB-11687) (MW), the National Science Foundation (MRI-0618148) (EJV), Oregon Nanoscience and Microtechnologies Institute (N00014-11-1-0193) (MW), Regione Piemonte (Italy) through the NANO-IGT Project (MB), Portland State University and the Oregon Opportunity for Biomedical Research for financial support of this work.
Footnotes
Electronic Supplementary Information (ESI) available: 1H NMR spectra of HoDOTA and HoDOTMA; crystallographic parameters, refinement details, and images. See DOI: 10.1039/b000000x/
DOTA = 1,4,7,10-tetraazacycldodecane-1,4,7,10-tetraacteate; DOTMA = 1R,4R,7R,10R-α,α′,α″, α‴-tetramethyl-1,4,7,10-tetraazcyclododecane-1,4,7,10-tetraacetate; Crystal data for C40H72ClHo2N8Na3O32, M = 1611.32, orthorhombic, space group C222 but pseudotetragonal and twinned, with a = 18.5845(3), b = 18.6061(6), c = 18.9964(4), α = 90.00, β = 90.00, γ = 90.00°, U = 6568.7(3)Å3, Dc = 1.545 gcm−3, λ(Mo-Ka) 0.71073 Å, Z = 4, µ = 2.541 mm−1. Data were collected on a Oxford Diffraction Gemini at 297(2) K The final full-matrix least-squares refinement converged to R1 = 0.0384 (7976 reflections, F2, I > 2σ(I)); R1 = 0.0484 and wR2 =.0991 for all 9231 data, 452 parameters, 0 restraints, goodness-of-fit (S) 1.147. CCDC. 920762.
Notes and references
- 1.Howard JAK, Kenwright AM, Moloney JM, Parker D, Woods M, Port M, Navet M, Rousseau O. Chem. Commun. 1998:1381–1382. [Google Scholar]
- 2.Aime S, Botta M, Ermondi G. Inorg. Chem. 1992;31:4291–4299. [Google Scholar]
- 3.Hoeft S, Roth K. Chem. Ber. 1993;126:869–873. [Google Scholar]
- 4.Aime S, Botta M, Fasano M, Marques MPM, Geraldes CFGC, Pubanz D, Merbach AE. Inorg. Chem. 1997;36:2059–2068. doi: 10.1021/ic961364o. [DOI] [PubMed] [Google Scholar]
- 5.Cosentino U, Villa A, Pitea D, Moro G, Barone V, Maiocchi A. J. Am. Chem. Soc. 2002;124:4901–4909. doi: 10.1021/ja017666t. [DOI] [PubMed] [Google Scholar]
- 6.Benetollo F, Bombieri G, Aime S, Botta M. Acta Cryst. C. 1999;C55:353–356. [Google Scholar]
- 7.Aime S, Botta M, Garda Z, Kucera BE, Tircso G, Young VG, Woods M. Inorg. Chem. 2011;50:7955–7965. doi: 10.1021/ic2012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botta M. Eur. J. Inorg. Chem. 2000:399–407. [Google Scholar]
- 9.Burgi HB, Dunitz JD. Struct. Correl. 1994;1:163–204. [Google Scholar]
- 10.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods M. J. Chem. Soc., Perkin Trans 2. 1999:493–504. [Google Scholar]
- 11.Kotek J, Rudovsky J, Hermann P, Lukes I. Inorg. Chem. 2006;45:3097–3102. doi: 10.1021/ic060006a. [DOI] [PubMed] [Google Scholar]
- 12.Vojtisek P, Cigler P, Kotek J, Rudovsky J, Hermann P, Lukes I. Inorg. Chem. 2005;44:5591–5599. doi: 10.1021/ic048190s. [DOI] [PubMed] [Google Scholar]
- 13.Webber BC, Woods M. Nature Chem. 2012 Submitted. [Google Scholar]
- 14.Rodriguez-Rodriguez A, Esteban-Gomez D, de Blas A, Rodriguez-Blas T, Fekete M, Botta M, Tripier R, Platas-Iglesias C. Inorg. Chem. 2012;51:2509–2521. doi: 10.1021/ic202436j. [DOI] [PubMed] [Google Scholar]
- 15.Piguet C, Geraldes CFGC. Handbook on the Physics and Chemistry of Rare Earths. 2003;33:353–463. [Google Scholar]
- 16.Aime S, Botta M, Parker D, Williams JAG. J. Chem. Soc., Dalton Trans. 1996:17–23. [Google Scholar]
- 17.Aime S, Barge A, Botta M, De Sousa AS, Parker D. Angew. Chem., Int. Ed. 1998;37:2673–2675. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673::AID-ANIE2673>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.