Abstract
The SH-2 containing inositol 5’ polyphosphatase 1 (SHIP1) is a multifunctional protein expressed predominantly, by hematopoietic cells. SHIP1 removes the 5’ phosphate from the product of PI3K, PI(3,4,5)P3, to generate PI(3,4)P2. Both PIP species influence the activity level of Akt, and ultimately regulate cell survival and differentiation. SHIP1 also harbors several protein interaction domains that endow it with many non-enzymatic cell signaling or receptor masking functions. In this review, we discuss the opposing roles of SHIP1 in cancer and in mucosal inflammation. On one hand, germline loss of SHIP1 causes myeloid lung consolidation and severe inflammation in the ileum, a phenotype that closely mimics human Crohn’s Disease and can be rescued by reconstitution with SHIP1 competent T cells. On the other, transient inhibition of the enzymatic activity of SHIP1 in cancer cell leads to apoptosis and enhances survival in lethal murine xenograft models. Overall, careful dissection of the different pathological mechanisms involved in several diseases provides novel opportunities for therapeutic intervention targeting SHIP1.
Introduction
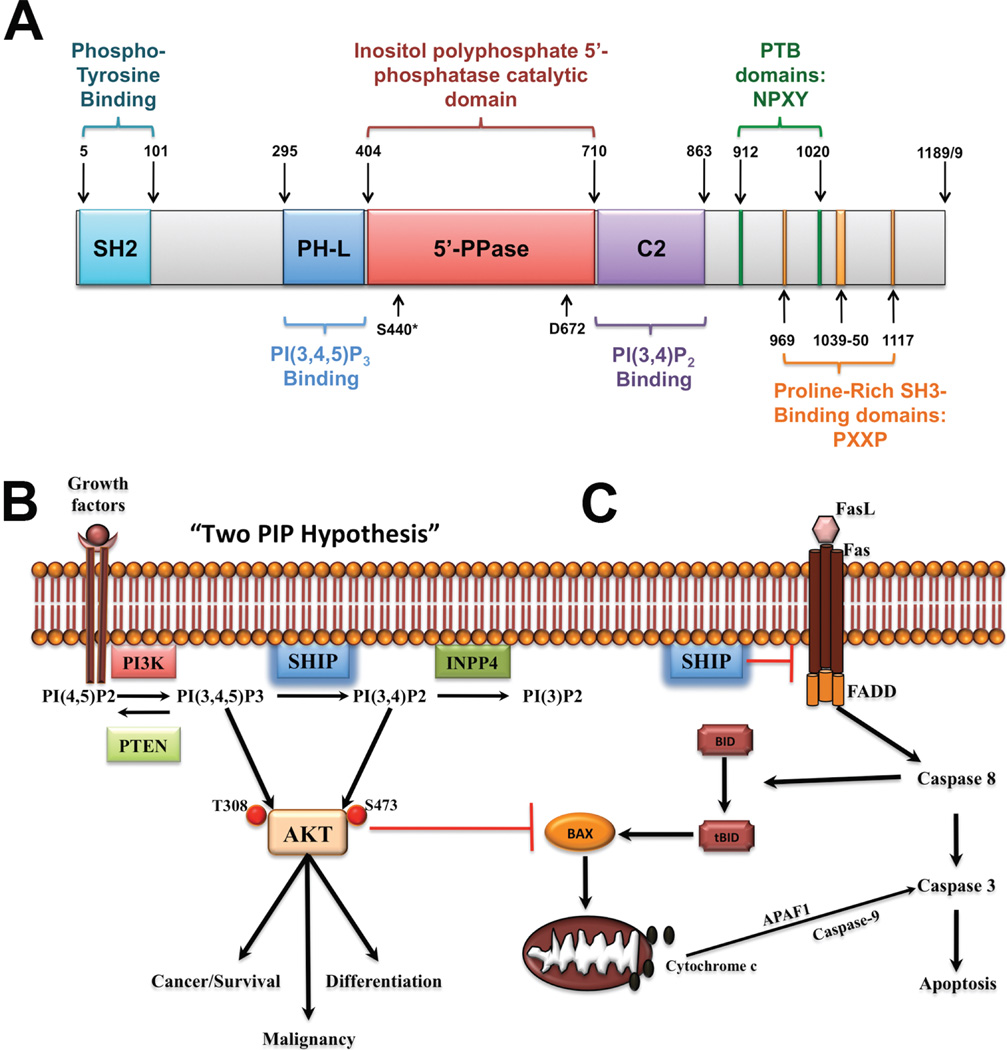
The SH2-containing inositol 5’-polyphosphatase (SHIP1) is expressed predominantly by cells in the hematopoietic compartment,1 but also by osteoblasts,2 and it is encoded by the INPP5D gene. The highly conserved enzymatic domain is centrally located within the protein and is flanked on the N-terminal side by a PH-like domain that binds the SHIP substrate phosphatidylinositol 3,4,5-triphosphate (PI(3,4,5)P3)3 and on the C-terminal side by a C2-domain that binds the product phosphatidylinositol 3,4 bisphosphate (PI(3,4)P2)4 (Fig.1A). By virtue of its dephosphorylation of the product of phosphatidylinositol 3-kinase (PI3K), SHIP is a key player in the inositol phospholipid signaling cascade that also involves the tumor suppressor PTEN, which reverses the PI3K reaction by removing the 3’-phosphate of PI(3,4,5)P3, and the INPP4A/B enzymes, which dephosphorylate the SHIP product, PI(3,4)P2, at the 4’ position to generate PI(3)P (Fig.1B).1 Moreover, non-enzymatic roles have been attributed to SHIP’s N-terminal SH2 domain and its C-terminal NPYX motifs and proline-rich regions. These C-terminal motifs are involved in interactions with proteins carrying phosphotyrosine-binding motifs (PTB), and for binding by SH3-domain containing proteins, respectively. SHIP can also be phosphorylated by cAMP-dependent PKA (Ser440, Fig.1A), and this increases its enzyme activity;5 however, the physiological role of this regulation remains to be determined. SHIP can also associate with receptor tails through its SH2 domain and mask key recruitment sites for other enzymes such as SHP1 or PI3K. Thus, SHIP1 can influence cell signaling in a manner that is independent of its enzymatic activity (Fig.1C).6,7 Because of its modular design, SHIP1 can have varied and disparate effects on cell signaling.
Fig. 1. SHIP1 and its role in cancer and mucosal inflammation.
A. SHIP1 is a 1189 amino acid (1188 aa for isoform b) protein consisting of several different domains, namely the central catalytic domain (5’-PPase) flanked by a PH-like (PH-L) and a C2 domain for binding of the substrate PI(3,4,5)P3 and product PI(3,4)P2 of the enzyme, respectively. The N-terminal region contains a SH2-domain, while the C-terminal “proline-rich” region contains PXXP motifs for interactions with SH3-containing proteins and two NPXY motifs for interaction with Phospho-Tyrosine Binding (PTB) protein. Phosphorylation of Ser440 by PKA has been shown to increase enzymatic activity and the aspartic acid at position 672 (D672) has been identified as a critical catalytic residue. B. The “two PIP hypothesis” suggests that both the substrate, PI(3,4,5)P3, and the product,PI(3,4)P2, of SHIP are necessary to fully activate Akt in order to achieve and maintain the malignant state. As such, selective SHIP1 or pan-SHIP1/2 inhibition can trigger cancer cell death both in vitro and in vivo. C. SHIP may also be involved in regulating Fas trimerization at the cell membrane and would thus serve a protective function against downstream Caspase 8 signaling. Selective SHIP inhibition may therefore have a dual role in triggering apoptosis in cancer cells, via reduced AKT activation and increased Caspase 8 activation. The latter mechanism also suggests that SHIP inhibition may prove useful in reducing inappropriate persistence of autoreactive effector T cells at mucosal surfaces by lifting the inhibition imposed on pathways downstream of Fas.
SHIP in cancer: the “two PIP hypothesis” of malignancy
Due to their ability to reduce PI(3,4,5)P3 levels at the plasma membrane, SHIP1, SHIP2 and PTEN are typically viewed as opposing the activity of the PI3K/Akt/mammalian Target of Rapamycin (mTOR) signaling axis that promotes cancer cell survival. However, emerging evidence suggests that in certain contexts, SHIP1 and SHIP2 may facilitate, rather than suppress, tumor cell survival as PTEN does.8,9 Indeed, while PTEN and the SHIP1/2 proteins dephosphorylate the direct product of PI3K, PTEN removes the phosphate from the D3 position of the inositol ring, while SHIP1/2 remove the D5 phosphate. This distinction is crucial as it enables SHIP1/2 and PTEN to have very different effects on Akt signaling. Consistent with this hypothesis, PI(3,4)P2 levels are increased in leukemic cells, and increased levels of PI(3,4)P2 in INPP4B (type II) mutant mice promote mammary epithelial cell transformation and tumorigenicity.10 This phenomenon can be explained by the “two PIP hypothesis”,1 where a certain amount of both PI(3,4,5)P3 and PI(3,4)P2 are required to promote and maintain the malignant state (Fig.1B). This is further supported by the fact that both SHIP1 agonistic4 and antagonistic8 compounds have been shown to kill multiple myeloma (MM) cells. We have also shown that a SHIP1-selective small molecule inhibitor, 3α-aminocholestane (3AC), reduced the viability of several acute myeloid leukemia cells (AML) cell lines, including KG-1 and C1498, in a dose-dependent manner while having no effect on leukemia cells that do not express SHIP1 such as K562.8 Hallmarks of apoptosis, such as cleavage of PARP, Caspase 3 and 9 and increased Annexin V staining were observed following treatment with 3AC, indicating that cell death pathways were triggered by 3AC. Recently, we and others have observed that SHIP1 may prevent oligomerization of Fas11, and thus SHIP1 inhibition may help increase Caspase 8 activation and promote apoptosis in cancer cells (Fig.1B) (Sudan, Fernandes, Srivastava, Kerr, unpublished data). In addition, SHIP1 inhibition, via 3AC treatment, was shown to abrogate MM growth in vivo and enhance survival, using a lethal xenograft model of MM in immunodeficient NOD/SCID/IL2RγC (NSG) mice.9 Significant reductions were observed in the tumor burden in 3AC-treated vs. vehicle control mice as measured by reduction of both human free lambda light-chain in the serum and percentage of circulating MM cells in the peripheral blood. Most importantly, pulsatile treatment with the 3AC SHIP1-inhibitor for several months failed to trigger the pathology or morbidity that is universally observed in mice with germline loss of the SHIP1 expression, including the lethal pulmonary pneumonia12 and Crohn’s like ileitis13 that afflict germline SHIP1−/− mice.
More recently, we demonstrated that pan-SHIP1/2–targeted inhibition might prove useful for treatment of various blood cancers, such as MM and AML, as wells as non-hematopoietic malignant cells that solely express SHIP2. Indeed, a dose-dependent decrease in viability was observed for both MCF-7 and MBA-MB-231 breast cancer cells following treatment with different pan-SHIP1/2 inhibitors.9 As observed for SHIP1-selective inhibition, pan-SHIP1/2 inhibition reduced basal and IGF-1-induced Akt activation, suggesting that targeting the SHIP1/2 proteins does indeed impact the Akt pathway downstream of PI3K (Fig.1B). Overall, we believe that selective and/or pan-SHIP1/2–inhibition may be a promising novel therapeutic approach to counter the PI3K/Akt/mTOR pathway that is frequently mutated in cancer and is critical for survival of malignant cells.
Role of SHIP in inflammation at mucosal surfaces
In the first paper describing germline Ship mutant mice, Helgason et al. noted a profound myeloid consolidation of the lungs that they proposed led to the early demise of the mice.12 We subsequently showed that this lethal disease could be cured by an allogeneic BM transplant from a SHIP competent donor, indicating that this pulmonary disease resulted from a defect in cellular processes confined to the hematolymphoid compartment and under the control of SHIP1.14 Consistent with this, we could recapitulate pulmonary consolidation in adult mice following induction of SHIP deficiency using an inducible Ship1 deletion model (MxCreShipflox/flox mice), demonstrating SHIP1 plays this role in the hematopoietic system throughout life, and not only during neonatal or juvenile life.8 The prevailing model to explain this lethal pneumonia was that it represented a myeloproliferative disease that impacts alveolar spaces due to decreased turnover of SHIP1 deficient myeloid subsets.12,15 However, this hypothesis was not directly tested in vivo. Subsequently we found that myeloid inflammatory disease also occurs in the small intestines of Ship1−/− mice,13 including two strains where Ship was mutated by targeting the promoter and first exon12,16 and a third strain that lacks the inositol phosphatase exons of SHIP1.17 This disease was highly demarcated as it was confined to the terminal portion of the ileum where lymphoid tissue is abundant in the intestine, but was not found in the proximal portion of the small intestine, large intestine or bowel.13 Thus, in most of its aspects the phenotype strongly resembles classic Crohn’s Disease (CD) as seen in human inflammatory bowel disease (IBD), and in particular those cases where inflammation has not propagated to the descending colon. Like the pulmonary inflammation seen in Ship−/− mice, histopathology and flow cytometry analysis of the small intestine indicated a profound inflammatory myeloid infiltrate was present in the terminal ileum that had a strong neutrophil component.13 However, a more intriguing observation was that both CD4+ and CD8+ T cell numbers in the small intestine lamina propria were greatly diminished in Ship1−/− mice,13 suggesting that Ship1−/− mice might have a selective deficiency of effector T cells at mucosal sites. T cells are now thought to play prominent roles in surveillance and immune responses to both commensal organisms and pathogens in the gut and lung. Hence, we proposed that the myeloid inflammatory disease in these mice results from a failure of this T cell function and consequently an over-response by SHIP1-deficient myeloid cells that also provide pathogen surveillance and protection at mucosal sites.13
To test the above hypothesis, we selectively reconstituted sub-lethally irradiated Ship−/− mice with SHIP1-competent, wild type T cell grafts. We find that if the T cell graft is given before 40 days of life, it was sufficient to rescue Ship1−/− mice from both pulmonary and gut inflammatory disease. In fact it endowed them a normal and healthful lifespan. These findings indicated SHIP1 plays a role in promoting the persistence of mature T cells in the periphery and at mucosal surfaces. We have confirmed this using both a small molecule inhibitor of SHIP1 and competition studies of wild type and Ship1−/− T cells in both immunocompetent and SCID hosts that lack an endogenous T cell compartment. These studies revealed that SHIP1 promotes the persistence of T cells in the recirculating T cell compartment represented by blood and spleen, but also in the more specialized mucosal T cell compartments such as lung and small intestine. Our results do not rule out roles for SHIP1-deficient myeloid cells in the inflammatory disease that impacts the lungs and gut. On the contrary, we believe that SHIP1-deficient myeloid cells ultimately cause the tissue destruction observed in germline mutant mice, but that their participation in this pathology is subsequent to, and a consequence of, a paucity of T effector function at these sites in Ship1−/− mice. These findings have important clinical implications as alterations in SHIP1/INPP5D expression or function may contribute to human CD. Indeed our analysis of a small cohort of CD patients indicates a subset of these patients (10–15%) have little or no detectable SHIP1 protein expression and enzyme activity in their peripheral blood mononuclear cells (Fuhler, Fernandes, Peppelenbosch, Kerr, unpublished data). Another study is currently being organized to confirm these initial findings in a larger patient cohort. The above findings also suggest a selective role for SHIP1 in the persistence of effector T cells at mucosal sites and one could consider using recently identified small molecule inhibitors of SHIP18,9 to target autoreactive T cells in other forms of IBD where SHIP1 deficiency does not play a role, such as ulcerative colitis. This latter molecular function of SHIP1 appears to involve protecting mucosal T cells from Caspase 8–mediated cell death triggered by Fas–FasL interaction (Park, Srivastava, Sudan, Kerr, unpublished data) (Fig.1C).
In sum, the recent findings described herein illustrate the diverse nature of SHIP1 interactions in various cellular and molecular contexts that are only beginning to be unraveled. Therefore, we believe that careful and continued dissection of the different pathological mechanisms involved in these diseases will provide novel opportunities for therapeutic intervention targeting SHIP1.
Acknowledgements
This work was supported in part by grants from the NIH (RO1 HL72523, R01 HL085580, R01 HL107127) and the Paige Arnold Butterfly Run. WGK is the Murphy Family Professor of Children’s Oncology Research, an Empire Scholar of the State University of NY and a Senior Scholar of the Crohn’s and Colitis Foundation of America. W.G.K. has patents, both pending and issued, concerning the analysis and targeting of SHIP1 in disease.
Footnotes
The other authors have no conflicts to disclose.
References
- 1.Kerr WG. Inhibitor and activator: dual functions for SHIP in immunity and cancer. Ann N Y Acad Sci. doi: 10.1111/j.1749-6632.2010.05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazen AL, et al. SHIP is required for a functional hematopoietic stem cell niche. Blood. 2009;113:2924–2933. doi: 10.1182/blood-2008-02-138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ming-Lum A, et al. A pleckstrin homology-related domain in SHIP1 mediates membrane localization during Fcgamma receptor-induced phagocytosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:3163–3177. doi: 10.1096/fj.11-201475. [DOI] [PubMed] [Google Scholar]
- 4.Ong CJ, et al. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood. 2007;110:1942–1949. doi: 10.1182/blood-2007-03-079699. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Walk SF, Ravichandran KS, Garrison JC. Regulation of the Src homology 2 domain-containing inositol 5'-phosphatase (SHIP1) by the cyclic AMP-dependent protein kinase. J Biol Chem. 2009;284:20070–20078. doi: 10.1074/jbc.M109.016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahle JA, et al. Inappropriate Recruitment and Activity by the Src Homology Region 2 Domain-Containing Phosphatase 1 (SHP1) Is Responsible for Receptor Dominance in the SHIPDeficient NK Cell. J Immunol. 2007;179:8009–8015. doi: 10.4049/jimmunol.179.12.8009. [DOI] [PubMed] [Google Scholar]
- 7.Peng Q, et al. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks R, et al. SHIP1 inhibition increases immunoregulatory capacity and triggers apoptosis of hematopoietic cancer cells. J Immunol. 2010;184:3582–3589. doi: 10.4049/jimmunol.0902844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuhler GM, et al. Therapeutic potential of SH2 domain-containing inositol-5'-phosphatase 1 (SHIP1) and SHIP2 inhibition in cancer. Mol Med. 2012;18:65–75. doi: 10.2119/molmed.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewinner C, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlier E, et al. SHIP-1 inhibits CD95/APO-1/Fas-induced apoptosis in primary T lymphocytes and T leukemic cells by promoting CD95 glycosylation independently of its phosphatase activity. Leukemia. 2010;24:821–832. doi: 10.1038/leu.2010.9. [DOI] [PubMed] [Google Scholar]
- 12.Helgason CD, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes & Development. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr WG, Park MY, Maubert M, Engelman RW. SHIP deficiency causes Crohn's disease-like ileitis. Gut. 60:177–188. doi: 10.1136/gut.2009.202283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghansah T, et al. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J Immunol. 2004;173:7324–7330. doi: 10.4049/jimmunol.173.12.7324. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes & Development. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JW, et al. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–2097. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson MC, et al. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]