Abstract
The production of hot embossed plastic microfluidic devices is demonstrated in 1–2 h by exploiting vinyl adhesive stickers as masks for electroplating nickel molds. The sticker masks are cut directly from a CAD design using a cutting plotter and transferred to steel wafers for nickel electroplating. The resulting nickel molds are used to hot emboss a variety of plastic substrates, including cyclo-olefin copolymer and THV fluorinated thermoplastic elastomer. Completed devices are formed by bonding a blank sheet to the embossed layer using a solvent-assisted lamination method. For example, a microfluidic valve array or automaton and a droplet generator were fabricated with less than 100 µm x-y plane feature resolution, to within 9% of the target height, and with 90±11% height uniformity over 5 cm. This approach for mold fabrication, embossing, and bonding reduces fabrication time and cost for research applications by avoiding photoresists, lithography masks, and the cleanroom.
Introduction
Microfluidic devices have been fabricated from silicon, glass, and poly(dimethyl siloxane) (PDMS), but there is now a move toward thermoset and thermoplastic materials, especially for commercial applications.1–3 The wide variety of polymers offers a choice of material properties for microfluidics-based sensors and analyzers, including hydrophobicity, flexibility, elasticity, adsorption, and light transmission.1–5 Furthermore, plastic chips can be integrated with electrodes6 and surface functionalized7 like those made from glass and PDMS.
Despite the practical and commercial advantages of plastic microfluidics, plastic materials are not commonly used in research applications. One possible explanation for this disconnect is the lack of simple, inexpensive, and rapid plastic prototyping methods. Hot embossing and injection molding machines for high throughput applications are expensive and not part of most microfabrication facilities. In addition, the fabrication of metal molds used in both systems is time consuming and does not lend itself to rapid prototyping. As a result, significant effort has gone into the development of alternative plastic microfluidics prototyping methods.8 Milled steel, aluminum, and brass,2,4,9 cast PDMS,10,11 epoxy,12,13 and photoresist14,15 have all been used as hot embossing molds for low throughput and prototyping applications. However, many of these methods still suffer from relatively long fabrication times and cannot withstand more than several dozen embossing cycles. Milled metal molds take significant time to fabricate, require high-precision milling machines, and can be relatively limited in resolution. Other plastic fabrication approaches, such as laser engraving,16 xurography,17,18 and thermoset polymer casting of SU-8 molds19,20 offer low production rates and limited materials.
As an alternative, we describe here a “sticker mask” fabrication method that offers rapid and inexpensive prototyping of nickel molds for hot embossing of microfluidic devices within 1–2 h starting from a CAD design. The sticker mask approach is based on cutting vinyl adhesive films with a plotter to generate an insulating mask on a steel wafer. The exposed designs are electroplated to form raised features, which are hot embossed using a hydraulic press with a simple heated platen insert followed by bonding to a featureless plastic sheet. Sticker mask microfabrication offers researchers access to a versatile and simple fabrication process for plastic microfluidic devices.
Experimental
Fabrication of nickel molds
A Graphtec Craft Robo Pro CE5000-40 Cutting Plotter (Graphtec America, Inc.) was used to plot CAD designs directly from a .dwg or .dxf file with a programmed step size of 10 µm. An 80 µm thick vinyl adhesive film (3M Scotchcal 220, Gerber Scientific Products, Inc.) was used as the positive electroplating mask. Following plotting, features to be plated up were removed manually using fine-tipped forceps. The plotted design was overlaid with laboratory tape prior to removal from the backing substrate. Feature heights greater than 80 µm were formed by layering one or more vinyl adhesive films over the first prior to cutting. Optimal electroplating uniformity was achieved by the addition of 500 µm wide perimeter boundary features within 500 µm of the designs.
Stainless steel sheets (0.030” thick 304 grade steel with a “mirror-like” finish; #9785K51, McMaster-Carr, Inc.) were machined into circular 90 mm diameter wafers and washed with isopropanol and distilled water. Following a nickel strike of the wafer at 235 A-m−2 for 2 min at 50–55 °C in a continually-stirred Wood’s Nickel Strike Solution (240 g-L−1 NiCl2•6 H2O, 25% v/v HCl) with an electrolytic nickel anode, the sticker mask was transferred to the steel wafer and the transfer tape or film was removed. The exposed features were electroplated to the desired height in a continually-stirred commercial bright nickel plating solution (#42027, Alfa Aesar, Inc.) at 55 °C. Multi-level features were fabricated by uncovering regions with the tallest features, and later removing the sticker mask from lower height features. For a repeatable and uniform plating rate, the sticker mask-coated wafer and the nickel anode were held in place in a custom acrylic manifold. A laboratory power supply (GPS-3030DD, Instek, Inc.) was used in current control mode to regulate the plating current density. Any remaining adhesive following sticker removal was dissolved with a 1:1 mixture of acetone and toluene. Care should be taken with handling and disposal of nickel salt solutions as nickel contact can cause allergic reactions in some people and ingestion or inhalation has been associated with increased cancer risk.
Hot embossing plastic microfluidic devices
Zeonor 1060R and 1420R cyclo-olefin copolymer (COC) sheets (1 mm thick) were obtained directly from Zeon Chemical, Inc. Extruded 250 µm thick THV500 samples were kindly provided by Dyneon, Inc. Hot embossing was performed in a hot embossing insert built in-house (see Fig. S1 and design details in ESI) attached to an H6231Z 10 Ton Hydraulic Press (Grizzly Industrial, Inc.). A sheet of polymer was sandwiched between the nickel mold and a sheet of glass and placed between the heated platens. Best results were obtained at pressures of 5–15 MPa for 0.5–2 min and >10 °C above the polymer’s glass transition temperature (Tg). Devices were cooled under pressure and de-embossed 10 °C below Tg.
Device assembly
Following drilling of via holes, devices were cleaned with distilled water and isopropyl alcohol and dried with nitrogen prior to bonding. Rapid bonding of the embossed COC sheets was accomplished using a solvent-assisted lamination approach modified from the work of Miserere et al.14 We identified o-xylene as an optimal solvent for bonding COC devices due to the absence of hazing or cracking, compared to cyclohexane21 and hexadecane,14 and low toxicity and carcinogenicity. Specifically, pipetting 1:1 o-xylene:isopropyl alcohol to cover the featureless polymer sheet and incubating for 30 s (Zeonor 1420R) or 10 s (Zeonor 1060R) resulted in a thin low-Tg layer that could be laminated to the embossed sheet at temperatures that would not distort the channels. The sheets were quickly dried with nitrogen prior to bonding at or slightly below the polymers’ Tg in a Peach 3500 Photo Pouch Laminator (PEACH 3500, Oregon Laminations, Inc.) at the slowest speed setting. THV500 devices were washed briefly with acetone and dried prior to laminator bonding. Additional experimental details can be found in the ESI.
Results and Discussion
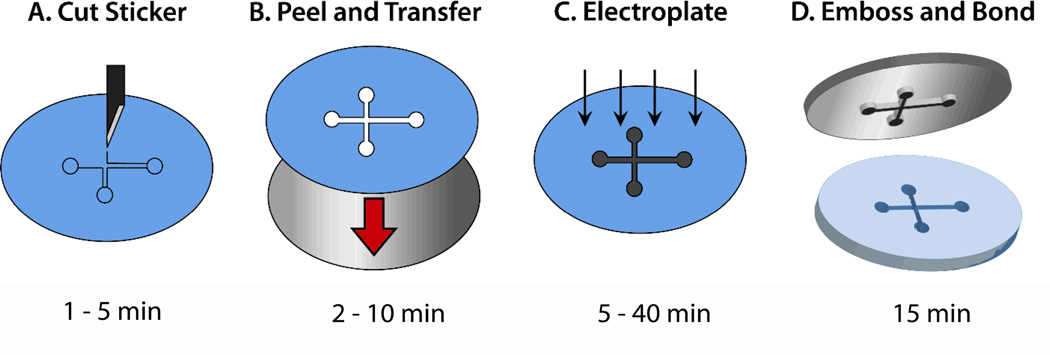
Nickel hot embossing molds for prototyping plastic microfluidic devices were fabricated using vinyl sticker patterns as shown in the workflow in Figure 1. In Fig. 1A, a cutting plotter translates the CAD design into a sheet of vinyl adhesive, and the cut features are removed. Fig. 1B shows the plotted sticker used as an insulating mask for electroplating a steel wafer. Electroplating the exposed features in a nickel sulfamate bath (Fig. 1C) forms raised structures. Fig. 1D shows hot embossing of the mold into plastic sheets to produce microfluidic devices using a hydraulic press with heated platen inserts as shown in Fig. S1. The embossed features are closed by bonding a sheet of featureless polymer using a solvent-assisted bonding method. The overall time required for producing functional microfluidic devices from digital CAD designs was as low as 1 h. Even the most complex and tall designs tested did not require more than 3 h. A single mold costs approximately $2 in materials: $1.50 per steel wafer (not including machining labor), $0.25 for each of the two vinyl adhesive sheets, and negligible cost for the electroplating chemicals per wafer. This is in contrast to recent polymer device fabrication methods that are either slow and expensive21 or relatively fast (<2 h starting with a prepared UV mask) but produce relatively weak and deformable molds that are limited to hot embossing only low Tg materials.14 The sticker mask approach resulted in durable nickel and steel molds that could be used to emboss devices into a wide range of polymers.
Figure 1.
Workflow for fabricating hot embossed plastic microfluidic devices. (A) The CAD design is cut into a vinyl sticker using a plotter, (B) transferred to a stainless steel wafer, and (C) electroplated to form the nickel mold. After sticker removal, (D) the nickel mold is hot embossed into the desired plastic. The entire process can take less than 1 h, depending on the complexity of the microfluidic design.
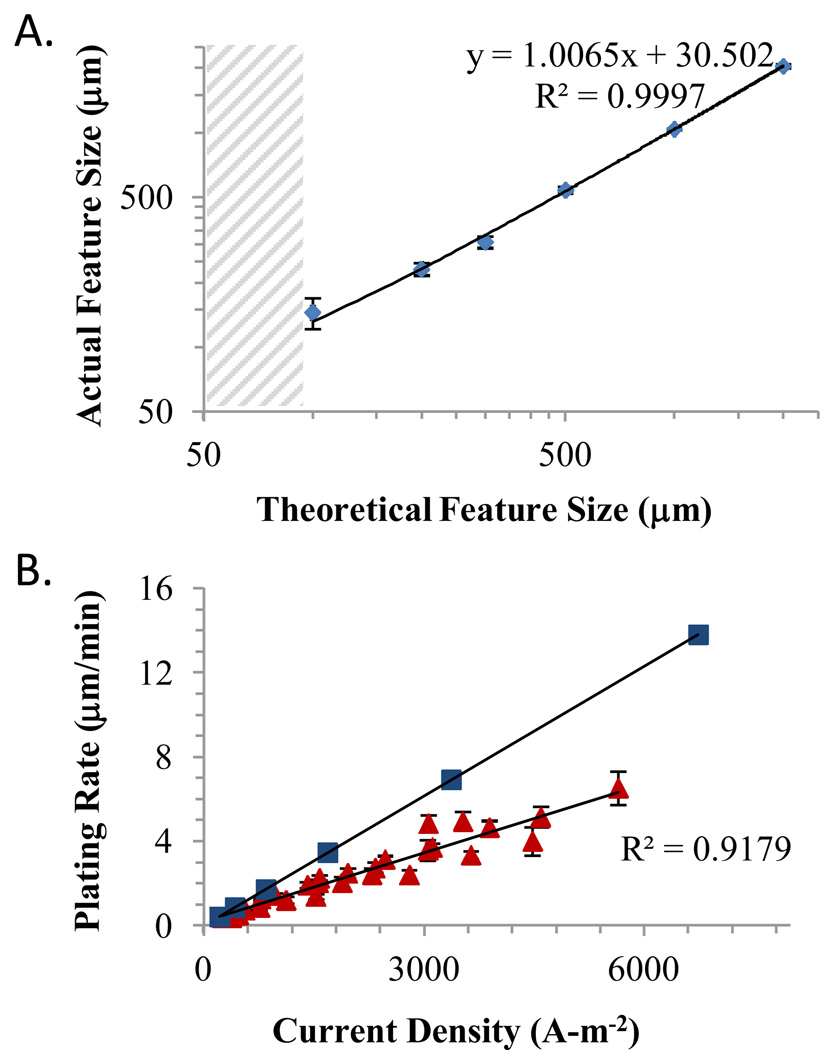
The plotting accuracy, electroplating rates, and feature quality for the sticker mask approach were characterized. Fig. 2A shows the relationship between theoretical feature dimensions in the CAD design and experimental feature dimensions of electroplated nickel molds. The slope of the regression line (1.006) and value of the correlation coefficient (0.999) indicate a high degree of plotting precision and accuracy over the range tested. We observed a real resolution limit of approximately 100 µm as shown by the inability to plot features below 100 µm. This could be due to slight lateral movement of the vinyl sheet during cutting as well as momentum of the blade assembly. The addition of small (~5–10 µm) rectangular features before and after any large steps or changes in direction improves the final design resolution when cutting features smaller than 200 µm by reducing blade momentum and minimizing travel distance between reference points. Multi-layer sticker masks only slightly decreased the resolution limit. Since many microfluidic devices consist of relatively wide channels, the 100 µm lateral resolution is not a significant drawback in such devices, and the tight control of feature height in a relatively rigid polymer is attractive for applications such as imaging-based cell assays22 or for control of flow-induced shear.23 For higher resolution features, nickel production molds can be fabricated using SU-8 photolithography with only minimal modifications to the workflow.
Figure 2.
(A) Plot showing a linear correspondence between final nickel feature resolution and the CAD design above 100 µm; below 100 µm the plotter fails to produce distinct features (gray hatching). The resolution is limited by the 10 µm resolution of the plotter and the stability of the vinyl sticker during cutting. (B) Plot of electroplating rate as a function of current density for a wide range of feature shapes and areas (triangles). The experimental plating efficiency is 53% relative to the theoretical limit (squares). All error bars show standard deviation of the mean.
Fig. 2B explores the dependence of electroplating rate as a function of current densities. The plating rate is expected to be proportional to the current density based on the following equation:
The rate is a function of the efficiency (ε), current (I, Amperes), molecular mass (A, g-mol−1), charge of the nickel ions (Q), Faraday’s constant (F, Coulomb-mol−1), nickel density (ρ, g-cm−3), and exposed surface area of the cathode (S, cm2). The 60,000 multiplier is used to convert the electroplating rate to µm-min−1. The slope of the data indicates an efficiency of 53% relative to the maximum theoretical plating rate across a wide range of feature sizes and currents tested. It is important to include the exposed wafer edges in the total surface area calculation (2.2 cm2 for the wafers used here), particularly in the case of small features, to accurately predict the plating rate. We were able to predict the final feature height to within 9% of the actual height and the observed height uniformity was 90±11% across 5 cm.
Reducing the lipping of plated features is important to achieve uniform feature heights. Lipping occurs due to greater electric field density at the edges of a conductive surface where electric field lines concentrate. To minimize this undesirable effect, boundary features were added at constant distances from all perimeter features in the design to improve the current density distribution of exposed cathode surfaces. The addition of boundaries was not necessary for high-density features and did not impede the fabrication of complex designs. Fig. S2 presents the degree of lipping as a function of the current density and distance of the boundary features. The plot shows that decreasing current density decreases lipping for all boundary distances, but features with boundary-feature gaps of 250 µm and 500 µm exhibit a 15% lipping at all current densities tested. SEM images showing improved electroplating quality are presented in Fig. S3. This finding indicates that adding boundary features produces reproducible feature heights and maintains mold quality while enabling fast plating rates. The optimal plating rate was found to be 1.5–3 µm/min. Nickel molds can also be subjected to chemical mechanical polishing to eliminate lipping.
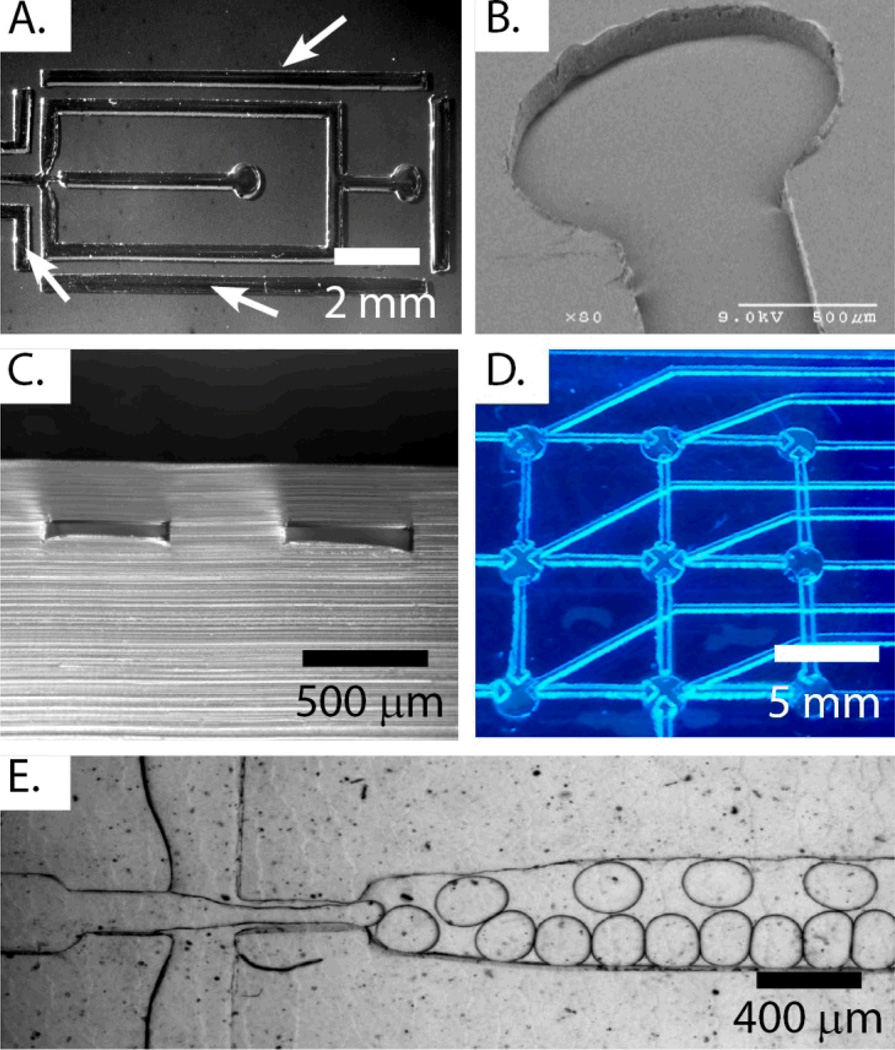
Fig. 3 presents examples of nickel molding and embossed features fabricated using sticker masks. Fig. 3A shows a droplet generator design with boundaries to produce uniform flow rates. Fig. 3B shows a clean flat channel embossed into COC from the mold in Fig. 3A. The nickel mold and embossed design enabled analysis of dimensional stability after hot embossing. For Zeonor 1060R, we observed dimensional fidelity of 101±1% in the x-y plane and 99±1% in the z-axis with respect to the nickel mold. Fig. 3C shows the cross section of a channel and nearby boundary formed by solvent-assisted lamination of an unfeatured 1420R COC wafer to the embossed design. Note the rectangular geometry of the left channel that indicates minimal channel collapse as a result of bonding. The ability of this method to bond extremely low aspect ratio features (< 1:100) in approximately 50 µm deep devices without channel collapse or distortion is demonstrated by the uniform fluorescence of the bonded design in Fig. S4A. A slight increase in fluorescence can be seen around the channel edges due to lipping as a result of not incorporating boundaries during electroplating.
Figure 3.
(A) Photograph of nickel mold incorporating electroplating boundaries, indicated by arrows. (B) SEM image of an embossed channel in COC. (C) Xylene-assisted lamination produces low aspect ratio channels in cyclo-olefin copolymer with minimal distortion as seen in this cross section. Note the slight asymmetry of the boundary (right feature) that is used to block stray electrical current to the fluidic channel during electroplating (left). The rapid prototyping method can be used to produce functional components, such as an automaton (D), consisting of an array of pneumatic valves, as well as a droplet generator nozzle (E) made from THV500 fluorinated elastomer.
The sticker mold fabrication method was used to create examples of microfluidic features for fluid handling. Pneumatically actuated valves play a critical role in fluid routing operations, including dilutions, combinations, volume splitting, and pumping.24,25 The plastic microfluidic automaton in Fig. 3D demonstrates the ability of the sticker fabrication method to produce pneumatic valves in COC. Fig. S4B presents a detailed view of a single four-way valve. Valves remained functional for several weeks of operation in pumping applications. Fig. 3E shows the production of 2 nL droplets of PCR mix in fluorinated oil at a rate of approximately 250 Hz in a droplet generator fabricated from the fluorinated elastomer THV500. Droplet generators are becoming widely adopted for digital PCR and single cell analysis26–28; easily fabricated disposable polymer droplet generators can be used to avoid cross contamination of samples and obviate the need for surface coating that degrades with use and can disrupt PCR reactions. Sticker masks result in durable nickel and steel molds that others have shown to last over 1,000 molding cycles21,29 and allow prototyping with the desired materials.
Conclusions
We have demonstrated and characterized a versatile approach for fabricating nickel molds for plastic device embossing. The sticker-based method can be used to produce microfluidic devices with 100 µm resolution and tight control of feature height. Boundaries incorporated around features improve the quality of electroplated designs and permit production of functional microfluidic components such as valves and droplet generator nozzles. The simplicity and speed of the sticker mold fabrication method make it well suited for rapid prototyping and the production of disposable plastic microdevices.
Supplementary Material
Acknowledgements
The authors would like to thank Paul Lum and Verena Charwat for helpful discussions. RN was supported by an NSF Graduate Research Fellowship, and NR was supported by a Haas Scholars Program grant. Fabrication was performed at the Biomolecular Nanotechnology Center at UC Berkeley. Work was supported by the trans-NIH Genes, Environment and Health Initiative, Biological Response Indicators of Environmental Systems Center Grant U54 ES016115-01 and by the Mathies Royalty Fund.
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/b000000x/
Notes and references
- 1.Fiorini GS, Chiu DT. BioTechniques. 2005;38:429–446. doi: 10.2144/05383RV02. [DOI] [PubMed] [Google Scholar]
- 2.Becker H, Locascio LE. Talanta. 2002;56:267–287. doi: 10.1016/s0039-9140(01)00594-x. [DOI] [PubMed] [Google Scholar]
- 3.Chin CD, Linder V, Sia SK. Lab Chip. 2012;12:2118. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 4.Leech PW. J. Micromech. Microeng. 2009;19:055008. [Google Scholar]
- 5.Toepke MW, Beebe DJ. Lab Chip. 2006;6:1484–1486. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- 6.Illa X, Ordeig O, Snakenborg D, Romano-Rodriguez A, Compton RG, Kutter JP. Lab Chip. 2010;10:1254–1261. doi: 10.1039/b926737a. [DOI] [PubMed] [Google Scholar]
- 7.Gubala V, Le NCH, Gandhiraman RP, Coyle C, Daniels S, Williams DE. Colloids Surfaces B: Biointerf. 2010;81:544–548. doi: 10.1016/j.colsurfb.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Jena RK, Yue CY, Lam YC, Tang PS, Gupta A. Sens. Act. B: Chem. 2012;163:233–241. [Google Scholar]
- 9.Hupert ML, Guy WJ, Llopis SD, Shadpour H, Rani S, Nikitopoulos DE, Soper SA. Microfluid Nanofluid. 2007;3:1–11. [Google Scholar]
- 10.Wang Y, Balowski J, Phillips C, Phillips R, Sims CE, Allbritton NL. Lab Chip. 2011;11:3089–3097. doi: 10.1039/c1lc20281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goral VN, Hsieh Y-C, Petzold ON, Faris RA, Yuen PK. J. Micromech. Microeng. 2011;21:017002. [Google Scholar]
- 12.Koerner T, Brown L, Xie R, Oleschuk RD. Sens. Act. B: Chem. 2005;107:632–639. [Google Scholar]
- 13.Svoboda M, Schrott W, Slouka Z, Přibyl M, Šnita D. Microelect. Eng. 2010;87:1527–1530. [Google Scholar]
- 14.Miserere S, Mottet G, Taniga V, Descroix S, Viovy J-L, Malaquin L. Lab Chip. 2012;12:1849. doi: 10.1039/c2lc21161k. [DOI] [PubMed] [Google Scholar]
- 15.Metwally K, Robert L, Queste S, Gauthier-Manuel B, Khan-Malek C. Microsyst. Tech. 2012;18:199–207. [Google Scholar]
- 16.Edwards TL, Harper JC, Polsky R, Lopez DM, Wheeler DR, Allen AC, Brozik SM. Biomicrofluidics. 2011;5 doi: 10.1063/1.3664694. 044115–044115–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartholomeusz DA, Boutte RW, Andrade JD. J. Microelectromech. Syst. 2005;14:1364–1374. [Google Scholar]
- 18.Sundberg SO, Wittwer CT, Gao C, Gale BK. Anal. Chem. 2010;82:1546–1550. doi: 10.1021/ac902398c. [DOI] [PubMed] [Google Scholar]
- 19.Fiorini GS, Jeffries GDM, Lim DSW, Kuyper CL, Chiu DT. Lab Chip. 2003;3:158–163. doi: 10.1039/b305074m. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, deMello AJ, Chang S-I, Hong J, O’Hare D. Lab Chip. 2011;11:4108–4112. doi: 10.1039/c1lc20603f. [DOI] [PubMed] [Google Scholar]
- 21.Mair DA, Rolandi M, Snauko M, Noroski R, Svec F, Fréchet JMJ. Anal. Chem. 2007;79:5097–5102. doi: 10.1021/ac070220w. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer AD, Vermeul K, Poot AA, Feijen J, Vermes I. Am. J. Physiol. Heart. Circ. Physiol. 2010;298:H719–H725. doi: 10.1152/ajpheart.00933.2009. [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF. Anal. Chem. 2004;76:5257–5264. doi: 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- 24.Jensen EC, Bhat BP, Mathies RA. Lab Chip. 2010;10:685–691. doi: 10.1039/b920124f. [DOI] [PubMed] [Google Scholar]
- 25.Grover WH, Skelley AM, Liu CN, Lagally ET, Mathies RA. Sens. Act. B: Chem. 2003;89:315–323. [Google Scholar]
- 26.Novak R, Zeng Y, Shuga J, Venugopalan G, Fletcher DA, Smith MT, Mathies RA. Angew. Chem. Int. Ed. 2011;50:390–395. doi: 10.1002/anie.201006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, Novak R, Shuga J, Smith MT, Mathies RA. Anal. Chem. 2010;82:3183–3190. doi: 10.1021/ac902683t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumaresan P, Yang CJ, Cronier SA, Blazej RG, Mathies RA. Anal. Chem. 2008;80:3522–3529. doi: 10.1021/ac800327d. [DOI] [PubMed] [Google Scholar]
- 29.Mair DA, Geiger E, Pisano AP, Fréchet JMJ, Svec F. Lab Chip. 2006;6:1346. doi: 10.1039/b605911b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.