Abstract
Background and objectives
Heterosexual HIV-1 serodiscordant couples are increasingly recognized as an important source of new HIV-1 infections in sub-Saharan Africa. A simple risk assessment tool could be useful for identifying couples at highest risk for HIV-1 transmission.
Methods
Using data from three prospective studies of HIV-1 serodiscordant couples from seven African countries and standard methods for development of clinical prediction rules, we derived and validated a risk scoring tool developed from multivariate modeling and composed of key predictors for HIV-1 risk that could be measured in standard research and clinical settings.
Results
The final risk score included age of the HIV-1 uninfected partner, married and/or cohabiting partnership, number of children, unprotected sex, uncircumcised male HIV-1 uninfected partner, and plasma HIV-1 RNA in the HIV-1 infected partner. The maximum risk score was 12, scores ≥5 were associated with an annual HIV-1 incidence of >3%, and couples with a score ≥6 accounted for only 28% of the population but 67% of HIV-1 transmissions. The area under the curve for predictive ability of the score was 0.74 (95% CI 0.70–0.78). Internal and external validation showed similar predictive ability of the risk score, even when plasma viral load was excluded from the risk score.
Conclusions
A discrete combination of clinical and behavioral characteristics defines highest-risk HIV-1 serodiscordant couples. Discriminating highest-risk couples for HIV-1 prevention programs and clinical trials using a validated risk score could improve research efficiency and maximize the impact of prevention strategies for reducing HIV-1 transmission.
Keywords: HIV-1 serodiscordant couples, HIV-1 acquisition, clinical prediction rule
Introduction
Of the nearly 2 million new HIV-1 infections in sub-Saharan Africa each year, a substantial proportion occur within stable, cohabiting heterosexual couples, making this population a priority for HIV-1 prevention research and implementation of effective HIV-1 prevention strategies [1–3]. As African countries are adopting couples HIV-1 counseling and testing as an HIV-1 prevention strategy, more couples of previously unknown serostatus are becoming aware of being HIV-1 serodiscordant [4, 5]. Couples aware of their serodiscordant status continue to face HIV-1 risk [6–10], and there is an urgent need to design optimal strategies for evaluation and delivery of HIV-1 prevention strategies for couples, particularly how to target prevention strategies to realize maximum population HIV-1 prevention benefits.
Risk factors for HIV-1 transmission in serodiscordant partnerships include high HIV-1 plasma concentrations in the HIV-1 infected partner, unprotected sexual activity, multiple partners and uncircumcised status for HIV-1 susceptible male partners [11–13]. A recent study showed that a risk algorithm, assessing the contribution of multiple risk factors, could be mathematically derived from the literature to identify partnerships at higher risk for transmission [14], but simple risk algorithms, for use in real-world settings and based on empiric data, have not been developed. While all serodiscordant couples are potentially at risk for HIV-1 transmission, defining those couples at the highest risk might permit more efficient recruitment of couples into clinical studies of novel prevention strategies and more cost-efficient, targeted delivery of expensive HIV-1 prevention interventions, such as earlier initiation of antiretroviral therapy (ART) for HIV-1 infected partners or antiretroviral pre-exposure prophylaxis (PrEP) for HIV-1 prevention in uninfected partners [15–18].
Clinical prediction rules, also known as clinical decision rules, are evidence-based assessment tools that use patient medical history, physical examination, and diagnostic test results to assist in medical decision-making [19, 20]. Clinical prediction rules are typically simple, efficient, and easy to implement and use in a clinical setting, but the methods for developing them can also be applied to assessing risk for prevention intervention [21–23]. Standardized, rigorous processes have been described for developing clinical prediction rules, including deriving and validating the prediction rule [23,24]. We used standard methods for development of clinical prediction rules to create and validate a risk-scoring tool to identify highest-risk HIV-1 serodiscordant couples.
Methods
We used data from three prospective studies in Africa of stable heterosexual HIV-1 serodiscordant couples to assess the relationship between clinical and behavioral variables and the risk of HIV-1 acquisition, focusing on variables that could be measured in a standard clinical or research setting.
Study population
Partners in Prevention HSV/HIV Transmission Study (derivation cohort)
Between November 2004 and April 2007, we enrolled 3408 heterosexual HIV serodiscordant couples from 7 African countries (Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, and Zambia) into the Partners in Prevention HSV/HIV Transmission Study, a randomized, double-blind, placebo-controlled clinical trial of herpes simplex virus type 2 (HSV-2) suppressive therapy to reduce HIV-1 transmission, as previously described [25]. Eligible couples were at least 18 years of age, reported ≥3 vaginal sex acts in the three months prior to enrollment, and intended to remain a couple. At enrollment, all HIV-1 infected partners were HSV-2 seropositive, had CD4 counts ≥250 cells/μL (making them ineligible for ART under national guidelines of the study countries at that time), and were not currently taking ART. HSV-2 suppressive therapy failed to reduce HIV-1 transmission within partnerships [26].
Couples Observational Study (validation cohort)
In a parallel study at two of the Partners in Prevention HSV/HIV Transmission Study sites (Kampala, Uganda and Soweto, South Africa), an additional 485 HIV-1 serodiscordant couples who were not participants in the Partners in Prevention HSV/HIV Transmission Study were enrolled into an observational study of immune correlates of HIV-1 protection [27]. Similar to the clinical trial cohort, participants were ≥18 years of age and sexually active and HIV-1 seropositive partners were not using ART.
Partners PrEP Study (validation cohort)
The Partners PrEP Study is a randomized, placebo-controlled, three-arm clinical trial that assessed the safety and efficacy of oral PrEP for the prevention of HIV-1 acquisition using the antiretroviral medication tenofovir (TDF), either alone or in combination with emtricitabine (FTC/TDF). Between July 2008 and November 2010, 4758 HIV-1 serodiscordant couples from nine sites in Kenya and Uganda were enrolled. Eligible couples were at least 18 years of age and sexually active, with the intention to remain a couple [28]. HIV-1 infected partners were not using ART, had CD4 counts ≥250 cells/mm3, and did not otherwise meet Kenyan or Ugandan guidelines for initiation of ART. The trial’s placebo arm was discontinued in July 2011 due to significant reduction in HIV-1 acquisition risk for both TDF and FTC/TDF [17]. For this analysis, we included only couples in the placebo arm.
In the Partners in Prevention HSV/HIV Study and the Couples Observational Study cohorts, HIV-1 uninfected partners were seen quarterly for HIV-1 serologic testing. In the Partners PrEP Study, testing was monthly.
Protection of Human Subjects
All participants received HIV-1 and risk-reduction counseling (both individual and as a couple), free condoms, and treatment for sexually transmitted infections (STIs), according to World Health Organization (WHO) guidelines. Written, informed consent was obtained from all participants. The study protocols were approved by the University of Washington Human Subjects Review Committee and ethical review committees at each of the study sites.
Laboratory methods
HIV-1 seroconversion of initially uninfected partners was determined by serologic testing using dual rapid HIV-1 antibody test with confirmatory HIV-1 EIA, Western blot, and RNA PCR. For HIV-1 infected partners, CD4 counts were quantified using standard flow cytometry and plasma HIV-1 RNA levels were quantified by PCR performed at the University of Washington using the COBAS Ampliprep/COBAS TaqMan real-time HIV-1 RNA assay, version 1.0 (Roche Diagnostics, Indianapolis, IN), with a lower limit of quantification of 240 copies/mL.
Risk score variables and data analysis
Our goal was to develop a risk score that could be calculated as a simple scorecard, aiming for three to ten categorical predictors, validated on external data sources. We used methods described by McGinn, et al. for developing clinical prediction rules using similar derivation and validation processes [23]. We derived the rule based on known and suspected predictors that were clearly defined and present in a significant proportion of our cohort. We validated the rule both internally and externally to determine the predictive ability of the rule.
The primary study outcome was HIV-1 seroconversion in previously HIV-1 seronegative participants. We did not utilize HIV-1 transmission linkage data available from viral sequencing [29] (i.e., to distinguish transmissions that occurred from the study HIV-1 infected partner versus an external partner), as we considered that any HIV-1 acquisition event, regardless of source, would be important for HIV-1 prevention programs.
From the Partners in Prevention HSV/HIV Transmission Study cohort, we identified potential predictors of HIV-1 acquisition based on characteristics known to be associated with HIV-1 risk that could also be feasibly collected from couples in general clinical and research settings. The list of variables considered included demographic (age, gender of the HIV-1 infected partner, number of children, marital status, cohabitation and duration of partnership), behavioral (frequency of sex, unprotected sex reported in the prior 30 days), clinical factors (male circumcision status for HIV-1 uninfected men, hormonal contraceptive use), and laboratory measures of HIV-1 disease stage in HIV-1 infected partners (plasma HIV-1 level and CD4 count) collected at study enrollment. We restricted our consideration to enrollment variables in order to mimic the type of cross-sectional information that would be available for performing a risk assessment in a standard clinical setting. We converted continuous predictors to categorical variables using optimal cut-points identified through signal detection ROC analysis, weighting false positives over false negatives [30]. We determined signal detection to be the appropriate method for selecting cut-points for categorizing, because it allows for higher-level interactions among all variables and uses recursive partitioning identifying subgroups at highest risk, thus reducing potential misclassification of more arbitrary categories.
We assessed the relationship between our defined predictor variables and HIV-1 infection risk. We censored couples’ follow-up at 12 months, anticipating that prevention programs would reevaluate couples’ risk at approximately annual intervals. Additionally, couples in which the HIV-1 infected partner started ART were censored at initiation since clinical studies and HIV-1 prevention programs would likely consider couples in which the infected member initiated ART to be receiving a highly-effective prevention intervention [15]. Potential predictors that were significantly associated with HIV-1 transmission risk in univariate comparisons or those predictors we selected a priori for evaluation (gender, unprotected sex, circumcision status and plasma HIV-1 RNA) were evaluated in a multivariate model. To determine the combination of variables that best predicted HIV-1 risk, potential predictors from the multivariate model were assessed in a fully stepwise sequence Cox proportional hazards model, where all predictors were evaluated at each step for inclusion or exclusion. We use the lowest Akaike Information Criterion (AIC) on all possible models from the final stepwise model to determine the predictors for the risk score. The score values for individual risk factors were obtained by dividing the coefficients from the hazard model for each predictor from the final proportional hazards model by the lowest coefficient among all predictors and rounding to the nearest integer. The sum of individual parameter score values for each predictor determined the final risk score for each couple. HIV-1 transmission incidence was calculated by risk score group. Due to the costs and limited availability of viral load assays in some settings, we also calculated HIV-1 incidence for risk score groups excluding viral load.
We used internal and external validation methods for assessing the robustness of our final risk score model. For internal validation, we used a 10-fold cross-validation of the final risk score and compared the area under the ROC curve (AUC) of our final model with the average AUC of the 10 different models for predictive ability and robustness. For external validation, we applied the risk scores separately to the Couples Observational Study cohort and the placebo arm of the Partners PrEP Study cohort.
All analyses were conducted using SAS (v.9.2, Cary, NC) and public domain ROC5 (Department of Veteran’s Affairs and the National Institute of Aging of the United States).
Results
Population
Of 3408 couples enrolled in the Partners in Prevention HSV/HIV Transmission Study, 61 were excluded because no follow-up visits were completed and 49 were excluded for missing predictor data. Of the remaining 3297 couples, most were married and cohabitating (Table 1). They reported a median of 4 (IQR 3–10) sex acts in the 30 days prior to enrollment with 35% reporting at least one unprotected sex act. The median number of children within the partnership was 1 (IQR 0–2), with 31% having no children together. Among HIV-1 infected partners, the median CD4 count was 462 cells/mm3 (IQR 347–631) and median plasma HIV-1 concentration was 11,746 copies/mL (IQR 2285–48,070) with 24% having a plasma HIV-1 concentration ≥50,000 copies/mL. Among HIV-1 uninfected men, 63% were uncircumcised. Retention of initially HIV-1 uninfected partners at 12 months was 92%. During 3126 person-years of follow-up, a total of 107 HIV-1 seroconversions occurred (incidence 3.4 per 100 person-years).
Table 1.
Enrollment characteristics
| Number (%)or median (IQR)
|
||||||
|---|---|---|---|---|---|---|
| Partners in Prevention HSV/HIV Transmission Study | Couples Observational Cohort Study | Partners PrEP Study, placebo arm | ||||
|
| ||||||
| Couples with HIV-1 acquisition, N=107 | Couples without HIV-1 acquisition, N=3190 | Couples with HIV-1 acquisition, N=15 | Couples without HIV-1 acquisition, N=461 | Couples with HIV-1 acquisition, N=57 | Couples without HIV-1 acquisition, N=1442 | |
|
| ||||||
| Couple characteristics | ||||||
|
| ||||||
| Female HIV-1 infected partner | 65 (60.8%) | 2153 (67.5%) | 3 (20.0%) | 246 (53.4%) | 27 (47.4%) | 880 (61.0%) |
| Married and/or cohabiting | 101 (94.4%) | 2921 (91.6%) | 14 (93.3%) | 434 (94.1%) | 57 (100.0%) | 1421 (98.5%) |
| Duration of partnership, years | 3 (1.3–6.1) | 5 (2.4–10.0) | 1.9 (1.2–4.4) | 4.4 (1.5–9.2) | 4.2 (1.0–9.2) | 7.4 (3.0–14.0) |
| Number of children within partnership | 1 (0–2) | 1 (0–3) | 1 (0–1) | 1 (0–3) | 1 (0–3) | 2 (1–4) |
| Number of sex acts in prior month | 6 (3–12) | 4 (3–9) | 3 (2–4) | 3 (1–4) | 4 (3–10) | 4 (3–8) |
| Any unprotected sex in prior month | 59 (55.1%) | 1101 (34.5%) | 4 (26.7%) | 69 (15.0%) | 23 (40.4%) | 378 (26.2%) |
|
| ||||||
| Characteristics of HIV-1 infected partner | ||||||
|
| ||||||
| Age, years | 30 (25–37) | 32 (27–38) | 29 (26–36) | 32 (26–38) | 32 (26–39) | 33 (27–39) |
| Effective contraceptive use (women)* | 14 (21.5%) | 391 (18.2%) | 0 (0.0%) | 110 (24.7%) | 8 (28.6%) | 260 (29.6%) |
| Plasma viral load, copies/mL | 44540 (7700– 119025) | 11388 (2184–45130) | 60615 (14670– 123320) | 21210 (3545–96655) | 30278 (11760– 123420) | 7669 (1534–31434) |
| CD4 count, cells/mm3 | 419 (330–557) | 463 (348–636) | 472 (228–655) | 391 (246–576) | 496 (356–626) | 500 (375–663) |
|
| ||||||
| Characteristics of HIV-1 uninfected partner | ||||||
|
| ||||||
| Age, years | 30 (26–38) | 34 (28–41) | 23 (19–28) | 31 (26–38) | 30 (25–37) | 34 (28–40) |
| Uncircumcised (men) | 41 (63.1%) | 965 (44.8%) | 0 (0.0%) | 110 (44.7%) | 14 (51.9%) | 414 (47.1%) |
| Effective contraceptive use (women)* | 7 (16.7%) | 156 (15.0%) | 3 (25.0%) | 25 (11.6%) | 16 (48.5%) | 216 (38.5%) |
Includes oral, implantable or injectable hormonal contraceptives, intrauterine device (IUD)and/or condoms
Risk score model
Development of the final risk score model is detailed in Table 2. In the stepwise Cox proportional hazards analysis, age of the HIV-1 uninfected partner, married and/or cohabiting partners, number of children, unprotected sex, uncircumcised status of male HIV-1 uninfected partners, and HIV-1 plasma viral load were retained in the final prediction model. Notably, gender was not determined to be a key predictor of HIV-1 risk and was not included in the final model, as gender effects on transmission risk were accounted for by other predictors, such as viral load.
Table 2.
Analysis of predictors and calculation of risk score
| Univariate analysis | Multivariate analysis* | Stepwise multivariate analysis** | Risk score*** | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Regression coefficient | ||
|
| ||||||||
| Female HIV-1 infected partner | 0.7 | 0.5–1.1 | 0.6 | 0.3–1.1 | ||||
| Age of HIV-1 infected partner | ||||||||
| 20 years or younger | 2.4 | 1.0–5.7 | 1.7 | 0.6–4.6 | ||||
| 21–30 years | 1.4 | 0.9–2.1 | 1.2 | 0.8–2.0 | ||||
| More than 35 years | ref | ref | ||||||
| Age of HIV-1 uninfected partner | ||||||||
| 20 years or younger | 5.5 | 2.8–10.8 | 3.3 | 1.5–7.2 | 4.5 | 2.2–9.1 | 1.51 | 4 |
| 21–35 years | 1.6 | 1.1–2.4 | 1.3 | 0.8–2.0 | 1.5 | 1.0–2.2 | 0.40 | 1 |
| More than 30 years | ref | ref | ref | |||||
| Married and/or cohabiting | 1.5 | 0.7–3.4 | 1.8 | 0.8–4.2 | 1.8 | 0.8–4.1 | 0.58 | 1 |
| Duration of partnership, years | 0.9 | 0.90–0.98 | ||||||
| Number of children | ||||||||
| 0 | 2.4 | 1.4–4.3 | 2.2 | 1.2–4.0 | 2.1 | 1.1–3.7 | 0.72 | 2 |
| 1–2 | 1.7 | 0.9–3.0 | 1.6 | 0.9–2.8 | 1.5 | 0.8–2.7 | 0.41 | 1 |
| 3 or more | ref | ref | ref | |||||
| Unprotected sex within partnership, prior 30 days | 2.3 | 1.6–3.4 | 2.2 | 1.5–3.2 | 2.2 | 1.5–3.2 | 0.78 | 2 |
| Number of sex acts within partnership, prior 30 days | 1.02 | 1.00–1.04 | ||||||
| Uncircumcised male HIV-1 uninfected partner | 1.4 | 1.0–2.1 | 1.9 | 1.1–3.1 | 1.5 | 1.0–2.3 | 0.44 | 1 |
| Effective contraceptive use | ||||||||
| HIV-infected women | 1.1 | 0.6–1.9 | ||||||
| HIV-uninfected women | 1.3 | 0.6–2.8 | 1.1 | 0.5–2.6 | ||||
| HIV-1 infected partner CD4 count (cells/mm3) | 1.0 | 0.99–1.0 | ||||||
| HIV-1 infected plasma viral load | ||||||||
| 50,000 copies or higher | 3.8 | 2.4–6.0 | 3.7 | 2.4–5.9 | 3.9 | 2.5–6.1 | 1.36 | 3 |
| 10,000 –49,999 copies | 1.6 | 0.9–2.7 | 1.5 | 0.9–2.5 | 1.5 | 0.9–2.6 | 0.43 | 1 |
| Less than 10,000 copies/mL | ref | ref | ref | |||||
Covariates selected for multivariate analysis were based on those factors that were selected a priori for evaluation (gender, unprotected sex, circumcision status and plasma HIV-1 RNA) and other factors that were statistically significant in univariate analysis
Covariates selected for the stepwise multivariate model based on lowest Akaike Information Criterion (AIC) score from stepwise procedure and not statistical significance of individual predictor
Points were assigned to each risk factor by dividing each coefficient from the stepwise proportional hazard model by 0.29 (the lowest coefficient value, corresponding to HIV-1 uninfected age 21–35 years) and rounding to the nearest integer
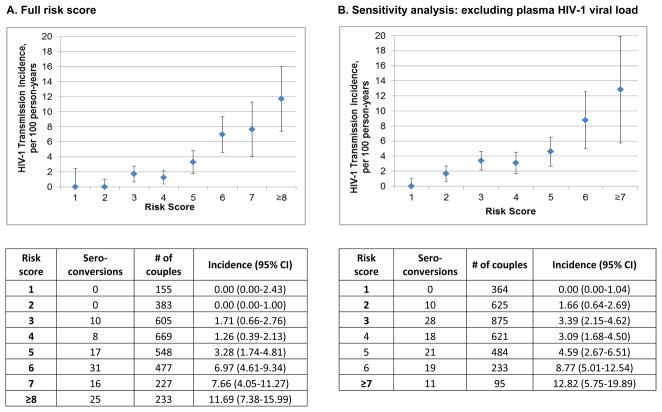
We calculated the total risk score for each couple by summing the individual parameter scores determined in the final risk model and the HIV-1 incidence for each risk score was generated (Figure 1A). Scores ≥5 were associated with an annual HIV-1 incidence of >3%. For example, a score of ≥6 identified 67% of the observed HIV-1 seroconversion events among only 28% of the total study population. In the risk score in which HIV-1 plasma viral load was excluded, the overall incidences were lower but followed a similar pattern to the full risk score (Figure 1B).
Figure 1.
Incidence of HIV-1 infection by risk score
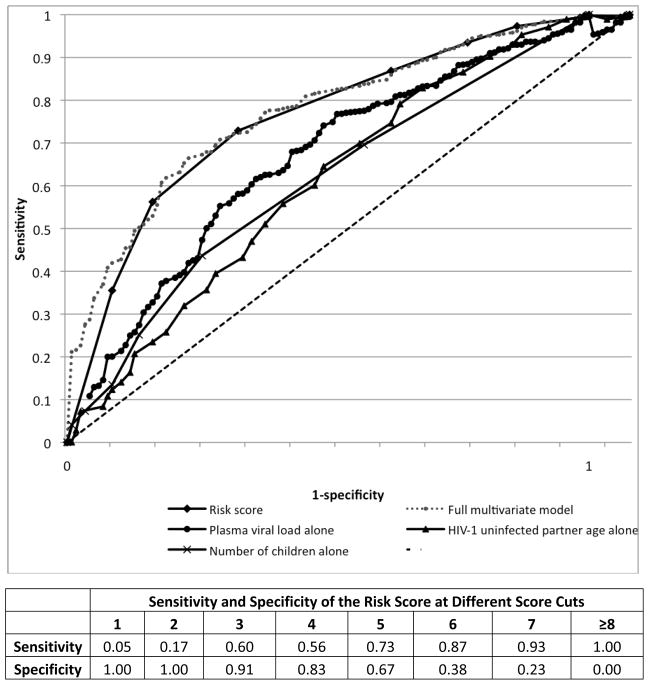
Compared to our risk score algorithm, we found that individual risk factors had more limited discriminatory potential in predicting HIV-1 seroconversion. Figure 2 shows ROC curves for the continuous predictors included in our model (plasma viral load, HIV-1 uninfected partner and number of children) along with the ROC curve for the composite risk score. In addition, Figure 2 depicts the ROC curve for the full multivariate model (from Table 2) compared with the composite risk score model based on the stepwise selection; the two curves essentially overlap, demonstrating that the more parsimonious risk score model captures essentially all predictive ability of the more complex model. Among binomial risk factors, unprotected sex alone predicted 55% of HIV-1 seroconversions, from 35% of the cohort (incidence 5.4 per 100 person-years), and uncircumcised status of male uninfected partners alone predicted 63% of male HIV-1 seroconversions, from 45% of the male cohort (incidence 4.3 per 100 person-years). Married and/or cohabiting partners made up 94% of HIV-1 seroconversions, but almost all couples (92%) were married and/or cohabiting.
Figure 2.
ROC curves comparing risk score to individual continuous predictors.
Model validation
The area under the curve (AUC) for the probability of the risk score to correctly predict HIV-1 acquisition was 0.74 (95% CI 0.70–0.78). Internal cross-validation showed the average AUC for 10 subsets analyzed was 0.73, similar to the AUC of the full dataset and indicating robust generalizability of the risk algorithm within the dataset.
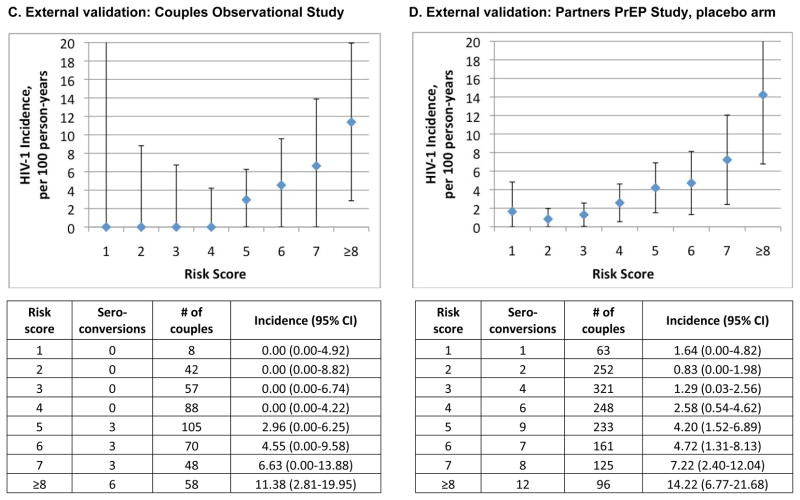
For external validation, we applied our risk score to the Couples Observational Study cohort and the placebo arm of the Partner PrEP Study (characteristics defined in Table 1). The observational cohort included 476 couples, of which 15 had an HIV-1 seroconversion event (incidence 3.2 per 100 person-years). Using the cutoff risk score of ≥6, we predicted 80% of seroconversions from 37% of the population (Figure 1C). No HIV-1 seroconversion events occurred among couples having a risk score ≤2. The AUC for the risk score applied to the Couples Observational Study cohort was 0.76 (95%CI 0.70–0.83) The Partners PrEP Study cohort included 1499 couples in the placebo arm, among whom 45 seroconverted in the first year of follow-up (incidence 2.6 per 100 person-years). A risk score cutoff of ≥6, which was observed in 25% of the cohort, predicted 55% of HIV-1 seroconversions (Figure 1D), with an AUC of 0.70 (95%CI 0.64–0.76).
Discussion
The results of this analysis demonstrate that a discrete set of factors, considered in combination and quantified to develop a risk score, can efficiently identify a subpopulation of stable HIV-1 serodiscordant couples at higher risk for HIV-1 transmission. The predictors selected for our final risk score model are well-established risk factors for HIV-1 and included factors measurable in clinical settings: plasma HIV-1 RNA concentrations, unprotected sex, young age, marital status, no or few children in the partnership, and uncircumcised status of HIV-1 uninfected men [11, 31–37]. Importantly, the combination of risk factors in a single algorithm allowed for more precise predictive capability than individual predictors. The score had good predictive ability in internal and external validation, which lends strength to our findings. To our knowledge, the model defined here is the first empirically-based risk assessment tool for identifying high-risk HIV-1 serodiscordant heterosexual couples, and it offers a simple, quantitative approach for defining couples at higher HIV-1 risk. Our findings are relevant to both clinical research studies (to improve efficiency of recruitment and predict anticipated HIV-1 incidence) and programmatic roll-out of new HIV-1 prevention strategies (to maximize cost-effectiveness by targeting those at greatest risk) [20–22].
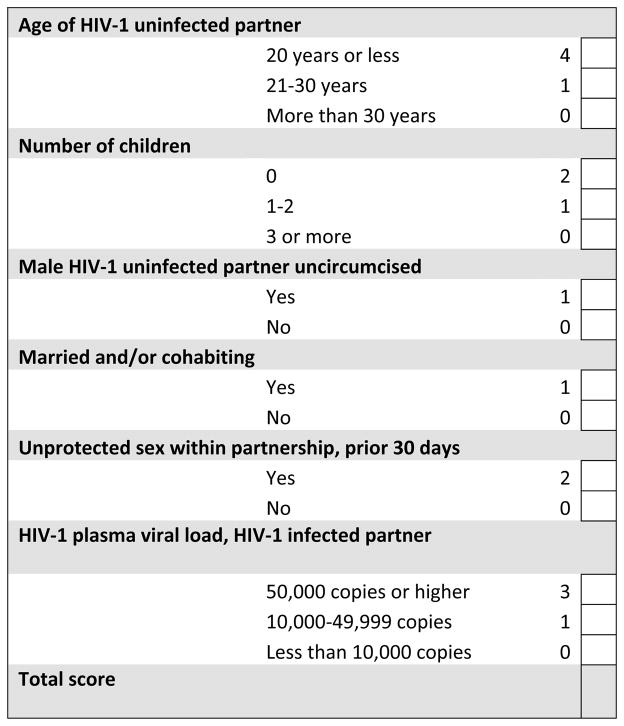
New HIV-1 prevention strategies, such as early ART initiation and PrEP, offer the potential to markedly decrease HIV-1 transmission. To have the greatest impact on preventing HIV-1 transmissions among HIV-1 serodiscordant couples, while containing costs, targeting couples at highest risk may be important [38]. Recent WHO guidance on HIV-1 prevention for HIV-1 serodiscordant couples recommended consideration of ART initiation for couples regardless of CD4 count as well as consideration of PrEP [39]. While ART for HIV-1 infected partners with immediate clinical need is required, many countries have not yet implemented earlier ART, or PrEP for couples, due to cost constraints. A risk score for couples allows for rapid risk assessment making the identification of target populations feasible in clinical settings. An example of the simplicity of the risk scoring tool is demonstrated by an example risk scoring card in Figure 3. Scores ≥5 were associated with an annual HIV-1 incidence of >3% in our cohorts (Figure 1), with higher incidences for scores of 6, 7 or ≥8. For HIV-1 prevention and research programs, determining a score cut-off to discriminate higher-risk couples for prioritized prevention interventions will need to take into account HIV-1 incidence, proportion of the population across different risk scores, and available resources.
Figure 3.
HIV-1 acquisition risk score worksheet.
In addition to programmatic roll-out, our results have relevance for recruitment for clinical research studies, such as large clinical trials of candidate prevention strategies for couples. HIV-1 incidence is the main determinant of the size of HIV-1 prevention efficacy trials, and novel methods for accurately estimating HIV-1 incidence for planning of trials are needed, to reduce the number of participants and follow-up time required to identify effective interventions [40]. Several HIV-1 prevention trials have ended with no discernible effect of an intervention, in part due to low HIV-1 incidence [41–43], and thus effective interventions may have been “missed” by trials that did not accurately anticipate HIV-1 incidence [43].
Although our risk score was derived from a study that was conducted in seven African countries, a limitation of our analysis is the lack of broad validation to different populations of couples; our research cohorts recruited couples in stable relationships with relatively low overall HIV incidence (~2–3% per year). However, our populations reflect the motivated subpopulation of HIV-1 serodiscordant couples who would present for research studies and to clinics to access novel HIV-1 prevention interventions – precisely the group for whom this scoring tool could be implemented. Our results do not derive from couples who are unaware of their serodiscordancy, who may face very high HIV-1 incidence [44], but such couples would also be unlikely to access prevention services – efforts to promote testing as a couple thus remain critically important. All HIV-1 infected partners in our derivation cohort were co-infected with HSV-2; however, HSV-2 seroprevalence is >80% among HIV-1 infected persons in sub-Saharan Africa [45]. Although HSV-2 seropositivity is a risk factor for HIV-1 acquisition [46], we did not include HSV-2 serostatus of HIV-1 uninfected partners as a potential predictor in our models because HSV-2 serologic testing is not broadly available in most clinical settings in Africa. We retained HIV-1 plasma viral load in our final model, given the importance of this factor in predicting HIV-1 transmission; however, we were able to identify a risk score that would sufficiently identify highest-risk couples even without the inclusion of plasma viral load for setting where viral load assays are not available. Notably, we previously reported that 30% of HIV-1 transmissions in our derivation cohort occurred from outside the study partnership [29], emphasizing that characteristics of the infected partner (like plasma HIV-1 levels) alone are likely not fully sufficient to predict transmission risk. Importantly, our risk score related predictor variables to all HIV-1 acquisitions, not just those determined to have occurred within the partnership, as ultimately HIV-1 prevention programs would want to prevent all new infections.
Operations research is needed to determine the feasibility of implementing this risk score in diverse research, clinical and HIV-1 testing settings and the impact on behaviors, costs, and programmatic and study efficiency. Further validation of our risk score in additional cohorts should be considered before widespread implementation. Importantly, this risk score was developed to identify couples at highest risk of HIV-1 transmission, but it is not necessarily a method for individual risk counseling. In our analyses, a low score did not indicate zero HIV-1 risk, and all serodiscordant couples should be counseled about risk-reduction strategies, including behavior change, condoms, and treatment of sexually transmitted infections that might facilitate HIV-1 transmission. Additionally, ongoing assessment of risk should be conducted among couples for changes in behavior and clinical progression that could impact HIV-1 transmission risk. Nonetheless, novel prevention strategies, such as PrEP, may have their greatest impact, as well as an appropriate balance of benefits versus potential toxicity, if targeted to those at greatest risk. Clinical research protocols frequently include behavioral risk characteristics in eligibility assessment, and evaluation of risk has been recommended in initial guidance documents related to PrEP for HIV-1 prevention [47].
To maximize use of resources, there is a crucial need to identify those subpopulations at highest risk for targeted prevention. Implementation of new prevention strategies and programmatic roll-out of interventions must consider efficient risk assessment that will target high-risk populations to achieve the greatest impact on reducing new HIV-1 infections. A simple, quantitative risk score could offer a robust, usable method for identifying HIV-1 serodiscordant couples at highest risk for HIV-1 acquisition.
Acknowledgments
Funding: The United States National Institutes of Health (grant R01-MH095507) and the Bill and Melinda Gates Foundation (grants #26469 and 41185).
We gratefully acknowledge the invaluable contributions of the HIV-1 serodiscordant couples that participated in this study. We thank the teams at the study sites and at the University of Washington for work on data and sample collection and management.
Partners in Prevention HSV/HIV Transmission Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins
Study sites and site principal investigators: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Jairam R. Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly R. Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Previous Presentation: This work was presented in part at the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, March 5-8, 2012.
Conflict of Interest: None of the authors have commercial or other conflicts of interest related to the content of this manuscript.
References
- 1.UNAIDS. AIDS epidemic update. 3 Vol. 304. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization; 2009. [Google Scholar]
- 2.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 3.Chemaitelly H, Cremin I, Shelton J, et al. Distinct HIV discordancy patterns by epidemic size in stable sexual partnerships in sub-Saharan Africa. Sex Transm Infect. 2012;88(1):51–57. doi: 10.1136/sextrans-2011-050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liechty CA. The Evolving Role of HIV Counseling and Testing in Resource-limited Settings: HIV Prevention and Linkage to Expanding HIV Care Access. Curr Infect Dis Rep. 2005;7(2):154–158. doi: 10.1007/s11908-005-0076-z. [DOI] [PubMed] [Google Scholar]
- 5.Grabbe KL, Bunnell R. Reframing HIV prevention in sub-Saharan Africa using couple-centered approaches. JAMA. 2010;304(3):346–347. doi: 10.1001/jama.2010.1011. [DOI] [PubMed] [Google Scholar]
- 6.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17(5):733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 7.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37(5):1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker SG, Bukusi EA, Odoyo J, et al. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med. 2011;12(5):316–321. doi: 10.1111/j.1468-1293.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser R, Bunnell R, Hightower A, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One. 2011;6(3):e17842. doi: 10.1371/journal.pone.0017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesh KK, de Bruyn G, Lurie MN, et al. Sexual Risk Behaviors Among HIV-Infected South African Men and Women with Their Partners in a Primary Care Program: Implications for Couples-Based Prevention. AIDS Behav. 2011 Apr 8; doi: 10.1007/s10461-011-9941-y. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 12.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300(5):540–549. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 14.Fox J, White PJ, Weber J, et al. Quantifying sexual exposure to HIV within an HIV-serodiscordant relationship: development of an algorithm. AIDS. 2011;25(8):1065–1082. doi: 10.1097/QAD.0b013e328344fe4a. [DOI] [PubMed] [Google Scholar]
- 15.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011 Aug 11;365 (6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten J, Celum C. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: The Partners PrEP Study. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July 2011; Rome, Italy. [Google Scholar]
- 18.WHO. Guidance on couples HIv testing and counseling and antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 19.Wasson JH, Sox HC, Neff RK, et al. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 20.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277(6):488–494. [PubMed] [Google Scholar]
- 21.Beattie P, Nelson R. Clinical prediction rules: what are they and what do they tell us? Aust J Physiother. 2006;52(3):157–163. doi: 10.1016/s0004-9514(06)70024-1. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro SE. Guidelines for developing and testing clinical decision rules. West J Nurs Res. 2006;28(2):244–253. doi: 10.1177/0193945905283722. [DOI] [PubMed] [Google Scholar]
- 23.McGinn TG, Guyatt GH, Wyer, et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009 Sep;36(9):547–555. doi: 10.1097/OLQ.0b013e3181a9cc41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lingappa JR, Kahle E, Mugo N, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the partners study. PLoS One. 2009;4(4):e5272. doi: 10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012 Jan;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell MS, Mullins JI, Hughes JP, et al. Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLoS One. 2011;6(3):e16986. doi: 10.1371/journal.pone.0016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiernan M, Kraemer HC, Winkleby MA, et al. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods. 2001;6(1):35–48. doi: 10.1037/1082-989x.6.1.35. [DOI] [PubMed] [Google Scholar]
- 31.Hira SK, Ngandu N, Wadhawan D, et al. Clinical and epidemiological features of HIV infection at a referral clinic in Zambia. J Acquir Immune Defic Syndr. 1990;3(1):87–91. [PubMed] [Google Scholar]
- 32.Beyrer C. HIV epidemiology update and transmission factors: risks and risk contexts--16th International AIDS Conference epidemiology plenary. Clin Infect Dis. 2007;44(7):981–987. doi: 10.1086/512371. [DOI] [PubMed] [Google Scholar]
- 33.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308(5728):1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 34.McClellan MK, Patel R, Kadzirange G, et al. Fertility desires and condom use among HIV-positive women at an antiretroviral roll-out program in Zimbabwe. Afr J Reprod Health. 2010;14(2):27–35. [PMC free article] [PubMed] [Google Scholar]
- 35.Heffron R, Were E, Celum C, et al. A prospective study of contraceptive use among African women in HIV-1 serodiscordant partnerships. Sex Transm Dis. 2010;37(10):621–628. doi: 10.1097/OLQ.0b013e3181e1a162. [DOI] [PubMed] [Google Scholar]
- 36.Gray RH, Li X, Kigozi G, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 37.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 38.Hallet TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modeling study. PLoS Med. 2011 Nov;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. Guidance on couples HIV testing and counseling including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. Geneva: World Health Organization; Apr, 2012. [PubMed] [Google Scholar]
- 40.GHPW Group, editor. Bill & Melinda Gates Foundation and Henry J. Kaiser Family Foundation. Global HIV Prevention Working Group; 2006. New approaches to HIV prevention: accelerating research and ensuring future access. GHPW Group, ed. [Google Scholar]
- 41.Lagakos SWGA, editor. Methodological challenges in biomedical HIV prevention trials. Washington, D.C: The National Academies Press; 2008. [Google Scholar]
- 42.Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3(1):e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padian NS, McCoy SI, Balkus JE, et al. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS. 2010;24(5):621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 45.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(S1):24–35. [PubMed] [Google Scholar]
- 46.Corey L, Wald A, Celum CL, et al. The effects of herpes simplex virus-1 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 47.CDC. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. Centers for Disease Control and Prevention. MMWR. 2011 Jan 28;60(03):65–68. [PubMed] [Google Scholar]