Abstract
Background
Altered ventilatory pattern and increased energy expenditure are facets of the complex cystic fibrosis (CF) phenotype. Whether these are inherent attributes of CF, secondary consequences of lung infection or other disease complications is not known.
Methods
Studies were performed in congenic C57BL/6J, F508del (Cftrtm1kth) and CF gut-corrected (F508del) mice. Ventilatory patterns were measured using whole-body plethysmography. Indirect calorimetry was used to determine oxygen consumption, carbon dioxide production and resting energy expenditure.
Results
CF mice (F508del and F508del gut-corrected) have a significantly faster respiratory rate and increased ventilatory pattern variability as compared to non-CF mice. F508del but not CF gut-corrected mice had significantly increased energy expenditure per gram body weight.
Conclusions
CF mice exhibit a faster, more variable ventilatory pattern. These changes were present in the absence of detectable infection or illness due to gastrointestinal obstruction. Increased resting energy expenditure does not completely account for these differences.
Keywords: Cystic Fibrosis, Ventilatory Pattern, Energy Expenditure, Respiratory Rate
1. Introduction
Cystic fibrosis (CF) patients exhibit increases in both respiratory rate (1–3) and energy expenditure (4). Changes in ventilatory pattern are thought to be related to the development of lung infection and disease progression; however, alterations in energy expenditure are associated with abnormal breathing patterns in other chronic pulmonary diseases (5). In CF, elevated resting energy expenditure has been reported independent of pulmonary function and nutritional status. The degree to which changes in ventilatory pattern are apparent prior to the development of lung infection and gut obstruction is not known. If alterations in ventilatory pattern manifest in the absence of infection then other mechanisms must contribute to their underlying etiology. To begin to address this knowledge gap, we tested the hypothesis that the absence of functional Cftr in mice will result in differences in respiratory rate, ventilatory pattern variability and energy expenditure.
Increased breathing rate in humans with CF is one component of ventilatory pattern that has been reported, but the mechanisms are not completely understood (1). Munder and colleagues (6) reported an increase in respiratory rate in CF mice with a hypomorphic mutation when compared to their wild type littermates. Development of infection and progression of lung disease are known to alter ventilatory patterns (7), but these may not be the only contributors to changes in breathing pattern in CF. There is evidence of a developmental role for CFTR in the lung (8) and diaphragm (9), which may influence pulmonary mechanics. Also, nitric oxide was reported to be decreased in the lungs of CF patients (10–12), and low levels of nitric oxide and subsequent reduction in airway relaxation have been linked to airway obstruction (13).
Another potential contributor to ventilatory pattern is energy homeostasis, in which breathing increases or decreases in response to energy demands for oxygen consumption and carbon dioxide production. Elevated energy expenditure has been reported in CF patients with good pulmonary function, nutritional status, and athletic fitness (4, 14) (15). For example, elevated energy expenditure was reported in 6 month old infants who were “clinically stable” with no progressive pulmonary disease (16). These findings suggest that elevated energy expenditure may be a “CF-specific” intrinsic defect affecting development of pulmonary disease and other co-morbidities. However, it is unknown if a relationship exists between energy expenditure and ventilatory pattern in CF.
As the CF mouse rarely shows signs of pulmonary infection, unless manipulated to do so, it provides a tool to examine the inherent differences in ventilatory pattern and energy expenditure in the absence of confounding factors such as infection. In addition, the capability to provide functional Cftr by transgene expression in the intestinal tract of CF mice permits characterization of the ventilatory pattern phenotype in the absence of illness due to gut obstruction. Further, CF mouse models are pancreatic sufficient and do not have apparent malabsorbtion (17) and thus serve as a model for the study of energy balance independent of malnutrition. Here we describe elevated respiratory rate and increased ventilatory pattern variability in CF mouse models, all in the absence of pulmonary infection, malnutrition and distress from gastrointestinal complications. Further, changes in resting energy expenditure do not completely account for these differences. The data provide compelling evidence that altered ventilatory pattern is an inherent feature of CF.
2. Methods
2.1 Mouse models
Two well-described mouse models of cystic fibrosis were examined: 1) F508del (Cftrtm1kth) the murine version of the most common human CF mutation (18), and 2) the so-called “gut-corrected” mice, (Tg(FABPCFTR)), in which the mice are homozygous for a mutation in the endogenous Cftr gene but expressing human CFTR from the rat fatty acid binding protein 1 promoter to prevent intestinal obstruction (19). To make a better comparison between our strains of mice we substituted the F508del mutation (Cftrtm1kth) for the null mutation reported for the original gut-corrected mouse (19). The F508del gut-corrected mouse with the F508del mutation displays a phenotype comparable to that reported for the null mutation (19, 20). Both of these CF mouse models are congenic on the C57BL/6J background, thus wild type C57BL/6J were used as controls. Each mouse model strain is backcrossed to the C57BL/6J background every five generations to account for possible genetic drift between strains. The Institutional Animal Care and Use Committee of Case Western Reserve University approved the experimental protocols. All mice used in the study were adults (at least 6 weeks old). Non-CF mice (n=11; 73% male) weighed 25±5g, F508del mice (n=11, 45% male) weighed 13±4g and FABP-F508del mice (n=6; 60% male) weighed 26±2g. All mice were allowed unrestricted access to chow (Harlan Teklad 7960; Harlan Teklad Global Diets, Madison, WI) and sterile water with an osmotic laxative, Colyte (Schwarz Pharma, Milwaukee, WI), and were maintained on a 12h light, 12h dark cycle at a mean ambient temperature of 22°C.
2.2 Whole-body plethysmography
Respiratory patterns were recorded in spontaneously breathing mice in a temperature equilibrated whole-body plethysmograph (Sampling rate = 200 Hz) following an acclimatization period as described previously (21). Pressure changes in the chamber were converted to signals representing ventilatory pattern, passed through a pre-amplifier (Max II, Buxco Electronics), acquired (Power1401, CED, Cambridge, UK) and stored with respiratory acquisition software (Spike 2, CED) for further analysis of breathing-pattern dynamics. Ventilatory pattern was quantified for 60 s epochs. Average values of three epochs are reported. Augmented breaths, sighs, swallows, obvious movements and gasps were excluded from the analysis, and animals were recorded under comparable conditions.
2.3 Indirect calorimetry
Oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio (RER) were determined using an Oxymax indirect calorimetry system (Columbus Instruments, Columbus, Ohio). Non-CF (n=4), F508del (n=4) and CF gut-corrected (n=3) mice were weighed and placed individually into enclosed plastic indirect calorimetry chambers, and given access to ad libitum water and food supply. The instrument was calibrated against a standard gas mixture containing defined quantities of oxygen, carbon dioxide, and nitrogen. After brief acclimatization and instrument calibration, collection of experimental data began and proceeded for ~24 hours under 12:12-h light-dark cycle at room temperature. Total energy expenditure was computed using a modified Weir equation (TEE = (3.815 + 1.232 × RER)×VO2).
2.4 Statistical Analysis
Linear and information theory-based analytical techniques were applied to stationary, three artifact-free epochs (60 s) of the raw plethysmography signal as described previously (22, 23). Respiratory cycle length (TTOT) was determined for each breath. Mean respiratory rate (RR) and coefficient of variation of respiratory cycle length (CV-TTOT = SD/mean) were calculated for each epoch and averaged to yield a value for each mouse.
Autocorrelation functions were computed for each epoch (22, 23) and showed characteristic relationships with the cycle length. We report the autocorrelation coefficient at the average respiratory cycle length as a measure of the strength of linear correlations in the time series.
An index of nonlinear complexity (22, 23) was generated by comparing the absolute value of statistically significant differences between the sample entropy of surrogate data sets and that of original data. As the breathing pattern is overall highly periodic, we computed the nonlinear complexity from adjacent points (separated by 5 ms) to one cycle length. Surrogate data sets (n=19) were constructed by shuffling the raw plethysmography recording while maintaining the amplitude distribution and the autocorrelation function of the original data set. This preserves linear correlations but destroys nonlinear relationships in the time series.
Data are presented as means ± standard deviation. A One-Way ANOVA was performed across the three groups (non-CF, F508del and F508del gut-corrected); if statistical significance was found (p<0.05), then an adjustment for multiple comparisons (Student-Newman-Keuls Method) was performed to determine significance among groups.
3. Results
3.1 Respiratory rate
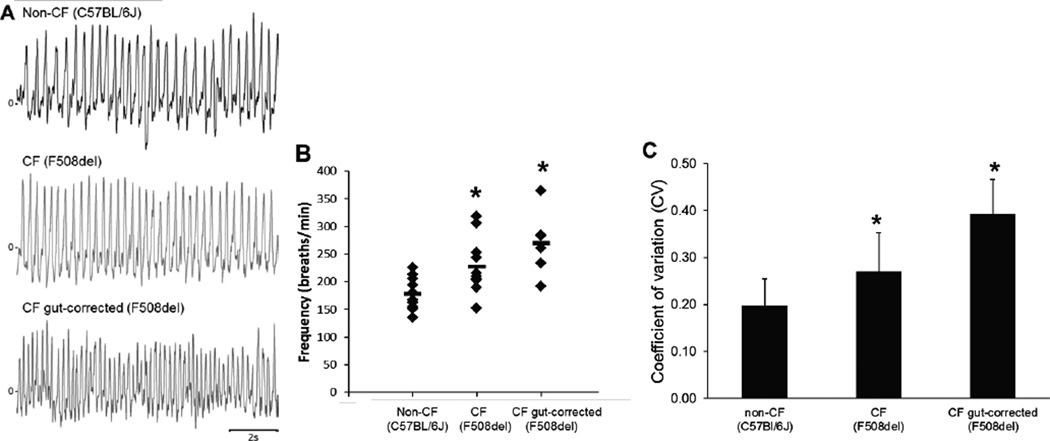
We used whole-body plethysmography to characterize breathing patterns in non- CF (C57Bl/6J) and CF (F508del and F508del gut-corrected) mice (Figure 1A). The F508del CF mice showed a significantly faster respiratory rate as compared to non-CF mice (227±50 vs. 179±28 breaths per minute; p=0.017; Figure 1B). Similarly, CF F508del gut-corrected mice had breathing frequencies (270±58 breaths per minute; Figure 1B) that were significantly faster than non-CF mice (p=0.001) and more similar to F508del CF mice (p=0.072). There were three CF mice (2 F508del and 1 F508del gut-corrected) with respiratory rates greater than 300 breaths per minute. Weight and age of these mice were statistically similar to others in their groups. To determine if these potential outliers were responsible for the statistical significance of the comparisons, analyses (One-Way ANOVA with Student-Newman-Keuls Method to account for multiple comparisons) were repeated with these three mice excluded. Results of this subgroup analysis were unchanged. Specifically, respiratory rates in non- CF mice remained significantly slower than F508del mice (208±29 breaths per minute; p=0.030) and F508del gut-corrected mice (251±39 breaths per minute; p<0.001).
Figure 1.
Ventilatory pattern and frequency are altered in CF mice. (A) Representative tracings of breathing pattern of non-CF (C57BL/6) mice (top), congenic CF F508del mice (middle) and congenic CF F508del gut-corrected mice (bottom). CF Mice (F508del and F508del gut-corrected) had a higher respiratory rate (B) and coefficient of variation of respiratory cycle length (C) than non-CF (C57BL/6) mice. *p<0.05 compared to non-CF (C57BL/6) mice.
These changes in respiratory rate were due to changes in the duration of both inspiratory and expiratory duration. Inspiratory time in non-CF mice (0.13±0.02 s) was significantly longer than F508del (0.10±0.02 s; p<0.001) and F508del gut-corrected (0.06±0.01 s; p<0.001) mice. Likewise, expiratory time tended to be longer in non-CF mice (0.21±0.04 s) than F508del (0.18±0.04 s; p=0.054) and F508del gut-corrected (0.15±0.02 s; p=0.014) mice.
3.2 Ventilatory pattern
F508del CF mice exhibited greater variability in their ventilatory patterns. Coefficient of variation (CV) of respiratory cycle length (Figure 1C) was significantly higher in both F508del (0.27±0.08; p=0.026) and F508del gut-corrected mice (0.39±0.07; p<0.001) than in non-CF mice (0.19±0.05). This increased variability was evident in the CV of the duration of inspiration and expiration. CV of inspiratory time in non-CF mice (0.15±0.05) was significantly lower than F508del (0.22±0.06; p=0.011) and F508del gut-corrected (0.39±0.03; p<0.001) mice. Likewise, CV of expiratory time was lower in non-CF mice (0.26±0.10) than F508del (0.39±0.11; p=0.046) and F508del gut-corrected (0.49±0.10; p<0.001) mice.
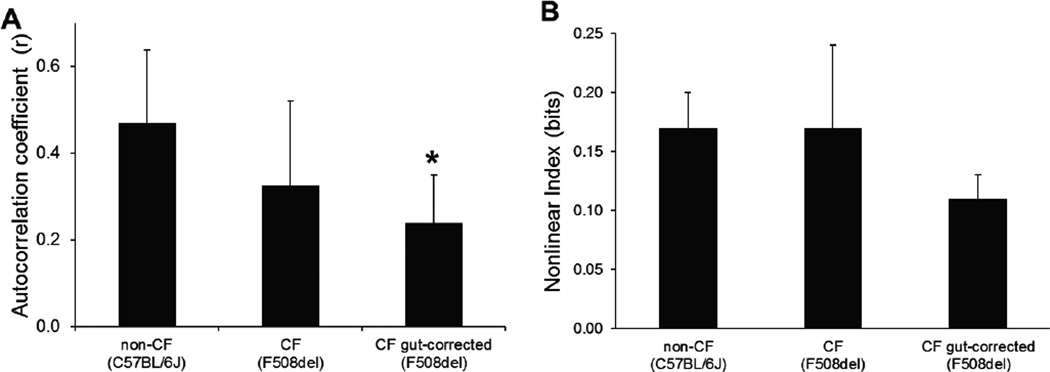
To further quantify differences in ventilatory pattern variability the autocorrelation coefficient was measured at a time lag corresponding to the average respiratory cycle length (Figure 2A). The autocorrelation coefficient tended to be lower in F508del (0.33±0.19; p=0.061) and F508del gut-corrected (0.24±0.11; p=0.035) mice as compared to non-CF controls (0.47±0.17). A measure of nonlinear complexity of the ventilatory waveform was similar between non-CF control mice (0.17±0.03 bits), F508del (0.17±0.07 bits) and F508del gut-corrected mice (0.11±0.02 bits) (Figure 2B, ANOVA p=0.073).
Figure 2.
(A) Autocorrelation coefficient was decreased at the average cycle length for CF mice as compared to non-CF mice. These data are consistent with the increase in the coefficient of variation. (B) An index of nonlinear complexity was similar between all three groups of mice. *p<0.05 compared to non-CF (C57BL/6) mice.
3.3 Energy Expenditure
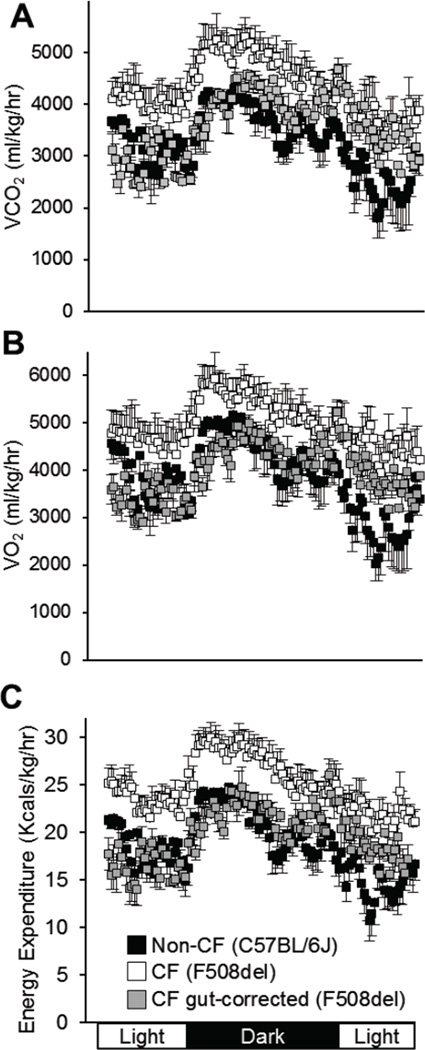
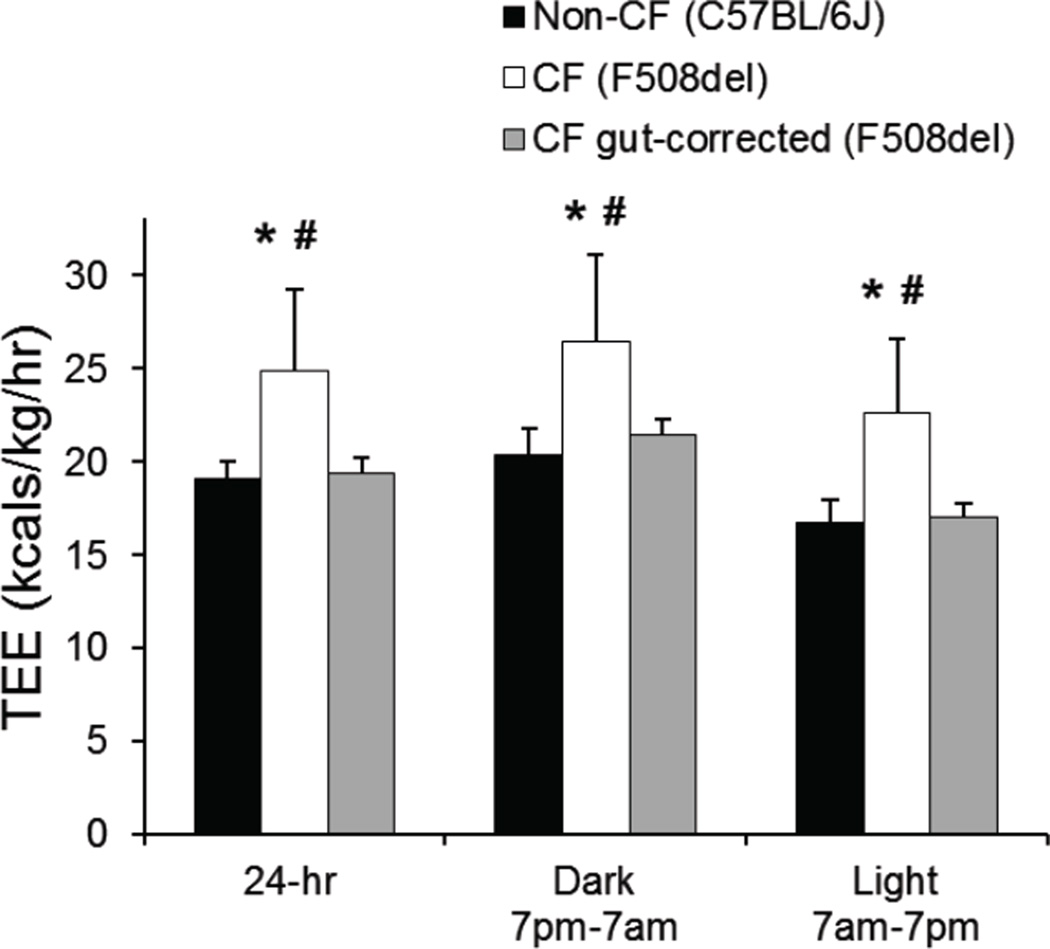
As alterations of respiratory pattern may be related to differences in energy expenditure, we measured carbon dioxide production and oxygen consumption, and calculated total energy expenditure per kilogram body weight over a 24-hour period. Overall patterns are shown in Figure 3. We found that F508del CF mice had significantly elevated average 24-hour total energy expenditure (F508del CF: 25.0±3.0 vs. non-CF: 19.1±0.9 Kcals/kg/hr, p=0.007, Figure 4). Interestingly, the F508del gut-corrected mice had 24-hour total energy expenditure (19.4±0.8 Kcals/kg/hr) which was significantly lower than F508del CF mice (p=0.006), and appeared more similar to non-CF mice (p=0.842). These relationships persisted when dark (active) and light (sleep) periods were analyzed separately (Figure 4).
Figure 3.
24-hour profiles for carbon dioxide production (A), oxygen consumption (B) and total energy expenditure (C) per kg body weight are shown for non-CF (C57BL/6) (black squares), CF F508del (white squares) and CF F508del gut-corrected (grey squares) mice. Data represent average values ± standard deviation for all animals at each time point. CF F508del mice had significantly elevated energy expenditure in both the dark and light phases, and across the 24- hour time period, as quantified in Figure 4.
Figure 4.
Average total energy expenditure per kg body weight is shown for the total 24-hour period, and for the dark (active) and light (rest) phases for non-CF (C57BL/6) (black bars), CF F508del (white bars) and CF F508del gut-corrected (grey bars) mice. Data represent average values ± standard deviation. *p<0.05 compared to non-CF (C57BL/6) mice; #p<0.05 compared to CF F508del gut-corrected mice.
4. Discussion
4.1 Rationale and results
Pulmonary pathology is the primary cause of CF-related morbidity and mortality, and identifying an animal model that spontaneously exhibits a pulmonary phenotype consistent with CF human disease continues to be a high priority. Although spontaneous pulmonary infections have not been reported in CF mice, these animals do exhibit altered airway mechanics including impaired relaxation, apparently due to decreased nitric oxide production (13). In addition, CF mice have increased airway resistance and decreased compliance (24), further demonstrating functional changes in the murine CF airway in the absence of infection and, presumably, inflammation. These findings suggest that although the CF mouse may not fully recapitulate CF pulmonary disease, it may still have significant utility in understanding intrinsic properties of CF pulmonary physiology.
The overall goal of the present study was to investigate correlates of human CF pulmonary phenotypes in CF mice. The mice utilized in this study were congenic in order to minimize variation from genetic background differences. Here we investigated the hypothesis that the absence or reduction of Cftr function in mice would result in differences in resting energy expenditure and ventilatory pattern. We found that, at rest and in the absence of intrinsic gut disease or detectable pulmonary infections, CF mice have a markedly faster breathing rate and greater breath-to-breath variability. CF mice also have significantly elevated resting energy expenditure as compared to their non-CF littermates. In contrast, F508del gut-corrected mice do not have an elevated total energy expenditure, with energy expenditure levels similar to control mice.
Munder and colleagues recently conducted a study of age-dependent susceptibility to Pseudomonas infection, and reported an increased respiratory rate in uninfected CF mice with a hypomorphic Cftr mutation using non-invasive head-out spirometry (6). Using whole-body plethysmography, we also observed an overall increased respiratory rate in F508del CF mice as compared to control mice. It is worth noting that the respiratory rates we observe in non-CF C57BL/6J mice are similar to those reported by others (25), though lower than those in the non-CF C57BL/6J group reported by Munder et al. (6). Though it is possible that differences in age, Cftr genotype or the head-out spirometry technique contribute to the overall differences in breathing rates, the results of both studies indicate that CF mice breathe faster than non-CF mice. This prompted us to apply additional techniques to quantify ventilatory pattern and to determine if increased breathing rate is associated with metabolic status.
4.2 Potential mechanisms
There are a number of potential mechanisms that might explain the observed differences in ventilatory pattern and energy expenditure. First, F508del mice exhibit a relatively strong propensity for intestinal obstruction, and are very small in both length and weight when compared to non-CF littermates (20). In order to determine if the increased respiratory rate observed in these mice was attributable to their overall health rather than a specific pulmonary manifestation, we examined the breathing rates of the F508del gut-corrected mice. These mice are completely protected from the obstruction phenotype of the F508del mouse, and overall are healthier (20). In our study, the F508del gut-corrected mice also exhibited an increased respiratory rate compared to non-CF mice, indicating that this phenotype is not a consequence of distress resulting from intestinal dysfunction.
Second, energy homeostasis can alter respiratory patterns, and we hypothesized that differences in metabolic rate might underlie the increase in respiratory frequency. F508del mice had higher resting energy expenditure than non-CF mice. Changes in cardiac output may alter energy expenditure. We did not quantify cardiac output, but studies in CF patients have not shown a cardiac limitation to exercise, but rather heart rate and ventilatory responses consistent with a pattern of ventilatory limitation (26, 27). Thus, it is unlikely that altered cardiac output in the CF mice is a key determinant of energy expenditure. However, we did not measure heart rate or LV stroke volume in our mice. Thus, our data cannot exclude the possibility that changes in circulation may contribute to the observed differences in energy expenditure.
It is also possible that increased work by respiratory muscles may contribute to this elevated energy expenditure. Cftr dysfunction in respiratory and skeletal muscles has been shown to contribute to altered metabolism and breathing rate (9, 28). The causal relationship between these differences in energy expenditure and ventilatory pattern remains to be determined. Interestingly, the higher resting energy expenditure observed in the F508del mice was not apparent in the F508del gut-corrected mice. Both CF mouse models (F508del and F508del gut-corrected) have increased respiratory rates, however only the F508del mice have elevated energy expenditure levels. Taken together, these findings suggest that increased energy expenditure is not solely responsible for the increased respiratory rate.
Systemic factors such as anemia (29) and fever (30) can increase respiratory rate. Further, anemia is a common complication of CF with an incidence that increases with age (31). Body temperature and hemoglobin levels were not measured in this study. Thus, we cannot exclude the possibility that changes in these physiologic parameters may contribute to the observed differences in ventilatory pattern.
Differences in pulmonary structure and function may also alter ventilatory pattern. The pulmonary and respiratory system in CF patients is characterized by many features, including mucus plugging, impaired mucociliary clearance, infection, inflammation, airway hyper-reactivity, reduced airway compliance, bronchiectasis and fibrosis. While mucus plugging appears to be an early manifestation of the CF airways, the temporal characteristics of the other traits is not clear as few studies are carried out in very young patients and thus are difficult to assess in the absence of lung infection. The airways of the CF mouse, in contrast, do not appear to display mucus plugging or impaired clearance, and no signs of bronchiectasis have been reported. Older CF mice do, however, develop signs of altered lung architecture, mount an inflammatory response that is slow to resolve, and even show signs of airway hyperreactivity (32, 33). However, the mice used in this study were young adults, suggesting that these changes were unlikely to fully explain the observed differences in ventilatory rate and pattern. In addition, mice utilized in this study showed no evidence of acute infection despite routine monitoring with post-mortem cultures of broncheoalveolar lavage fluid. It is possible, however, that alterations in airway physiology may contribute to the observed phenotype. For example, iNOS expression is decreased in murine and human CF epithelial cells (34). Studies in CF mice (13) identified decreased nitric oxide (NO) levels, impaired smooth muscle relaxation and increased airway resistance, which can potentially contribute to changes in frequency and ventilatory pattern.
Finally, CFTR is widely, if not ubiquitously expressed, and the F508del mice used in this study had systemic reduction of functional Cftr. Thus, it is possible that this reduced function in extra-pulmonary cell types may contribute to the observed phenotype. Bonora and colleagues (35) found that CF mice have a normal ventilatory response to hypercapnia and decreased ventilatory response to hypoxia, suggesting that neuronal ventilatory drive may be altered. However, these studies were conducted in a different mouse strain (129/BC) with a different Cftr mutation (S489X) than the mice used in our study. The importance of Cftr function in neurons or muscle cells responsible for orchestration of the ventilatory pattern will be the focus of future studies.
4.3 Clinical implications of ventilatory pattern in CF
In addition to having a faster breathing rate, both F508del and F508del gut-corrected mice exhibit a higher coefficient of variation of respiratory cycle length than non-CF mice. Moreover, the autocorrelation coefficient measured at the average respiratory cycle length was lower for the CF than for the non-CF mice. The decrease in autocorrelation coefficient, a measure of linear correlation within the ventilatory waveform, is consistent with the observed increase in coefficient of variation of respiratory cycle length. Nonlinear determinants of variability can also contribute to differences in ventilatory pattern, and changes in nonlinear variability have been described with the development of respiratory disease (21). However, no significant differences in nonlinear complexity were detected. Taken together, these findings suggest that changes in linear structure contribute to the greater breath-to-breath variation in CF mice.
The identification of differences in respiratory pattern in CF mice has potential clinical implications. More frequent, variable breaths might lead to heterogeneity in the inflation of distal lung units with the possibility of promoting regions of alveolar collapse and subsequently more anaerobic conditions for bacterial growth. More frequent breaths would also have a desiccating effect on already reduced airway surface liquid, further exacerbating the CF airway phenotype. The relationship between increased respiratory rate and CF disease progression remains to be determined. Even in the absence of a clinical mechanism, ventilatory pattern differences could be a useful marker to quantify disease severity or measure the efficacy of pharmaceutical interventions, with the potential advantage that respiratory rate measurements may be more sensitive to sub-clinical changes in overall health.
4.4 Conclusions
Increased respiratory rate has been observed in CF patients with advanced stages of lung disease and has been thought to be attributable to infection, fibrosis, or exercise (1–3). We have observed increased respiratory rates in CF mice at rest and in the absence of detectable infection or fibrosis. It is unlikely that increased resting energy expenditure is solely responsible for these differences, as F508del gut-corrected mice breathe fast, but do not have increased energy expenditure. These data suggest that altered ventilatory pattern variability, including markedly increased respiratory rate, may be in part an inherent result of the underlying genetic defect in CF, rather than a symptomatic reaction to acute pulmonary pathology. In addition, tracking changes in ventilatory pattern may hold promise as a novel biomarker to augment current well-established screening and surveillance methods for CF lung disease progression.
Acknowledgements
The authors would like to thank Thomas E. Dick for his assistance with data interpretation and David Nethery for his assistance with data analysis. We gratefully acknowledge the Case Mouse Metabolic Phenotyping Center (U24 DK76174) and thank Alma Wilson and Molly Halligan of the Case Western Reserve University CF animal core for their work maintaining the mouse colony.
Financial support: This research was supported by grants from the National Institutes of Health (RR-032425 Hodges and Drumm; DK27651 Drumm), a grant from the Cystic Fibrosis Foundation (R447-CR11 Drumm), and Award I01BX000873 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (Jacono).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, et al. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010 Sep;65(9):808–814. doi: 10.1136/thx.2009.131409. [DOI] [PubMed] [Google Scholar]
- 2.Keochkerian D, Chlif M, Delanaud S, Gauthier R, Maingourd Y, Ahmaidi S. Breathing pattern adopted by children with cystic fibrosis with mild to moderate pulmonary impairment during exercise. Respiration. 2008;75(2):170–177. doi: 10.1159/000097772. [DOI] [PubMed] [Google Scholar]
- 3.Hart N, Polkey MI, Clement A, Boule M, Moxham J, Lofaso F, et al. Changes in pulmonary mechanics with increasing disease severity in children and young adults with cystic fibrosis. Am J Respir Crit Care Med. 2002 Jul 1;166(1):61–66. doi: 10.1164/rccm.2112059. [DOI] [PubMed] [Google Scholar]
- 4.Vaisman N, Pencharz PB, Corey M, Canny GJ, Hahn E. Energy expenditure of patients with cystic fibrosis. J Pediatr. 1987 Oct;111(4):496–500. doi: 10.1016/s0022-3476(87)80107-5. [DOI] [PubMed] [Google Scholar]
- 5.Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med. 2005 Dec;99(Suppl B):S3–S10. doi: 10.1016/j.rmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Munder A, Wolbeling F, Kerber-Momot T, Wedekind D, Baumann U, Gulbins E, et al. Acute intratracheal Pseudomonas aeruginosa infection in cystic fibrosis mice is age-independent. Respir Res. 2011;12:148. doi: 10.1186/1465-9921-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bien MY, Hseu SS, Yien HW, Kuo BI, Lin YT, Wang JH, et al. Breathing pattern variability: a weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med. 2004 Feb;30(2):241–247. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JC, Lundblad LK, Bates JH, Levitzky M, Larson JE. The "Goldilocks effect" in cystic fibrosis: identification of a lung phenotype in the cftr knockout and heterozygous mouse. BMC Genet. 2004 Jul 27;5:21. doi: 10.1186/1471-2156-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010 Jun;67(6):802–808. doi: 10.1002/ana.21982. [DOI] [PubMed] [Google Scholar]
- 10.Balfour-Lynn IM, Laverty A, Dinwiddie R. Reduced upper airway nitric oxide in cystic fibrosis. Arch Dis Child. 1996 Oct;75(4):319–322. doi: 10.1136/adc.75.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotsch J, Demirakca S, Terbrack HG, Huls G, Rascher W, Kuhl PG. Airway nitric oxide in asthmatic children and patients with cystic fibrosis. Eur Respir J. 1996 Dec;9(12):2537–2540. doi: 10.1183/09031936.96.09122537. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, Nordvall SL, Weitzberg E, Kollberg H, Alving K. Exhaled nitric oxide in paediatric asthma and cystic fibrosis. Arch Dis Child. 1996 Oct;75(4):323–326. doi: 10.1136/adc.75.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mhanna MJ, Ferkol T, Martin RJ, Dreshaj IA, van Heeckeren AM, Kelley TJ, et al. Nitric oxide deficiency contributes to impairment of airway relaxation in cystic fibrosis mice. Am J Respir Cell Mol Biol. 2001 May;24(5):621–626. doi: 10.1165/ajrcmb.24.5.4313. [DOI] [PubMed] [Google Scholar]
- 14.Bowler IM, Green JH, Wolfe SP, Littlewood JM. Resting energy expenditure and substrate oxidation rates in cystic fibrosis. Arch Dis Child. 1993 Jun;68(6):754–759. doi: 10.1136/adc.68.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvadurai HC, Allen J, Sachinwalla T, Macauley J, Blimkie CJ, Van Asperen PP. Muscle function and resting energy expenditure in female athletes with cystic fibrosis. Am J Respir Crit Care Med. 2003 Dec 15;168(12):1476–1480. doi: 10.1164/rccm.200303-363OC. [DOI] [PubMed] [Google Scholar]
- 16.Davies PS, Erskine JM, Hambidge KM, Accurso FJ. Longitudinal investigation of energy expenditure in infants with cystic fibrosis. Eur J Clin Nutr. 2002 Oct;56(10):940–946. doi: 10.1038/sj.ejcn.1601441. [DOI] [PubMed] [Google Scholar]
- 17.Bederman I, Perez A, Henderson L, Freedman JA, Poleman J, Guentert D, et al. Altered de novo lipogenesis contributes to low adipose stores in cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol. 2012 Aug;303(4):G507–G518. doi: 10.1152/ajpgi.00451.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr, et al. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995 Oct;96(4):2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994 Dec 9;266(5191):1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 20.Hodges CA, Grady BR, Mishra K, Cotton CU, Drumm ML. Cystic fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2011 Sep;301(3):G528–G536. doi: 10.1152/ajpgi.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE. Lung and brainstem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol. 2011 Sep 30;178(3):429–438. doi: 10.1016/j.resp.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhingra RR, Jacono FJ, Fishman M, Loparo KA, Rybak IA, Dick TE. Vagal-dependent nonlinear variability in the respiratory pattern of anesthetized, spontaneously breathing rats. J Appl Physiol. 2011 Jul;111(1):272–284. doi: 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MADG Frank J Jacono, Wilson Christopher G, Dick Thomas E, Loparo Kenneth A. Data aquisition and complex systems analysis in critical care: developing the intensive care unit of the future. Journal of Healthcare Engineering. 2010. 2010 Sep;1(3):337–355. [Google Scholar]
- 24.Cohen JC, Lundblad LK, Bates JH, Levitzky M, Larson JE. The "Goldilocks effect" in cystic fibrosis: identification of a lung phenotype in the cftr knockout and heterozygous mouse. BMC Genet. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, PHS.] 2004 Jul 27;5:21. doi: 10.1186/1471-2156-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman L, Haines A, Klann K, Gallaugher L, Salibra L, Han F, et al. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol. 2004 Nov;97(5):1787–1795. doi: 10.1152/japplphysiol.01394.2003. [DOI] [PubMed] [Google Scholar]
- 26.Marcotte JE, Grisdale RK, Levison H, Coates AL, Canny GJ. Multiple factors limit exercise capacity in cystic fibrosis. Pediatr Pulmonol. 1986 Sep-Oct;2(5):274–281. doi: 10.1002/ppul.1950020505. [DOI] [PubMed] [Google Scholar]
- 27.Lands LC, Heigenhauser GJ, Jones NL. Cardiac output determination during progressive exercise in cystic fibrosis. Chest. 1992 Oct;102(4):1118–1123. doi: 10.1378/chest.102.4.1118. [DOI] [PubMed] [Google Scholar]
- 28.Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009 Jul;5(7):e1000586. doi: 10.1371/journal.pgen.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumgart HL, Altschule MD. Clinical significance of cardiac and respiratory adjustments in chronic anemia. Blood. 1948 Apr;3(4):329–348. [PubMed] [Google Scholar]
- 30.Nijman RG, Thompson M, van Veen M, Perera R, Moll HA, Oostenbrink R. Derivation and validation of age and temperature specific reference values and centile charts to predict lower respiratory tract infection in children with fever: prospective observational study. BMJ. 2012;345:e4224. doi: 10.1136/bmj.e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Drygalski A, Biller J. Anemia in cystic fibrosis: incidence, mechanisms, and association with pulmonary function and vitamin deficiency. Nutr Clin Pract. 2008 Oct-Nov;23(5):557–563. doi: 10.1177/0884533608323426. [DOI] [PubMed] [Google Scholar]
- 32.Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, et al. Lung disease in mice with cystic fibrosis. J Clin Invest. 1997 Dec 15;100(12):3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004 Apr;164(4):1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998 Sep 15;102(6):1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonora M, Bernaudin JF, Guernier C, Brahimi-Horn MC. Ventilatory responses to hypercapnia and hypoxia in conscious cystic fibrosis knockout mice Cftr. Pediatr Res. 2004 May;55(5):738–746. doi: 10.1203/01.PDR.0000117841.81730.2B. [DOI] [PubMed] [Google Scholar]