Abstract
Accumulating evidence demonstrates a functional role for the hippocampus in mediating relapse to cocaine-seeking behavior and extinction-induced inhibition of cocaine seeking, and dentate gyrus neurogenesis in the hippocampus may have a role. Here, we tested the hypothesis that disruption of normal hippocampal activity during extinction alters relapse to cocaine-seeking behavior as a function of dentate gyrus neurogenesis. Adult rats were trained to self-administer cocaine on a fixed-ratio schedule, followed by extinction and cocaine-primed reinstatement testing. Some rats received low frequency stimulation (LFS; 2 Hz for 25 min) after each extinction session in the dorsal or ventral hippocampal formation. All rats received an injection of the mitotic marker 5-bromo-2′-deoxyuridine (BrdU) to label developing dentate gyrus neurons during self-administration, as well as before or after extinction and LFS. We found that LFS during extinction did not alter extinction behavior, but enhanced cocaine-primed reinstatement. Cocaine self-administration reduced levels of twenty-four day old BrdU cells and dentate gyrus neurogenesis, which was normalized by extinction. LFS during extinction prevented extinction-induced normalization of dentate gyrus neurogenesis and potentiated cocaine-induced reinstatement of drug seeking. LFS inhibition of extinction-induced neurogenesis was not due to enhanced cell death, revealed by quantification of activated caspase3 labeled cells. These data suggest that LFS during extinction disrupts hippocampal networking via disrupting neurogenesis and also strengthens relapse-like behaviors. Thus, newly born dentate gyrus neurons during withdrawal and extinction learning facilitate hippocampal networking that mediates extinction-induced inhibition of cocaine seeking and may play a key role in preventing relapse.
Keywords: Low-frequency stimulation, Self-administration, Dentate gyrus, BrdU, Ki-67, activated caspase3
Introduction
The hippocampus is recognized as an important structure in learning and memory (Bast, 2007; Churchwell et al., 2010). In this context, the hippocampus plays a crucial role in the recall of fear extinction through its direct and indirect (via the medial prefrontal cortex [mPFC]) influences on the amygdala (Bruchey et al., 2007; Hugues et al., 2006; Hugues et al., 2004; Santini et al., 2001). Consistent with this role, electrophysiological findings have revealed that fear extinction is accompanied by increases in synaptic efficacy in dorsal and ventral hippocampal projections to the mPFC (Deschaux et al., 2011; Farinelli et al., 2006; Hugues and Garcia, 2007). Additionally, artificial disruption of hippocampal synaptic activity by either low-frequency stimulation [LFS; an invasive procedure that produces long-term depression (LTD); distinct from other invasive and noninvasive procedures such as intracranial injection of local anesthetic lidocaine noted for its inhibitory effects on long-term potentiation (LTP; (Smith et al., 1993)) and repetitive transcranial magnetic stimulation, noted for producing LTP-like phenomena (Wang et al., 2011), administered immediately after extinction impairs the retrieval of extinction memory (Deschaux et al., 2010; Farinelli et al., 2006; Garcia et al., 2008). These data support a role for intact hippocampal synaptic networks (i.e., hippocampal networking) in fear extinction memory. However, whether hippocampal networking, which is recruited during extinction following drug self-administration (del Olmo et al., 2006), plays a role in the recall of drug extinction memories remains to be determined.
The hippocampal formation receives projections from the mesocorticolimbic dopamine system and is involved in relapse to cocaine-seeking behavior (Kalivas and McFarland, 2003; Neisewander et al., 2000; Noonan et al., 2010; Noonan et al., 2008). The dorsal and ventral hippocampus is thought important for the association between cocaine and contexts that facilitate the development and maintenance of cocaine seeking and taking (Atkins et al., 2008; Fuchs et al., 2005; Lasseter et al., 2010; Rogers and See, 2007). Functional inactivation of the ventral hippocampus or ventral subiculum (an output region of the ventral hippocampus) attenuates cocaine-seeking behavior (Rogers and See, 2007; Sun and Rebec, 2003). Conversely, the reinstatement of extinguished cocaine-seeking behavior can be elicited by electrical stimulation of the ventral subiculum (Vorel et al., 2001). These studies demonstrate that the reinstatement of cocaine-seeking behavior critically relies on the functional integrity of the ventral hippocampus. Therefore, identifying the mechanisms that contribute to the neuroplasticity of hippocampal networks is critical for understanding drug-associated memories and relapse to drug seeking.
Spontaneous neurogenesis, a form of neuroplasticity in the hippocampal dentate gyrus, has been implicated in the maintenance of hippocampal networking (Aimone et al., 2006; Clark et al., 2012; Lacefield et al., 2012) and facilitates certain hippocampus-dependent behaviors (Deisseroth et al., 2004; Feng et al., 2001; Kim et al., 2011; Schmidt-Hieber et al., 2004). Particularly relevant is the hypothesized role for dentate gyrus neurogenesis in cocaine seeking and self-administration (Garcia-Fuster et al., 2011; Garcia-Fuster et al., 2010; Mustroph et al., 2011; Noonan et al., 2008). Such changes in the levels of spontaneous plasticity in the dentate gyrus induced by cocaine exposure may predict behavioral outcomes produced by cocaine (Recinto et al., 2011; Sudai et al., 2011).
Therefore, the present study tested the hypothesis that extinction normalizes cocaine-induced decreases in the levels of neurogenesis, and that disruption of extinction-induced neuroplastic changes by LFS in the hippocampus impairs the retrieval of extinction memory and enhances cocaine-primed reinstatement of cocaine seeking.
Methods
Animals
All of the animal procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines. Forty-one adult male Wistar rats (Charles River, Hollister, CA), weighing 280–300 g at the time of surgery, were separated into six groups (Fig. 1A-F). They were housed in groups of two or three in plastic cages with a 12 h/12 h light/dark cycle with lights on at 8:00 PM. Food and water were available ad libitum except during behavior testing.
Figure 1.
Timeline of cocaine self-administration and location of cannula placements for LFS. (A-F) Schematic representation of experimental procedures: (A) cocaine-naive rats; (B-F) cocaine rats. All cocaine rats self-administered cocaine (0.5 mg/kg; indicated by vertical lines) in 1 h sessions under an FR1 schedule followed by FR2 and FR5 schedules. Both cocaine-naïve and cocaine self-administering rats received one injection of BrdU (indicated by a syringe) to label neural progenitors in the S phase of the cell cycle. Cocaine self-administering rats were divided into four groups: (B) continued cocaine self-administration, in which BrdU was injected and self-administration was continued; (C) pre-extinction group, in which BrdU was injected before extinction sessions began; (D) post-extinction group, in which BrdU was injected after eight extinction sessions; (E) ventral LFS group, in which animals received LFS in the ventral hippocampus after each extinction session, and BrdU was injected after the last LFS into the ventral hippocampus; (F) dorsal LFS group, in which animals received LFS in the dorsal hippocampus after each extinction session, without receiving BrdU injection. (G, H) Schematic representation of LFS injection cannula placements within the dorsal and ventral hippocampus. The symbols (closed red circles) on the schematics represent the most ventral point of the infusion cannulae tracts for rats that received unilateral LFS (Paxinos and Watson, 1997). Numbers below each schematic indicate the distance from bregma in millimeters.
Surgery and apparatus
Thirty-five rats underwent surgery for intravenous catheters for cocaine self-administration (Fig. 1B-F). The rats were anesthetized with 2–3% isoflurane and implanted with a silastic catheter (0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning, Midland, MI) into the right external jugular vein under aseptic conditions (Vendruscolo et al., 2011). During self-administration sessions, each rat was placed in a standard operant chamber that was placed in a light- and sound-attenuating cubicle (28 × 26 × 20 cm; Med Associates, St. Albans, VT). The front door and back wall of the chamber were made of transparent plastic, and the other walls were opaque metal. The chamber had two retractable response levers mounted on one side of the opaque walls and a food hopper located between the levers. A stimulus light was mounted above each lever. A drug injection was delivered by a syringe pump (Razel Scientific Instruments, Georgia, VT) located on top of the cubicle. Experimental sessions were controlled and recorded by a computer with a custom interface and software in the experimental room.
Following intravenous surgeries, twenty-two rats were placed in a stereotaxic frame for unilateral (all in the right hemisphere) stimulating electrode implantation in area CA1 of the dorsal hippocampus according to coordinates 3.2 mm posterior to bregma, 1.6 mm lateral from midline, and 2.5 mm from dura or in area CA1 of the ventral hippocampus according to the coordinates 6.0 mm posterior to bregma, 5.2 mm lateral from midline, and 7.0 mm from dura (n = 11 dorsal hippocampus, n = 6 received LFS; n = 11 ventral hippocampus, n = 6 received LFS; Fig. 1G, H (Paxinos and Watson, 1997)). These electrodes were made of twisted silver wires (90 mm diameter) that were insulated except at the tip. The entire miniature system was fixed onto the skull by means of three screws and dental cement. For LFS, some rats with electrodes (dorsal or ventral hippocampus, n = 6 each) were placed into a gray plastic cylindrical cage (22 cm diameter, 30 cm height) connected to a stimulator and subjected to hippocampal LFS (train of 100-ms pulses of 500 μA at 2 Hz for 25 min). These LFS parameters were based on our previous studies that demonstrated their capacity to induce fear return (Deschaux et al., 2010; Farinelli et al., 2006; Garcia et al., 2008).
Self-administration procedure
After surgery and recovery, thirty-five rats were trained to self-administer 0.5 mg/kg per 100 μl cocaine in 1 h sessions (baseline sessions) under an FR1 schedule (i.e., every active lever press was reinforced with a dose of cocaine) for 29 sessions. A 20 s timeout period, during which responses had no scheduled consequences, followed each cocaine infusion. When the animals achieved stable responding under the FR1 schedule (i.e., less than 15% variation in response rates over three consecutive days), they were switched to an FR2 and then FR5 schedule until they achieved at least 3 days of stable performance according to the same criterion as the FR1 schedule (Fig. 1b-f). A group of rats continued cocaine self-administration until the end of the study, whereas another group of rats underwent an extinction phase after cocaine self-administration, during which cocaine was replaced with saline. Daily 1 h extinction sessions were conducted until responding was less than 25% of the response rate maintained during cocaine self-administration. Immediately after each extinction session, the rats were subjected to hippocampal LFS as described above. Following the extinction phase, cocaine priming-induced reinstatement of drug-seeking behavior was assessed by an intravenous administration of 0.1 ml cocaine (0.5 mg/kg) or saline before the beginning of the reinstatement session (1 h). The reinstatement session was identical to the self-administration session except that cocaine was not delivered. Each rat was submitted to only one reinstatement session followed the next day by re-exposure to an extinction session (1 h, FR5). Thirty-one rats successfully completed the self-administration study.
BrdU injections and perfusions
Thirty rats from five experimental groups were injected with BrdU, (Group A-cocaine-naive control, n = 10; Group B- continued cocaine, n = 5; Group C- pre-extinction, n = 4; Group D- post-extinction without LFS, n = 5 (note: only n=5 rats from a group of n=10); Group E- post-extinction with LFS in the ventral hippocampus (VH), n = 6) received one intraperitoneal injection of 150 mg/kg BrdU (Boehringer Mannheim Biochemica) dissolved in 0.9% saline and 0.007N NaOH at 20 mg/ml. We did not detect BrdU labeled cells in six rats; 4 from naïve controls and 2 from LFS-VH group. LFS-dorsal hippocampus group did not receive any BrdU injections. BrdU was injected in subgroups of cocaine rats to label S-phase cells (1) 16–22 h after the self-administration session during acute withdrawal, after which the rats continued self-administration for an additional 20 sessions (Group B), (2) 16–22 h after the last self-administration session during acute withdrawal, after which the rats learned to extinguish operant behavior when cocaine was substituted for saline for 20 sessions (Group C, pre-extinction group), (3) 2–3 h after 10 extinction sessions without LFS (Group D, post-extinction group), and (4) 2–3 h after 10 extinction sessions with LFS in the ventral hippocampus (Group E, LFS group). Perfusions were performed 20–24 days after the BrdU injections (Fig. 1a-f). After the last experimental session, rats with patent catheters were anesthetized using chloral hydrate (14 mg/kg, intravenous), and the rats without patent catheters were anesthetized using chloral hydrate (240 mg/kg, intraperitoneal) ((Lasseter et al., 2010); note: unpublished observations demonstrate that the route of administration of chloral hydrate did not produce acute effects on surviving BrdU cells in adult Wistar rats). The rats were then transcardially perfused with phosphate-buffered saline (15 ml/min over 2 min) and 4% paraformaldehyde (15 ml/min over 20 min). The brains were dissected and postfixed in 4% paraformaldehyde at 4oC for 16–20 h. Prior to sectioning in the coronal plane at a thickness of 40 μm on a freezing microtome, the entire right hemisphere of each brain was marked. The sections through the brain were collected in nine vials and one-ninth of the brain region was used for immunohistochemical analysis.
Cannula placements, antibodies, immunohistochemistry, microscopic analysis, and cellular quantification
Hippocampal sections were slide mounted, stained with Vector FastRed, and visualized under light microscopy to verify cannula placements. The most ventral portion of each cannula tract was mapped onto schematics of appropriate plates from the rat brain atlas (Fig. 1g, h (Paxinos and Watson, 1997)).
The following primary antibodies were used for immunohistochemistry (IHC): Ki-67 (1:1000), BrdU (1:400), Neuronal nuclease (NeuN, 1:50), and AC-3 (1: 500). The left (unmarked) and right (marked) hemispheres of every ninth section through the hippocampus were slide mounted, coded, and dried overnight before IHC. After incubating with primary antibodies (BrdU, Ki-67, AC-3), the sections were incubated with biotinylated secondary antibody (1:200) followed by amplification with an avidin-biotin complex, and staining was visualized with nickel-3′,3-diaminobenzidine (DAB). Visual cell quantification was performed with a light microscope (Zeiss) by an observer blind to the experimental groups. For BrdU/NeuN colabeling IHC, six bilateral sections that contained the dentate gyrus were used for confocal analysis from each rat from each group.
For IHC, immunoreactive cells in each hemisphere (ipsilateral and contralateral to the LFS site) in the subgranular zone (SGZ; i.e., cells that touched and were within three cell widths inside and outside the hippocampal granule cell-hilus border for Ki-67) or granule cell layer (GCL; mature BrdU, AC-3) were visually quantified with a Zeiss Axiophot photomicroscope at 400× magnification using the optical fractionator method, in which sections through the dentate gyrus (-1.4 to -6.7 mm from bregma (Paxinos and Watson, 1997)) were examined. Cells in the SGZ and GCL from each hemisphere were summed and multiplied by 9 to give the total number of cells (Eisch et al., 2000). For BrdU phenotype analysis, every 27th section through the hippocampus was triple-labeled with BrdU (CY2), NeuN (CY3), and glial fibrillary acidic protein (GFAP; CY5). All BrdU-immunoreactive cells in the GCL, approximately 15 BrdU-immunoreactive cells from each rat were scanned and analyzed for BrdU/NeuN or BrdU-alone labeling (cocaine naive: 11 ± 3; cocaine: 14 ± 3; pre-extinction: 11 ± 3; post-extinction without LFS: 9 ± 3; post-extinction with LFS: 17 ± 4). All labeling was visualized and analyzed using a confocal microscope (LaserSharp 2000, version 5.2, emission wavelengths 488, 568, and 647 nm; Bio-Rad Laboratories). The percentage of BrdU-immunoreactive cells that were NeuN-positive or GFAP/NeuN-negative in relation to the total number of BrdU cells was analyzed from each rat.
Statistical analysis
The results are expressed as mean ± SEM. The number of lever presses was analyzed using a two-way repeated-measures analysis of variance (ANOVA), with electrical stimulation as the between-group variable and time as the repeated measure. Fischer’s Least Significant Difference test was used for post hoc comparisons. The number of lever presses for the control group was compared between the extinction and reinstatement periods using Student’s paired t-test. One-way ANOVA was used to determine differences in the levels of BrdU, Ki-67, and AC-3 cells (dependent variables). Two-way ANOVA was used to determine hemisphere differences in levels of BrdU cells (treatment x hemisphere differences). Students-t-test was performed to determine differences between levels of BrdU cells ipsilateral and contralateral sides of LFS treatment. Statistical analyses were performed using SPSS software. The accepted level of significance for all of the tests was p ≤ 0.05.
Results
Cocaine self-administration and extinction
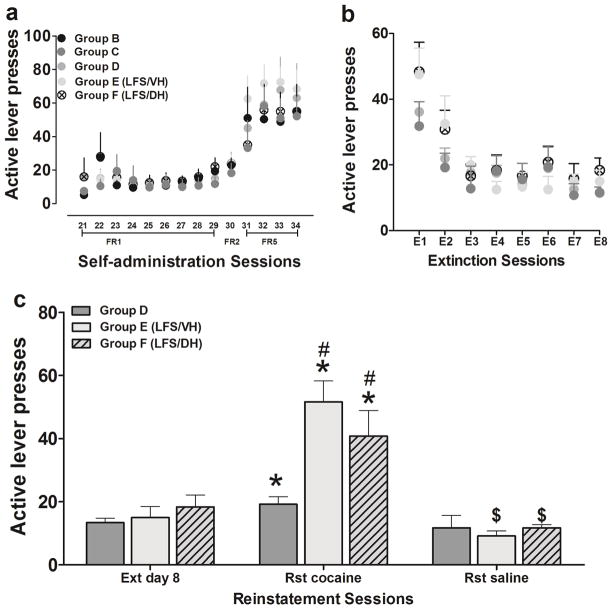
Rats in Groups B-F (Fig. 1) acquired cocaine self-administration without a significant difference in the rate of acquisition and maintenance over the entire self-administration period (Fig. 2a). The active lever presses for 0.5 mg/kg cocaine per infusion, i.v., on a fixed-ratio 5 (FR5) schedule on day 4 were the following: Group B, 54 ± 16 (n = 5); Group C, 53 ± 21 (n = 4); Group D, 51 ± 9 (n = 10); Group E-ventral hippocampus LFS, 55 ± 11 (n = 6); Group F-dorsal hippocampus LFS:, 68 ± 11 (n = 6); Fig. 2a, n.s.. Following self-administration, Groups C-E (Fig. 1) underwent extinction sessions in their operant chambers. During the extinction sessions, cocaine was no longer available. The cocaine-paired lever presses across all extinction groups were the following on the day after the first LFS session: Group C-19 ± 4 (n = 4) and Group D- 21 ± 3 (n = 10) without LFS; Group E-ventral hippocampus LFS, 30 ± 5 (n = 6); Group F-dorsal hippocampus LFS, 32 ± 8 (n = 6); Fig. 2b, n.s. Responses during the sessions gradually declined to the criterion (significant main effect of day, F7,192 = 23.81, p < 0.001), which was reached with 2–4 days of training (p < 0.05 vs. day 1). Extinction behavior did not significantly differ between groups (Fig. 2b).
Figure 2.
Cocaine-paired lever responding during self-administration, extinction, and reinstatement testing (a-c). (a) Rats from all groups did not significantly differ in rates of cocaine self-administration over FR schedules of reinforcement. (b) Rats from all groups did not significantly differ in cocaine-paired lever responses during extinction sessions. The latency to reach extinction also did not significantly differ between groups. (c) Disruption of hippocampal activity by LFS in the dorsal or ventral hippocampus enhances cocaine priming-induced reinstatement of cocaine-seeking behavior. The bar graphs (Group D without LFS, gray bars; Group E with LFS in the ventral hippocampus, light gray filled); Group F with LFS in the dorsal hippocampus, light gray hatched depict non-reinforced, cocaine-paired lever responses (mean ± SEM) during testing in extinction (Ext), cocaine-primed (cocaine) and saline-primed (saline) reinstatement (Rst). n = 4–10 each group. Data are expressed as mean ± SEM. * significant difference relative to responding during extinction (p < 0.05); # significant difference compared with Group D during reinstatement (p < 0.05); $ significant difference relative to cocaine-primed treatment (p < 0.01).
LFS after extinction enhances reinstatement of cocaine seeking
Rats that did not receive LFS during the extinction sessions (Group D) and rats that received LFS in the dorsal or ventral hippocampus after the extinction sessions (Group E and F) showed significant cocaine-primed reinstatement, but rats that received LFS in the ventral or dorsal hippocampus after the extinction sessions (Group E and F) exhibited dramatically more reinstatement (Fig. 2c). Two-way ANOVA revealed a significant effects of time (F2,45 = 32.7, p < 0.001) and treatment (F2,45 = 5.3, p < 0.01) and a significant time × treatment interaction (F4,45 = 5.1, p < 0.001). Post hoc comparisons indicated that rats in the three groups showed significant cocaine-primed reinstatement after 0.5 mg/kg cocaine priming compared with the last extinction session (p < 0.05). Moreover, the number of lever presses increased in the groups subjected to LFS in the ventral (p < 0.01) and dorsal (p < 0.05) hippocampus compared with the non-LFS group (Fig. 2). No group differences in lever presses were found after the saline challenge.
Cocaine self-administration reduces levels of spontaneous neurogenesis in the dentate gyrus
After four FR5 sessions of cocaine self-administration, neural progenitors in the synthesis (S) phase of the cell cycle in the dentate gyrus were labeled with one injection of a saturating dose of BrdU (Fig. 1B). In parallel, cocaine-naïve, age-matched rats were also injected with BrdU (Fig. 1A). Although it could be argued that this group of rats may not represent suitable ‘control’ conditions (e.g. intravenous surgery and post-surgery maintenance) compared to all other groups, unpublished observations demonstrated that invasive procedures such as intravenous surgery and antibiotic maintenance post-surgery does not significantly alter labeling and survival of BrdU cells. Therefore we are confident that the levels of BrdU cells in drug-naïve controls provide a sufficiently valid comparison for evaluating the effects of cocaine, extinction, and LFS during extinction on survival of BrdU cells. Cocaine self-administering rats continued on the FR5 schedule for 20 additional sessions and were euthanized 16 to 22 h after the last self-administration session. Because the BrdU labeled cells were 20 days old at the time of euthanasia, BrdU labeling experiments determined whether cocaine self-administration decreases the number of newly born neurons in the dentate gyrus.Mature BrdU cells were round and less clustered and presented more punctate BrdU staining (Fig. 3a, b), typical of neural progenitor cells 4 weeks after BrdU injection (Cameron and McKay, 2001; Dayer et al., 2003). One-way ANOVA (total BrdU cells) demonstrated a significant effect of cocaine on levels of neurogenesis (F4,24 = 3.6, p < 0.05). Two-way ANOVA (BrdU cells per hemisphere) did not detect a significant cells x treatment interaction (F4,38 = 0.04, p = 0.9) or effect of hemisphere (F4,38 = 0.006, p = 0.93), but showed a significant effect of cocaine self-administration on both hemispheres (F4,38 = 5.6, p < 0.01). The post hoc analysis revealed a significant decrease in the number of BrdU cells compared with cocaine-naive controls, an effect that was seen combined (p < 0.05; Fig. 3d) and individually in each hemisphere (Fig. 3c; ipsilateral side, p < 0.05; contralateral side, p < 0.05). The confocal analysis of BrdU cells showed that cocaine self-administration did not significantly alter the percentage of BrdU cells that became granule cell neurons in the dentate gyrus (Fig. 3e).
Figure 3.
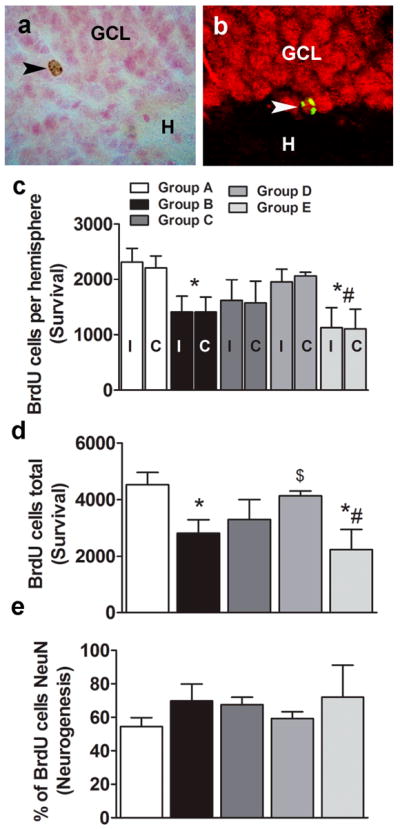
Effects of cocaine self-administration, extinction learning, and LFS during extinction learning on dentate gyrus neurogenesis. Photomicrograph of DAB-labeled (a) or fluorescent labeled, single z-stack image (BrdU, CY2, green; NeuN, CY3, red) (b) mature BrdU cell in the granule cell layer of the hippocampal dentate gyrus labeled with a BrdU antibody. Images presented are at 400x magnification. BrdU-labeled 20- to 24-day-old surviving cells are visible as oval-to-round-shaped cells. BrdU-labeled cells colocalized with NeuN cells, suggesting maturation and differentiation into granule cell neurons. GCL, granule cell layer; H, hilus. Images are 400× magnification. (c-e) Quantitative analysis of BrdU cell counts from cocaine-naive and cocaine rats from serial coronal sections. Total number of BrdU cells per hemisphere ipsilateral (I) or contralateral (C) to the LFS site (c) or combined (d). Ratio of BrdU-labeled cells colabeled with NeuN analyzed by confocal analyses (e). n = 4–6 each group. Data are expressed as mean ± SEM. *p < 0.05, compared with Group A (control); #p < 0.05, compared with Group D (non-LFS group); $p < 0.05, compared with Group B (continued cocaine group).
Extinction after cocaine self-administration normalizes cocaine-induced reduction of the number of mature BrdU cells, and LFS during extinction prevents extinction-induced normalization of neurogenesis
Because cocaine self-administration reduced the number of newly born dentate gyrus neurons, we next determined whether extinction during withdrawal following cocaine exposure alters the survival of newly born dentate gyrus neurons. Twenty four-day-old BrdU cells were quantified from Group C and D extinction groups (Fig. 1). One-way ANOVA revealed a significant effect of extinction on the cocaine-induced reduction of the levels of BrdU cells (F4,24 = 3.6, p < 0.05). The post hoc analysis revealed a significant difference between Group B (cocaine group) and Group D (post-extinction group, p < 0.05; Fig. 3c, d). No significant differences were observed between Group B (cocaine group) and Group C (pre-extinction group). These data suggest that extinction did not alter the maturation and survival of newly born cells labeled before the commencement of extinction, whereas extinction enhanced certain developmental stages of neural progenitors and normalized the levels of neurogenesis.
Rats that received LFS in the ventral hippocampus were injected with BrdU 2–3 h after LFS after the last extinction session (Fig. 1d vs. 1e) to label S-phase cells. Low-frequency stimulation occurred unilaterally, and brain tissue was marked to differentiate the ipsilateral and contralateral hemispheres. Twenty-four-day-old BrdU cells were quantified and the levels of mature BrdU cells were compared between each hemisphere and between groups. Student’s t-test did not detect significant differences in the number of mature BrdU cells between the hemispheres in LFS-exposed rats (Fig. 3c). One-way ANOVA (total BrdU cells) demonstrated a significant effect of LFS on the extinction-induced normalization of neurogenesis (F4,24 = 3.6, p < 0.05). Two-way ANOVA (BrdU cells per hemisphere) did not detect a significant cells x treatment interaction (F4,38 = 0.04, p = 0.9) or effect of hemisphere (F4,38 = 0.006, p = 0.93), but showed a significant effect of LFS on both hemispheres (F4,38 = 5.6, p < 0.01). The post hoc analysis demonstrated a significant decrease in the number of BrdU cells ipsilateral and contralateral to the LFS hemisphere compared with cocaine-naive controls (Group A, p < 0.001; Fig. 3c, d) and non-LFS rats (Group D, p < 0.01).
Subsequent experimental analysis verified whether extinction enhanced proliferation. The analysis of the levels of the endogenous cell proliferation marker Ki-67 (Fig. 4, inset) in all groups (Group A-F; Fig. 1A-F) demonstrated that extinction learning enhanced the net proliferation of neural progenitors in the dentate gyrus (F5,28 = 13.13, p < 0.005). The post hoc analysis indicated a significantly higher number of Ki-67 cells in Group C compared with all other groups (p < 0.05; Fig. 4). Group B also displayed a higher number of Ki-67 cells compared with Groups D, E, and F (p < 0.05)
Figure 4.
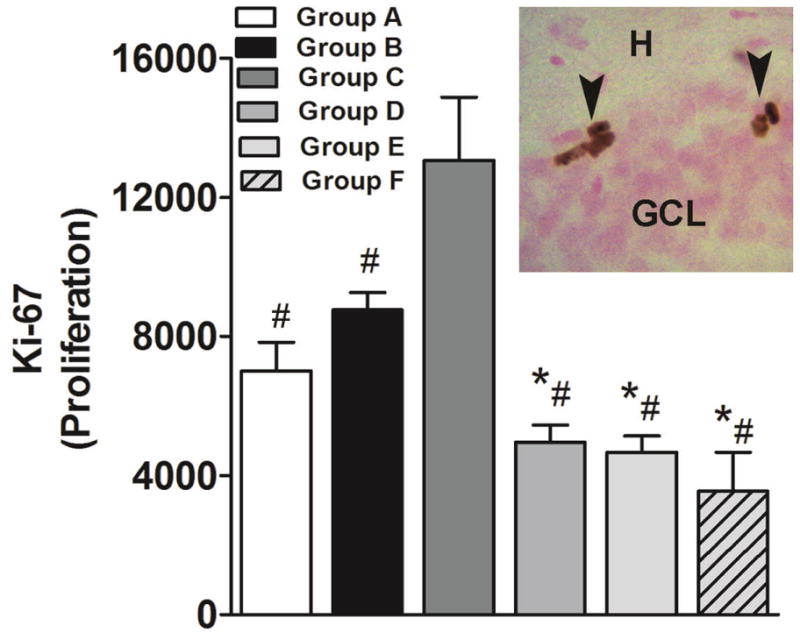
Effects of cocaine self-administration, extinction, and LFS during extinction on cell proliferation. (Main panel) Quantitative analysis of the number of DAB-labeled Ki-67-immunoreactive cells in the subgranular zone of the dentate gyrus of the hippocampus. (Inset; image presented is at 400x magnification) Photomicrograph of DAB-labeled Ki-67-immunoreactive cells. GCL, granule cell layer; H, hilus. Image is 400× magnification. n = 4–6 each group. Data are expressed as mean ± SEM. *p < 0.05, compared with Group B; #p < 0.05, compared with Group C.
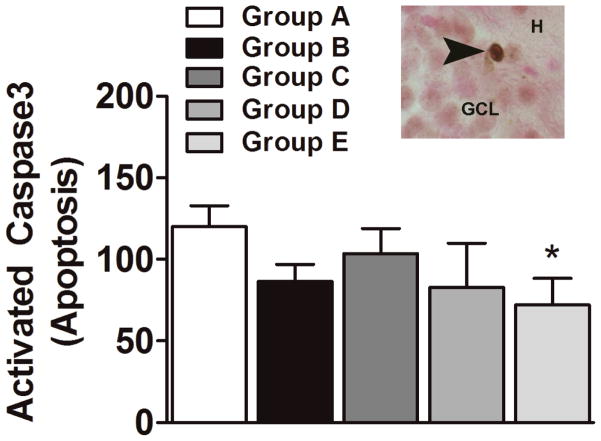
Cocaine self-administration and LFS inhibit spontaneous neurogenesis in the dentate gyrus without increasing apoptosis in the dentate gyrus
To determine whether enhanced apoptosis contributed to the cocaine- and LFS-induced reduction of the number of newly born neurons in the dentate gyrus, activated caspase 3 (AC-3)-labeled apoptotic cells were quantified. One-way ANOVA showed no significant difference in the levels of apoptosis in any of the experimental groups (Fig. 5). Unpaired t-tests demonstrated a significant difference between Group A and Group E (p = 0.05).
Figure 5.
Effects of cocaine self-administration, extinction, and LFS during extinction on cell death. (Main panel) Quantitative analysis of the number of DAB-labeled activated caspase 3 (AC-3)-immunoreactive cells in the granule cell layer of the dentate gyrus of the hippocampus. (Inset; image presented is at 400x magnification) Photomicrograph of DAB-labeled AC-3-immunoreactive cells. GCL, granule cell layer; H, hilus. Image is 400× magnification. n = 4–6 each group. Data are expressed as mean ± SEM. *p < 0.05, compared with Group A.
Discussion
The present study demonstrated that the hippocampus regulates the consolidation of the extinction of cocaine-seeking behavior. The LFS-induced disruption of hippocampal (dorsal or ventral) function during extinction did not impair extinction. However, LFS in the dorsal or ventral hippocampus during extinction was sufficient to potentiate relapse to cocaine-seeking behavior triggered by low-dose cocaine priming injections. Low-frequency stimulation in the ventral hippocampus during extinction prevented the extinction-induced normalization of hippocampal neurogenesis, thus providing a putative mechanism for LFS-induced alterations in hippocampal network function and plasticity. Furthermore, the analysis of developmental stages of hippocampal neurogenesis demonstrated that extinction increased the levels of neural progenitors in the dentate gyrus to normalize the levels of neurogenesis reduced by cocaine self-administration. Altogether, these results indicate that extinction modulates hippocampal networking to produce a form of behavioral inhibition that attenuates cocaine-seeking behavior, partly mediated by neurogenesis, and these effects are abolished by LFS, which disrupts the hippocampal synaptic network.
Inactivation of hippocampal networking by LFS during extinction was sufficient to facilitate relapse to cocaine seeking triggered by cocaine priming without altering cocaine seeking during extinction. These results suggest that the effects of LFS were associated with the motivational responses to cocaine itself (Larson et al., 2011; Sutton et al., 2003). In contrast, LFS disrupts the consolidation of extinction in cued fear conditioning (Deschaux et al., 2010; Farinelli et al., 2006; Garcia et al., 2008). These differences could involve paradigm differences between extinction learning in fear conditioning and extinction learning in cocaine-seeking tests. For example, differences in the latency to extinguish fear learning vs. operant self-administration behavior or differences in the neuroanatomical substrates involved in the extinction of fear vs. drug memories could play a role (Burke et al., 2006; Maren, 2011). Therefore, the lack of an effect of LFS on cocaine seeking during extinction may be explained by the hypothesis that decreases in the number of lever presses during extinction is more likely attributable to a neurobiological interaction between cocaine history and LFS than a mnesic effect per se.
The dorsal and ventral hippocampus (but not dentate gyrus) is required for the expression of drug context-, cue-, and cocaine priming-induced cocaine-seeking behavior in rats (Fuchs et al., 2005; Lasseter et al., 2010; Rogers and See, 2007). Additionally, electrical stimulation of glutamate fibers in the ventral subiculum induces drug context-induced cocaine seeking (Vorel et al., 2001), while inactivation of the ventral subiculum attenuates cue-induced and drug-primed reinstatement (Sun and Rebec, 2003). Interestingly, neonatal ventral hippocampal lesions produce long-lasting changes in reward sensitivity to saccharin, ethanol, cocaine, and methamphetamine. For example, neonatal ventral hippocampal lesions increase rates of acquisition, consumption, and seeking of sucrose-sweetened ethanol solutions, as well as cocaine and methamphetamine intake, and also promote relapse to cocaine seeking during adulthood (Berg et al., 2011; Brady et al., 2008; Chambers and Self, 2002). These findings suggest that disruption of the hippocampus enhances addiction liability via influencing vulnerability to drug-seeking behavior. In contrast, other studies have reported that inactivation of either the dorsal or ventral subiculum had no effect on cue- or drug priming-induced reinstatement (Black et al., 2004). These discrepancies may be attributable to the fact that the hippocampus projects to the mPFC (a brain region involved in both drug seeking and fear conditioning (Peters et al., 2009)) and that many other brain regions display synaptic changes following hippocampal train stimulation (e.g., amygdala (Maren and Fanselow, 1995), nucleus accumbens (Dong et al., 2007), and thalamus (Hugues and Garcia, 2007)). The present findings demonstrate that disruption of hippocampal activity by LFS in both the dorsal and ventral hippocampus equally facilitate relapse to cocaine seeking triggered by cocaine priming, perhaps by disrupting the consolidation of drug extinction memories. However, some caution is warranted when interpreting our cocaine- and saline-induced reinstatement procedures, as the sessions were not counter balanced. Importantly, higher levels of cocaine-paired lever responses in response to cocaine- versus saline-primingsuggest that the reinstatement effect is specific to cocaine-seeking responses. Nevertheless, these results are consistent with previous reports on fear extinction that showed LFS-induced disruption of the consolidation of fear extinction memory (Deschaux et al., 2010; Garcia et al., 2008). These findings suggest that the disruption of extinction recall by hippocampal LFS could be provoked by direct changes in hippocampal outputs and that the hippocampus proper (i.e., CA regions) modulates the recall of cocaine-associated extinction processes via its direct projections to other limbic regions. Altogether, these data support an important role for the dorsal and ventral hippocampus, via neurogenesis, in the mediation of the extinction-induced inhibition of relapse to cocaine seeking (Deisseroth et al., 2004; Feng et al., 2001; Kim et al., 2011; Schmidt-Hieber et al., 2004).
The dentate gyrus in the hippocampus harbors neural stem cells, and these cells undergo a process of neuronal development during adulthood to produce dentate gyrus granule cell neurons (Abrous et al., 2005). Moreover, recent evidence demonstrated functional incorporation of newly born dentate gyrus neurons into hippocampal circuitry. These newly born neurons assist with the plasticity, activity, and functional networking of the adult hippocampus (Aimone et al., 2011; Lacefield et al., 2012; Song et al., 2012). Furthermore, cocaine exposure affects spontaneous neurogenesis in the dentate gyrus of the hippocampus (Andersen et al., 2007; Dominguez-Escriba et al., 2006; Lloyd et al., 2010; Yamaguchi et al., 2004). Notably, reinforcing doses of cocaine decrease neurogenesis in the dentate gyrus, and withdrawal from cocaine self-administration reduces net proliferation and produces aberrant maturation and survival of neural progenitors, perhaps mediated by the altered expression of molecular markers associated with the propensity for cocaine abuse (Garcia-Fuster et al., 2011; Garcia-Fuster et al., 2010; Noonan et al., 2008; Sudai et al., 2011). Additionally, recent causal evidence demonstrated a role for dentate gyrus neurogenesis in cocaine taking and seeking behaviors (Noonan et al., 2010). Our work adds to the growing number of studies on cocaine exposure and hippocampal neurogenesis in demonstrating that cocaine self-administration reduces survival of BrdU cells and neurogenesis, and that the reduction is uniform in both hemispheres. For example, our findings show that cocaine self-administration does not alter the intrinsic hemispheric relationship in neurogenesis in the dentate gyrus (Urrea et al., 2007). The results also demonstrate that extinction (i.e., diminished cocaine seeking in the absence of cocaine) increases neurogenesis when compared with continued cocaine self-administration and that the increase is equal in both hemispheres. This observation is important because intrinsic hemispheric differences in levels of cell proliferation are noted in other proliferative regions of the brain (e.g. medial prefrontal cortex) and pharmacological treatments have abolished these intrinsic differences (Czeh et al., 2007). The effects of extinction and protracted abstinence on survival of neural progenitors were abolished by LFS uniformly in both hemispheres, suggesting that disruption of hippocampal networking affected developmental processes related to hippocampal neural progenitors ipsilateral and contralateral to the site of stimulation (Chun et al., 2009; Guo et al., 2012).
The effects of cocaine on BrdU survival and neurogenesis were independent of the effects on net proliferation as cocaine self-administration did not reduce levels of Ki-67 cells, suggesting that cocaine influenced later stages of neuronal development (Noonan et al., 2008). Conversely, extinction increased levels of net proliferation, and it can be hypothesized that the higher number of proliferating progenitors generated during extinction normalized the levels of neurogenesis during abstinence. Moreover, we show that extinction-induced increases in proliferation were not evident during protracted abstinence (weeks after extinction terminated). This suggests that the enhanced proliferation during extinction is transient and could have contributed to enhanced survival during protracted abstinence seen in cocaine-exposed animals that did not receive LFS during extinction compared with continuously cocaine-exposed animals. The effects of extinction on proliferation in cocaine-exposed animals that received LFS during extinction were probably inhibited by LFS during extinction, and these changes could have contributed to the reduced survival during protracted abstinence compared with cocaine-exposed animals without LFS during extinction. However, the present data do not provide evidence for immediate effects of LFS during extinction on extinction-induced proliferation and differentiation of neural progenitors. Nevertheless, these findings suggest that the normalization of cocaine-impaired neurogenesis in the dentate gyrus may help reverse aberrant neuroplasticity in the hippocampus during abstinence and thus may help reduce vulnerability to relapse to cocaine seeking (Mandyam and Koob, 2012; Noonan et al., 2010).
We also investigated whether the LFS-induced inhibition of the proliferation and survival of neural progenitors was attributable to enhanced cell death. Programmed cell death (i.e., apoptosis) was quantified in the granule cell layer of the hippocampus to assess this possibility. The present study demonstrates significant reduction in the levels of AC-3 cells in LFS rats, suggesting that cell survival mechanisms (such as alterations in apoptosis) could compensate for the reduced levels of spontaneous neurogenesis produced by LFS.
Hippocampal memory processes are induced by ongoing neuronal plasticity, such as LTP (Bliss and Collingridge, 1993), LTD (Dudek and Bear, 1992), and depotentiation (Staubli and Chun, 1996), which are believed to represent the cellular mechanisms that underlie information storage in the adult hippocampus (Heynen et al., 1996). Cocaine self-administration facilitates the induction and maintenance of different forms of LTP in the hippocampus, and these changes strengthen during withdrawal (Thompson et al., 2004) and are reduced, although they persist, after extinction (del Olmo et al., 2006). However, facilitatory mechanisms of LTP in the hippocampus can be altered by subsequent LFS in the hippocampus (Bashir and Collingridge, 1994), a process that produces depotentiation due to LTD (Abraham, 1996), and assists with erasing new memories (Huang and Hsu, 2001). Furthermore, LFS after extinction can enhance dopamine activity (resulting from cocaine exposure)-mediated LTD in the hippocampus (Mu et al., 2011), suggesting an additive effect to LFS-induced depotentiation. Therefore, considering that LTP is a major candidate for the processes underlying hippocampal learning and memory, the correlation between extinction and enhanced neurogenesis suggests a close relationship between LTP and neurogenesis. In support of this hypothesis, several studies suggest bi-directional interactions between LTP and dentate gyrus neurogenesis. Neurogenesis contributes to LTP induction (Derrick et al., 2000; Farmer et al., 2004; Snyder et al., 2001; van Praag et al., 1999) and, conversely, LTP induction promotes dentate gyrus neurogenesis (Chun et al., 2009; Chun et al., 2006). Furthermore, LTD induction (through LFS) before LTP induction in perforant path synapses significantly suppresses LTP-induced neurogenesis (Chun et al., 2009). These findings suggest that LFS during extinction may inhibit neurogenesis (new memories) associated with extinction through LTD-dependent mechanisms (Quirk and Mueller, 2008) and thereby enhance the recall of drug-associated memories. Because newly born granule cell neurons in the dentate gyrus of the hippocampus modulate the processing of the overall tone of dentate gyrus activity and maintain hippocampal networking (Lacefield et al., 2012), depotentiation induced by LFS in the CA1 region may be assisted by the reduced survival of newly born granule cell neurons.
In summary, our findings define a novel and distinct impact of the disruption of hippocampal activity on relapse to cocaine-seeking behavior, with spontaneous neurogenesis in the adult dentate gyrus being decreased by this disruption of normal hippocampal activity. These studies indicate a critical role of spontaneous neurogenesis in the amelioration of relapse to cocaine-seeking behavior.
Acknowledgments
Research was supported by National Institutes of Health grants DA022473 (CDM) and DA004398 (GFK) from the National Institute on Drug Abuse and the funding from ABMRF the foundation for alcohol research (CDM) and Pearson Center for Alcoholism and Addiction Research (GFK). LDA was supported by an AMGEN scholarship. We acknowledge the excellent technical assistance of Siddharth Iyengar, Wednesday Bushong, and Jan Kirby Zabala from the Life Sciences Summer Internship Program at The Scripps Research Institute and Airee Kim and Mathew Soleiman from the independent study program at the University of California, San Diego, for assistance with immunohistochemistry. We appreciate the technical support of Robert Lintz and Yanabel Grant and the editorial assistance of Michael Arends. We thank Dr. Scott Edwards for helpful comments on the manuscript. This is publication number 21637 from The Scripps Research Institute.
References
- Abraham WC. Induction of heterosynaptic and homosynaptic LTD in hippocampal sub-regions in vivo. J Physiol Paris. 1996;90:305–306. doi: 10.1016/s0928-4257(97)87902-8. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Perry JC, Bignotto M, Perez-Mendes P, Cinini SM, Mello LE, Tufik S. Influence of chronic cocaine treatment and sleep deprivation on sexual behavior and neurogenesis of the male rat. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1224–1229. doi: 10.1016/j.pnpbp.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Collingridge GL. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp Brain Res. 1994;100:437–443. doi: 10.1007/BF02738403. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Berg SA, Czachowski CL, Chambers RA. Alcohol seeking and consumption in the NVHL neurodevelopmental rat model of schizophrenia. Behav Brain Res. 2011;218:346–349. doi: 10.1016/j.bbr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behav Brain Res. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145:423–437. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Gugsa N, Schoenbaum G. Prior cocaine exposure disrupts extinction of fear conditioning. Learn Mem. 2006;13:416–421. doi: 10.1101/lm.216206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Sun W, Jung MW. LTD induction suppresses LTP-induced hippocampal adult neurogenesis. Neuroreport. 2009;20:1279–1283. doi: 10.1097/WNR.0b013e3283303794. [DOI] [PubMed] [Google Scholar]
- Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, Deyoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012 doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- del Olmo N, Miguens M, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Solis JM, Ambrosio E. Enhancement of hippocampal long-term potentiation induced by cocaine self-administration is maintained during the extinction of this behavior. Brain Res. 2006;1116:120–126. doi: 10.1016/j.brainres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Derrick BE, York AD, Martinez JL., Jr Increased granule cell neurogenesis in the adult dentate gyrus following mossy fiber stimulation sufficient to induce long-term potentiation. Brain Res. 2000;857:300–307. doi: 10.1016/s0006-8993(99)02464-6. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Motanis H, Spennato G, Moreau JL, Garcia R. Re-emergence of extinguished auditory-cued conditioned fear following a sub-conditioning procedure: effects of hippocampal and prefrontal tetanic stimulations. Neurobiol Learn Mem. 2011;95:510–518. doi: 10.1016/j.nlm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Thevenet A, Spennato G, Arnaud C, Moreau JL, Garcia R. Low-frequency stimulation of the hippocampus following fear extinction impairs both restoration of rapid eye movement sleep and retrieval of extinction memory. Neuroscience. 2010;170:92–98. doi: 10.1016/j.neuroscience.2010.06.067. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Dong Z, Cao J, Xu L. Opiate withdrawal modifies synaptic plasticity in subicular-nucleus accumbens pathway in vivo. Neuroscience. 2007;144:845–854. doi: 10.1016/j.neuroscience.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recallof fear extinction independently of prefrontalcortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ, Akil H. Decreased Proliferation of Adult Hippocampal Stem Cells During Cocaine Withdrawal: Possible Role of the Cell Fate Regulator FADD. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, Tang S, Liu H, Jiang J. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci. 2001;12:51–68. doi: 10.1515/revneuro.2001.12.1.51. [DOI] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction Infusion of a Mitogen-Activated Protein Kinase Inhibitor Into the Medial Prefrontal Cortex Impairs Memory of the Extinction of Conditioned Fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kim WR, Christian K, Ming GL, Song H. Time-dependent involvement of adult-born dentate granule cells in behavior. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Balest ZR, Corotto FS, Smeyne RJ. Cocaine selectively increases proliferation in the adult murine hippocampus. Neurosci Lett. 2010;485:112–116. doi: 10.1016/j.neulet.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31:4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1997. 3° edition ed. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of Neural Progenitors in the Hippocampus Predicts Memory Impairment and Relapse to Drug Seeking as a Function of Excessive Methamphetamine Self-Administration. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Smith DA, Browning M, Dunwiddie TV. Cocaine inhibits hippocampal long-term potentiation. Brain Res. 1993;608:259–265. doi: 10.1016/0006-8993(93)91466-6. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Song J, Christian K, Ming GL, Song H. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol. 2012 doi: 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y, Roth-Deri I, Kinor N, Aharoni S, Ben-Tzion M, Yadid G. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol. 2011;16:251–260. doi: 10.1111/j.1369-1600.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A, Friedman E, Quartarone A, Ghilardi MF. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J Neurosci. 2011;31:11044–11054. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]