Abstract
Background:
It is believed to be the standard of care to obtain a blood culture in a child who is hospitalized for pneumonia. In recent years, many studies have questioned the utility of this practice in the presence of age appropriate immunization. We conducted this study to determine the current prevalence of bacteremia in children with uncomplicated pneumonia and the utility of obtaining blood cultures in these children.
Objective:
To evaluate the risk of bacteremia in hospitalized young children with pneumonia.
Methods:
This was a retrospective review from July 2003 until July 2008. The setting was the pediatric emergency department of an urban teaching hospital. The study population included children under 36 months of age who had been fully immunized and who had been hospitalized with radiographic evidence of uncomplicated pneumonia. Excluded were children who were currently using antibiotics or who had used antibiotics within the previous 48 hours, as well as children with immunodeficiency status such as sickle cell anemia, immunoglobulin deficiency, or children on steroid therapy.
The radiologist’s interpretation of each chest radiograph was reviewed and recorded. The variables studied were age (in months), gender, race, birth history, pneumococcal vaccination status, appearance on arrival, temperature on arrival, respiratory rate, oxygen saturation, white blood cell (WBC) count, neutrophil count, band count, and urine culture. The chi-square test and logistic regression were used to analyze the data.
Results:
A blood culture was obtained in 535 children hospitalized with radiographic pneumonia. Bacteremia was present in 12 children (2.2%). All organisms isolated from the blood cultures were considered contaminants.
Conclusion:
Children hospitalized with uncomplicated pneumonia have a low rate of positive blood cultures. None of the variables studied predicted bacteremia. The absence of true-positive cultures among the organisms isolated suggests little value in obtaining blood cultures in children hospitalized due to uncomplicated pneumonia.
Keywords: pneumonia, blood Culture
Introduction
Pneumonia is a common reason for hospitalization in the pediatric population. In industrialized nations, the annual incidence of pneumonia is estimated to be 33 per 10,000 in young children.1 Blood cultures are frequently obtained in the management of children hospitalized for pneumonia. Studies have shown a low prevalence of bacteremia in the presence of pneumonia.2,3 Although studies have shown a continued decline in rates of bacteremia, the practice of obtaining a blood culture has remained unchanged. We conducted this study to determine the current prevalence of bacteremia and the utility of blood cultures in children with uncomplicated pneumonia who are otherwise healthy and fully immunized.
Methods
Study design
This retrospective cohort study included children up to 36 months of age with uncomplicated pneumonia who were hospitalized and had blood cultures drawn prior to receiving antibiotics. This study was conducted in an urban hospital between July 1, 2003, and June 30, 2008. The institutional review board of this hospital approved this study. During the study period, it was a common practice to obtain a blood culture in children with pneumonia requiring hospitalization. Blood cultures were obtained by either physicians or nurses using sterile techniques, and the cultures were then inoculated into pediatric blood culture bottles (Pedi-BacT, bioMérieux, Durham, NC). The microbiology laboratory used a microbial detection system (BacT/Alert, bioMérieux) to process all blood cultures. Bottles identified as positive were immediately removed from the instrument, and an aliquot was taken for gram stain and subculture. Bacterial isolates were identified by conventional procedures and cultures were allowed to grow for 5 days before a final positive confirmation.
Participants
We identified children with pneumonia using the ICD-9 diagnosis code. Children up to 36 months of age were included if all of the following pertained: (1) there was radiographic evidence of pneumonia that had been interpreted by an attending radiologist, (2) the child was hospitalized, (3) the child had been fully immunized, (4) the child had no chronic condition (including sickle cell disease, neuromuscular disorder, congenital heart disease, and cystic fibrosis) or immunodeficienty state, and (5) a blood culture had been obtained. Exclusion criteria included the following: (1) the child had complicated pneumonia (eg, mutilobar pneumonia, empyema, or effusion), (2) the child was currently using or had used antibiotics within the previous 48 hours, or (3) the child used steroids.
Measured outcomes and protocol
Bacteremia was defined as growth of any pathogenic organism on blood culture. The bacteria that were considered pathogenic included Streptococcus pneumoniae, Staphylococcus aureus, group A streptococci, Enterococcus species, Neisseria meningitidis, Enterobacteriaceae, Salmonella species, Moraxella catarrhalis, Pseudomonas species, Hemophilus influenzae, Campylobacter species, and Escherichia coli. The bacteria that were considered contaminant included coagulase-negative Staphylococcus species, β-hemolytic streptococci, Micrococcus species, Clostridium species, Corynebacterium species, and Neisseria species other than Neisseria meningitidis or Neisseria gonorrhoeae.
To determine possible predictors of bacteremia, the following variables were studied: age (in months), gender, race, birth history, pneumococcal vaccine status, appearance on arrival, temperature on arrival, respiratory rate, oxygen saturation, white blood cell (WBC) count, neutrophil count, band count, and urine culture.
Statistical methods
Both the chi-square test and logistical regression were used to analyze the data. Statistical significance was determined a priori as P < 0.05 by chi-square test. Only these variables were entered into a logistic regression model predicting blood culture positivity.
Results
Blood cultures were obtained in 535 children with radiographic pneumonia (Table 1). Of the 535 children, 313 (58.5%) were male. The mean patient age was 14.6 ± 7.9 months. Most children were 3 months or older (96.6%). Race and birth history had no significance in the results of the blood cultures (P = 0.28 and P = 0.97 respectively). The mean temperature was 100.6 °F ± 0.08 °F, and 143 patients (26.7%) had a temperature of 102 °F or higher at initial evaluation. The rate of contamination was 100% in the positive cultures. There were no statistically significant differences in age or gender between patients who were later identified as having bacteremia and those with negative cultures. There was no difference in mean temperature between patients with bacteremia or contaminated blood cultures (101.52 °F ± 0.48 °F) and those with negative blood cultures (100.58 °F ± 0.08 °F, P = 0.32). There were no statistically significant differences between children with positive blood culture and those without as can be seen from the white blood cell count (12.52 ± 0.28 vs. 15.05 ± 2.38, P = 0.58), neutrophil count (57.23 ± 0.88 vs. 63.75 ± 6.49, P = 0.33), band count (4.60 ± 0.33 vs. 6 ± 1.72; P = 0.56), respiratory rate (44.02 ± 0.60 vs. 45.67 ± 2.96, P = 0.93), and O2 saturation (96.68 ± 0.11 vs. 96.41 ± 0.80, P = 0.68). The only variable that came close to statistical significance was appearance on arrival. Looking ill on arrival predicted 33% (4/12) of bacteremic patients (P = 0.056) (Table 2). All the subsequent blood cultures obtained in these children were negative for pathogenic bacteria.
Table 1.
Demographics of patients who participated in the study.
| Blood culture |
Negative
|
Total negative |
Positive
|
Total positive | Total general | ||
|---|---|---|---|---|---|---|---|
|
Gender
|
Gender
|
||||||
| M | F | M | F | ||||
| Age (avg months) | 14.38 | 15.21 | 16 | 17 | |||
| Race | |||||||
| Hispanic | 226 | 152 | 378 | 6 | 4 | 10 | 388 |
| African-American | 72 | 60 | 132 | 2 | 2 | 134 | |
| Other | 9 | 4 | 13 | 13 | |||
| Total general | 307 | 216 | 523 | 6 | 6 | 12 | 535 |
Table 2.
Statistical significance of variables for bacteremia.
| Variable | P value |
|---|---|
| Age | 0.9 |
| Gender | 0.42 |
| Race | 0.28 |
| Birth history | 0.97 |
| Appearance on arrival | 0.05 |
| Pneumococcal vaccine | 0.93 |
| O2 saturation | 0.68 |
| Temperature on arrival | 0.32 |
| Respiratory rate | 0.93 |
| WBC count | 0.58 |
| Neutrophil count | 0.32 |
| Band count | 0.56 |
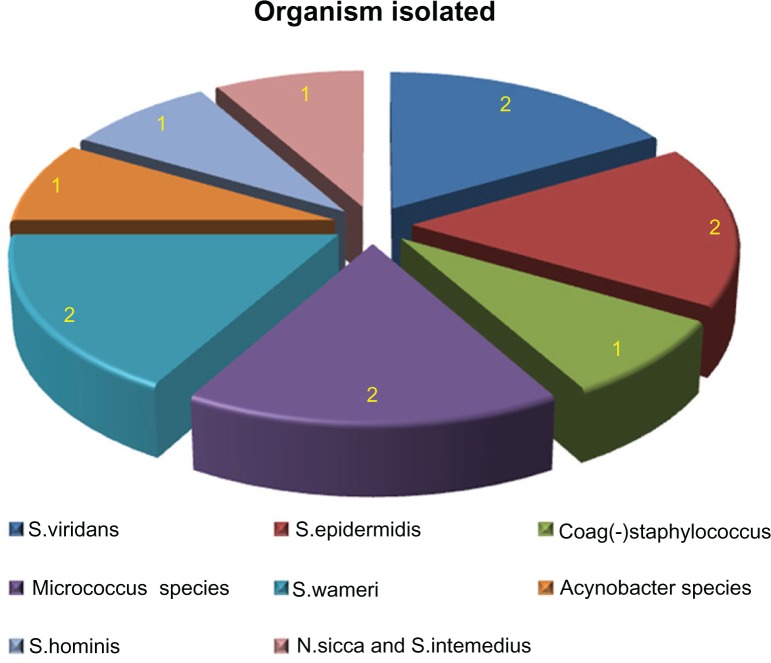
The organisms isolated from the blood cultures were Streptococcus viridians (2), Staphylococcus epidermidis (2), Staphylococcus warneri (2), Micrococcus species (2), Staphylococcus hominis (1), staph coagulase (-) (1), Acinobacter species (1) and Neisseria sicca (1). (Figure 1) No child required respiratory support and there was no mortality.
Figure 1.
Organisms isolated from blood cultures.
Discussion
This study addresses the current risk of bacteremia in uncomplicated pneumonia in young, otherwise healthy children. We evaluated the utility of blood culture in children with uncomplicated pneumonia. The prevalence of bacteremia in this study was one of the lowest reported rates of bacteremia in children with pneumonia. Previously published studies have reported bacteremia rates of 1.2% to 2.7%. The low bacteremia rate shown in our study is related to several factors. Before the introduction of routine immunization with Haemophilus influenzae type b (Hib) vaccine and pneumococcal conjugate vaccine, the prevalence of occult bacteremia was 5% in otherwise healthy children.4,5 Prior to 2000, bacteremia rates of 1.9% and 1.6% were reported in children with pneumonia.6,7 The widespread use of pneumococcal conjugate vaccine has further reduced the risk of occult bacteremia.8–11 The efficacy of the current pneumococcal conjugate vaccine (PCV7) against invasive disease caused by vaccine serotypes is 97.4%.12 The successful implementation of PCV has made occult bacteremia less common.13
Since many underlying cardiopulmonary disorders and other medical conditions may predispose to pneumonia, we excluded children with these conditions from this study. The purpose of this study was to determine the risk of bacteremia in otherwise healthy children who have been fully immunized.
In this study, all 12 cases of bacteremia were false positives due to contaminants. Studies have shown that drawing blood cultures from young uncooperative children may influence the rate of contamination.14 A contamination rate of 2% to 3% has been considered acceptable.15 These false positive or contaminant blood cultures may lead to unnecessary diagnostic evaluation and treatment and prolonged hospitalization.16–18 In this cost-conscious era, it is important to look at the additional costs associated with contaminated blood cultures.
We can also conclude that if the child is completely immunized, the majority of blood culture results will be false positive. Our findings are in agreement with the previous report of 80% false positive blood culture.19
Limitations
This study has several limitations. Children with pneumonia of viral etiology may have been included. This may lead to underestimating the overall risk of bacteremia in children with pneumonia. The study did not deal with the subgroup of patients who initially might have had uncomplicated pneumonia but later developed complicated pneumonia. In these patients, we cannot comment on the utility of blood cultures.
The results of this study may not be generalizable, as treating physicians at our institution may be overly conservative in admitting patients to the hospital because of poor compliance and follow-up. These biases may have had major effects on our results, thus rendering our results not generalizable.
Since obtaining a blood culture was a prerequisite for study inclusion, we did not include children with pneumonia in whom a blood culture was not obtained. In addition, we only included those children who were hospitalized and not those who were treated as outpatients.
Conclusion
This study indicates that children with uncomplicated pneumonia who are immunocompetent and fully immunized are at low risk for bacteremia. In this study, all positive blood cultures were contaminants. This finding also calls into question the utility of blood cultures in children with uncomplicated pneumonia.
Footnotes
Author Contributions
Conceived and designed the experiments: MW, AMP, ML. Analysed the data: AMP, MW, ML, JB. Wrote the first draft of the manuscript: AMP, MW. Contributed to the writing of the manuscript: ML, EE. Agree with manuscript results and conclusions: AMP, MW, ML. Jointly developed the structure and arguments for the paper: MW, ML. Made critical revisions and approved final version: MW, AMP, ML, EE. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Harris M, Clark J, Coote N, et al. British Thoracic Society Standards of Care Committee British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey RW, Bowman MJ, Smith GA. Utility of blood cultures in pediatric patients found to have pneumonia in the emergency department. Ann Emerg Med. 1996;27:721–5. doi: 10.1016/s0196-0644(96)70189-0. [DOI] [PubMed] [Google Scholar]
- 4.McGowan JE, Jr, Bratton L, Klein JO, Finland M. Bacteremia in febrile children seen in a “walk-in” pediatric clinic. N Engl J Med. 1973;288(25):1309–12. doi: 10.1056/NEJM197306212882501. [DOI] [PubMed] [Google Scholar]
- 5.Teele DW, Pelton SI, Grant MJ, et al. Bacteremia in febrile children under 2 years of age: results of cultures of blood of 600 consecutive febrile children seen in a “walk-in” clinic. J Pediatr. 1975;87(2):227–30. doi: 10.1016/s0022-3476(75)80584-1. [DOI] [PubMed] [Google Scholar]
- 6.Alpern ER, Alessandrini EA, Bell LM, Shaw KN, McGowan KL. Occult bacteremia from a pediatric emergency department: current prevalence, time to detection, and outcome. Pediatrics. 2000;106(3):505–11. doi: 10.1542/peds.106.3.505. [DOI] [PubMed] [Google Scholar]
- 7.Lee GM, Harper MB. Risk of bacteremia for febrile young children in the post-Haemophilus influenzae type b era. Arch Pediatr Adolesc Med. 1998;152(7):624–8. doi: 10.1001/archpedi.152.7.624. [DOI] [PubMed] [Google Scholar]
- 8.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004;113:443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 11.Whitney CG, Farley MM, Hadler J, et al. Decline in Invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 12.Shah SS, Alpern ER, Zwerling L, McGowan KL, Bell LM. Risk of bacteremia in young children with pneumonia treated as outpatients. Arch Pediatr Adolesc Med. 2003;157(4):389–92. doi: 10.1001/archpedi.157.4.389. [DOI] [PubMed] [Google Scholar]
- 13.Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med. 2004;158:671–5. doi: 10.1001/archpedi.158.7.671. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovsky M, Press J, Peled N, Yagupsky P. Blood culture contamination in pediatric patients: young children and young doctors. Pediatr Infect Dis J. 2006;25(7):611–4. doi: 10.1097/01.inf.0000220228.01382.88. [DOI] [PubMed] [Google Scholar]
- 15.Strand CL, Wajsbort RR, Sturmann K. Effect of iodophor vs. iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269:1004–6. [PubMed] [Google Scholar]
- 16.Richter SS, Beekman SE, Croco JL, et al. Minimizing the workup of blood culture contaminants: Implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol. 2002;40:2437–44. doi: 10.1128/JCM.40.7.2437-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates DW, Goldmann L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265:365–9. [PubMed] [Google Scholar]
- 18.Thuler LC, Jenicek M, Turgeon JP, Rivard M, Lebel P, Lebel MH. Impact of a false positive blood culture result on the management of febrile children. Pediatr Infect Dis J. 1997;16(9):846–51. doi: 10.1097/00006454-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sard B, Bailey MC, Vinci R. An analysis of pediatric blood cultures in the postpneumococcal conjugate vaccine era in a community hospital emergency department. Pediatr Emerg Care. 2006;22(5):295–300. doi: 10.1097/01.pec.0000215137.51909.16. [DOI] [PubMed] [Google Scholar]