SUMMARY
We have previously shown that benzamidine-type compoundscan inhibit the activity of arginine-specific cysteine proteinases (gingipainsHRgpA and RgpB); well-known virulence factors of Porphyromonas gingivalis. They also hinder in vitro growth of this important period on to pathogenic bacterium. Apparently growth arrest is not associated with their ability to inhibit these proteases, as pentamidine, which is a 20-fold less efficient inhibitor of gingipainthan 2,6-bis-(4-amidinobenzyl)-cyclohexanone (ACH), blocked P. gingivalis growth far more effectively. To identify targets for benzamidine-derived compounds other than Arg-gingipains, and to explain their bacteriostatic effects, P. gingivalis ATCC 33277 and P. gingivalis M5-1-2 (clinical isolate) cell extracts were subjected to affinity chromatography using a benzamidine-Sepharose column to identify proteins interacting withbenzamidine. In addition to HRgpA and RgpB the analysis revealed heat-shock protein GroELas another ligand forbenzamidine. To better understand the effect ofbenzamidine-derived compounds on P. gingivalis, bacteria were exposed to benzamidine, pentamidine, ACH and heat, and the expression of gingipains and GroEL was determined. Exposure to heat and benzamidine-derived compounds caused significant increasesin GroEL, both at the mRNA and protein levels. Interestingly, despite the fact that gingipains were shown to be the main virulence factors in a fertilized egg model of infection, mortality rates were strongly reduced, not only by ACH, but also bypentamidine, a relatively week gingipain inhibitor. This effect may depend not only ongingipain inhibition but also oninteraction of benzamidine derivatives with GroEL. Therefore these compounds may find use in supportive periodontitis treatment.
Keywords: Porphyromonas gingivalis, benzamidine derivatives, Arg-gingipains, fertilized egg model
INTRODUCTION
A limited number of bacterial species have been associated with periodontitis, among which Porphyromonas gingivalis, an anaerobic Gram-negative bacterium, is most strongly associated withdisease progression (Genco, 1996; Lamont & Jenkinson, 2000; Ezzo & Cutler, 2003). In the last few years a major focus of P. gingivalis research has been the link between periodontal and systemic diseases, e.g. atherosclerotic cardiovascular disease, preterm birth, and diabetes (Hayashi et al., 2010). Moreover, P. gingivalis expresses a peptidylarginine deiminase that is able to citrullinate proteins, and may provide antigens that drive autoimmune responses in rheumatoid arthritis (Wegner et al., 2010). Amongstits virulence factors, cysteine proteinases referred to as gingipains are considered as major contributors to P. gingivalis pathogenic potential (Guo et al., 2010). Indeed, gingipains are responsiblefor at least 85% of the proteolytic activity of this bacterium (Potempa et al., 1997). While gingipain with arginine-Xaa specificity (Arg-gingipains, Rgps) originate from two different genes, rgpA and rgpB, the lysine-Xaa specific gingipain (Lys-gingipain, Kgp) is derived from a single gene, kgp (Mikolajczyk-Pawlinska et al., 1998). The gingipains contribute to P. gingivalis virulence in multiple ways, for example, by enhancing vascular permeability gingipains generate gingival crevicular fluid (Rubinstein et al., 2001), providing nutrients and essential growth factors for the bacterium. This effect is enhanced bydegradation of intracellular adhesion-molecule-1, and junction proteins on human epithelial cells (Katz et al., 2002). At the same time, by efficient degradation ofmultiple components of the complement system, gingipains exert resistance to the antibacterial activity of complement (Popadiak et al., 2007). Simultaneously, the cleavage of immunoglobulin G1 and G3 at the hinge region by Lys-gingipainmay abrogate the affects of acquired immunity (Vincents et al., 2011).
Antibiotics are widely used as an adjunct therapy in severe cases of P. gingivalis-associated periodontitis. Unfortunately, a side effect of such treatmentis the risk ofdeveloping antibiotic resistant isolates. In these circumstances, the application of gingipain-specific inhibitors to reduce P. gingivalis virulence seems to be an attractive alternative to antibiotic treatment (Travis & Potempa, 2000). To this end, benzamidine derivatives have previously been found to inhibit the amidolytic activities and growth of P. gingivalis. In addition, treatment of the bacterium with these compounds promoted phagocytosis by polymorphonuclear neutrophils. The inhibition of hemagglutination, and enhancement of phagocytosisby individual compounds, strongly correlated with the potency of given compound (measured as the Ki-values) to inhibitthe amidolytic activity of Rgps. Surprisingly, however, pentamidine, a relatively weak protease-inhibitor (high Ki-value), was the most effective compound for growth inhibition (Eick et al., 2003). In this follow-up study we show that benzamidine derivatives bind not only to Arg-gingpains, but also to the chaperone GroEL, and increase expression of the latter protein. Usinga fertilized egg modelof infection we show that these compounds effectively reduce the lethality of P. gingivalis during disease causation.
METHODS
Benzamidine derivatives
Benzamidine (Sigma-Aldrich, Taufkirchen, Germany), pentamidine (Sigma-Aldrich) and 2,6-bis-(4-amidinobenzyl)-cyclohexanone (synthesized by J Stürzebecher, Institute of Vascular Biology and Medicine, University Hospital of Jena) were chosen from a panel of compounds with benzamidine-like structures (Figure 1). The inhibition constants (Ki) against purified RgpB were 40.3 μM for pentamidine, 0.51 μM for 2,6-bis-(4-amidinobenzyl)-cyclohexanone and 277 μM for benzamidine. Ki-values against HRgpA were 2.9 μM for 2,6-bis-(4-amidinobenzyl)-cyclohexanone, 107 μM for pentamidine and 536 μM for benzamidine. No benzamidine derivative impacted Lys-gingipainactivity (Ki> 1000 μM). In all experiments inhibitors were used at a final concentration of 20 μM.
Fig. 1.
The structures of benzamidine (benzam.), 2,6-bis-(4-amidinobenzyl)-cyclohexanone (2,6b(4ab)ch) and pentamidine (pentam.).
Bacterial strains
P. gingivalis ATCC 33277 was purchased from the German strain collection DSMZ (Braunschweig, Germany). P. gingivalis M5-1-2 strain is a clinical isolate obtained from a patient with severe chronic periodontitis. In the plated sample of subgingival plaque derived from this patient, P. gingivalis compromised 64% of the discernable anaerobic microbiota. Bacterial strains were grown anaerobically on Schaedler agar (Oxoid) supplemented with 8% sheep blood, 2.5 μg/ml menadione; and in Schaedler broth (Oxoid) supplemented with 2.5 μg/mL menadione.
For growth inhibition experiments, 1 × 106 bacteria/ml were added to Schaedler broth supplemented with 2.5 μg/mL menadione and 20 μM of inhibitor. After incubation for 24 h and 48 h at 37°C, the number of colony forming units (cfu) was determined. These experiments were repeated in triplicate. Statistical analysis was performed using Student’s t-test.
Purification of Arg-gingipains by means of an affinity chromatographyona benzamidine-Sepharose column
p-aminobenzamidine (375 mg) was ligated with CH-Sepharose 4B (5 g; Pharmacia Fine Chemical, London, GB) using 1.853 g carbodiimide according to manufacturer’s protocol. P. gingivalis strains were grown in batch culture (3 L) and harvested by centrifugation (5000 g, 30 min at 4°C). The cell free culture fluid was precipitated using acetone, and the protein pellet was resuspendedin 20 mMBis-Tris, 150 mM NaCl, 5mM CaCl2, and 0.02% NaN3 at pH 6.8. The solution was then dialyzed against the same buffer containing 1.5 mMaldrithiole (two changes). Dialyzed fractions were clarified by centrifugation (13,000 × g, 15 min at 4°C), concentrated by lyophilization and applied to a column (1.3 cm × 13 cm) of p-aminobenzamidine-Sepharose equilibrated with the Bis-Tris buffer. After eluting non-binding proteins, a linear NaCl gradient (from 0 to 3 MNaCl), and then a benzamidine gradient from 0 to 2 M was applied at a flow rate of 0.8 ml/min. Arginine- and lysine-specific amidolytic activities in each fraction were assayed at 37°C with 0.5 mM N-α-benzoyl-DL-arginine-p-nitroanilide (BApNA) or N-p-Tosyl-Gly-Pro-Lys-p-nitroanilide, respectively, in 1.0 ml of 0.2 M Tris-HCL, 0.1 M NaCl, 5 mM CaCl2, 10 mM cysteine, pH 7.6. Fractions with the highest activity in the p-aminobenzamidine-Sepharose flow-through (pool I), NaCl gradient (pool II), and benzamidineelution (pool III) were combined. The collected fractions were desalted using Sephadex G-25-columns equilibrated with 0.05 M Tris-HCL, 1 mM CaCl2, 0.02% (w/v) NaN3 (pH 7.4) and freeze-dried.
Proteins from each pool were analyzed by sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) according to Laemmli under non-reduced conditions using 10% polyacrylamide gels. Gels were stained usinga silver staining kit (GE Healthcare, Amersham, UK).
For analysis of N-terminal amino acid sequences, protein separated by SDS-PAGE were electro transferred onto a polyvinylidenedifluoride (PVDF) membrane. Protein bands were visualized by staining with SYPRO Ruby blot stain (Bio-Rad, Hercules, CA). The stained bands were excised and N-terminal amino acid sequence determined by automated Edman degradationat Leibniz Institute for Age Research, Jena.
Expression of genes encoding GroEL and gingipains
P. gingivalis strains were precultivated in Schaedler broth supplemented with 2.5 μg/ml menadione for 24 h. After this time, the bacterial suspension was divided into five samples in microcentrifuge tubes. Benzamidine, pentamidine and 2,6-bis-(4-amidinobenzyl)-cyclohexanone were added to each sample (to a final concentration of 20 μM). Four samples (three with inhibitors, one without) were incubated at 37°C, while a fifth sample without inhibitor was exposed to 45°C in an anaerobic atmosphere for 1 h.
Total RNA from approximately 2 × 108 bacterial cells was purified using anRNeasy kit (Qiagen GmbH, Hilden, Germany) and cDNA was synthesized from 100 ng of total RNA using anOmniscript kit (Qiagen) according to the manufacturer’sinstructions. Real-time PCR was carried out in a reaction volume of 20 μL, consisting of 2 μL of cDNA solution and 18 μl of reaction mixture, containing 2 μL 10×PCR buffer, 2.75 mM MgCl2, 0.2 mM nucleotides, 0.5 μM of each primer, 10−4 SybrGreen and 1 U Taq polymerase (Fermentas Life Sciences, St. Leon-Rot, Germany) using a RotorGene 2000 device (Corbett Research, Sydney, Australia). The oligonucleotide primers were as follows:rgpA (accession: U15282) 5′-TAT CCT TCG TGA TGT GCG TG-3′ (forward), 5′-GCT GTA ACG GGA GAA GCA AT-3′ (reverse); rgpB (accession: U85038) 5′-CAT TCT CCT CTC TGT TGG GA-3′ (forward), 5′-CGT AGG GGA TTT GAT CAG GA-3′ (reverse); kgp (accession: U54691) 5′-TCA AGC AGT TCG ATG CAA GC-3′ (forward), 5′-ACT TGG GTC AGT TCT TGT CC-3′ (reverse); groEL (accession: D17342) 5′-TGC CGT TAA AGT TAC CCT CG-3′ (forward), 5′-CAC TTC CTT AGC CAT ACC TG-3′ (reverse). The housekeeping gene sod (accession: M60401) 5′-AAT TCC ACC ACG GTA AGC AC-3′ (forward), 5′-TTC TCG ATG GAC AGT TTG CC-3′ (reverse) served as control. All primers were designed using the DNASIS program (Hitachi Software EMEA, Tokyo, Japan). Cycling conditions comprised of an initial denaturation step at 94°C for 5 min, followed by 40 cycles of 95°C for 15s, 58°C for 20s, 72°C for 20s. The amountof gingipain and stress gene mRNA was quantified in relation to sod mRNA levels. All experiments were performed in triplicate. Statistical analysis was performed using Student’s t-test for paired samples, samples after exposure to heat or benzamidine derivatives were compared with those without any exposure.
Effect of inhibitors on GroELprotein levels
P. gingivalis strains were grown in Schaedler broth supplemented with 2.5 μg/ml menadionefor 24 h, and exposed to inhibitors and stress as described above. Bacteria were collected by centrifugation and pellets containing approximately 2 × 109 bacteria were suspended with 500 μl of sample buffer (0.125 M Tris-HCl, 20% glycerol, 4% SDS), before being subjected to SDS-PAGE (10% polyacrylamide including 0.1% SDS) under non-reduced conditions. To avoid proteolytic degradation of proteins during boiling, all samples were treated with 0.05 mMFFRck. Next, resolved proteins were transferred (Mini Trans-Blot System; Bio-Rad) onto nitrocellulose membranes (GE Healthcare) for immunoblotting. Nonspecific bindingsites on the membranes were blocked overnight in 5% skimmed milk (BD, Franklin Lakes, NJ). Blots were then probed with a monoclonal mouseantibody against GroEL (Sigma-Aldrich), followed bygoat anti-mouse IgG horseradish peroxidase conjugated antibodies (Dako Deutschland GmbH, Hamburg, Germany). The blots were developed using the ECL Plus (GE Healthcare) substrate kit according to the manufacturer’s protocol. Chemiluminescence of immunoreactive bands was recorded on X-ray films (Kodak, Rochester, NY). All experiments were run in quadruplicate.
Testing the effect of benzamidine-derivatives on P. gingivalis virulence using an egg model of infection
Fertile White Leghorn eggs were set in a humidified, self-turning incubator maintained at 37.8°C. Dose-lethality curves were established by i.v. inoculation through the chorioallantoin vein of 12-day-old chicken embryos with increasing numbers of P. gingivalis in Schaedler broth to determine LD50 and LD90 values. Based on the results of these experiments, fertilized eggs (24 for each inoculum) were inoculated with 2 × 106, 4 × 106, and 1 × 107 of P. gingivalis alone (12 eggs), or together with 20 μMof benzamidine derivatives. The viability of chicken embryos was checked hourly for the first 5 h by candling, and then by daily candling for up to 5 days post-infection. After finishing experiments, randomly selected eggs were checked for the presence of viable bacteria by plating of allantoic fluid on Schaedler agar.
To determineif gingipains by themselves are pathogenic for fertilized eggs in this model, purified HRgpA, RgpB and Kgpat concentrations ranging from 1 nM to 100 nM were injected into eggs in the same manner as live bacteria. Six eggs were used for each group in these experiments.
RESULTS
Growth inhibition by benzamidine inhibitors
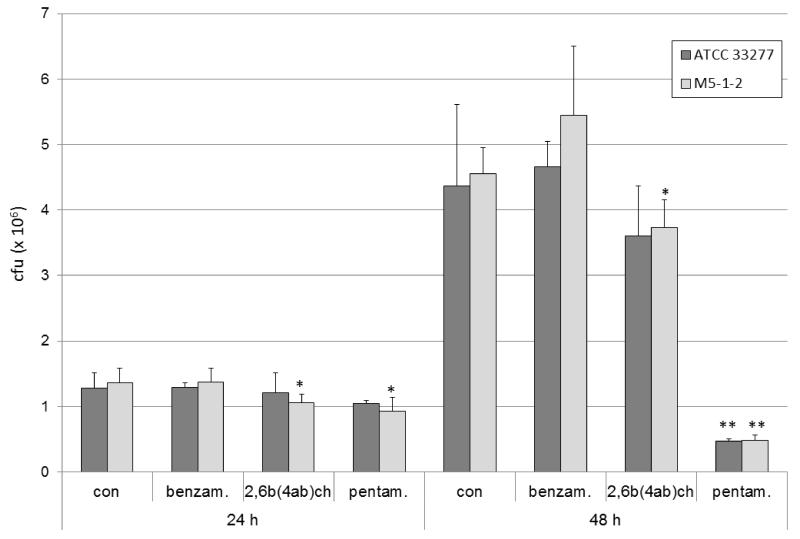
We have previously shown that benzamidine-derived compounds effected P. gingivalis growth (Eick et al., 2003). Here we have confirmed this data by showing that benzamidine-derived inhibitors at 20 μM concentration reduced the numbers of viable P. gingivalis in batch cultures in a time-dependent manner. Specifically, after 24 h, only a slight reduction has occurred (p<0.05 for P. gingivalis M5-1-2 and both inhibitors), howeverby 48 h cfunumbers were decreased by around 20%in the presence of 2,6-bis-(4-amidinobenzyl)-cyclohexanone (p<0.05 for P. gingivalis M5-1-2), and by 90% when treated with pentamidine (both strains p<0.01). Interestingly, benzamidine did not notably influence the growth of bacterial strains tested. Additionally, the effect of inhibitors was apparently strain-independent (Fig. 2).
Fig. 2.
Growth inhibition of P. gingivalis ATCC 33277 and P. gingivalis M5-1-2 (clinical isolate) by 20 μM of benzamidine (benzam.), 2,6-bis-(4-amidinobenzyl)-cyclohexanone (2,6b(4ab)ch), and pentamidine (pentam.).
*p<0.05, **p<0.01 compared to control
Separation of proteins on p-aminobenzamidine-Sepharose
To identify proteins which interact with benzamidine-derivatives, P. gingivalis culture medium was subjected to the affinity chromatography using p-aminobenzamidine-Sepharose. The chromatography resolved arginine-specific amidolytic activity into three peaks, one in the flow-through, a second eluted with 3 M NaCl (pool II) and a third, with the highest activity, eluted at 2 M benzamidine (pool III). In fractions eluted with salt and benzamidine the lysine-specific activity was very lowsuggestingthat Kgp did not bind to the benzamidine (Figure 3).
Fig. 3.
Arginine- and lysine specific amidolytic activities separated by affinity chromatography on a benzamidine-Sepharose column
The molecular massof proteins obtained by chromatography on benzamidine-Sepharose, and estimated by SDS-PAGE, was in the range of 15 kDato 98 kDa. Proteins were subsequently identified by NH2-terminal sequence analysis, revealing the sequence YTPVEEKQNG in the presence of HRgpA (primary accession number P28784) in a 98-kDa band in pool II. On the other hand, an NH2-terminal sequence of YTPVEEKENG was determined for 15-kDa, 35-kDa, and 48-kDa proteins in pool III, clearly indicating the presence ofRgpB (primary accession number P95493). Most interestingly, however, was an NH2-terminal sequenceof AKEIKFDME identified by analysis of a 60-kDa protein in pool IIthat was unambiguously identified as GroEL (primary accession number P42375). GroELis a ubiquitous protein in bacterial species, and belongs to the heat shock protein (Hsp) 60 family.
Interaction with GroEL
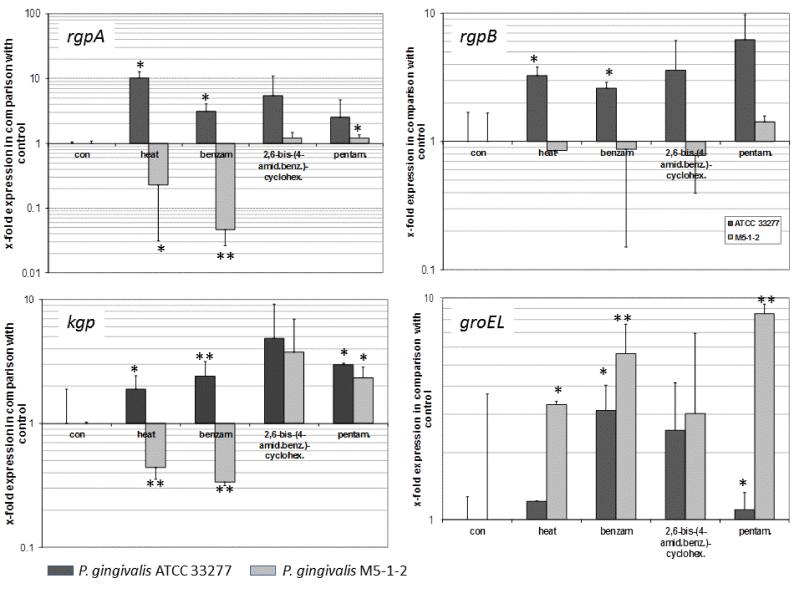
Interaction of p-aminobenzamidine with GroEL suggests that benzamidine-derived compounds may affect expression of GroEL in P. gingivalis. To verify this assumptionwe analysed expression of groEL in P. gingivalis exposed to heat and benzamidine-derivatives. Heat stress (1 h at 45°C) increased the mRNA expression of groEL in M5-1-2 strain of P. gingivalis (p<0.05). Similarly, supplementation of cultures with benzamidine and pentamidineled to a significant increase in mRNA levels for groEL. This effect was always slightly more pronounced in the clinical isolate M5-1-2 (p<0.01) than in the reference strain ATCC 33277 (p<0.05).
Changes in gingipain gene expressions in response to stress and benzamidine-derivatives appeared to be strain dependent. Specifically, strain ATCC 33277 responded to heat stress and the presence of benzamidine with increased expression of all gingipaingenes (rgpA and rgpB; p<0.05, kgp;p<0.05, p<0.01). Conversely, heat stress and benzamidine decreased mRNA levels of both rgpA (p<0.05, p<0.01) and kgp (each p<0.01) in strain M5-1-2. Pentamidine increased the mRNA expression of rgpA and kgp (each p<0.05; Figure 4).
Fig. 4.
Effect of heat shock (1h) and exposure to benzamidine (benzam.), 2,6-bis-(4-amidinobenzyl)-cyclohexanone (2,6-bis-(4-amid.benz.)-cyclohex.), and pentamidine (pentam.) on expression of the gingipains (rgpA, rgpB, kgp) and groEL in P. gingivalis ATCC 33277 and P. gingivalis M5-1-2 (clinical isolate); (samples normalized to sod expression). Results are presented in comparison to P. gingivalis which was grown at 37°C without the addition of benzamidine derivatives (control – set to 1).
*p<0.05, **p<0.01 compared to control
Abundance of the GroEL protein in both P. gingivalis strains was enhanced by heat stress, 2,6-bis-(4-amidinobenzyl)-cyclohexanoneand pentamidine. Densitometry scanning of bands intensity allowed calculation of the relative protein contents of individual immunoreactive bands. This analysis revealedGroEL increased by 100% and 300% in the ATCC 33277 and the M5-1-2 strains after heat exposure and by 50% and 100% after treatment to the benzamidine derivatives. Surprisingly, in the M5-1-2 strain additional bands with a lower molecular mass were immuno reactive with anti-GroEL antibodies, suggestingpartial degradation of this protein (Figure 5).
Fig. 5.
Representative Western blot illustrating the effect of heat shock (1h) and exposure to benzamidine (benzam.), 2,6-bis-(4-amidinobenzyl)-cyclohexanone (2,6b(4ab)ch) and pentamidine (pentam.) on the abundance of GroELin strains ATCC 33277 (A) and M5-1-2 (B). Samples were normalized so that the same amount of protein was loaded in each lane.
Virulence in chicken embryos
Gingipains are essential P. gingivalis virulence determinants. Therefore we tested if benzamidine-derived compounds are able to attenuate P. gingivalis virulence using a fertilized egg model. Most changes in viability of the eggs were observed 3 h after inoculation. Following that, this time-point was chosen for determining the LD50 and LD90. For both M5-1-2 and ATCC 33277 strains of P. gingivalis the LD90 of circa1 × 107 bacteria was determined. Conversely, the LD50 of 4 × 106 bacteria was determined for the ATCC 33277 strain, and 2 × 106 bacteria for the clinical isolate M5-1-2 (Table 1). P. gingivalis was cultured from the chorioallantoic fluid of all infected eggs in which embryos were killed, whilst no P. gingivalis cells could be detected from any viable egg at the end of a 5 dincubation time.
Table 1.
Percentages of viable eggs (mean and standard deviation) at different times after inoculation with P. gingivalis ATCC 33277 and P. gingivalis M5-1-2 (clinical isolate). Each group consisted of 12 fertilized eggs. The values represent results from three independent experiments each meaning at all 252 eggs were included in the assays.
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | 24 h | |
|---|---|---|---|---|---|---|---|
| Controls | 100 | 100 ± 0 | 100 ± 0 | 1002 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| ATCC 33277 | |||||||
| 2 × 106 | 100 | 95 ± 7 | 85 ± 14 | 78 ± 19 | 77 ± 21 | 75 ± 23 | 72 ± 24 |
| 4 × 106 | 100 | 87 ± 13 | 70 ± 28 | 35 ± 28.5 | 23 ± 34 | 23 ± 34 | 23 ±34 |
| 107 | 100 | 60 ± 40 | 17 ± 37 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| M5-1-2 | |||||||
| 2 × 106 | 100 | 870 ± 19 | 75 ± 26.08 | 57 ± 38 | 48 ± 45 | 48 ± 45 | 43 ± 66 |
| 4 × 106 | 100 | 72 ± 18 | 57 ± 25 | 27 ± 39 | 22 ± 40 | 17 ± 29 | 13 ± 22 |
| 107 | 100 | 38 ±40 | 22 ±40 | 12 ± 18 | 7 ± 9 | 5 ± 7 | 3 ± 7 |
The addition of benzamidine derivatives reduced the number of eggskilled, whilst benzamidine itself was only slightly effective. Dependent on the time-point20 μM 2,6-bis-(4-amidinobenzyl)-cyclohexanone reduced lethality of the ATCC 33277 strain by 38-71% and those of the M5-1-2 strain by 5-71%. The respective values for pentamidine were 13 – 67% for the ATCC 33277 strain and 17-38% for the M5-1-2 strain (Table 2).
Table 2. Effect of benzamidine and benzamidine-derivatives (applied at 20 μM concentration) on embryo mortality induced by inoculation of fertilized eggs with P. gingivalis(4 × 106cfu/egg).
Results are shown as: numbers of dead eggs in the presence of inhibitor/number of dead eggs in the absence of inhibitor(% of reduction of dead eggs by addition of an inhibitor)
| Number of dead eggs with/without inhibitor | Total number of eggs with/without inhibitor |
||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 h | 1 h | 2 h | 3 h | 5 h | 24 h | ||
| 2,6-bis-(4-amidinobenzyl)-cyclohexanone* | |||||||
| Controls | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 24/24 |
| ATCC 33277 | 0/0 (0%) | 2/4 (50%) | 2/7 (71%) | 10/16 (38%) | 10/18 (45%) | 11/18 (39%) | 24/24 |
| M5-1-2 | 0/0 (0%) | 2/7 (71%) | 6/11 (45%) | 16/17 (6%) | 17/19 (11%) | 21/22 (5%) | 24/24 |
| Pentamidine | |||||||
| Controls | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 24/24 |
| ATCC 33277 | 0/0 (0%) | 1/3 (67%) | 5/8 (38%) | 13/15 (13%) | 14/18 (22%) | 16/19 (16%) | 24/24 |
| M5-1-2 | 0/0 (0%) | 5/6 (17%) | 7/10 (30%) | 12/18 (33%) | 13/21 (38%) | 16/21 (24%) | 24/24 |
| Benzamidine | |||||||
| Controls | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 0/0 (0%) | 24/24 |
| ATCC 33277 | 0/0 (0%) | 2/3 (33%) | 5/7 (24%) | 15/15 (0%) | 19/19 (0%) | 18/18 (0%) | 24/24 |
| M5-1-2 | 0/0 (0%) | 5/7 (29%) | 10/10 (0%) | 17/18 (6%) | 20/20 (0%) | 19/19 (0%) | 24/24 |
None of the inhibitors by themselveswere toxic to eggs
Of note, separate injections of each gingipain at 100 nM concentrations were 100% lethal to inoculatedeggsby 2 h. Lower concentrations of Arg-gingipains (1 and 10 nM) did not kill eggs up to 5 h post-inoculum, and only one embryo was dead after 24h. In contrast, Kgpat 10 nM exerted a strong lethal effect, killing 5 out of the 6 inoculated eggs within 2h after injection (Table 3).
Table 3. Effect of gingipains on the lethality of fertilized eggs.
Shown are the cumulative numbers (%) of dead eggs after inoculation withHRgpA, RgpB and Kgp. For each, 6 eggs were tested.
| Gingipain | Concen- tration |
Dead eggs after | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 24 h | |||
| Control | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 | |
| HRgpA | 100 nM | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 |
| 10 nM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 | |
| 1 nM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 6 | |
| RgpB | 100 nM | 3 (50%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 |
| 10 nM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 6 | |
| 1 nM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) | 6 | |
| Kgp | 100 nM | 1 (17%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 |
| 10 nM | 0 (0%) | 0 (0%) | 2 (33%) | 3 (50%) | 3 (50%) | 5 (83%) | 6 | |
| 1 nM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 | |
Discussion
The in vitro egg model detailed herein has previously been used forthe testing of virulence for Haemophilus influenzae (Melhus et al., 1998) andStreptococcus pyogenes (Schmidt et al., 2001). In this system the antimicrobial activity relies largely on phagocytosis, since the phagocytic system is fully developed in 12-day-old chicken embryos (Schmidt et al., 1993; Schmidt et al., 2001). At this time, however, lymphocytes are not detectable (Schmidt et al., 1993). Here weshow that P. gingivalis was lethal to fertilized eggs, however far greaterbacterial counts were needed to kill eggs in comparison to S. pyogenes (Schmidt et al., 1997); indicating that P. gingivalis is less virulent in this model of infection. Chicken embryos were generally immune to inoculation with up to 1 × 106 bacteria, although interestingly, when eggs were inoculated with higher doses, surviving embryos were free ofviable P. gingivalis cells, indicating the presence of effective mechanismsfor bacteria killing. This result also suggests that the total clearance of infection is necessary for embryo survival. The application of 100 nM of each gingipain resulted in the immediate death of embryos, arguing for high toxicity of these proteolytic enzymes in this model. Using 10 nM gingipains we shown that Lys-gingipain was much more lethal, killing 5 of 6 embryos (83%), than Arg-gingipains, which killed only 1 of 6 (17%) eggs. It must be kept in mind, however, that in human in gingival crevicular fluid concentrations of Arg-gingipains (up to 1 μM) is much higher than that of Lys-gingipain (up to 10 nM) (Guentsch et al., 2011; Guentsch et al., 2012). One can only speculate as to how gingipain kills embryos in this infection system. For example, the gingipain lethal effect may be related to cleavage of cell adhesion molecules, exerted by HRgpA, RgpB and Kgp (Sheets et al., 2006), or the degradation of hemoglobin by Kgp (Curtis et al., 2002). Importantly, the time- and dose-dependent lethal effects exerted by gingipains and P. gingivalis on chicken embryos were similar. At high concentrations, the embryos had already died after two hours, while at low concentrations this process was extended to 24 hours. This underlines once more that gingipains are one of the most essential virulence factors of P. gingivalis.
Benzamidine-type inhibitors modulatedthe virulence properties of P. gingivalis, with the chicken embryo model proving to be a suitable system for investigatingthe influence of inhibitors on bacterial virulence. In this model benzamidine showed no strong protective effect on embryokilling by P. gingivalis. Conversely, both derivatives, 2,6-bis-(4-amidinobenzyl)-cyclohexanone and pentamidine, substantially reduced the lethality of P. gingivalis strains. Of note, lethality was not completely eliminated, leading to a conclusionthat the inhibitors do not block all virulence related activities, as a number of bacterial cells remained viable. This contention is supported by the fact that inhibitors block the activity of Arg-gingipains, but not Lys-gingipain. Interestingly, the significant difference inKi-values for Rgps inhibition by 2,6-bis-(4-amidinobenzyl)-cyclohexanone and pentamidinedid not corresponded to their protective effect. The latter compound, although a much weaker inhibitor of Rgps than the 2,6-bis-(4-amidinobenzyl)-cyclohexanone, yielded the same efficient protection of embryo as 2,6-bis-(4-amidinobenzyl)-cyclohexanone. This suggests that the pentamidine-dependent protective effect against P. gingivalis in fertilized eggsrelies on interaction of this compound with target(s) other than Rgps. The protective effect may be exerted by benzamidine and benzamidine derivatives ability to enhanced phagocytotic capacity of chicken phagocytes as it was found for P. gingivalis clearance by human peripheral blood neutrophils (Eick et al., 2003). The presence of a fully developed phagocytic system in fertilized eggs supports this contention.
The purification of Arg-gingipains by means of a benzamidine-Sepharose column confirmed that these inhibitors have affinity to Arg-gingipains. The binding was very strong, especially for RgpB, which was found in pool III eluted from the column with 2 M benzamidine. In pool II, HRgpA, and surprisingly GroEL (Hsp60), were identified as the main proteins. Hsp60 is a conserved molecular chaperone present in eubacteria as well as in eukaryotic mitochondria, suggesting that eukaryotic Hsp60 has aeubacterial origin (Viale & Arakaki, 1994). Because benzamidine is a polar molecule unable to penetrate lipid membranes we limited our analysis to cell free culture medium. GroEL of period on to pathogens was purified from cell lysates (Hinode et al., 1995) and in A. actinomycetemcomitans GroEL-like protein was found in membrane, cytoplasmic and periplasmic cell fractions (Paju et al., 2000). Conversely, however, in Helicobacter pylori an etiologic agent in chronic gastritisa GroEL homolog was found in the extracellular fraction (Vanet & Labigne, 1998). Also our results indicate a possible extracellular release of GroEL by P. gingivalis, which needs to be verified in follow-up studies. GroEL is present in all strains of P. gingivalis tested to date (Lu & McBride, 1994) (Vayssier et al., 1994), and may play some role in the pathogenesis of periodontitis. For example, P. gingivalis GroEL interacts with Toll-like receptors 2 and 4 (Argueta et al., 2006). Additionally, the molecular mimicry between GroEL of P. gingivalis and human Hsp60 is apparently responsible for the immunological cross-reactivity of serum antibodies against GroEL with Hsp60 (Tabeta et al., 2000). Recently it was shown that atherosclerotic patients contain serum antibodies cross-reacting with peptide 19 of P. gingivalis GroEL, suggesting the peptide is an immuno reactive epitope in the association of periodontitis and atherosclerosis (Choi et al., 2011). Seroreactivity against this peptide was also predominant in rheumatoid arthritis patients with ongoing periodontitis (Jeong et al., 2012). Moreover, P. gingivalis GroEL has been discussed as a candidate for vaccine formulation in preventing periodontal disease (Choi et al., 2005; Lee et al., 2006).
The treatment of P. gingivalis with benzamidine and benzamidine-derived inhibitors enhanced the mRNA expression of groEL in the compound- and strain-dependent manner. Protein expression was not in exact accordance with the mRNA levels. Weak correlation between mRNA expression and protein levelis not uncommon since translation of mRNA is regulated by many factors independently of gene transcription. Moreover, mRNA as well as proteins can be degraded (Picard et al., 2009). Accordingly we found additional bands in immunoblots of the clinical strain which may indicate a cleavage and or degradation of GroEL. The transcriptional response of gingipain genes in P. gingivalis exposed to heat and benzamidine derivatives was seemingly strain-dependent. This finding correlates with previous observations showing a differential response of the gingipain genes to heat-shock in different strains. Inthe ATCC 33277 strain, heat shockstimulated expression of Arg-gingipains (Shelburne et al., 2005), whereas in W50, as with our M5-1-2 strain, this stress negatively impacted levels of Arg-gingpain mRNA (Percival et al., 1999).
Pentamidine is a clinical drug of choice in the treatment of human protozoal infections (Werbovetz, 2006). I n addition, in an aerosol form, pentamidine is used to prevent Pneumocystis jiroveci pneumonia, a serious health risk for immunocompromised patients (Marras et al., 2002). Although pentamidinebinds to DNA (Edwards et al., 1992) and RNA (Sun & Zhang, 2008), the precise mechanism of itsantimicrobial activity is still unknown. Here we show that pentamidine and 2,6-bis-(4-amidinobenzyl)-cyclohexanone can be used to abrogate P. gingivalis virulence. In this case, the protective effect might beexerted by cumulative effect ofinhibition of Arg-gingipains, binding to GroEL, and stimulation of phagocytosis. Yet unknown factors induced by benzamidine derivatives may also contribute to quenching of the P. gingivalis pathogenicity. In this context, topical application of pentamidine and 2,6-bis-(4-amidinobenzyl)-cyclohexanone might be a promising developmentfor supportive periodontitis treatment.
Acknowledgement
This paper is dedicated to JörgStürzebecher (Institute of Vascular Biology and Medicine, University Hospital of Jena) who passed away in 2008. JörgStürzebecher encouraged us to investigate the effect of these inhibitors on gingipains. He designed and purifiedthe Arg-gingipains by means of an affinity chromatography on a benzamidine-Sepharose column. We are grateful for his providing the compounds and all his helpful advice.
Further, the authors would like to thank Claudia Ranke and Katja Sack for excellent technical assistance. Dr Eckhard Birch-Hirschfeld, University Hospital of Jena, is acknowledged for synthesizing primers. Furthermore, we are indebted to Bernhard Schlott (Leibniz Institute for Age Research, Jena) for N-terminal sequencing.
Most of the study was funded by the participating departments, and supported by IUVENTUS Plus (0221/IP1/2011/71, MNiSW, Warszawa, Poland), along withgrants from: the National Institutes of Health (Grant DE 09761, USA), National Science Center (2011/01/B/NZ6/00268, Kraków, Poland), the European Community (FP7-HEALTH-2010-261460 “Gums&Joints”), MNiSW (2136/7. PR UE/2011/2, Warszawa, Poland) and the Foundation for Polish Science (TEAM project DPS/424-329/10). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (grant No: POIG.02.01.00-12-064/08 – ”Molecular biotechnology for health”).
Footnotes
The authors declare that there are no conflicts of interest in this study.
References
- Argueta JG, Shiota S, Yamaguchi N, Masuhiro Y, Hanazawa S. Induction of Porphyromonas gingivalis GroEL signaling via binding to Toll-like receptors 2 and 4. Oral Microbiol Immunol. 2006;21:245–251. doi: 10.1111/j.1399-302X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Lee SY, Kim K, Choi BK. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res. 2011;46:240–245. doi: 10.1111/j.1600-0765.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- Choi JI, Choi KS, Yi NN, et al. Recognition and phagocytosis of multiple period on to pathogenic bacteria by anti-Porphyromonas gingivalis heat-shock protein 60 antisera. Oral Microbiol Immunol. 2005;20:51–55. doi: 10.1111/j.1399-302X.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Aduse Opoku J, Rangarajan M, et al. Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun. 2002;70:6968–6975. doi: 10.1128/IAI.70.12.6968-6975.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KJ, Jenkins TC, Neidle S. Crystal structure of a pentamidine-oligonucleotide complex: implications for DNA-binding properties. Biochemistry. 1992;31:7104–7109. doi: 10.1021/bi00146a011. [DOI] [PubMed] [Google Scholar]
- Eick S, Pfister W, Sturzebecher U, Jarema S, Sturzebecher J. Inhibitors of benzamidine type influence the virulence properties of Porphyromonas gingivalis strains. Acta Biochim Pol. 2003;50:725–734. [PubMed] [Google Scholar]
- Ezzo PJ, Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003;32:24–35. doi: 10.1046/j.0906-6713.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C, Gudino CV, Gibson FC, 3rd, Genco CA. Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. 2010;25:305–316. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinode D, Grenier D, Mayrand D. Purification and characterization of a DnaK-like and a GroEL-like protein from Porphyromonas gingivalis. Anaerobe. 1995;1:283–290. doi: 10.1006/anae.1995.1028. [DOI] [PubMed] [Google Scholar]
- Katz J, Yang QB, Zhang P, et al. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect Immun. 2002;70:2512–2518. doi: 10.1128/IAI.70.5.2512-2518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000;15:341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yi NN, Kim US, et al. Porphyromonas gingivalis heat shock protein vaccine reduces the alveolar bone loss induced by multiple period on to pathogenic bacteria. J Periodontal Res. 2006;41:10–14. doi: 10.1111/j.1600-0765.2005.00832.x. [DOI] [PubMed] [Google Scholar]
- Lu B, McBride BC. Stress response of Porphyromonas gingivalis. Oral Microbiol Immunol. 1994;9:166–173. doi: 10.1111/j.1399-302x.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Marras TK, Sanders K, Lipton JH, et al. Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transpl Infect Dis. 2002;4:66–74. doi: 10.1034/j.1399-3062.2002.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- Melhus A, Hermansson A, Forsgren A, Prellner K. Intra- and interstrain differences of virulence among nontypeable Haemophilus influenzae strains. APMIS. 1998;106:858–868. [PubMed] [Google Scholar]
- Mikolajczyk-Pawlinska J, Kordula T, Pavloff N, et al. Genetic variation of Porphyromonas gingivalis genes encoding gingipains, cysteine proteinases with arginine or lysine specificity. Biol Chem. 1998;379:205–211. doi: 10.1515/bchm.1998.379.2.205. [DOI] [PubMed] [Google Scholar]
- Paju S, Goulhen F, Asikainen S, et al. Localization of heat shock proteins in clinical Actinobacillus actinomycetemcomitans strains and their effects on epithelial cell proliferation. FEMS Microbiol Lett. 2000;182:231–235. doi: 10.1111/j.1574-6968.2000.tb08900.x. [DOI] [PubMed] [Google Scholar]
- Percival RS, Marsh PD, Devine DA, et al. Effect of temperature on growth, hemagglutination, and protease activity of Porphyromonas gingivalis. Infect Immun. 1999;67:1917–1921. doi: 10.1128/iai.67.4.1917-1921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Dressaire C, Girbal L, Cocaign-Bousquet M. Examination of post-transcriptional regulations in prokaryotes by integrative biology. C R Biol. 2009;332:958–973. doi: 10.1016/j.crvi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Potempa J, Travis J, Gao XP. Mechanisms mediating Porphyromonas gingivalis gingipain RgpA-induced oral mucosa inflammation in vivo. Infect Immun. 2001;69:1199–1201. doi: 10.1128/IAI.69.2.1199-1201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KH, Gerlach D, Gubbe K, et al. Virulence of group A streptococci in fertile hens eggs is mainly effected by M protein and streptolysin O. Int J Med Microbiol. 2001;291:45–56. doi: 10.1078/1438-4221-00102. [DOI] [PubMed] [Google Scholar]
- Schmidt KH, Podbielski A, Reichardt W, Gubbe K, Amberg C. Virulence of Streptococcus pyogenes for chicken embryos after isogenic inactivation of different streptococcal pathogenicity factors. Adv Exp Med Biol. 1997;418:793–795. doi: 10.1007/978-1-4899-1825-3_187. [DOI] [PubMed] [Google Scholar]
- Schmidt KH, Wiesner J, Gerlach D, et al. Susceptibility of chicken embryos to group A streptococci: correlation with fibrinogen binding. FEMS Immunol Med Microbiol. 1993;7:231–240. doi: 10.1111/j.1574-695X.1993.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Fletcher HM, Casiano CA. Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect Immun. 2006;74:5667–5678. doi: 10.1128/IAI.01140-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne CE, Gleason RM, Coulter WA, Lantz MS, Lopatin DE. Differential display analysis of Porphyromonas gingivalis gene activation response to heat and oxidative stress. Oral Microbiol Immunol. 2005;20:233–238. doi: 10.1111/j.1399-302X.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhang Y. Pentamidine binds to tRNA through non-specific hydrophobic interactions and inhibits aminoacylation and translation. Nucleic Acids Res. 2008;36:1654–1664. doi: 10.1093/nar/gkm1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–293. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J, Potempa J. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim Biophys Acta. 2000;1477:35–50. doi: 10.1016/s0167-4838(99)00278-2. [DOI] [PubMed] [Google Scholar]
- Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssier C, Mayrand D, Grenier D. Detection of stress proteins in Porphyromonas gingivalis and other oral bacteria by western immunoblotting analysis. FEMS Microbiol Lett. 1994;121:303–307. doi: 10.1111/j.1574-6968.1994.tb07117.x. [DOI] [PubMed] [Google Scholar]
- Viale AM, Arakaki AK. The chaperone connection to the origins of the eukaryotic organelles. FEBS Lett. 1994;341:146–151. doi: 10.1016/0014-5793(94)80446-x. [DOI] [PubMed] [Google Scholar]
- Vincents B, Guentsch A, Kostolowska D, et al. Cleavage of IgG1 and IgG3 by gingipain K from Porphyromonas gingivalis may compromise host defense in progressive periodontitis. FASEB J. 2011;25:3741–3750. doi: 10.1096/fj.11-187799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbovetz K. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr Opin Investig Drugs. 2006;7:147–157. [PubMed] [Google Scholar]