Abstract
Purpose
To compare ovarian morphology in adolescent girls with and without polycystic ovary syndrome (PCOS) using magnetic resonance (MR) imaging.
Materials and Methods
In 21 adolescent girls (12–18 years) without and 19 adolescents with PCOS (diagnosis based on excessive hair growth and irregular menstrual cycles) ovarian volume, antral follicle count (AFC) per ovary, and follicle size were evaluated. MR imaging was performed at 1.5 T or 3 T and axial or angled-axial single-shot echo-train spin echo images of 6 mm slice thickness were acquired. In a subset of subjects, 2 mm images were also obtained. PCOS and non-PCOS groups were compared using mixed affects regression.
Results
Mean AFC per ovary and ovarian volume were substantially greater in PCOS subjects compared to non-PCOS subjects. Mean follicle size was similar between groups. Follicles exceeding 10 mm were seen in 2/19 PCOS subjects versus 9/21 non-PCOS subjects. Consistently higher follicle counts were detected in images obtained at 2 mm compared to 6 mm slice thickness.
Conclusion
In adolescence MR imaging of the ovary reveals distinct differences between girls with and without PCOS. MR imaging may help evaluate young patients in whom transvaginal ultrasound is contraindicated.
Keywords: Magnetic Resonance, Ovarian Follicles, Polycystic Ovary Syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of reproductive aged women, affecting 5–10% of this population (1). The presenting features are excessive hair growth, irregular menstrual bleeding, and bilaterally enlarged polycystic ovaries. While these symptoms usually emerge at or soon after puberty, often the diagnosis is not made until early adulthood which may delay initiation of treatment (2). While ovarian morphology alone is neither necessary nor sufficient for the diagnosis of polycystic ovary syndrome (PCOS), imaging evidence of typical ovarian morphology is an important part of current diagnostic algorithms. Accepted criteria based on transvaginal ultrasound include: ≥ 12 follicles 2–9 mm in diameter per ovary and ovarian volume > 10 cm3 (3, 4). These values were obtained from studies of pelvic ultrasound examinations performed in adult women with PCOS (5–7). However, in adolescent girls with suspected PCOS, transvaginal ultrasound may be contraindicated, and given the high prevalence of obesity in this population, transabdominal ultrasound may be non-diagnostic. Magnetic resonance (MR) imaging features of adult PCOS women have been described and include enlarged ovaries with numerous small follicles and increased dark central stroma on T2-weighted images (8, 9). Comparable studies in adolescent girls are lacking. In a pilot study of young girls with PCOS, MR imaging provided improved visualization of ovarian morphology and revealed greater follicle number than that detected by transabdominal ultrasound (10). Precise diagnostic criteria, including follicle count threshold, have not been established for MR imaging in adults or adolescents. This study compares MR imaging features of the ovary in adolescent girls with and without PCOS.
Materials and Methods
Subjects
Nineteen adolescent girls with PCOS and 21 aged-matched normal controls with regular menstrual cycles were recruited for study. The diagnosis of PCOS was based on clinical and/or biochemical evidence of hyperandrogenism and irregular menstrual bleeding, according to the 1990 National Institutes of Health Conference on PCOS and consistent with the 2003 Rotterdam PCOS Consensus Workshop (3, 4, 11). Hyperandrogenism was based on the presence of hirsutism and/or elevated serum testosterone level. The normal adolescent girls had regular menstrual cycles and no evidence of excessive hair growth. The mean chronological age of the PCOS group was 15.8 ± 0.4 years compared to 15.4 ± 0.3 years for the normal controls.
All adolescents were euthyroid, without evidence of hyperprolactinemia or adrenal enzyme deficiency. No subjects were on any medications. Informed consent was obtained from the legal guardian of each subject less than 18 years of age and from those subjects 18 years or older before participation in this study. Informed assent was obtained from study subjects less than 18 years of age. The study had been previously approved by the Institutional Review Board.
Procedures
Normal control subjects were scheduled for imaging study during the follicular phase of their menstrual cycle (within the first 13 days prior to ovulation). PCOS subjects were scheduled for the study on an arbitrary day. On the day of study a physical exam, urine pregnancy test, and MRI was done on each subject.
MRI
All subjects were examined on a Symphony 1.5 T MR imaging system (Siemens Medical Systems, Malvern, PA, USA) or a Signa 3 T Twin Speed MR imaging system (GE Medical Systems, Milwaukee, IL, USA), using a phased array torso coil placed over the pelvis. Images of the ovaries were obtained in axial, coronal, and sagittal planes using T2-weighted, single-shot echo-train spin-echo images (SSFSE/HASTE, effective TE approximately 80 msec), with a slice thickness of 6 mm and spacing of 6 mm. In a subset of participants, the same sequence was also obtained using 2 mm slice thickness and spacing of 2 mm. Field of view and matrix varied between subjects.
MR images were archived digitally on an Agfa Healthcare IMPAX® picture archiving and communication system (PACS) network (Agfa Corporation, Ridgefield Park, NJ) and reviewed in a blinded fashion by one of the authors, a radiologist with expertise in pelvic imaging. Axial T2-weighted single-shot echo-train spin-echo images at 6 mm slice thickness were used for follicle evaluation. A separate blinded reading was done using images at 2 mm slice thickness in the subset of patients imaged at both 6 mm and 2 mm slice thickness. Ovarian dimensions and follicle diameters were measured using electronic calipers on the PACS system monitor. Ovarian volume was calculated with the prolate ellipse formula: D1xD2xD3x0.523, where D1 was the longitudinal diameter, D2 was the anteroposterior diameter, and D3 was the transverse diameter of the ovary. Ovarian diameter measurements recorded were the maximum orthogonal measurements of the ovary as best determined using all three imaging planes.
Statistical analysis
Mixed effects regression was used to model ovary volume, total follicle count and average follicle size as a function of group (PCOS versus control) with a random (subject-specific) intercept fitted to the data. This is a regression-based version of a t-test which accounts for within-patient dependence in the ovary information. Correlation between 2mm and 6mm follicle counts was computed and a paired t-test was used to evaluate systematic shifts between the measurements.
Results
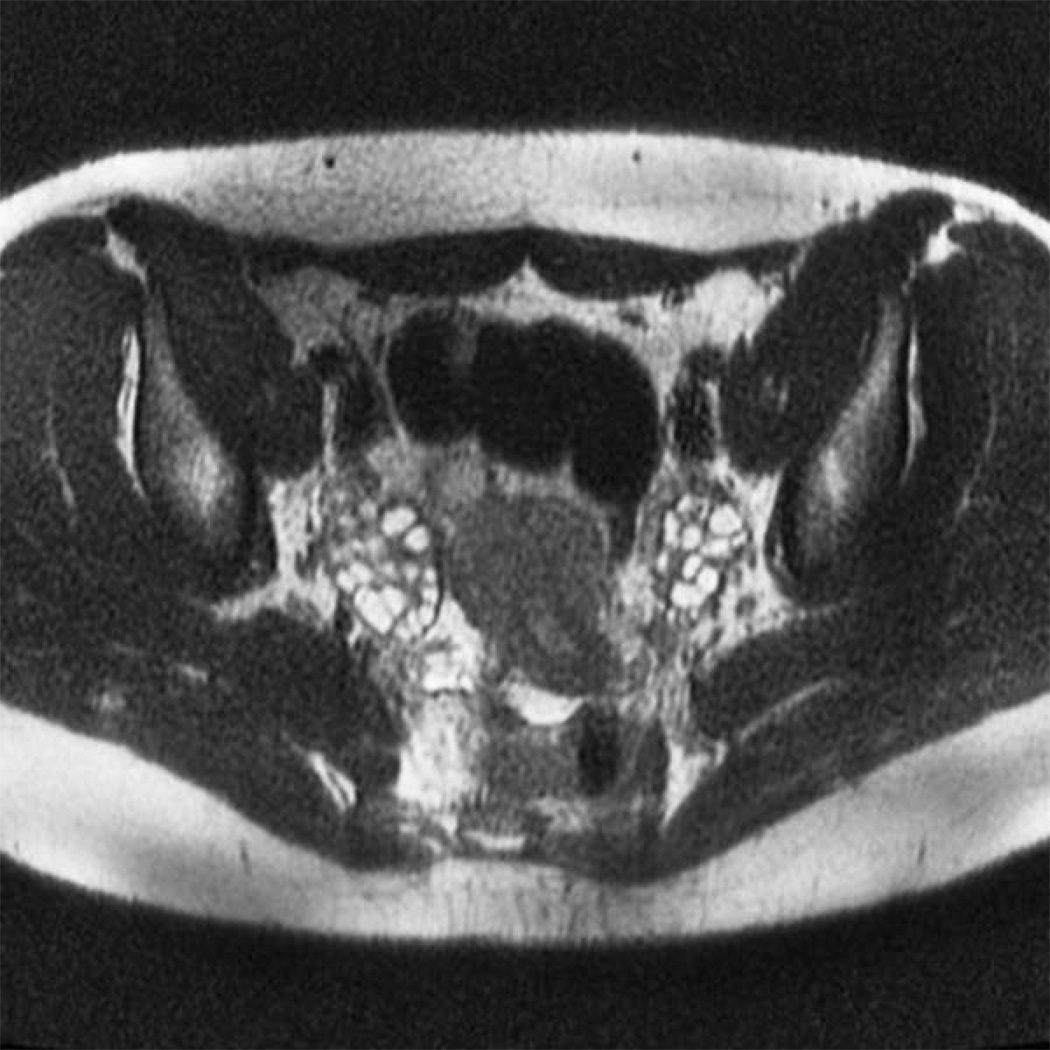
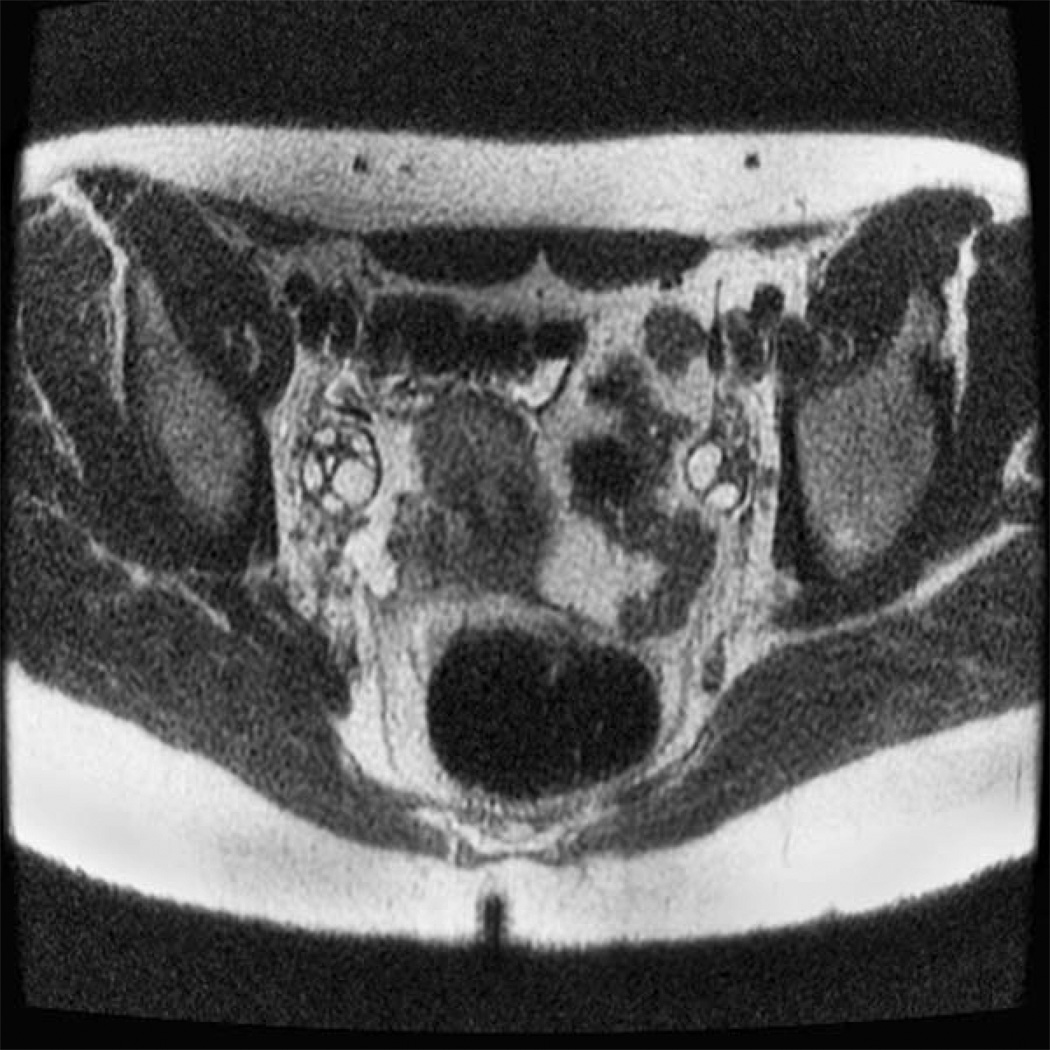
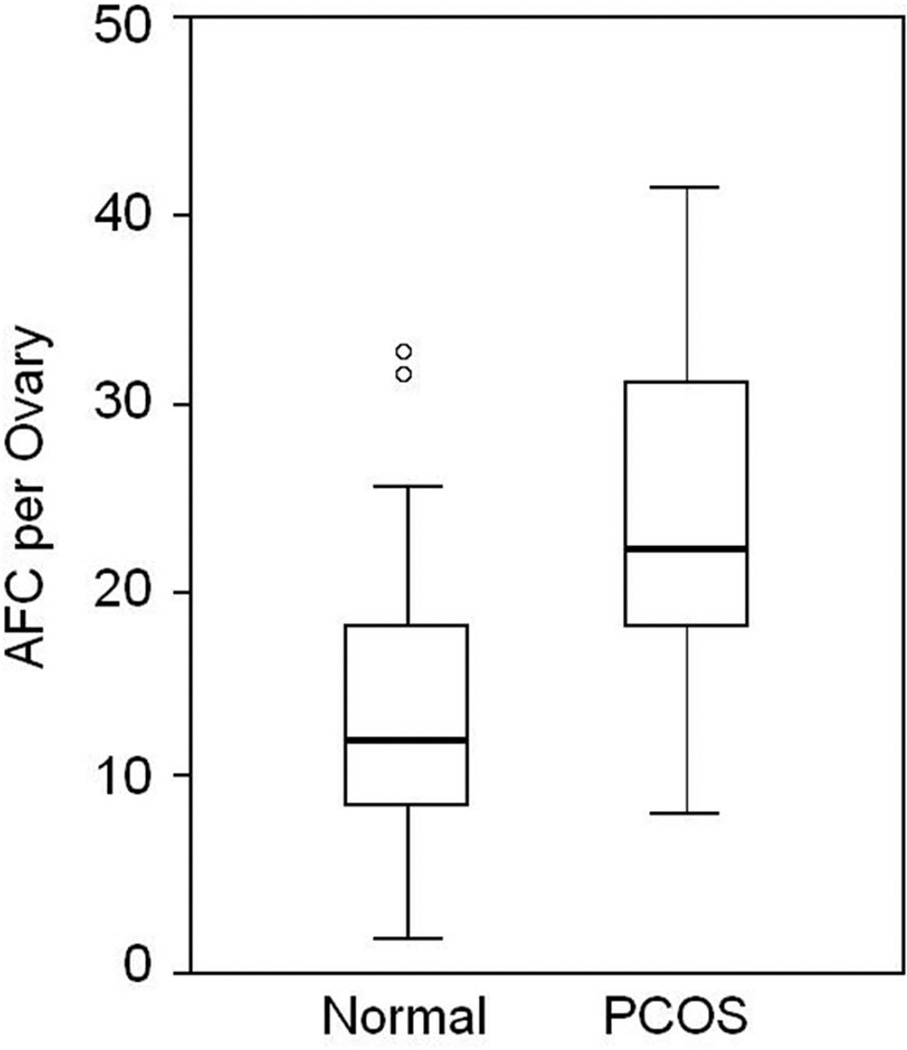
A total of 38 ovaries from adolescent PCOS girls (fig 1) and 42 ovaries from normal controls (fig 2) were analyzed for antral follicle number and ovarian volume. In PCOS subjects the mean (± SD) antral follicle count (AFC) per ovary was 24.0 ± 8.7 compared to 13.9 ± 7.1 in age-match normal controls (p = 0.0001) (fig 3). The median AFC and range of values were 22 (9–41) for girls with PCOS and 12 (4–33) in normal adolescents.
Figure 1.
Axial T2-weighted single-shot echo-train spin-echo image in an adolescent girl with polycystic ovary syndrome.
Figure 2.
Axial T2-weighted single-shot echo-train spin-echo image in an adolescent girl with normal ovaries.
Figure 3.
Box-and-whisker plot showing follicle number per ovary (FNPO) in normal adolescent girls and adolescent girls with polycystic ovary syndrome (PCOS). Horizontal small bars represent the 5–95th percentile range and the boxes indicate the 25–75th percentile range. The horizontal line within each box corresponds to the median. O, values beyond the 95th percentile.
Average follicle sizes were the same in both PCOS and normal girls. For all subjects distribution of follicle number grouped according to size revealed considerably more small antral follicles (2–5 mm diameter) compared to larger sized follicles (6–9 mm diameter). In adolescent girls with PCOS the AFC in both size categories were greater than those observed in normal controls (Table 1). Follicles larger than 10 mm diameter were present in 2/19 PCOS subjects compared to 9/21 non-PCOS subjects.
Table 1.
Mean (± SD) and median (range) follicle number per ovary (FNPO) in adolescent girls with PCOS and normal girls.
| FNPO Mean (SD) |
FNPO Median (Range) |
|
|---|---|---|
| PCOS | ||
| 2–5 mm | 22.0 ± 8.4 | 21.5 (9 – 37) |
| 6–9 mm | 4.1 ± 3.4 | 4.0 (0 – 16) |
| 2–9 mm | 24.0 ± 8.7 | 22 (9 – 41) |
| Normal | ||
| 2–5 mm | 12.0 ± 7.4 | 10 (2 – 31) |
| 6–9 mm | 1.7 ± 2.0 | 1 (0 –10) |
| 2–9 mm | 13.9 ± 7.1 | 12 (4 – 33) |
| All Subjects | ||
| 2–5 mm | 16.7 ± 9.3 | 16 (2 –37) |
| 6–9 mm | 2.2 ± 2.8 | 1 (0 –16) |
| 2–9 mm | 18.7 ± 9.3 | 18 (4 – 41) |
The mean (± SD) ovarian volume was 15.1 ± 5.6 ml in PCOS subjects compared to 5.9 ± 3.0 ml in normal adolescents (p=.0001) (fig 4). The median values for ovarian volume were 15.1 ml (3.9–29.0) and 5.3 ml (1.5–13.1) for PCOS girls and normal controls, respectively.
Figure 4.
Box-and-whisker plot showing ovarian volume in normal adolescent girls and adolescent girls with polycystic ovary syndrome (PCOS). Horizontal small bars represent the 5–95th percentile range and the boxes indicate the 25–75th percentile range. The horizontal line within each box corresponds to the median. O, values beyond the 95th percentile.
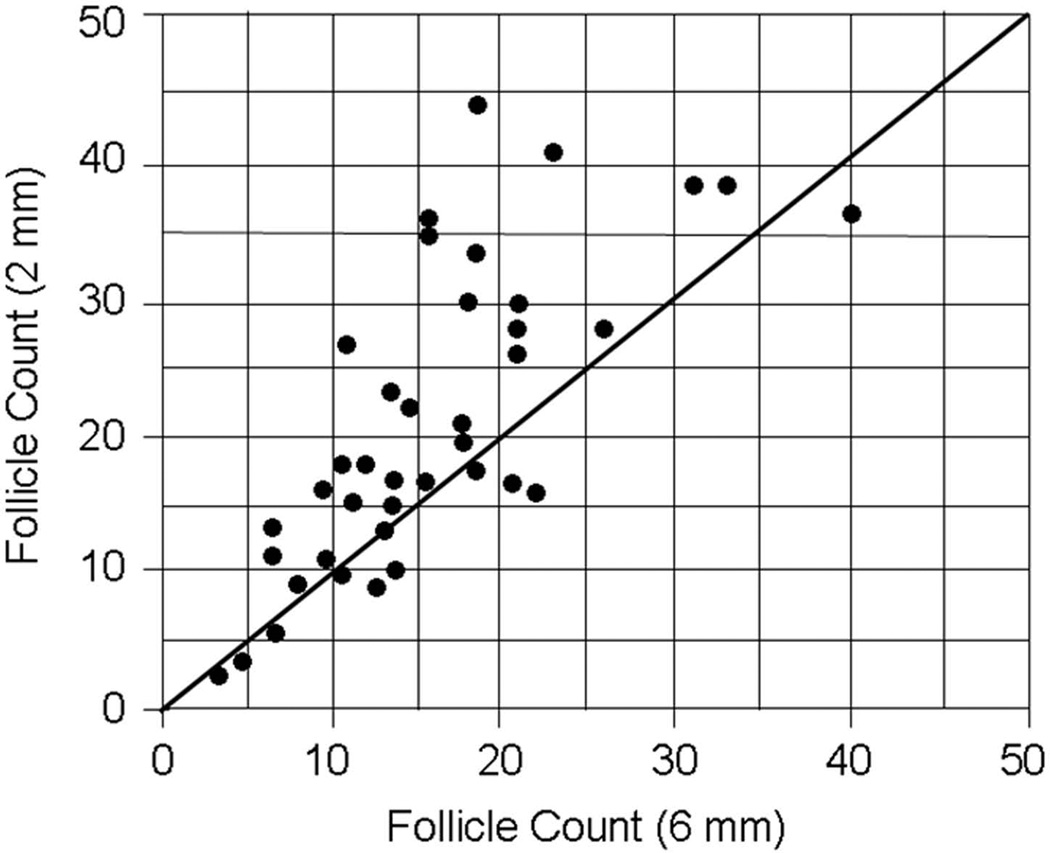
In 22 patients, images were obtained in the same plane using 6 mm and 2 mm slice thickness and follicle counts of 44 ovaries were compared (fig 5). The 6 mm counts tended to be lower in the same patients using a conservatively adjusted t-test due to the fact that there were 22 rather than 44 unique patients. Average follicle count was 15.8 + 7.4 at 6 mm and 20.5 + 10.5 at 2 mm slice thickness (p = 0.006). Median values were 14 (4–40) at 6 mm slice thickness and 18 (3–44) at 2 mm slice thickness.
Figure 5.
Correlation between total ovarian follicle counts using 2 mm sections and 6 mm sections in normal adolescents (n = 15) and adolescent girls with PCOS (n =7). (r value = 0.73, p <0.001)
Discussion
The results of this study have provided a quantitative description of ovarian antral follicle number and ovarian volume in normal adolescent girls as well as those with PCOS as determined by magnetic resonance imaging. These morphometric measures were greater in adolescents with PCOS compared to values observed in age-matched normal controls. Notably, the mean and median total antral follicle count (AFC) per ovary in PCOS girls exceeded those reported for most adult women with this disorder. In addition, we determined antral follicle number in normal adolescent girls.
In adolescence, there a paucity of imaging literature that addresses the morphological appearance of the ovary. In most instances, the data has been generated by ultrasonography using the abdominal approach as endovaginal application may not be suitable in girls that are virginal. However, clarity of imaging may be impeded by excess abdominal and pelvic adipose in obese individuals. As a result, most studies have reported ovarian volume and not the actual number of antral follicles in both normal and PCOS girls. In general, accumulated evidence suggests that ovarian size in girls with PCOS may be slightly smaller, 6–12 ml, than those of adult PCOS, 12–15 ml (5, 12–20). By comparison, in normal adolescents ovarian volumes by ultrasound have been shown to be similar to that of adult women (5, 12–21). One recent publication reported that in PCOS girls the mean volume of the ovary, 19.2 ml, was larger than previously noted by others (22). In the same study, the ovarian volume of normal girls was comparable to adult women.
The findings of the current study have revealed that in adolescent girls with PCOS mean ovarian size was slightly greater than most values previously reported for this disorder, although individual values comprised a wide range. In normal girls the ovarian volume was considerably smaller than that of adolescent PCOS and commensurate with values described by other investigators (12–14, 21).
The increased number of antral follicles in adolescent girls with PCOS was consistent with our earlier findings of greater follicle number compared to that of normal controls, as expected (10). Notably, the mean (± SD) AFC of 24.0 ± 8.7 per ovary was greater than the majority of values reported for either normal or PCOS adult women (5, 15, 16, 18–20). One study identified 29.8 ± 11.5 follicles per ovary in women with PCOS using 3-D ultrasound (17). In our normal subjects, the mean antral follicle number of 12.0 ± 7.4 per ovary was somewhat surprising as this figure exceeded those previously recorded for normal adults by nearly 2-fold. Notably, the increment in follicle number was largely attributable to 2–5 mm follicles in both normal and PCOS adolescents, which for diagnostic purpose underscores the need to carefully document the quantity of small sized follicles. The discordance between our findings in adolescents and those noted in adults may be attributed to MR technology, which offers fine resolution and high sensitivity to small fluid-containing structures such as ovarian follicles on T2-weighted echo-train spin-echo images (10).
MRI evaluation of polycystic ovarian morphology in adult women has been reported previously (8, 23, 24). Similar to findings on ultrasound, MRI findings overlap between subjects with and without clinical and biochemical evidence of PCOS. Barber et al. found that a threshold of 12 ovarian follicles is appropriate for detection of PCOS using MRI, however several subjects in that series had greater than 35 follicles seen within a single ovary. Our data support a higher threshold, which may reflect the adolescent population and/or improved delineation of individual follicles by MRI compared to ultrasound. The latter explanation is supported by our finding of slightly higher follicle counts using 2 mm slices when compared to 6 mm slices in the same subjects. Recent studies using current high-resolution transvaginal ultrasound have prompted some authors to revisit the 12 follicle threshold described in the Rotterdam criteria. One study found a threshold value of 19 to yield the best compromise between sensitivity and specificity, and concluded that the higher value was a reflection of the greater resolving power of modern ultrasound equipment (25). Similarly, our finding of higher follicle counts may reflect the greater resolving capability of MRI compared to earlier ultrasound equipment.
Alternatively, the relative increase of follicle number in normal adolescence may represent physiological growth which suggests that during puberty through adolescence there is continued and, perhaps, a relative excess of antral follicle formation. It has been shown that from early to late puberty there is a modest increase in the number of antral follicles up to an average of approximately 6 per ovary which may have resulted from rising serum gonadotropin levels (26). It is apparent that this morphological transformation of increasing ovarian follicle number continues into adolescence as indicated by our findings. Subsequently, in early adulthood the AFC declines to achieve normal values. The mechanism by which the ovary undergoes these changes is unknown.
Our findings may have relevance to the diagnosis of PCOS in adolescent girls. The current diagnostic criteria for PCOS include distinctive features of ovarian morphology as defined by the 2003 Rotterdam consensus (4). Namely, the number of antral follicles measuring 2–9 mm in diameter are equal to or greater than 12 and/or an increased ovarian volume of greater that 10 ml. These criteria were based primarily on ultrasonographic findings in adult women with PCOS. While the number of subjects in the current study was limited, the application of adult values to detect a polycystic ovary, at least by follicle number, may not be appropriate for adolescent girls. Moreover, threshold values based on previous ultrasound data may not be appropriate for MRI, which is a modality well-suited for adolescents in whom transvaginal imaging is contraindicated. Clearly, additional studies are needed to corroborate the current findings and potentially to re-consider morphological criteria for polycystic ovaries in adolescent girls.
Limitations of the current study include lack of uniformity of MR equipment and protocols due in part to the use of two different MR systems and vendors. Parameters were not identical however images appeared qualitatively similar and comparable sequences were used for measurements. In addition, the present results are based on a single blinded reader and interobserver variability can thus not be addressed. While the reader was experienced in the interpretation of gynecological MR images, the process of counting and measuring individual follicles was completed manually. Given the high reported interobserver variation in follicle count based on ultrasound (27), it is possible that results based on MRI may also be prone to interobserver variation. Standardized training and/or automated techniques would presumably improve reliability and optimize the utility of MRI for the quantitative assessment of polycystic ovaries.
In summary, these results demonstrate that adolescent girls with PCOS exhibit greater ovarian follicle number and volume compared to those of normal girls using MRI, a distinction similar to observations in adults based on ultrasound data. However, the mean follicle number in the adolescent population using MRI appears to be much higher than values reported for adult women using ultrasound. Thus, the current criteria for polycystic ovaries may not be suitable for adolescent girls who may be best evaluated with MRI rather than transvaginal ultrasound.
Acknowledgments
Grant Support: This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD12303-28) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (RJC).
References
- 1.Brown MA, Chang RJ. Polycystic ovary syndrome: clinical and imaging features. Ultrasound Q. 2007;23:233–238. doi: 10.1097/ruq.0b013e318159927f. [DOI] [PubMed] [Google Scholar]
- 2.Chang R. Recommendations for the Early Recognition and Prevention of PCOS. In: Dunaif A, Chang R, Franks S, Legro R, editors. Polycystic Ovary Syndrome: Current Concepts and Controversies, From the Ovary to the Pancreas. Totowa: Humana Press; 2008. pp. 147–157. [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 5.Pache TD, Wladimiroff JW, Hop WC, Fauser BC. How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology. 1992;183:421–423. doi: 10.1148/radiology.183.2.1561343. [DOI] [PubMed] [Google Scholar]
- 6.van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril. 1997;67:452–458. doi: 10.1016/s0015-0282(97)80068-4. [DOI] [PubMed] [Google Scholar]
- 7.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 8.Barber TM, Alvey C, Greenslade T, et al. Patterns of ovarian morphology in polycystic ovary syndrome: a study utilising magnetic resonance imaging. Eur Radiol. 2010;20:1207–1213. doi: 10.1007/s00330-009-1643-8. [DOI] [PubMed] [Google Scholar]
- 9.Hauth EA, Umutlu L, Libera H, Kimmig R, Forsting M. Magnetic resonance imaging of the pelvis in patients with polycystic ovary syndrome. Rofo. 2009;181:543–548. doi: 10.1055/s-0028-1109179. [DOI] [PubMed] [Google Scholar]
- 10.Yoo RY, Sirlin CB, Gottschalk M, Chang RJ. Ovarian imaging by magnetic resonance in obese adolescent girls with polycystic ovary syndrome: a pilot study. Fertil Steril. 2005;84:985–995. doi: 10.1016/j.fertnstert.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 12.Gulekli B, Turhan NO, Senoz S, Kukner S, Oral H, Gokmen O. Endocrinological, ultrasonographic and clinical findings in adolescent and adult polycystic ovary patients: a comparative study. Gynecol Endocrinol. 1993;7:273–277. doi: 10.3109/09513599309152512. [DOI] [PubMed] [Google Scholar]
- 13.van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil Steril. 2000;74:49–58. doi: 10.1016/s0015-0282(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 14.Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 15.Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 16.Hahn S, Tan S, Elsenbruch S, et al. Clinical and biochemical characterization of women with polycystic ovary syndrome in North Rhine-Westphalia. Horm Metab Res. 2005;37:438–444. doi: 10.1055/s-2005-870236. [DOI] [PubMed] [Google Scholar]
- 17.Allemand MC, Tummon IS, Phy JL, Foong SC, Dumesic DA, Session DR. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril. 2006;85:214–219. doi: 10.1016/j.fertnstert.2005.07.1279. [DOI] [PubMed] [Google Scholar]
- 18.Lam PM, Johnson IR, Raine-Fenning NJ. Three-dimensional ultrasound features of the polycystic ovary and the effect of different phenotypic expressions on these parameters. Hum Reprod. 2007;22:3116–3123. doi: 10.1093/humrep/dem218. [DOI] [PubMed] [Google Scholar]
- 19.Lam P, Raine-Fenning N, Cheung L, Haines C. Three-dimensional ultrasound features of the polycystic ovary in Chinese women. Ultrasound Obstet Gynecol. 2009;34:196–200. doi: 10.1002/uog.6442. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Fu Q. Three-dimensional transrectal ultrasonography in adolescent patients with polycystic ovarian syndrome. Int J Gynaecol Obstet. 2007;98:34–38. doi: 10.1016/j.ijgo.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Hart R, Sloboda DM, Doherty DA, et al. Prenatal determinants of uterine volume and ovarian reserve in adolescence. J Clin Endocrinol Metab. 2009;94:4931–4937. doi: 10.1210/jc.2009-1342. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab. 2006;91:3786–3790. doi: 10.1210/jc.2006-0835. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell DG, Gefter WB, Spritzer CE, et al. Polycystic ovaries: MR imaging. Radiology. 1986;160:425–429. doi: 10.1148/radiology.160.2.3726121. [DOI] [PubMed] [Google Scholar]
- 24.Kimura I, Togashi K, Kawakami S, et al. Polycystic ovaries: implications of diagnosis with MR imaging. Radiology. 1996;201:549–552. doi: 10.1148/radiology.201.2.8888256. [DOI] [PubMed] [Google Scholar]
- 25.Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 26.Cacciatore B, Leminen A, Ingman-Friberg S, Ylostalo P, Paavonen J. Transvaginal sonographic findings in ambulatory patients with suspected pelvic inflammatory disease. Obstet Gynecol. 1992;80:912–916. [PubMed] [Google Scholar]
- 27.Lujan M, Chizen D, Peppin A, Dhir A, Pierson R. Assessment of ultrasonographic features of polycystic ovaries is associated with modest levels of inter-observer agreement. J Ovarian Res. 2009;2:6. doi: 10.1186/1757-2215-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]