Abstract
Case Description
A 16-year-old spayed female cat evaluated for lagophthalmos and chronic exposure keratitis in both eyes.
Clinical Findings
Ophthalmic examination revealed upper and lower eyelid entropion of the left eye (OS) and markedly decreased retropulsion, restricted eye movement, marked episcleral congestion, and severe keratitis of both eyes (OU). Magnetic resonance imaging of both orbits revealed extensive, irregular, contrast-enhancing tissue without evidence of osteolysis considered compatible with diffuse inflammatory tissue. Feline herpesvirus DNA was not detected in conjunctival samples.
Treatment and Outcome
Partial temporary tarsorrhaphies were placed OU and the cat was treated with topically administered erythromycin ointment OU, orally administered famciclovir and prednisolone, and sublingually administered buprenorphine. Little improvement was noted after 2 weeks. Six weeks after presentation, a left exenteration was performed and histopathology was consistent with idiopathic sclerosing orbital pseudotumor (ISOP). Ten weeks after presentation, the patient presented for weight loss and jaw pain. Computed tomography demonstrated disease progression in the right orbit and the patient was euthanized. Histopathology of the decalcified skull revealed an aggressive and highly infiltrative mass involving the right orbit with extension to the maxilla, hard palate, nasal cavity and gingiva most consistent with feline restrictive orbital myofibroblastic sarcoma (FROMS).
Clinical Relevance
Clinical data from this patient support the reclassification of ISOP as FROMS. MRI and CT may provide supportive evidence for FROMS but histopathology is necessary for definitive diagnosis. Aggressive and early surgical treatment, including bilateral exenteration, with adjunctive radiotherapy and/or chemotherapy should be considered for patients with FROMS.
CASE REPORT
A 16-year-old spayed female Siamese cat was referred to the Ophthalmology Service of the University of California, Davis Veterinary Medical Teaching Hospital in January 2008 for evaluation and treatment of chronic changes in both eyes. Prior to referral, the patient had been treated for 3 months by a veterinary ophthalmologist for presumed herpetic keratitis, lagophthalmos with exposure keratitis, bullous keratopathy, and external ophthalmoplegia in both eyes (OU). At presentation, the patient was receiving vidarabine ophthalmic ointment (1/4″ strip, q 8 h, OU), gentamicin ophthalmic ointment (1/4″ strip, q 8 h, OU), sodium chloride ophthalmic ointment (1/4″ strip, q 8 h, OU), and 0.14 mg/kg buprenorphine (0.6 mg PO, q 12 h).
On physical examination, the patient was obese (BCS 6/9, weight 4.4 kg) with a grade II/VI parasternal heart murmur and an unkempt haircoat with mild scaling. On ophthalmic examination, both eyes had an incomplete palpebral reflex that resulted in lagophthalmos. Medial superior and inferior entropion were noted in the left eye (OS) and marked episcleral congestion was present OU. Both corneas were diffusely edematous and infiltrated by large caliber blood vessels that supplied large axial regions of raised dense granulation tissue admixed with multifocal regions of ulceration and corneal bullae (Figure 1). The globes were markedly resistant to retropulsion such that the third eyelids could not be protruded for examination. Forced duction of both globes revealed substantial mechanical restriction of movement in all directions. Detailed examination of intraocular structures was precluded by the marked corneal changes; however both irides were noted as blue. Schirmer tear test values were decreased at 7 mm/min OS and 10 mm/min in the right eye (OD; reference range, 11-23 mm/min).1 Intraocular pressures were within the reference range (15-25 mm Hg)2 at 21 mm Hg OS and 18 mm Hg OD.
Figure 1.
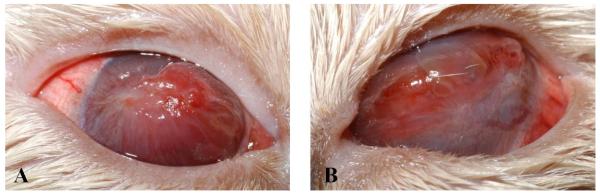
Frontal view of the right (A) and left (B) eye of a cat with marked episcleral congestion, dense corneal vascularization, edema, and granulation tissue with multifocal regions of ulceration and bullae formation. Medial superior and inferior entropion is present in the left eye (B).
Results of a complete blood count were unremarkable. Serum biochemical analysis showed mild hypochloremia (115 mmol/L; reference range 117-126 mmol/L) and increased bicarbonate concentration (24 mmol/L; reference range 15-21 mmol/L). Total thyroxine concentration (2.3 μg/dL) and free thyroxine concentration (1.22 μg/dL) were within the reference ranges of 1.1 to 3.9 and 0.52 to 1.39 μg/dL, respectively. Analysis of urine collected by cystocentesis revealed a urine specific gravity of 1.018 with mild proteinuria likely associated with collection method since 40-60 red blood cells per high power field were also noted. Thoracic radiographs confirmed that the patient was obese but were otherwise unremarkable. Ultrasound of the abdomen revealed slightly small kidneys (length of left kidney 3.38 cm, length of right kidney 3.43 cm) with diminished corticomedullary junction definition and minimal pelvic dilation. Echocardiography showed mild hypertrophy of the left ventricular papillary muscles. Magnetic resonance imaging (MRI) of the brain and orbits was performed with pulse sequences as described in Table 1 and revealed mild thickening of the soft tissues lining the retrobulbar spaces and signal reduction and partial effacement of the retrobulbar fat bilaterally. No apparent abnormal signal intensity was observed on T1, T2, or proton density weighted images (Figure 2: A, B). The thickened retrobulbar tissues were intermediate in signal intensity between muscle and cortical gray matter and hypointense relative to fat on each of these sequences. Following intravenous administration of 0.2 mL/kg gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals Inc., Wayne, NJ), marked enhancement of the sclera, episclera and tissues lining the retrobulbar spaces was observed OU; more notably OS (Figure 2: C). Heterogeneous, increased signal intensity was present in the left retrobulbar space on short tau inversion recovery (STIR) images (Figure 2: D). No orbital bone lysis was noted. The extensive irregular contrast enhancing tissue was considered most compatible with diffuse inflammation. Thus, the clinical diagnosis at this stage was idiopathic sclerosing orbital pseudotumor (ISOP).
Table 1.
Pulse Sequence Parameters for MRI
| Pulse Sequence | Plane | TR (ms) |
TE (ms) |
TI (ms) |
FOV (cm) |
Acquisition matrix |
|---|---|---|---|---|---|---|
| T1 weighted SE | Dorsal | 400 | 18 | - | 14 × 14 | 256 × 192 |
|
| ||||||
| T1 weighted SE | Transverse | 433 | 11 | - | 12 × 12 | 256 × 224 |
| T2 weighted FSE | Dorsal | 3350 | 87.1 | - | 14 × 14 | 256 × 256 |
| T2 weighted FSE | Transverse | 2967 | 95.1 | - | 12 × 12 | 256 × 224 |
| PD weighted FSE | Transverse | 2967 | 27.2 | - | 12 × 12 | 256 × 224 |
| STIR FSE | Dorsal | 3700 | 44.5 | 150 | 18 × 18 | 256 × 256 |
All images were obtained with a slice thickness of 3 mm and slice interval of 0.5 mm. Post-contrast T1-weighted images were obtained with chemical fat saturation. SE, spin echo; FSE, fast spin echo; PD, proton density; STIR, short tau inversion recovery; TR, repetition time; TE, echo time; TI, inversion time; FOV, field of view.
Figure 2.
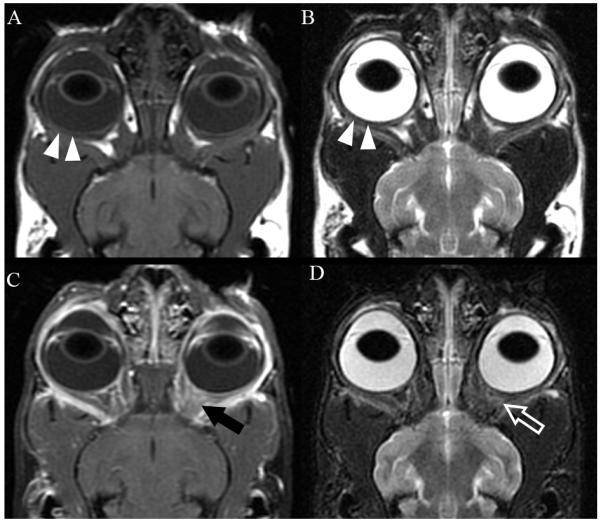
Dorsal plane magnetic resonance images of the retrobulbar spaces of both eyes of the cat shown in figure 1. All images are oriented with the left side of the cat to the right of the image. Pre-contrast (A) T1-weighted (TR = 400 ms; TE = 18 ms) and (B) T2-weighted (TR = 3350 ms; TE = 87.1 ms) images. Note mild thickening of the episclera and adjacent retrobulbar soft tissues (arrowheads). (C) Post-contrast T1-weighted image with chemical fat saturation (TR = 400 ms; TE = 18ms) demonstrating marked contrast enhancement of the retrobulbar tissues, especially on the left (black arrow). (D) STIR image (TR = 3700 ms, TE = 44.5 ms, and TI = 150 ms) depicting heterogeneous, increased signal intensity of the left retrobulbar soft tissues (open arrow). TR, repetition time; TE, echo time.
Following MRI and while still under general anesthesia, tissue samples of ventromedial palpebral conjunctiva were collected from both eyes for feline herpesvirsus type 1 (FHV-1) PCR and peribulbar tissues were collected from the lateral equatorial region of the left globe for fungal culture, aerobic and anaerobic bacterial cultures, and histopathology. A partial (lateral) temporary tarsorrhaphy was performed in both eyes to protect the corneas from exposure and the cat recovered uneventfully from general anesthesia. The cat was discharged from hospital and the owners were instructed to apply erythromycin ointment (1/4″ strip, q 6 h, OU). Prednisolone (1.1 mg/kg PO q 12 hours) was prescribed because most human and some feline patients with orbital pseudotumors respond to immunosuppressive medications.3,4 Famciclovir (85 mg/kg PO q 8 hours) was prescribed because the cat had been previously diagnosed with presumed herpetic keratitis, because prednisolone may cause reactivation of FHV-15, and because herpesviruses have been associated with idiopathic orbital pseudotumors in some human patients.6,7 Buprenorphine (0.14 mg/kg) was continued sublingually as needed (about every 8 to 12 hours) for analgesia. The owners were instructed to keep an Elizabethan collar in place all times and return for reevaluation in 2 weeks. Following discharge from hospital, FHV-1 DNA was not detected using the polymerase chain reaction8 in either of the conjunctival samples. Histologic examination of the sample of the left periocular tissue revealed moderate chronic lymphocytic and neutrophilic conjunctivitis without evidence of neoplasia or intranuclear inclusion bodies. No aerobic or anaerobic bacteria or fungus were cultured from the periocular sample collected from the left eye.
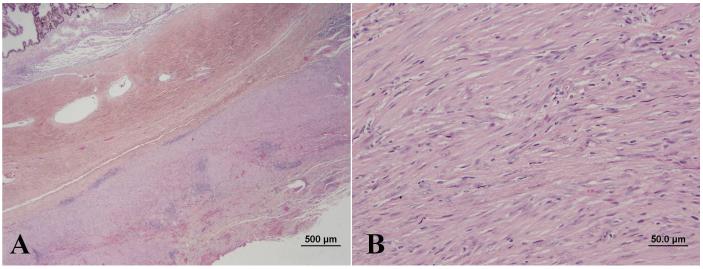
On reexamination 2 weeks following initial presentation, improvement was not detected. For this reason and due to negative PCR results and absence of intranuclear inclusion bodies consistent with FHV-1 on conjunctival histology, famciclovir was discontinued. All other medications were continued as before. Five weeks after initial presentation, the cat was reexamined, no improvement was noted, and the prednisolone dose was tapered. Six weeks following initial presentation, the left eye appeared very uncomfortable and orbital exenteration was performed. The globe and orbital tissues were fixed in 10% formalin, embedded in paraffin sectioned at 5 μm, and stained with hematoxylin and eosin. Histologic examination of the globe and orbital tissues revealed highly cellular spindle cell proliferation with abundant collagen in the episclera and surrounding facial planes of the extraocular muscles, Tenon’s capsule and orbital adipose tissue. The eyelid dermis was also distorted by bland appearing spindle cells. There was a moderate lymphoplasmacytic infiltration of the conjunctival epithelium as well as numerous areas of spindle cell proliferation distorting the conjunctival substantia propria. The axial cornea was diffusely ulcerated and markedly thickened by granulation tissue in the anterior stroma and edema in the posterior stroma. A mild lymphoplasmacytic infiltrate was present in the anterior uvea and a fibrous, spindle cell membrane was found on the anterior surface of the iris. These findings were consistent with feline idiopathic sclerosing orbital pseudotumor syndrome involving the skin, conjunctiva, episclera and facial planes of the orbit, along with chronic ulcerative and stromal keratitis, and chronic anterior uveitis (Figure 3).
Figure 3.
Section of the sclera and adnexa (A) demonstrating infiltration by a marked collagen producing spindle cell infiltrate (B).
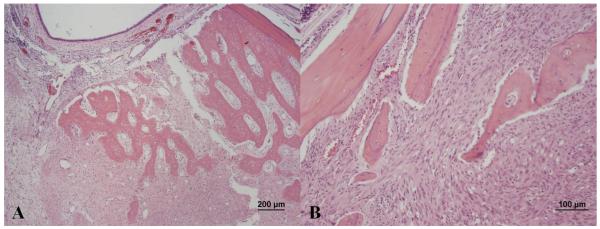
Ten weeks following initial presentation, the patient presented to the Dentistry and Oral Surgery Service of the University of California Davis Veterinary Medical Teaching Hospital for weight loss and apparent difficulty eating. On physical examination, the patient had lost 25% of its initial body weight (BCS 4/9, weight 3.3 kg), had a grade III/VI parasternal heart murmur, and severe ulcerative keratitis and conjunctivitis OD. The surgical wound following enucleation OS had healed appropriately and no gross abnormalities were observed on left orbital palpation. Oral examination revealed appropriate occlusion, severe generalized gingivitis, and apparent pain upon opening the mouth. No oral masses or ulceration were noted. Thoracic radiographs revealed no cardiovascular or pulmonary abnormalities. Computed tomography (CT) of the brain and orbits prior to and following intravenous administration of a contrast agent (2.75 mL/kg iopamidol 370 mg/mL; Isovue 370, Bracco Diagnostics, Princeton, NJ) showed a contrast-enhancing right retrobulbar mass causing regional destruction of the adjacent right frontal, palatine, lacrimal, and zygomatic bones, as well as the alveolar bone surrounding the right caudal maxillary premolar teeth (Figure 4). The mass was extended to the caudoventral aspect of the right side of the nasal cavity, the palatine tissues, the rostral aspect of the nasopharynx, and the right caudal infraorbital foramen. Based on its CT appearance, the mass was believed to be aggressive in nature and likely neoplastic. Within the left orbit, a large cystic structure with a thick peripheral region of contrast enhancement was thought to be inflammatory tissue or residual or recurrent pseudotumor. Due to the patient’s discomfort and poor prognosis, the owners elected euthanasia and a complete necropsy was performed. Histopathology of the decalcified skull revealed a marked, neoplastic spindle cell population and collagen deposition extending into eyelids, orbit, superficial dermis of the haired skin surrounding the eyelid margin, and conjunctival epithelium OD. In addition, neoplastic spindle cells invaded deep tissues of the right orbit with extension into bones of the maxilla, hard palate, gingiva and nasal cavity (Figure 5). Greater than 75% of the neoplastic cells demonstrated strong intracytoplasmic labeling for smooth muscle actin (SMA) and vimentin which in combination with the fibroblastic phenotype of the neoplastic cells is consistent with a myofibroblastic sarcoma.9 In addition, S-100 labeling was weakly positive in the nucleus and cytoplasm of greater than 75% of neoplastic cells, and glial fibrillary acidic protein (GFAP) labeling was weakly positive in the cytoplasm of less than 25% of neoplastic cells. No cells were labeled with Melan A or CD18. The pathologic diagnosis was feline restrictive orbital myofibroblastic sarcoma (FROMS).9 Within the superficial aspect of the left orbit, an extensive spindle cell rich granulation tissue, elements of suture material, lymphoplasmacytic inflammation, and small areas of granulomatous inflammatory infiltrate were noted. While most of the lesions in this region were consistent with postsurgical inflammation, the extensive spindle cell proliferation could not be definitively characterized as non-neoplastic. Other necropsy findings included small intestinal T-cell lymphoma; unilateral thyroid adenoma and mononuclear thyroiditis; left ventricular myocardial fibrosis and left atrioventricular valvular endocardiosis; hepatocellular lipidosis; mild interstitial nephritis and tracheitis; and gastric spiral bacteria.
Figure 4.
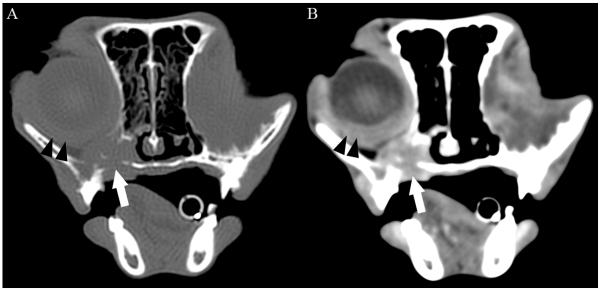
Transverse 2mm collimated CT images of the orbits obtained 10 weeks after initial diagnosis and 4 weeks after exenteration of the left orbit of the cat shown in figure 1. Images are displayed with the left of the cat to the right of the image. (A) Pre-contrast image at the level of the right globe reconstructed with a bone filter and displayed with window width = 1500 HU and window level = 300 HU. (B) Post-contrast image reconstructed with a detail filter and displayed with a window width = 300 HU and window level = 100 HU. Note the contrast-enhancing soft tissue mass ventral to the right globe (white arrow) associated with osteolysis of the ventral and medial aspects of the orbit, palatine bone, and alveolar bone of the maxilla. The mass was contiguous with the episcleral and retrobulbar tissues caudally. The sclera, episclera, and retrobulbar tissues were thickened and markedly contrast enhancing (black arrowheads). The left eye was absent and replaced by a central non-contrast enhancing cystic structure (not shown) and thickened, contrast-enhancing peripheral soft tissues. HU, Hounsfield units.
Figure 5.
Photomicrograph of the right nasal cavity from the cat in Figure 1. Note the marked osteolysis of the nasal turbinates (A) in association with a bland-appearing population of neoplastic spindle cells with moderate collagen production (B).
DISCUSSION
This report presents clinical, cross sectional imaging (MRI and CT), histologic, immunohistochemical, microbiologic (bacterial, fungal, and virologic), therapeutic, and complete postmortem findings of a cat with FROMS. Although this condition has been reported in cats9 and this cat is briefly decribed as Case #2 in that series, the present study adds novel information or reinforces earlier hypotheses regarding this disease. For example, this is the first report to the authors’ knowledge to show MRI images from a cat with FROMS. Comparison of these with MRI findings from humans affected with similar conditions suggests that this imaging mode can be useful in the diagnosis of FROMS in cats. Second, the present report contains, to the authors’ knowledge, the first record of testing for presence of FHV-1 DNA and treatment of this condition with a systemic antiviral drug. Although orbital pseudotumors in some human patients have been associated with herpesviruses,6,7 the failure to detect FHV-1 DNA using PCR in conjunctival tissue from this cat and the poor response to famciclovir (in combination with immunosuppressive doses of prednisolone) suggest that this case was not herpetic in origin. Third, the response of the patient in the current report to exenteration of one orbit suggests that aggressive surgical therapy should be considered reasonably early in the treatment of FROMS. Finally, this case presents strong support for the suggested reclassification of this condition as an aggressive neoplasm (FROMS) rather than its original description as an inflammatory condition .9-11
As part of a thorough diagnostic evaluation, the brain and orbits of the cat in the present study were initially imaged with MRI, which showed mild thickening of the retrobulbar tissues; attenuation of the retrobulbar fat; heterogeneous, increased STIR signal intensity; and marked contrast enhancement of the sclera, episclera and tissues lining the retrobulbar spaces. These MRI abnormalities were most obvious on dorsal plane images acquired parallel to the optic nerves. Although these finding are similar to reported CT findings,9,10 MRI is recognized as providing superior visualization of the globe, optic nerve, and of intracranial extension of lesions, as well as permitting better characterization of lesions, including tumors, based on the signal intensity differences among various tissues and increased prominence of lesions following fat suppression and administration of contrast.12 In particular, the current report highlights the importance of including fat suppression techniques when disease of the orbit, retrobulbar space, or optic nerve is suspected since, prior to contrast administration in the present study, abnormal signal intensity was observed on only the STIR images in this cat. Pre- and post-contrast T1-weighted images with chemical fat saturation are an alternative method of fat suppression and facilitate the detection of contrast enhancing disease in the retrobulbar space. To the authors’ knowledge, orbital myofibroblastic sarcomas have not been reported in human patients; however, the clinical and radiological features of FROMS early in the disease course are similar to idiopathic orbital pseudotumor in humans and thus can be compared. In people, MRI is reported to be more specific than CT for detecting idiopathic orbital pseudotumor because, as in the present case, the fibrous nature of the mass causes low signal intensity on pre-contrast T1-weighted and T2-weighted images.13 Additionally, in humans with idiopathic orbital pseudotumor, contrast administration aids identification of infiltrated, streaky fat and extraocular muscle and optic nerve enlargement.14 Although bone erosion is rare in humans with orbital pseudotumor, bone sclerosis occurs in a small percentage of cases and is usually associated with the sclerosing subset of idiopathic orbital pseudotumor.14 By contrast, CT and histology in the present case late in the disease course confirmed extensive destruction of several bones of the right orbit with invasion of the right nasal cavity, nasopharynx and caudal infraorbital foramen. In addition, the CT scan revealed palatine and alveolar bone involvement despite the oral mucosa appearing intact on examination. Although these changes may have been less obvious on MRI than CT, this suggests dramatic and rapid disease progression in the ten weeks between performance of the MRI and CT.15 Such extensive and rapid tissue destruction is more consistent with neoplastic than inflammatory tissue and supports the diagnosis of FROMS rather than ISOP.
Histopathologic and immunohistochemical findings9 for tissue from within the right orbit of the cat in the present study also support a neoplastic (FROMS) rather than an inflammatory (ISOP) diagnosis despite its bland cellular features. Consistent with other cats in a series of FROMS,9 the cat in the present study had a population of collagen-producing spindle cells that infiltrated tissues such as the adnexa, extraocular muscles, and gingiva.9 The degree of bone destruction in the present case as well as the clinical evidence of rapid disease progression over the final 10 weeks of this cat’s life demonstrates the aggressive nature of this tumor.9 In addition, strong intracytoplasmic immunohistochemical labeling for vimentin (a mesenchymal cell marker) and smooth muscle actin in combination with the fibroblastic phenotype of the tumor cells was helpful in characterizing the tumor in the present study as a myofibroblastic sarcoma.9 The weak labeling for S-100 in the cytoplasm and nucleus of most neoplastic cells was not surprising given that many tissues of the head originate from the neural crest.9 Inconsistent GFAP labeling of FROMS has been noted previously and is attributed to presence of fragments of peripheral nerves containing nonmyelinating Schwann cells.9 Not surprisingly, cells within the myofibroblastic sarcoma from the cat in the present study did not label with Melan A and CD18 which are markers for melanocytes and activated inflammatory cells, respectively.9
Treatment of patients diagnosed with ISOP or FROMS using antiviral agents has not been previously described. In the current report, therapy with an antiviral agent was elected based on the cat’s historical diagnosis of herpetic keratitis, the concurrent use of immunosuppressive doses of prednisolone, and the observation that several human herpetic viruses have been associated with orbital pseudotumors in humans.6,7 In one case, HSV-1-induced retinal necrosis in an adult female human led to a severe inflammatory orbitopathy, optic neuritis, and proptosis which eventually responded to antiviral and anti-inflammatory therapy but left the patient blind.7 In the present case, famciclovir was selected because oral administration of this drug has been shown to be without recognized side effects and remarkably efficacious in the treatment of client-owned cats with suspected herpetic disease and cats experimentally infected with FHV-1.16-18 In the current report, the cat’s clinical signs did not improve following treatment with oral famciclovir (along with prednisolone). In addition, the conjunctival samples from this cat failed to reveal the presence of intranuclear inclusion bodies or FHV-1 DNA when tested using PCR. While FHV-1 did not appear to play a role in this patient’s ocular disease, it may be worthwhile to explore the relationship between FROMS and FHV-1 further by testing orbital tissue obtained from FROMS patients for FHV-1 DNA by PCR. The lack of improvement with systemically administered antibiotics, anti-inflammatory agents, and immunosuppressive drugs is typical of cats with ISOP or FROMS9,10,11, although reports exist of some cats responding transiently to oral steroids.4 Although, the cat in the present study did not have any histologic or microbiologic evidence of bacterial or fungal disease, histopathology, microbial culture, and molecular techniques are still warranted in patients with suspected ISOP or FROMS because of clinical similarities between this condition and other granulomatous diseases of the feline orbit such as those due to fungal organisms.19-21 In addition, an inflammatory pseudotumor of the left hind paw has been reported in a cat with cutaneous mycobacteriosis.22
Optimal management of low-grade myofibroblastic sarcomas in humans includes surgical excision with wide margins sometimes along with adjunctive radiotherapy or chemotherapy with doxorubicin and adriamycin.23,24 Some data from the present study support this approach. At initial presentation, the cat in the present study had virtually identical clinical findings in both orbits and worse MRI changes on the left. Following exenteration of the left orbit, imaging and histologic features of disease at that location were markedly different from those at initial presentation and from those in the right orbit at necropsy. The orbit that did not undergo exenteration underwent rapid and extensive tissue destruction with neoplastic extension to the maxilla, hard palate, nasal cavity and gingiva within 10 weeks. Meanwhile, the orbit that underwent exenteration showed no evidence of bony destruction and contained evidence of spindle cell proliferation that could not be definitively characterized as neoplastic. The observation that many patients with FROMS appear painful and are sometimes blind due to extensive ulcerative corneal disease with or without perforation also supports surgical management. Finally, there is some evidence that FROMS progresses from unilateral to bilateral orbital involvement despite enucleation of the initially affected eye and that most owners elect euthanasia when involvement of the second orbit occurs.9,10 Adjunctive therapy may also be indicated. A recent review article reported improvement in clinical signs following radiation therapy of a cat with ISOP but noted that the follow-up period was too short to determine if radiation therapy would manage the condition long-term.4 Taken together, these disease features along with the condition’s lack of response to medical therapy alone9-11 suggest aggressive and early therapy should be considered in patients with FROMS. However, FROMS often is not easily recognized as an aggressive neoplastic disease early in its course. Findings from the present study suggest that feline patients with orbital disease should undergo MRI with fat suppression techniques such as STIR, orbital or eyelid biopsy (with even bland spindle-cell populations creating a high degree of suspicion regarding FROMS), and immunohistochemical analysis of eyelid or orbital tissues relatively early in the disease course.9 Further, this case report reinforces the reclassification of ISOP to FROMS.
Acknowledgements
The authors thank Dr. Christopher Reilly for photographing the histopathologic samples. This study was supported in part by the National Institute of Health K08 EY021142.
Footnotes
The present address for Dr. Thomasy, Dr. Arzi, and Dr. Cissell is Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California-Davis, Davis, CA 95616.
Dr. Lo’s present address is Grayscale Veterinary Teleradiology, Austin TX 78720.
Dr. Jose Vilches-Moure’s present address is Graduate Group in Comparative Pathology, University of California Davis, Davis, CA 95616.
REFERENCES
- 1.Veith LA, Cure TH, Gelatt KN. The Schirmer tear test—in cats. Modern Veterinary Practice. 1970;51:48–49. [Google Scholar]
- 2.Miller PE, Pickett JP, Majors LJ, et al. Evaluation of two applanation tonometers in cats. American Journal of Veterinary Research. 1991;52:1917–1921. [PubMed] [Google Scholar]
- 3.Jacobs D, Galetta S. Diagnosis and management of orbital pseudotumor. Current Opinion in Ophthalmology. 2002;13:347–351. doi: 10.1097/00055735-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 4.van der Woerdt A. Orbital inflammatory disease and pseudotumor in dogs and cats. Veterinary Clinics of North America Small Animal Practice. 2008;38:389–401. vii–viii. doi: 10.1016/j.cvsm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Hickman MA, Reubel GH, Hoffman DE, et al. An epizootic of feline herpesvirus, type 1 in a large specific pathogen-free cat colony and attempts to eradicate the infection by identification and culling of carriers. Lab Anim. 1994;28:320–329. doi: 10.1258/002367794780745038. [DOI] [PubMed] [Google Scholar]
- 6.Lexa FJ, Galetta SL, Yousem DM, et al. Herpes zoster ophthalmicus with orbital pseudotumor syndrome complicated by optic nerve infarction and cerebral granulomatous angiitis: MR-pathologic correlation. American Journal of Neuroradiology. 1993;14:185–190. [PMC free article] [PubMed] [Google Scholar]
- 7.Tornerup NR, Fomsgaard A, Nielsen NV. HSV-1--induced acute retinal necrosis syndrome presenting with severe inflammatory orbitopathy, proptosis, and optic nerve involvement. Ophthalmology. 2000;107:397–401. doi: 10.1016/s0161-6420(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 8.Westermeyer HD, Thomasy SM, Kado-Fong H, et al. Assessment of viremia associated with experimental primary feline herpesvirus infection or presumed herpetic recrudescence in cats. American Journal of Veterinary Research. 2009;70:99–104. doi: 10.2460/ajvr.70.1.99. [DOI] [PubMed] [Google Scholar]
- 9.Bell CM, Schwarz T, Dubielzig RR. Diagnostic features of feline restrictive orbital myofibroblastic sarcoma. Veterinary Pathology. 2011;48:742–750. doi: 10.1177/0300985810369900. [DOI] [PubMed] [Google Scholar]
- 10.Billson FM, Miller-Michau T, Mould JR, et al. Idiopathic sclerosing orbital pseudotumor in seven cats. Veterinary Ophthalmology. 2006;9:45–51. doi: 10.1111/j.1463-5224.2005.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SA, van der Woerdt A, Bartick TE. Retrobulbar pseudotumor of the orbit in a cat. Journal of the American Veterinary Medical Association. 2000;216:356–358. doi: 10.2460/javma.2000.216.356. 345. [DOI] [PubMed] [Google Scholar]
- 12.Weber AL, Sabates NR. Survey of CT and MR imaging of the orbit. European Journal of Radiology. 1996;22:42–52. doi: 10.1016/0720-048x(96)00737-1. [DOI] [PubMed] [Google Scholar]
- 13.Ding ZX, Lip G, Chong V. Idiopathic orbital pseudotumour. Clinical Radiology. 2011;66:886–892. doi: 10.1016/j.crad.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Aviv RI, Miszkiel K. Orbital imaging: Part 2. Intraorbital pathology. Clinical Radiology. 2005;60:288–307. doi: 10.1016/j.crad.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Dennis R. Use of magnetic resonance imaging for the investigation of orbital disease in small animals. Journal of Small Animal Practice. 2000;41:145–155. doi: 10.1111/j.1748-5827.2000.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomasy SM, Lim CC, Reilly CM, et al. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. American Journal of Veterinary Research. 2011;72:85–95. doi: 10.2460/ajvr.72.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Thomasy SM, Maggs DJ. Treatment of ocular nasal, and dermatologic disease attributable to feline herpesvirus 1 with famciclovir. Veterinary Ophthalmology. 2008;11:418. [Google Scholar]
- 18.Malik R, Lessels NS, Webb S, et al. Treatment of feline herpesvirus-1 associated disease in cats with famciclovir and related drugs. Journal of Feline Medicine and Surgery. 2009;11:40–48. doi: 10.1016/j.jfms.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith LN, Hoffman SB. A case series of unilateral orbital aspergillosis in three cats and treatment with voriconazole. Veterinary Ophthalmology. 2010;13:190–203. doi: 10.1111/j.1463-5224.2010.00780.x. [DOI] [PubMed] [Google Scholar]
- 20.Barrs VR, Halliday C, Martin P, et al. Sinonasal and sino-orbital aspergillosis in23 cats: aetiology, clinicopathological features and treatment outcomes. Veterinary Journal. 2012;191:58–64. doi: 10.1016/j.tvjl.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Parkes HM, Shilton CM, Jerrett IV, et al. Primary ocular melioidosis due to a single genotype of Burkholderia pseudomallei in two cats from Arnhem Land in the Northern Territory of Australia. Journal of Feline Medicine and Surgery. 2009;11:856–863. doi: 10.1016/j.jfms.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MA, Fales WH, McCracken WS, et al. Inflammatory pseudotumor in a cat with cutaneous mycobacteriosis. Veterinary Pathology. 1999;36:161, 163. doi: 10.1354/vp.36-2-161. [DOI] [PubMed] [Google Scholar]
- 23.Takahama A, Jr., Nascimento AG, Brum MC, et al. Low-grade myofibroblastic sarcoma of the parapharyngeal space. International Journal of Oral and Maxillofacial Surgery. 2006;35:965–968. doi: 10.1016/j.ijom.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Mentzel T, Dry S, Katenkamp D, et al. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. American Journal of Surgical Pathology. 1998;22:1228–1238. doi: 10.1097/00000478-199810000-00008. [DOI] [PubMed] [Google Scholar]