Abstract
Introduction
Nearly 40% of adults aged 65 and older in the United States consume alcohol. Research in older adults has largely examined potential health effects of a moderate drinking lifestyle. Examination of acute effects in this population is generally lacking. To investigate alcohol-induced alteration of electrophysiological correlates of attention in this population, we employed a covert attentional task. We hypothesized that moderate alcohol administration as well as older age would reduce P3 amplitude and increase latency. We anticipated an interaction such that, relative to their age-matched controls, older adults receiving alcohol would be more affected than their younger counterparts.
Methods
Participants included healthy older (aged 50–67; n = 20; 9 men) and younger (aged 25–35; n = 12; 5 men) moderate drinkers. Participants received either a moderate dose of alcohol (breath alcohol concentration [BrAC] ~50 mg/dl) or a placebo beverage. Following absorption, the task was administered and neurophysiological measures were obtained. P3 amplitude and latency were separately subjected to ANOVA across cue conditions using age and dose as independent variables.
Results
As predicted, P3 amplitude in older adults was significantly lower than younger adults across cue conditions. An age by alcohol interaction was detected, revealing that older adults receiving alcohol showed lower P3 amplitudes than any other group. An age effect for P3 latency was found, with older adults having longer latencies than their younger counterparts. A significant age by alcohol interaction for P3 latency was detected, revealing that older adults receiving alcohol displayed delayed P3 latencies relative to older adults receiving placebo. In contrast, younger adults receiving alcohol had reduced latency compared to those receiving placebo although this effect did not reach significance.
Conclusions
Results suggest that older adults demonstrated alcohol related shifts in P3 characteristics during an intentional attention task, whereas younger adults failed to demonstrate this pattern.
Keywords: moderate alcohol, aging, attention, neurophysiology, P3
Introduction
Approximately 67% of the US population endorses at least occasional alcohol consumption, including 40% of older adults aged 65+ (Gallup, Inc., 2011; Substance Abuse and Mental Health Services Administration, 2010). An aging “baby boomer” population, accompanied by extensions in retirement age and social engagement (both associated with alcohol use) suggest future increases in the proportion of older adults endorsing current alcohol consumption (Adams et al., 1990; Zucker, 1998). There is some research on moderate drinking in older adults, yet such work has largely examined potential health effects of a moderate drinking lifestyle (e.g., Balsa et al., 2008; Mukamal and Rimm, 2008). Empirical literature examining moderate drinking as an event is generally lacking with notable exceptions (e.g., Tupler et al., 1995; Vogel-Sprott and Barret, 1984).
Moderate alcohol doses producing blood alcohol concentrations below intoxicating levels have been associated with impairment in a variety of cognitive and behavioral measures (for reviews see Holloway, 1994; Moskowitz and Robinson, 1988). Findings are generally confined to populations of younger, college-aged drinkers or animal models. Although census projections predict over 72 million Americans will be 65 or older by 2030 (US Census Bureau, 2008), and age-associated differences in alcohol metabolism are recognized (Meier and Seitz, 2008), moderate alcohol effects have not been systematically studied in older drinkers.
Age-related cognitive effects, similar to those of low-to-moderate doses of alcohol, are frequently subtle and often difficult to detect via gross behavioral measures (e.g., Drag and Bielauskas, 2010; Holloway, 1994). Thus, we took advantage of changes in brain electrophysiology which more sensitively index such effects (Leuthold and Sommer, 1998). A number of electrophysiological examinations of both aging and acute alcohol have revealed significant alteration in various event related potential (ERP) components. The P3 component was of particular interest due to its presumed reflection of processes related to working memory and contextual updating (Polich, 2004; Porjesz and Begleiter, 1982). P3 disruptions following acute alcohol include increased latency and decreased amplitude (Iragui et al., 1993; Rohrbaugh et al., 1987; for review see Oscar-Berman and Marinkovic, 2007; Polich and Kok, 1995). Similar shifts are noted in aging populations (e.g., Hirayasu et al., 2000; for review see Kok, 2000; Kugler et al., 1993). We are unaware of studies reporting electrophysiological measures following moderate doses of alcohol concurrently in older and younger social drinkers.
To address this gap in understanding, we employed a modified version of the covert visual attention task used by Posner and colleagues (e.g, Hillyard et al., 1995; Luck et al., 1994; Posner, 1995). The task required participants to shift attention to detect the presence and location of targets preceded by spatial cues. We hypothesized negative effects of moderate alcohol (~50 mg/dl) and older age on the P3. Of particular interest was the potential alcohol by age interaction. Though guiding data are lacking in the wider literature, previous work from our laboratory (Gilbertson et al., 2009; 2010) led us to hypothesize that acute alcohol and older age would induce greater-than-additive electrophysiological disruption.
Materials and Methods
Screening
Volunteers were recruited via flyer, word-of-mouth, and radio/newspaper advertisement, and were reimbursed for screening and experimental participation. All procedures were approved by the Medical Institutional Review Board of the University of Kentucky.
Interested volunteers provided written informed consent, then completed a brief screening session, during which they completed packets of questionnaires. Questionnaires addressed basic demographic information such as age and education. Information concerning health status, medication history, substance use (nicotine, alcohol and illicit drugs), alcohol consumption over the past 6 months (quantity-frequency index [QFI]; Cahalan et al., 1969), state anxiety (Spielberger State Anxiety Inventory [AI]; Spielberger, 1983), and general intellectual ability (Shipley Institute of Living Vocabulary and Abstraction scales [SILS-V, SILS-A]; Zachary, 1986) were also obtained. Volunteers were also screened using standard, age-appropriate measures for depressive symptomatology (Beck Depression Inventory, 2nd ed. [BDI-II], Beck et al., 1996; Geriatric Depression Scale [GDS], Yesavage et al., 1982). Psychiatric symptomatology was assessed using a computerized version of the National Institute of Mental Health Diagnostic Interview Schedule (Robins et al., 1995).
Exclusionary criteria included evidence of a past or current Axis I disorder including drug/alcohol abuse or dependence (including nicotine). Significant medical conditions (e.g., cardiovascular, pulmonary, or hepatic conditions such as stroke, hypertension, or untreated diabetes) or history of neurological trauma (e.g., seizure disorder, head injury resulting in prolonged unconsciousness), which might confound interpretation of results or contraindicate low dose alcohol consumption, were also exclusionary. Individuals meeting eligibility requirements were invited to participate in experimental sessions.
Participants
Participants included older (aged 50–67; n = 20; 9 men) and younger (aged 25–35; n = 12; 5 men) moderate drinkers without histories of substance abuse disorders who consumed at least one drink/month for the preceding 6 months. Due to the paucity of literature describing effect sizes in older individuals and the greater variability anticipated in this group (Gazzaley and d’Esposito, 2007; Salthouse, 2011; Wilson et al., 2002), older participants were deliberately oversampled. Participants included Caucasians (87.5%), African-Americans (9.38%), and undeclared/others (3.13%). All female participants of childbearing potential tested negative for pregnancy. All participants were negative when tested via urinalysis for tetrahydrocannabinal, cocaine, benzodiazepines, morphine, and amphetamines. All participants displayed breath alcohol concentrations (Intoxilyzer, Model 400, CMI, Inc., Owensboro, KY) of 0.00 mg/dl at initiation of study sessions. This sample partially overlapped with that reported inSklar et al. (2012).
Experimental Timeline/Procedure
Experimental sessions were initiated at approximately 11:15 AM. Prior to the session, participants provided written informed consent. Following a 2 h fast, participants consumed a standard lunch (~500 kcal). Participants moved to a shielded testing area for task practice and electrode cap application, during which they consumed a snack (~115 kcal). Beverages were delivered at minutes 0 and 30 (see below). Testing was initiated at minute 35. Breath alcohol concentration (BrAC) measurements were interspersed between trial blocks. Upon completion of the experiment, participants were debriefed regarding the alcohol content of their beverages, informed of their current BrAC, and transported home upon registering ≤ 10 mg/dl.
Acute Alcohol Administration
Participants received either a moderate dose of alcohol or a placebo beverage. Beverage administration was conducted via randomized, double-blind procedure. Dosing calculations for initial beverages were determined using a modification of Widmark formula (Watson et al., 1981) to achieve an initial BrAC of ~40 mg/dl, selected due to its approximation of a typical social drinking situation. Thirty minutes following consumption, booster beverages containing half the original alcohol dose (or vehicle) were administered (except younger women, for whom full-dose boosters were required to achieve target BrACs; Gilbertson et al., 2009) resulting in moderate BrACs of ~50 mg/dl. Standard administration procedures (e.g., Fillmore et al., 2000) were utilized; 200-proof (100%) medical-grade alcohol was delivered in a sugar-free, non-caffeinated citrus soda, mixed in a 1:3 ratio. Placebo beverages contained only soda, with a negligible amount of alcohol floated on the surface. Both beverages were delivered ice-cold, misted with alcohol to enhance placebo effectiveness, and divided evenly into two glasses. Participants were instructed to consume each glass in no more than 2 minutes.
Attentional Testing
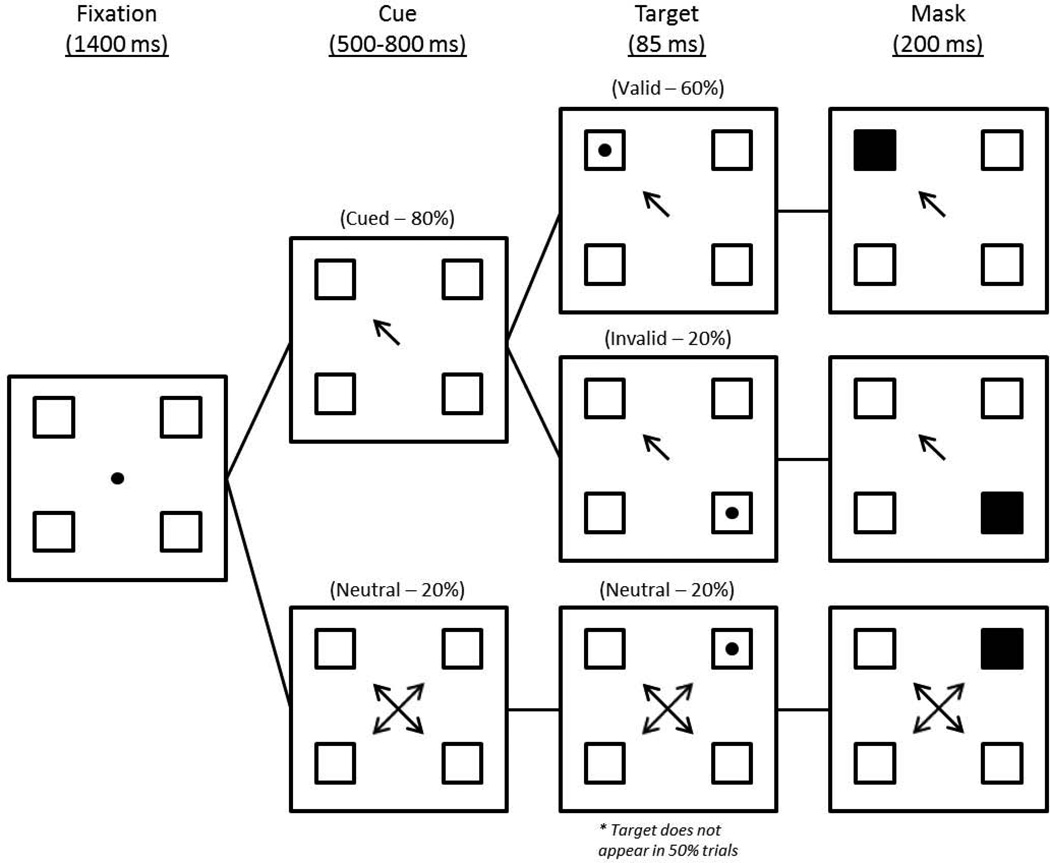
A covert attentional task developed by Posner (1980) and modified by Luck and colleagues (1994) was used (see Figure 1). Targets were presented in one of four squares (1.2” × 1.2”) distributed 6.1” from a central fixation point. Each trial occurred in four discrete phases, beginning with a fixation phase (1400 ms) in which participants focused on the central point. A cue phase followed, in which a central cue of variable duration (500–800 ms) pointed to a single box or all four boxes (neutral); the cue remained on the screen for the duration of the trial. A target phase (85 ms) followed in which a target (a solid dot) appeared in one of the four squares (50% of trials), or did not appear. In the final masking phase (200 ms), the target-containing square was obscured. Mask position in non-targeted trials was distributed equally across the four squares. The task was divided into four blocks and required approximately 30–45 min to complete. P3s were analyzed only for those trials containing a target. Sixty percent of trials were cued validly (target appears in the indicated quadrant), 20% cued invalidly (the target appears in an un-indicated quadrant), with 20% neutral (arrows pointed to all four boxes). Participants indicated the presence or absence of a target by pressing one of two buttons. The hand (left vs. right) used to indicate target presence/absence was counterbalanced without regard to participant handedness in order to avoid contaminating a particular response by using only the non-dominant hand. The distribution of those responding to target presence or absence with dominant vs. non-dominant hands was equal. Participants were seated 70 cm from the color monitor. To reduce head movement and encourage central fixation, a chin rest attached to the table and adjusted to allow clear view of the monitor was used. Only trials with correct responses were used in analyses.
Figure 1. Attentional Task Schematic.
A schematic depiction of the attentional task (based on Luck et al., 1994). Although all three cue conditions are depicted, the 50% of trials not containing targets are ommitted.
Brain Electrophysiology
ERP data were collected over the course of the task. Participants were fitted with a 64-electrode montage embedded in an elastic cap (Electro-Cap International, Inc., Eaton, OH), outfitted based on the standard international 10/20 system. The center forehead site (FPz) functioned as a ground, with a linked earlobe reference. Two electrodes, placed above and below the outer canthus of the left eye, monitored eye movement and blinks. Impedance was maintained at or below 5 kΩ. Amplifier gain was set at 10,000× with a high pass filter cutoff of .15 Hz and a low pass filter cutoff of 50 Hz. Sampling rate was 500 Hz. Offline, data were filtered using a 30 Hz low pass digital filter and 24 dB attenuation. The ocular artifact reduction function included with NeuroScan 4.3 (Compumedics USA, Inc., Charlotte, NC) was used to correct for myogenic artifacts associated with eye movements and blinks. We then separated the continuous EEG into 1100 ms epochs locked to target presentation, including a 100 ms baseline period. Epochs containing artifacts (±75 µV) at any electrode were eliminated prior to averaging, as were trials with incorrect responses. Following artifact removal, an average of 233 trials (per electrode, per person) remained available for analysis. P3 peak detection in averaged waveforms was performed automatically by identifying the maximal positive deflection in the window from 250 to 600 ms. Data reduction, peak identification and statistical analyses were conducted with NeuroScan 4.3 software and SAS 9.3 (SAS Institute, Cary NC).
Results
Demographics
Results of demographic analyses are depicted in Table 1. Across groups, participants appeared well matched on appropriate demographic variables. Scores which were not directly comparable (i.e., BDI / GDI) were within normal limits.
Table 1.
Demographic Comparisons by Age and Alcohol Group
| OLDER | YOUNGER | |||
|---|---|---|---|---|
| ALCOHOL (n=11) |
PLACEBO (n=9) |
ALCOHOL (n=7) |
PLACEBO (n=5) |
|
| Variable | M (SD) | M (SD) | M (SD) | M (SD) |
| Age (yrs) | 57.24 (6.77) | 54.11 (5.55) | 29.14 (4.37) | 27.20 (1.79) |
| Education (yrs) | 16.60 (3.44) | 16.67 (1.66) | 19.75 (3.81) | 17.20 (1.30) |
| BDI-II / GDS | 6.64 (8.14) | 4.44 (2.51) | 7.14 (8.65) | 4.40 (4.72) |
| AIa | 47.36 (5.16) | 44.44 (10.04) | 46.57 (6.05) | 46.80 (6.34) |
| SILS-Vb (mental age) | 18.64 (2.08) | 18.91 (1.05) | 17.99 (1.68) | 18.04 (0.67) |
| SILS-Ab (mental age) | 17.47 (1.60) | 17.04 (1.98) | 19.24 (1.41) | 17.30 (3.04) |
| QFIc (oz. absolute EtOH/day) | 0.44 (0.47) | 0.63 (0.52) | 0.92 (0.96) | 0.49 (0.28) |
| BMId (kg/m2) | 29.30 (9.62) | 28.2 (5.59) | 25.86 (5.58) | 28.50 (6.76) |
Anxiety Inventory (age corrected; Spielberger, 1983;
Shipley Institute of Living Scale (Zachary, 1986);
Quantity Frequency Index (Cahalan et al. 1969);
Body Mass Index (weight [kg] / height [m]2)
Breath Alcohol Concentration
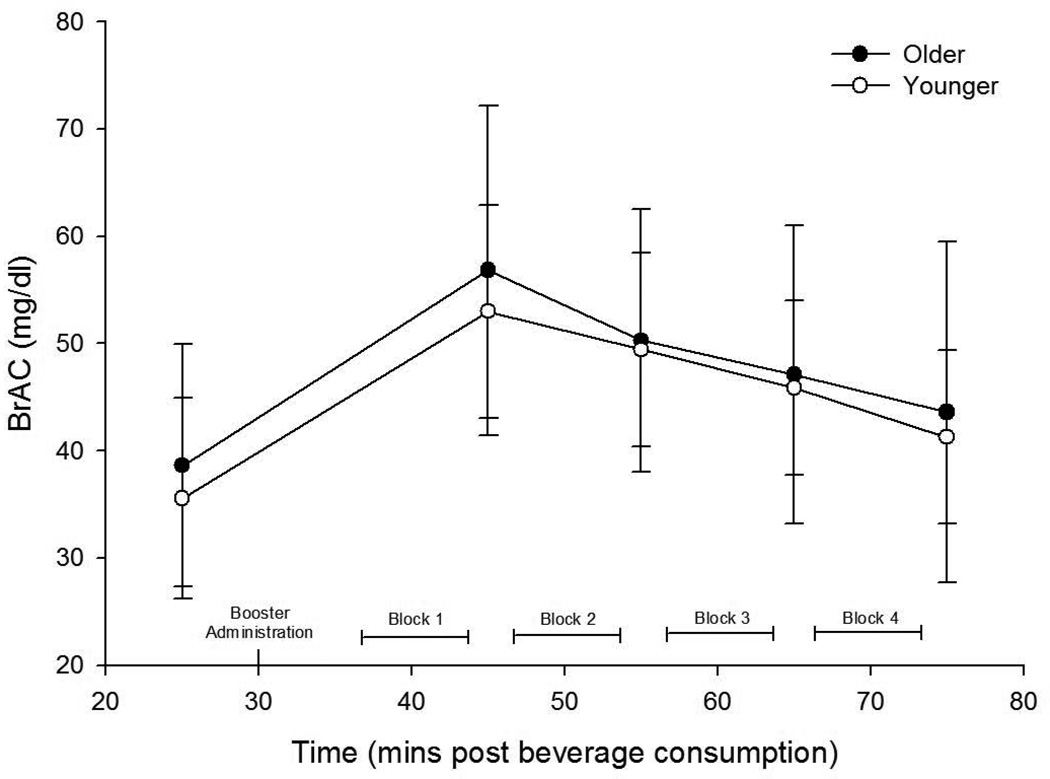
Mean BrACs for participants receiving alcohol were 37 ± 13, 55 ± 13, 50 ± 11, 47 ± 12, and 43 ± 13 mg/dl, respectively for 25, 45, 55, 65 & 75 minutes post-administration. Paired t-tests comparing BrAC values between younger and older participants (see Figure 2, below) indicated no significant differences at any time point (all ps > .57).
Figure 2. Mean BrACs for Older and Younger Participants Receiving Alcohol.
Mean BrACs (±SD) for both older and younger participant groups receiving alcohol. BrACs were measured at 25, 45, 55, 65, & 75 minutes post-administration between the four 8-minute blocks of the task. Paired t-tests indicated no significant differences at any time point (all ps > .57).
Preliminary/Guiding Analyses
As this task was a centrally-focused task of visual attention, hemispheric differences were not relevant to our particular research questions. Because there was no lateral component to the task, for comparison purposes our analyses focused on several central sites, including the parietal (Pz) and occipital (Oz) midline sites, although the central midline (Cz) site was considered in initial analyses. Results from Cz were similar to other electrodes in overall pattern (using all participants/groups), although of the 16 dependent measures of interest, only one achieved statistical significance. For the sake of clarity these results were omitted.
To guide analyses of primary interest, the effects of cue condition were investigated with two initial repeated-measures ANOVA ([Valid vs. Neutral vs. Invalid] × [Pz vs. Oz]), conducted separately for amplitude and latency to determine whether P3 parameters varied as a function of cue condition, electrode site, or their interaction. These initial 3 × 2 ANOVA included all participants. Amplitude analysis indicated a main effect of cue condition, F(2,104)=7.24, p=.001, but no electrode site main effect or interaction. Latency analysis revealed neither main effects nor an interaction. To determine whether interpretation of P3 analyses were confounded by age-related anterior shifts in maximal P3 amplitude (e.g., Fabiani et al., 1998), an additional repeated measures ANOVA ([Valid vs. Neutral vs. Invalid] × [Pz vs. Oz vs. Cz] × [Younger vs. Older]) was conducted. Results revealed no significant age group by electrode interaction, which would be expected if such a shift were present in older participants (F<1).
Consistent with convention, further 2-way ANOVAs (age [Older vs. Younger] × alcohol [Placebo vs. Alc]) were performed separately for each condition. However, planned comparisons (described below) were not contingent on their outcome. Tests indicated no violations of homogeneity of variance for any electrophysiological measure at any electrode site. All parametric analyses in the current work utilized Type III sums of squares, minimizing the inflation of Type I errors due to unequal ns (Tabachnick & Fidell, 1989). A summary of F and p values for all 2 × 2 ANOVA is presented in Table 2, and discussed below.
Table 2.
Summary of (2 × 2) ANOVA Results by Cue Condition and Electrode.
| Amplitude | Latency | |||||
|---|---|---|---|---|---|---|
| Valid |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
| Pz |
8.10 (.008)* |
0.77 (.388) |
0.76 (.392) |
5.22 (.030)* |
0.21 (.649) |
0.93 (.343) |
| Oz |
4.66 (.039)* |
0.82 (.374) |
4.81 (.037)* |
2.25 (.145) |
0.13 (.717) |
3.61 (.068)† |
| Neutral |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
| Pz |
7.74 (.010)* |
0.49 (.491) |
3.78 (.062)† |
3.16 (.086)† |
0.31 (.581) |
1.83 (.187) |
| Oz |
13.27 (.001)* |
0.40 (.532) |
3.56 (.070)† |
3.72 (.064)† |
0.94 (.340) |
13.67 (<.001)* |
| Invalid |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
Age F (p) |
Alc F (p) |
Age × Alc F (p) |
| Pz |
7.87 (.009)* |
1.61 (.210) |
2.10 (.016)* |
6.71 (.015)* |
0.65 (.427) |
0.65 (.427) |
| Oz |
13.93 (<.001)* |
1.15 (.293) |
5.31 (.029)* |
2.00 (.168) |
0.89 (.353) |
0.91 (.349) |
p <.05;
.05> p <.1; df=(1,28) for main effects; df=(3,28) for interactions.
Planned comparisons based on our hypotheses were utilized to examine age (comparing the [Older/Placebo vs. Younger/Placebo] and [Older/Alc vs. Younger/Alc] groups) and alcohol effects (comparing [Older/Placebo vs. Older/Alc] and [Younger/Placebo vs. Younger/Alc] groups). These planned comparisons were specifically selected based on our hypotheses; other possible comparisons (i.e. [Older/Alc vs Younger/Placebo] were excluded.
Amplitudes
Valid Cue Condition
2 × 2 ANOVAs detected an interaction at Oz, F(1,28)=4.81, p=.037. Significant age effects, with older subjects demonstrating lower P3 amplitudes (relative to younger individuals) were noted at Pz, F(1,28)=8.10, p=.008 and Oz, F(1,28)=4.66, p=.039.
Planned comparisons indicated that for both electrodes, younger and older subjects receiving alcohol differed; amplitudes were lower among Older/Alc subjects than Younger/Alc subjects, at both Pz t(17)=2.83, p=.008 and Oz, t(17)=3.32, p=.002. Additionally, Older/Alc individuals displayed lower amplitudes at Oz, relative to Older/Placebo participants, t(19)=3.32, p=.017. All other ps ≥ .423.
Neutral Cue Condition
2 × 2 ANOVAs revealed trend-level interactive effects at electrodes Pz, F(1,28)=3.78, p=.062 and Oz, F(1,28)=3.56, p=.070. Age main effects, with older subjects demonstrating lower P3 amplitudes relative to younger individuals, were noted at Pz, F(1,28)=7.74, p=.010 and Oz, F(1,28)=13.27, p=.001.
Planned comparisons indicated decreases in P3 amplitude among Older/Alc participants, relative to Younger/Alc participants, t(17)=3.61, p=.001 and t(17)=4.22, p<.001, for Pz and Oz, respectively. Additionally, Older/Alc individuals displayed lower amplitudes at Oz, relative to Older/Placebo participants, t(19)=2.07, p=.048. All other ps ≥ .255.
Invalid Cue Condition
2 × 2 ANOVAs revealed interactive effects at electrodes Pz, F(1,28)=2.10, p=.016 and Oz, F(1,28)=5.31, p=.029. Significant age effects, with older subjects demonstrating reduced P3 amplitudes, were noted at both Pz, F(1,28)=7.87, p=.009, and Oz, F(1,28)=13.93, p<.001.
Planned comparisons revealed Older/Alc participants displayed reduced P3 amplitude relative to Older/Placebo at both electrodes, t(19)=2.23, p=.034 and t(19)=2.77, p=.010, for Pz and Oz, respectively. Furthermore, decreases in P3 amplitude were noted among Older/Alc participants, relative to Younger/Alc participants, t(17)=3.24, p=.003 and t(17)=4.61, p<.001, at Pz and Oz, respectively. All other ps ≥ .353.
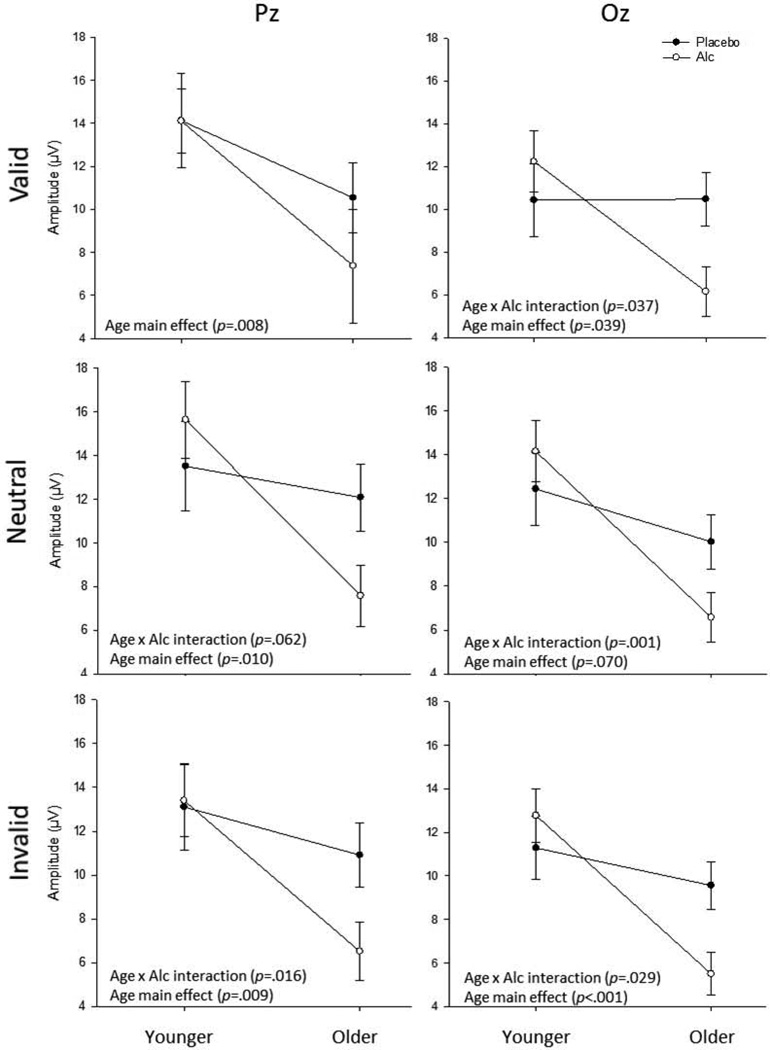
Alcohol by age interactions on P3 amplitude are depicted in Figure 3, below, separated by cue condition and electrode.
Figure 3. Age by Alcohol Amplitude Interactions by Cue Condition and Electrode Site.
Depictions of age by alcohol interactions (and SEs) on P3 amplitude, across all cue conditions and electrode sites. Age main effects were consistently noted, such that age was associated with reduced amplitude. Age by alcohol interactions were noted for all conditions at Oz, and for neutral and invalid conditions at Pz. Post hoc comparisons suggested these interactions were due to pronounced effects of alcohol among older individuals (see text for details).
Latencies
Valid Cue Condition
2 × 2 ANOVAs yielded a trend-level interaction at Oz, F(1,28)=3.61, p=.068. Analysis also revealed age effects, with older subjects demonstrating reduced P3 latencies at Pz, F(1,28)=5.22, p=.030.
Planned comparisons indicated Older/Alc participants demonstrated higher P3 latencies relative to Younger/Alc, at both Pz, t(17)=2.48, p=.019 and Oz, t(17)=2.59, p=.015. All other ps ≥ .164.
Neutral Cue Condition
2 × 2 ANOVAs revealed an interactive effect at Oz, F(1,28)=13.67, p<.001. Age effects, such that older participants displayed increased P3 latencies, were noted at both Pz, F(1,28)=3.16, p=.086 and Oz F(1,28)=3.72, p=.064.
Planned comparisons indicated that at each electrode, Older/Alc individuals displayed increased P3 latencies relative to Younger/Alc participants, t(17)=2.39, p=.024 and t(17)=4.29, p<.001, for Pz and Oz, respectively. Additionally, Older/Alc individuals displayed longer latencies relative to Older/Placebo participants at Oz, t(19)=3.83, p<.001. Additionally, a trend was noted such that Younger/Alc participants appears to have shorter P3 latencies, relative to Younger/Placebo individuals at Oz, t(10)=1.72, p=.097. All other ps ≥ .128.
Invalid Cue Condition
Contrary to prior analysis, 2 × 2 ANOVAs revealed only a main effect of age, such that older individuals displayed increased P3 latencies at electrode Pz, F(1,28)=6.71, p=.015.
Planned comparisons indicated differences between Older/Placebo and Younger/Placebo individuals at Pz, such that older participants displayed increased P3 latency, t(13)=2.25, p=.033. All other ps ≥ .147.
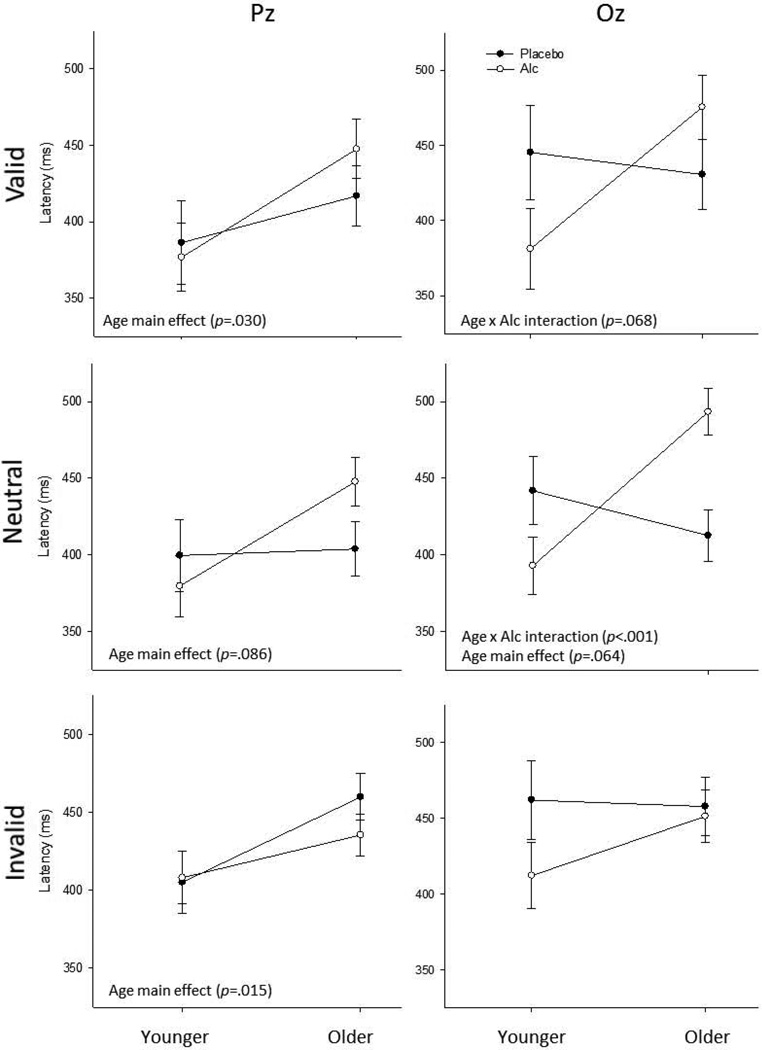
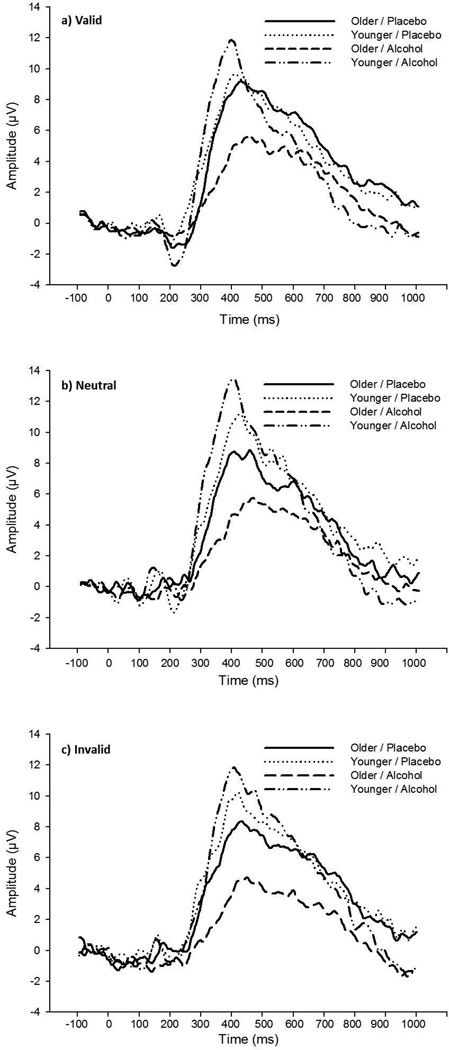
Alcohol by age interactions on P3 latency are depicted in Figure 4, below, separated by cue condition and electrode. Grand average waveforms for each group, measured at electrode Oz, are presented in Figure 5, below, separated by cue condition.
Figure 4. Age by Alcohol Latency Interactions by Cue Condition and Electrode Site.
Depictions of age by alcohol interactions (and SEs) on P3 latency, across all cue conditions and electrode sites. Age main effects were consistently noted, although subsequent analyses suggested these effects were driven primarily by the interactions. Age by alcohol interactions were noted in three cases; subsequent analyses (see text for details) suggested the interactions may have reflected both alcohol-associated decrements among older individuals and alcohol-associated facilitation among younger participants.
Figure 5. Grand Average Waveforms by Group (Measured at Oz).
Grand average waveforms at Oz, presented by age/alcohol groups for each cue condition.
N2 Component
An intepretational concern for these analyses was that P3 differences may have been driven by alterations in earlier ERP components. In order to investigate this concern, we analyzed the N2 component in a manner consistent with the 2 × 2 ANOVA described above. No significant main effect or interaction was noted for age or alcohol, at either electrode.
Behavior
To aid interpretation of these electrophysiological data, gross behavioral measures from the Posner task were analyzed. Initial 2 × 2 × 3 RM ANOVA (age [Older vs. Younger] × alcohol [Placebo vs. Alc] × condition [Valid vs. Invalid vs. Neutral]) were performed for both reaction time (ms) and task accuracy (correct/total trials). The reaction time analysis indicated an effect of validity condition, F(2,46)=7.68, p=.011, and an effect of age, with younger individuals responding more quickly, F(1,23)=50.43, p<.001; no age by alcohol interaction was noted. The ANOVA for accuracy failed to detect either main effects or interactions for age or alcohol. All other ps ≥ .074.
Paired t-tests indicated times on the valid condition (M=507, SD=902) were faster than in the neutral condition (M=546, SD=97), t(26)=8.49, p<.001, which were faster than in the invalid condition (M=576, SD=95), t(26)=4.36, p<.001. The accuracy analysis again indicated an effect of validity condition, F(2,46)=7.64, p=.005, but failed to detect any between-subjects effects. Accuracy in the valid (M=.838, SD=.109) and neutral (M=.807, SD=.155) conditions were equivalent, and each had greater accuracy than the invalid condition (M=.743, SD=.191), t(26)=3.04, p=.005 and t(26)=2.98, p=.006, respectively.
To investigate alcohol and age effects further, 2 × 2 ANOVA (age [Older vs. Younger] × alcohol [Placebo vs. Alc]) were performed for both reaction time and task accuracy, in each validity condition. A main effect of age on reaction time, such that younger individuals responded more quickly across all conditions, F(1,23)=11.21, p=.003; F(1,23)=6.81, p=0.16; and F(1,23)=4.99, p=.035, for valid, neutral, and invalid, respectively. No effects of age or alcohol were noted for task accuracy, and no interactive effects were noted for either behavioral measure.
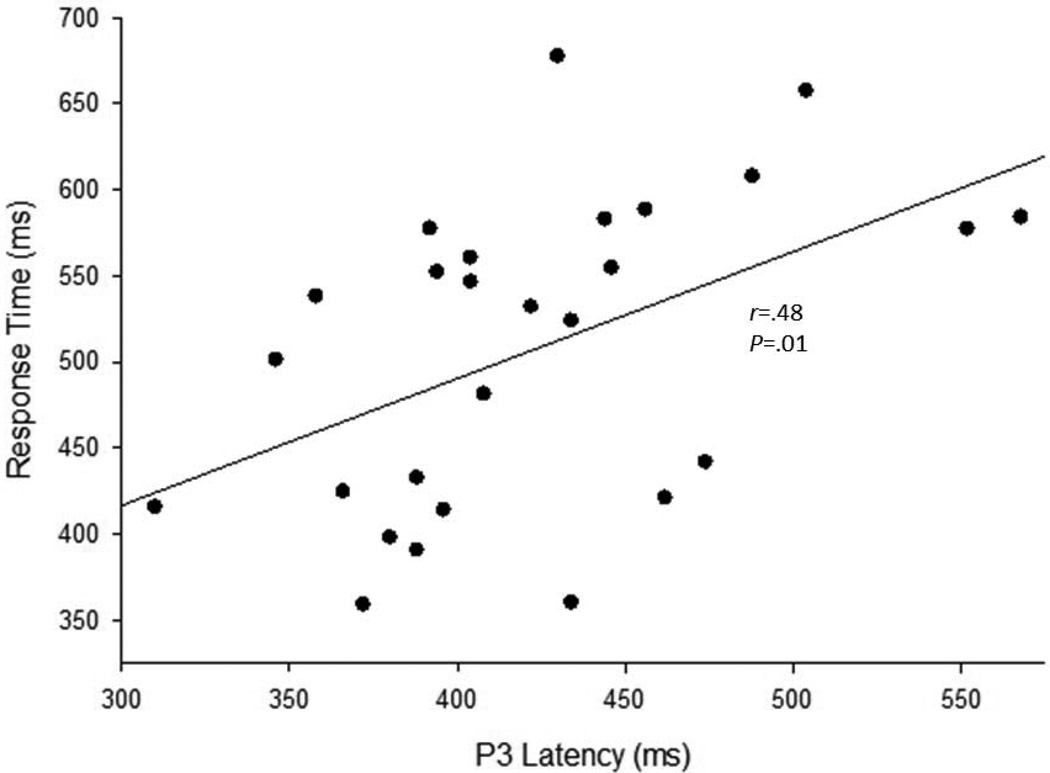
Correlation analyses were performed, comparing accuracy and reaction time with P3 peak and latency (Pz). Positive correlations between reaction time and P3 latency were noted across all validity conditions (r=.48, p=.010; r=.40, p=.039; r=.38, p=.053, for valid, neutral, and invalid conditions, respectively). See Figure 6 for a scatterplot of this relationship in the valid condition.
Figure 6. Scatterplot of Reaction Time and P3 Latency (For Valid Condition).
Scatterplot of task reaction time and P3 latency for the valid condition measured at Pz and including all groups (r=.48, p=.010).
Discussion
Based on previous literature (Friedman, 2003; Polich and Criado, 2006; Rohrbaugh et al., 1987), it was hypothesized that both acute alcohol and increased age would be associated with reductions in P3 amplitude and increases in P3 latency. Although our age-related hypothesis was supported, evidence for the hypothesized alcohol main effect was lacking. An age by alcohol interaction was also tentatively hypothesized. This interaction was noted across a variety of cue conditions and electrode sites; older individuals receiving alcohol displayed P3 decrements beyond those associated with age alone.
Examination of P3 amplitudes among placebo participants revealed significant differences between age groups only rarely, however visual observation of the pattern indicated consistent effects across conditions (see Figure 3). To further describe these outcomes, mean effect sizes were calculated and averaged across cue condition and electrode (Cohen’s d; Cohen, 1988). These estimates revealed moderate age effects (d=.49). Examination of alcohol effects revealed no meaningful pattern among younger individuals (d=.32), despite large effects among older participants (d=1.11). This reinforces our interpretation that the interaction between age and alcohol on P3 amplitude was driven primarily by alcohol effects in older participants. Results from analysis of the Cz electrode suggested that the observed age by alcohol effects were not localized to only Oz and Pz. At Cz, consistent, albeit non-significant, alcohol effects were noted among older individuals (ds=.78, .69 and .90 for valid, invalid and neutral cues, respectively).
As shown in Figure 4, age-associated differences in latency among placebo participants were not observed. A pattern of age by alcohol interactions was again noted for P3 latency. However, effect size analysis indicated they may have been driven by a moderate reduction in latency under alcohol for younger adults (d=.75). In contrast, alcohol prolonged P3 latency in older individuals (d=.64).
The subtle effects of age, although initially surprising, may be related to the fact that although our inclusionary criteria for the older group included individuals between 50 and 75 years of age, the average age of this group in our final sample was approximately 55 years. Thus, while P3 declines across the lifespan are marked (Rossini et al., 2007), it is perhaps unsurprising that only moderate decrements were noted in this relatively young group of older adults.
The lack of a robust alcohol main effect was also unexpected, given that previous work in younger adults (i.e., Rohrbaugh et al., 1987) has demonstrated latency increases of approximately 25 ms and amplitude reductions of approximately 1–2 µV at similar acute doses. Observations that acute low-dose alcohol administration may improve performance on some tasks among younger individuals (Linnoila and Mattila, 1973; Lloyd and Rogers, 1997; Sklar et al., 2011; Sklar et al., 2012) suggest that such alcohol-related discrepancies in the literature may be largely due to task selection. This discrepancy should be addressed in future studies with a broader range of tasks.
Cue Conditions
The consistency of the age and alcohol main effects and their interaction across cue conditions suggests the robustness of these findings. One empirical question of this work involved the effects of varied cue conditions in the elicitation of P3s, particularly insofar as cue conditions might interact with age and/or alcohol. Thus, conditions were analyzed separately, both for theoretical reasons (Luck et al., 1994), and due to the main effect of cue condition noted in the guiding analyses. Relative to neutrally cued conditions, invalid cues are noted to delay the latency of early ERP components (Luck et al., 1994). This delay is taken to reflect the cost of attentional redirection to the correct spatial location (Posner, 1980), suggesting P3 decrements during invalid trials should be evident. This prediction was partially supported; invalid cues generated the lowest amplitude P3s (M=9.71 µV), followed by valid (M=10.07 µV) and neutral (M=10.82 µV) conditions. However, this was a weak effect (d=.23), and no differences in latency as a function of cue conditions were noted. This was somewhat puzzling, as behavioral analyses revealed a significant effect of validity condition on reaction time, and further, reaction times and P3 latencies were correlated across conditions. Potential faciliatory effects of validly cued trials were also of interest, however no evidence of such facilitation was noted.
Caveats / Considerations
Several limitations to the current study bear consideration. These results should not yet be considered evidence of generalized effects of age and alcohol on electrophysiological indices of attention. The attentional task used in this study provides an interesting context for this investigation. However, replication of age by alcohol effects in well-validated tasks more commonly used to elicit P3s (e.g., auditory oddball task) would aid generalization.
This design was not adequately powered to detect gender differences. Numerous gender-associated differences in response to alcohol (e.g., Nolen-Hoeksema and Hilt, 2006) suggest that incorporating gender into future investigations will be particularly important in further describing the complex effects of alcohol across the lifespan. Furthermore, gender is known to affect P3 characteristics, with women producing higher amplitudes than men (Polich & Kok, 1995). Women were over-represented in this sample (55% and 58% of the older and younger participants, and 50% and 61% of the placebo and alcohol-treated participants, respectively, were female). No significant differences in the distribution of women across age group or dose assignement were noted (χ2s<0.4). Given the similarities in gender composition across groups, any bias related to gender should have been distributed equally across groups. Preliminary gender analyses revealed no differences in either peaks or latencies. However, the possible influence of gender-driven differences in P3s cannot be completely discounted.
It is conceivable that the observed P3 results may have been related to effects on preceding ERP components. To address this concern, the N2 component was analyzed in a similar manner, conducting age by alcohol ANOVA for each electrode and cue condition. No differences were noted. Thus, preceding components seem unlikely to have confounded the observed P3 differences. Although this cursory N2 analysis was useful for interpretational purposes, neither N2 nor other, earlier components (i.e., N1, P1, P2) were a primary concern of the work, which focused on the P3 as a reflection of higher-order working memory and attentional processes.
Finally, the exclusionary criteria used in this study ensured that participants were generally healthy; older participants in particular, may have differed in health status from a general age-matched population. Although this potential difference should be noted when considering applicability, that these effects were noted among such a healthy group of older individuals suggests they may be even more pronounced in the general population.
Summary
In sum, these results suggest the pattern of reduced/delayed P3s in older participants was driven by the effect of alcohol, implying that while older individuals may demonstrate subtle P3 decrements due to age alone, such differences become marked under the effects of even moderate alcohol consumption. Critically, this interaction was marked despite the relatively young age (~55) of “older” participants, suggesting alcohol effects on attentional processing at older ages may be even more pronounced.
Substantial literatures have separately considered the effects of both age and acute alcohol on P3 function, however few studies have directly addressed acute effects in older individuals, and fewer still have included young adults for comparison. Thus, while novel and provocative, these findings should be interpreted cautiously. For instance, effects may be sensitive to task demand; replication in additional tasks with varying difficulty and/or sensory modality would be useful. Finally, these neurophysiological decrements may be compounded by additional disruption in numerous situations (e.g., dual-task or divided attention conditions, medication-alcohol interactions, concomitant age-associated cognitive decline). Given these obvious public health implications, further investigation is warranted.
Acknowledgements
Funding was provided by NIAAA R03 AA014039 (SJ Nixon, PI) and the University of Kentucky Department of Psychology. Special thanks to Dr. Mark Fillmore for help with protocol development and study design.
References
- Adams WL, Garry PJ, Rhyne R, Hunt MS, Goodwin JS. Alcohol intake in the healthy elderly, changes with age in a cross-sectional and longitudinal study. J Am Geriatr Soc. 1990;38:211–216. doi: 10.1111/j.1532-5415.1990.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Balsa AI, Homer JF, Fleming MF, French MT. Alcohol consumption and health among elders. Gerontologist. 2008;48:622–636. doi: 10.1093/geront/48.5.622. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. Second Edition. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Cahalan D, Cissin L, Crossley H. American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6) New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol affects processing of ignored stimuli in a negative priming paradigm. J Stud Alcohol. 2000;61:571–578. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Friedman D. Cognition and aging: a highly selective overview of event-related potential (ERP) data. J Clin Exp Neuropsychol. 2003;25:702–720. doi: 10.1076/jcen.25.5.702.14578. [DOI] [PubMed] [Google Scholar]
- Gallup Inc. Alcohol and Drinking. 2011 [Google Scholar]
- Gazzaley A, D'Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Boissoneault J, Prather R, Nixon SJ. EEG correlates of moderate alcohol consumption in older and younger social drinkers. Paper presented at: Research Society on Alcoholism; San Antonio. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: Perceived impairment versus psychomotor performance. J Stud Alcohol Drugs. 2009;70:242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Mangun GR, Woldorff MG, Luck SJ. Neural systems mediating selective attention. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: The MIT Press; 1995. pp. 665–681. [Google Scholar]
- Hirayasu Y, Samura M, Ohta H, Ogura C. Sex effects on rate of change of P300 latency with age. Clin Neurophysiol. 2000;111:187–194. doi: 10.1016/s1388-2457(99)00233-3. [DOI] [PubMed] [Google Scholar]
- Holloway FA. Low-Dose Alcohol Effects on Human Behavior and Performance: A Review of Post-1984 Research. Washington, DC: Federal Aviation Administration; 1994. Pub. No. 94-35919. [Google Scholar]
- Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on event-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30:10–22. doi: 10.1111/j.1469-8986.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kugler CF, Taghavy A, Platt D. The event-related P300 potential analysis of cognitive human brain aging: a review. Gerontology. 1993;39:280–303. doi: 10.1159/000213544. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. Postperceptual effects and P300 latency. Psychophysiology. 1998;35:34–46. [PubMed] [Google Scholar]
- Linnoila M, Mattila MJ. Interaction of alcohol and drugs on psychomotor skills as demonstrated by a driving simulator. Br J Pharmacol. 1973;47:671P–672P. [PMC free article] [PubMed] [Google Scholar]
- Lloyd HM, Rogers PJ. Mood and cognitive performance improved by a small amount of alcohol given with a lunchtime meal. Behav Pharmacol. 1997;8:188–195. [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Robinson CD. National Highway Traffic Safety Administration Report No. DOT HS 807 280. Washington, DC: Department of Transportation; 1988. Effects of Low Doses of Alcohol on Driving-Related Skills: A Review of the Evidence. [Google Scholar]
- Mukamal KJ, Rimm EB. Alcohol consumption: risks and benefits. Curr Atheroscler Rep. 2008;10:536–543. doi: 10.1007/s11883-008-0083-2. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychol. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am. 2004;15:133–161. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potential deficits in alcoholism and aging. Alcohol Clin Exp Res. 1982;6:53–63. doi: 10.1111/j.1530-0277.1982.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Attention in Cognitive neuroscience: an overview. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 615–624. [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, Compton W. The Diagnostic Interview Schedule, Version IV. St. Louis: Washington University; 1995. [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff B, Lane EA, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical Substrates of Age-Related Cognitive Decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar AL, Boissoneault J, Morey T, Chernoby G, Nixon SJ. Acute effects of low-dose alcohol consumption on psychomotor performance [abstract] Paper presented at: Research Society on Alcoholism (RSA) Atlanta, GA: 2011. [Google Scholar]
- Sklar AL, Gilbertson RJ, Boissoneault J, Prather R, Nixon SJ. Differential Effects of Moderate Alcohol Consumption on Performance Among Older and Younger Adults. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01833.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Mental Health Findings, NSDUH Series H-42, HHS Publication No. (SMA) 11-4667. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Second Edition. Glenview, Illinois: HarperCollins; 1989. [Google Scholar]
- Tupler LA, Hege S, Ellinwood EH., Jr Alcohol pharmacodynamics in young-elderly adults contrasted with young and middle-aged subjects. Psychopharmacology (Berl) 1995;118:460–470. doi: 10.1007/BF02245947. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. National Population Projections. 2008 [Google Scholar]
- Vogel-Sprott M, Barrett P. Age, drinking habits and the effects of alcohol. J Stud Alcohol. 1984;45:517–521. doi: 10.15288/jsa.1984.45.517. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J Stud Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Zucker RA. Developmental aspects of aging, alcohol involvement, and their interrelationship. In: Gomberg ESL, Hegedus AM, Zucker RA, editors. Alcohol Problems and Aging. Bethesda: National Institute on Alcohol Abuse and Alcoholism; 1998. pp. 3–23. [Google Scholar]