Abstract
Proteus mirabilis, named for the Greek god who changed shape to avoid capture, has fascinated microbiologists for more than a century with its unique swarming differentiation, Dienes line formation and potent urease activity. Transcriptome profiling during both host infection and swarming motility, coupled with the availability of the complete genome sequence for P. mirabilis, has revealed the occurrence of interbacterial competition and killing through a type VI secretion system, and the reciprocal regulation of adhesion and motility, as well as the intimate connections between metabolism, swarming and virulence. This Review addresses some of the unique and recently described aspects of P. mirabilis biology and pathogenesis, and emphasizes the potential role of this bacterium in single- species and polymicrobial urinary tract infections.
In Homer’s Odyssey, Proteus was pursued by many for his ability to foretell the future to anyone capable of capturing him, but he changed shape to evade his pursuers. The name Proteus was first used in bacterial nomenclature by Hauser in 1885 to describe a shape-shifting bacterium isolated from putrefied meat1. Proteus mirabilis, a Gram-negative, dimorphic, motile member of the family Enterobacteriaceae, has fascinated scientists for more than 125 years owing to its ability to differentiate from short rods into elongated, multinucleate swarm cells that express thousands of flagella2. Members of the genus Proteus are widely distributed in nature and can be isolated from soil, stagnant water, sewage and the intestinal tract3. P. mirabilis is a leading agent of pyelonephritis, urolithiasis, prostatitis and catheter-associated urinary tract infections (CAUTIs) and causes approximately 3% of all nosocomial infections and up to 44% of CAUTIs in the United States4–6.
CAUTIs are the most common health care-associated infections worldwide, accounting for up to 40% of hospital- acquired infections7. The duration of catheterization is the most important risk factor associated with CAUTI development, as roughly 10–50% of patients undergoing short-term catheterization (1–7 days) develop only bacteriuria, whereas essentially all patients catheterized for 28 days or longer develop a CAUTI8. CAUTIs are thought to be caused by self-inoculation of the catheter; indeed, for P. mirabilis, the strains causing bacteriuria in a given patient match the faecal isolates from that patient9.
P. mirabilis is generally not the first organism found on the catheter surface, but it is common in long-term catheterization10,11. When P. mirabilis colonizes a catheter, the bacterial cells develop in protected communities known as biofilms. The biofilms that are initially formed on catheters tend to be monomicrobial, but rapidly become polymicrobial during long-term catheterization, with up to 72% of catheters being colonized by two or more species12. Catheter bio-films and urine samples taken from patients undergoing long-term catheterization frequently contain combinations of P. mirabilis, Morganella morganii, Providencia stuartii, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae5,7,13–16. Data compiled over the past 30 years reveal that, depending on the study parameters and sample population, up to 77% of CAUTIs are polymicrobial, and P. mirabilis is generally the most common organism isolated from these infections13–18.
P. mirabilis has many virulence factors that contribute to the establishment of a UTI in a mouse model of infection19. The bacterium expresses fimbriae, or bacterial appendages tipped with adhesive proteins, that mediate attachment to cells in the urinary tract and probably also to the catheter. In addition, P. mirabilis produces urease, which hydrolyses urea to carbon dioxide and ammonia. This reaction provides an abundant source of nitrogen for the bacterium, but also causes the formation of crystalline biofilms that block the catheter. Furthermore, P. mirabilis can differentiate into swarm cells that contribute to the establishment of infection by migrating along the catheter. Inside the urinary tract, the bacterium has mechanisms to scavenge nutrients and evade the host immune response, and is capable of reciprocal expression of fimbriae (for adherence) and flagella (for motility when it needs to ascend the urinary tract).
There have been many recent advances in our understanding of P. mirabilis pathogenicity, including the publication of the complete 4.06 Mb genome sequence of a CAUTI isolate (P. mirabilis str. HI4320)20, the identification of novel pathogenicity factors by signature-tagged mutagenesis21–23, and the elucidation of the intricate connections between metabolism, swarming motility, urease activity and nitrogen availability in the establishment and persistence of P. mirabilis UTIs. This Review aims to address the most recent findings concerning P. mirabilis pathogenicity and to place these findings in the context of CAUTI progression, identifying the stage of infection (for example, initial entry to the urinary tract, ascending the tract and immune evasion) to which they contribute.
Furthermore, given the growing appreciation in the field for the polymicrobial nature of many infections, it is imperative to consider how knowledge gained from single-species investigations can be applied to the study and treatment of polymicrobial infection. Although few studies have addressed polymicrobial CAUTIs to date, P. mirabilis is frequently isolated from these infections. It is therefore important to consider how P. mirabilis and other common causes of CAUTIs interact on catheters and during infections, and how these interactions influence the course of an infection. The final section of this Review discusses P. mirabilis pathogenicity factors in the context of polymicrobial infection and considers ways in which this organism interacts with other uropathogens.
Entry to the urinary tract
To establish a CAUTI, P. mirabilis must first gain access to the urinary tract. Although the catheter provides a direct route to the bladder, P. mirabilis needs to adhere tightly to the catheter to resist the flow of urine and must traverse this surface or disrupt the flow of urine to gain entry to the bladder.
Adherence to catheters
P. mirabilis readily colonizes the lumen and external surface of all current catheter types, and adherence is enhanced in the presence of urine24–28. P. mirabilis encodes 17 putative fimbrial operons, the most encoded by any sequenced bacterial species20. Five fimbrial types have been characterized and reviewed29 (TABLE 1), but little is known about the contribution of fimbriae to catheter adherence and colonization. P. mirabilis expresses mannose-resistant Klebsiella-like (MRK) fimbriae, a type that mediates catheter attachment by P. stuartii30 and Proteus penneri31 and might therefore contribute to colonization by P. mirabilis. The ambient-temperature fimbriae (ATFs) of P. mirabilis do not contribute to colonization of the mouse urinary tract but are optimally expressed at ambient temperature and could therefore influence catheter colonization32,33.
Table 1.
Fimbriae of Proteus mirabilis
| Type of fimbriae | Contribution to catheter colonization | Adherence capabilities in vitro | Contribution to UTI pathogenesis |
|---|---|---|---|
| ATFs | Possible | Unknown | Not associated |
| MRK fimbriae | Likely | Bowman’s capsules (kidney) | Kidney colonization |
| MRP fimbriae | Unknown | Kidney tubular cells, and epithelial cells from urine | Bladder and kidney colonization, and reciprocal regulation of motility |
| NAFs | Unknown | Uroepithelial cells | Unknown |
| PMFs | Unknown | Uroepithelial cells | Bladder colonization |
ATFs, ambient-temperature fimbriae; MRK, mannose-resistant Klebsiella-like; MRP, mannose-resistant Proteus-like; NAFs, non-agglutinating fimbriae; PMFs, Proteus mirabilis fimbriae; UTI, urinary tract infection.
Swarming motility
An overview of P. mirabilis swarming motility is presented in BOX 1. Swarming describes flagellum- dependent movement across a surface, in contrast to swimming through liquid or soft agar. This form of motility allows P. mirabilis to migrate across catheters, gaining entry to the urinary tract4,34,35. Swarming is required for migration across most catheter types, as non-swarming mutants traverse only hydrogel-coated latex catheters, by swimming through the water-filled channels in the matrix36. In addition, P. mirabilis catheter biofilms contain protruding swarm cells37. Although the role of these biofilm-associated swarm cells is unclear, they might seed dispersal from the catheter to the urinary tract37.
Box 1. Proteus mirabilis swarming motility.
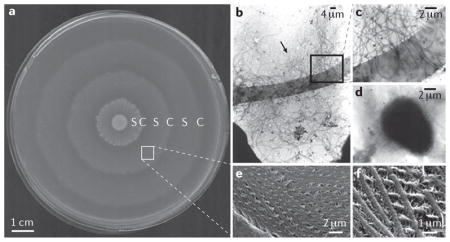
Unlike species that swarm on 0.45% agar, such as Escherichia coli, Proteus mirabilis swarming motility occurs on 1.5% agar at both 30 °C and 37 °C, resulting in a characteristic bull’s eye pattern (see the figure, part a). This pattern is caused by sequential rounds of swarm cell differentiation, swarming colony migration (designated S in part a), and consolidation with de-differentiation back to a swimmer-cell morphology (designated C in part a). On a solid surface, P. mirabilis differentiates into swarm cells that are multinucleate, 20–50-fold elongated and express thousands of flagella (see the figure, part b (arrow) and, at higher magnification, part c)2,73. In liquid culture, P. mirabilis cells are rod shaped, 1–2 μm in length and peritrichously flagellated (see the figure, part d). Recent work allowed visualization of the helical connections formed in swarm rafts during migration, revealing that these connections consist of interwoven flagellar filaments from adjacent swarm cells (see the figure, part e and, at higher magnification, part f))36. It is not yet clear how P. mirabilis coordinates the formation of these structures, but they seem to be required for normal swarming motility.
Although consolidation was once considered a resting stage, P. mirabilis is considerably more metabolically active during consolidation than it is during swarming, and overall gene expression is also higher127,128. The genes that are most highly upregulated during consolidation include those encoding proteins involved in amino acid import and synthesis, peptide uptake, central metabolic pathways, peptidoglycan remodelling and cell wall synthesis, as well as stress response proteins, proteases and many flagellar proteins. By contrast, few genes are upregulated in swarm cells compared to in consolidated cells, and swarming can occur in the absence of protein synthesis. This indicates that swarming is almost entirely devoted to flagellum- mediated motility, whereas consolidation seems to be the active stage during which P. mirabilis prepares for the next round of motility128.
Parts b–d are reproduced, with permission, from REF. 73 © (1999). Parts e and f are reproduced, with permission, from REF. 36 © (2004).
Most strains of P. mirabilis are unable to differentiate into swarm cells in liquid medium without the addition of a thickening agent, so it is thought that differentiation is triggered by surface contact or inhibition of flagellar rotation38. Several other factors have been implicated in the regulation of swarming motility (and are reviewed elsewhere39,40), including the upregulator of flagellar master operon (Umo) proteins, the regulator of colanic acid capsule synthesis (Rcs) phosphorelay, the RppAB two-component system, the carbon storage regulator (CsrA) homologue RsmA, Lon protease, the tyrosine decarboxylase DisA and leucine-responsive regulator (Lrp). An overview of the role of these factors in regulating swarm cell differentiation is presented in BOX 2. Interestingly, Lrp is part of a family of transcription factors that link gene regulation to metabolism, and its activity in P. mirabilis is regulated by leucine, isoleucine, serine, histidine, threonine and methionine41,42. Although glutamine does not regulate Lrp activity, this amino acid also appears to promote swarm cell differentiation43, and additional metabolic intermediates that promote swarming under normally non-permissive conditions have been identified (C.E.A. and H.L.T.M., unpublished observations). Thus, the decision to swarm appears to be influenced by metabolic status and the presence of specific amino acids, including glutamine and histidine, which are two of the most concentrated amino acids in normal human urine44.
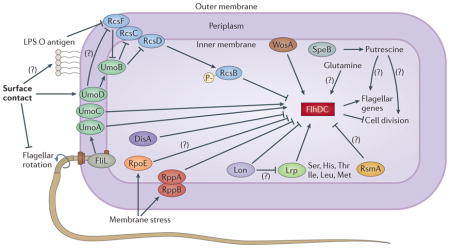
Box 2. Swarm cell differentiation in Proteus mirabilis.
Recent studies concerning Proteus mirabilis swarm cell differentiation have revealed a complex regulatory network, with most factors acting on the flagellar transcriptional regulator (FlhDC). Some of these factors alter gene expression, and others modify protein level; in the figure, all factors are shown as affecting FlhDC, for simplicity. The regulator of colanic acid capsule synthesis (Rcs) phosphorelay, composed of a response regulator (RcsB), a sensor kinase (RcsC), an outer-membrane activator (RcsF) and a phosphotransferase (RcsD; also known as RsbA), ultimately results in phosphorylated RcsB, which represses flhDC113,129,130. When P. mirabilis contacts a surface under conditions that are favourable for swarming, this signal is sensed and propagated by some combination of the inhibition of flagellar rotation, FliL, WosA and changes in the cell wall involving the O antigen of lipopolysaccharide (LPS)38,131–133. The signal is relayed by inhibition of RcsF and increased upregulator of flagellar master operon D (UmoD) activity, leading to activation of UmoB and, consequently, a reduction in phosphorylated RcsB, alleviating flhDC repression132,134. The importance of the Rcs phosphorelay in the regulation of swarm cell differentiation is further underscored by the finding that disruption of rcsD alleviates the requirement for surface contact and allows the formation of elongated cells in liquid culture113. In addition to the Rcs system, Lon protease negatively regulates swarming by degrading FlhD and possibly also leucine-responsive regulator (Lrp)135. RppA negatively regulates flagellin synthesis by decreasing expression of flhDC as well as modulating LPS synthesis136,137, and RNA polymerase σ-factor RpoE may respond to membrane stress sensed by the RppAB system by decreasing expression of flhDC136. The putative tyrosine decarboxylase DisA also negatively regulates swarming by decreasing expression of the class 2 and class 3 flagellar genes138. The carbon storage regulator (CsrA) homologue RsmA inhibits swarming when overexpressed, possibly by regulating flhDC expression40,139. The amino acid glutamine promotes swarm cell differentiation under normally non-permissive conditions, and the polyamine putrescine (generated by agmatinase (SpeB)) is required for swarming, although their mechanisms of action are not yet known43,120. Thus, initiation of swarming requires the integration of numerous signals and is intimately connected to the metabolic status of the bacterium, membrane integrity, and cell wall changes associated with surface contact.
Urease and catheter encrustation
The urease gene cluster of P. mirabilis, which comprises ureRDABCEFG, produces a cytoplasmic nickel metalloenzyme that is positively regulated by UreR and repressed by the nucleoid- associated protein H-NS45–52. UreR and H-NS compete for the same regulatory region upstream of ureD, resulting in tight control of urease expression: H-NS represses transcription of the ure operon at ambient temperature, but a shift to host body temperature and high concentrations of urea alleviate H-NS transcriptional repression, allowing UreR to activate urease expression47. Urease contributes to catheter colonization by hydrolysing urea to ammonia and carbon dioxide, thereby increasing the pH and facilitating the precipitation of polyvalent ions in urine, leading to the formation of struvite (magnesium ammonium phosphate) and apatite (calcium phosphate) crystals19,53,54. Bacterial adherence typically occurs when the urine pH increases to ~8.2 and crystals deposit on the catheter27.
The crystals accumulate within biofilms on the catheter and eventually block the catheter lumen, obstructing urine flow and leading to complications such as incontinence and painful distension of the bladder (caused by urinary retention)55. This in turn leads to vesicoureteral reflux, bacteriuria, ascending infection, pyelonephritis and possibly septicaemia56. P. mirabilis str. HI4320 generally causes urinary stones and severe pyelonephritis in experimental UTIs, and urease is expressed by this strain within the bladder and kidneys57. Furthermore, mutation of the gene encoding the major structural subunit of urease, UreC, severely reduces P. mirabilis colonization of the bladder and kidneys, prevents stone formation and limits kidney damage, indicating that urease is a major contributing factor to both the severity and persistence of P. mirabilis-mediated UTIs58–60.
Ascending UTIs and pathogenesis
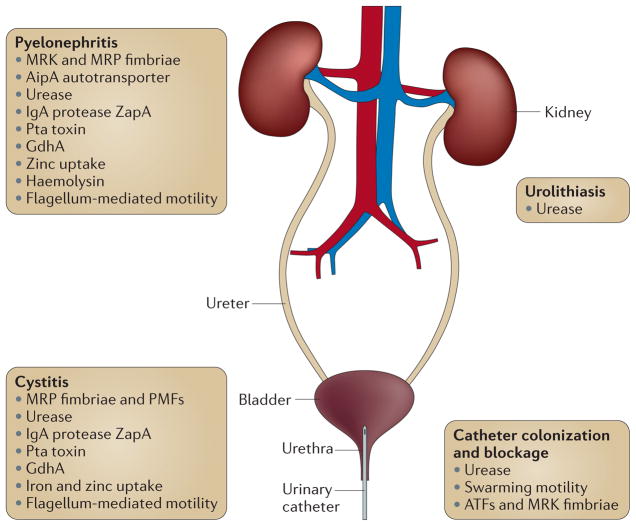
The urinary tract consists of two kidneys, two ureters, the bladder and the urethra (FIG. 1). Catheterization provides a direct route to the bladder, and the combined actions of fimbriae, adhesins, swarming motility and urease promote entry of P. mirabilis to the urinary tract, but the bacterium then faces new challenges when it is inside the host. To establish a UTI, P. mirabilis must adhere to the uroepithelial cells lining the bladder. The bacterium also generally does not stay confined to the bladder, but ascends the ureters to infect the kidneys. Therefore, P. mirabilis must modulate the expression of the adherence and motility factors that are important for ascension and must produce specialized virulence factors for survival within the urinary tract. A mouse model of an ascending UTI, in which P. mirabilis is introduced into the bladder via a catheter that is inserted into the urethra and carefully removed immediately following infection, is commonly used to study the contribution of these virulence factors to infection. This model allows for direct infection of the bladder, but the initial inoculum does not reach the kidneys unless the bacteria actively ascend.
Figure 1. Ascending urinary tract infection and Proteus mirabilis virulence factors.
An overview of key Proteus mirabilis virulence factors that contribute to catheter colonization and blockage, infection of the bladder (cystitis) and kidneys (pyelonephritis), and to the formation of urinary stones (urolithiasis). A full list of P. mirabilis pathogenicity factors and their contribution to infection is provided in TABLE 2. ATFs, ambient- temperature fimbriae; GdhA, glutamate dehydrogenase; IgA, immunoglobulin A; MRK, mannose-resistant Klebsiella-like; MRP, mannose-resistant Proteus-like; PMFs, P. mirabilis fimbriae; Pta, Proteus toxin agglutinin; ZapA, serralysin.
Fimbriae and adhesins
Although P. mirabilis fimbriae and adhesins are important determinants of pathogenicity, as they promote adherence to the uroepithelium, most studies suggest that individual P. mirabilis fimbriae and adhesins are necessary, but not sufficient, to establish a UTI. For instance, the autotransporter AipA mediates adhesion to human kidney and bladder epithelial cell lines and contributes to colonization of the kidneys and spleen in a mouse model, but an aipA mutant still colonizes the bladder and kidneys at relatively high levels61. Even with the loss of two distinct fimbrial types, P. mirabilis retains the ability to adhere to bladder cells and establish a UTI, albeit at a reduced level62. Therefore, determining the role of individual fimbrial types is complex.
Despite these challenges, recent work has elucidated the contributions of several fimbria types to UTIs (TABLE 1). Non-agglutinating fimbriae (NAFs; also known as uroepithelial cell adhesin (UCA) fimbriae) facilitate adherence to uroepithelial cell lines63,64, MRK fimbriae mediate adherence to Bowman’s capsules of the kidney glomeruli63, and mannose-resistant Proteus-like (MRP) fimbriae mediate adherence to the lumen and cytoplasm of tubular cells in the kidney and to epithelial cells present in urine63. P. mirabilis fimbriae (PMFs) are also thought to contribute to colonization of the bladder, but are not necessary for kidney colonization65,66. However, the exact contribution of PMFs to infection remains unclear, as expression of the major structural subunit (PmfA) is lower during infection than in non-infecting bacteria67.
MRP fimbriae are the best studied fimbrial type of P. mirabilis, and they contribute to colonization of the urinary tract, as disruption of the mrp operon decreases bacterial load in the urine, bladder and kidneys68,69. MRP fimbriae are subjected to phase variation (FIG. 2), and phase variation in vivo favours the expression of these fimbriae70. Furthermore, inhibiting mrp expression results in a slight defect in colonization, whereas constitutive mrp expression allows the bacteria to out-compete the wild-type strain in the bladder, indicating that MRP fimbriae provide an advantage during bladder colonization71. Along with the finding that MRP fimbriae are present in 94% of P. mirabilis isolates obtained from the mouse bladder during infection, these results strongly indicate that MRP fimbriae contribute to colonization of the bladder and probably the entire urinary tract72.
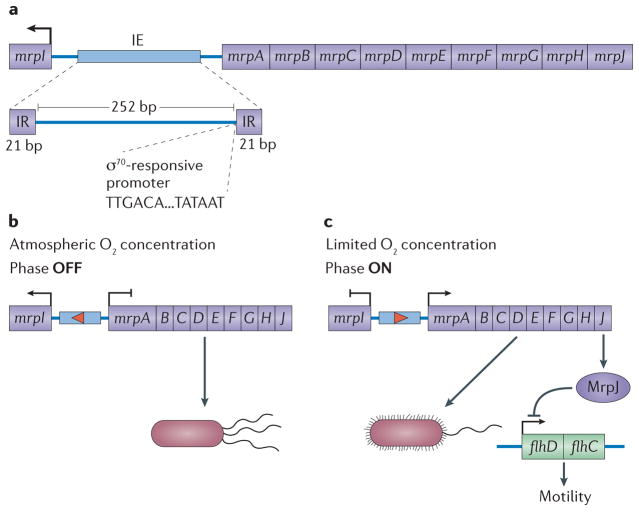
Figure 2. The mannose-resistant Proteus-like fimbrial operon: organization and phase variation.
a. The mannose-resistant Proteus-like (mrp Nature) fimbrial operon consists of mrpI, an intergenic region containing an invertible element (IE), and mrpABCDEFGHJ. The 252 bp IE is flanked by 21 bp inverted repeats (IRs) and contains an RNA polymerase σ70-responsive promoter that is predicted to drive transcription of mrpABCDEFGHJ when the IE is in the ON position, resulting in the formation of MRP fimbriae. A recombinase encoded by mrpI is divergently transcribed from the rest of the operon (using a separate promoter). When expressed, MrpI reverses the orientation of the IE and the promoter contained within it, driving phase variation. As MrpI is the sole recombinase in Proteus mirabilis71, the IE can be phase locked by mutating mrpI. b. Aerobic conditions favour mrpI expression and the active conversion of fimbriate bacteria to the non-fimbriate form77. c. Reduced oxygen levels favour expression of the mrp operon, including mrpJ, which represses motility by inhibiting expression of the flagellar transcriptional regulator (flhDC) operon77.
Reciprocal regulation of fimbriae and flagella
Bacteria generally do not express adherence factors at the same time as flagella, suggesting that there is an underlying mechanism of reciprocal regulation2,73. For instance, P. mirabilis swarm cells express thousands of flagella but few fimbriae, and expression of the mrp operon is highest when the expression of flagellar genes is reduced67,74. Recent work determined that the last gene in the mrp operon, mrpJ, encodes a transcriptional regulator that directly represses motility by binding to the promoter region of the flagellar transcriptional regulator (flhDC) operon75,76. Furthermore, locking the invertible element (IE) of the mrp operon in the OFF position, and thus inhibiting mrpJ expression, favours faster swarming motility72. Interestingly, P. mirabilis str. HI4320 encodes 14 additional mrpJ paralogues, ten of which are associated with other fimbrial operons76. Overexpression of most of these paralogues similarly represses motility and, for some paralogues, inhibits swarm cell differentiation76, indicating that P. mirabilis is exquisitely committed to regulating the expression of fimbriae and flagella. The proteins encoded by all 15 mrpJ-type genes contain a conserved DNA-binding domain, although it is not known whether all paralogues bind the flhDC promoter76. MrpJ might also contribute to phase variation of the fimbrial mrp operon77, providing another layer of complexity.
Further insight into the reciprocal expression of MRP fimbriae and flagella comes from analysis of gene expression in a mouse UTI model. Transcriptome profiling revealed that, during infection, the most highly upregulated P. mirabilis genes are components of the mrp operon, whereas genes involved in motility are highly down-regulated67. An analysis of temporal expression, however, showed that the mrp operon is highly expressed at days 1 and 3 post-infection but is not as highly expressed by day 7, whereas the expression of genes encoding flagellar components is decreased for only 1 day following infection67. This finding suggests that the expression of MRP fimbriae is crucial for early stages of infection but decreases over time, possibly to allow for flagellum- mediated motility. This is a noteworthy possibility, as swarm cells seem to be rare during the early stages of a UTI, but might have a crucial role in later stages of infection78–80.
Metabolic requirements for infection
On the basis of in vivo gene expression and other study data, P. mirabilis nitrogen metabolism appears to be crucial for the establishment of a UTI. During experimental infection, P. mirabilis increases glutamate dehydrogenase (gdhA) expression and decreases expression of glutamine synthetase (glnA)67. GdhA mediates the conversion of ammonia and α-ketoglutarate to glutamate when ammonia is abundant, whereas GlnA is part of the GS–GOGAT system (glutamine synthase–glutamine oxoglutarate amino-transferase system) for the high-affinity conversion of limited amounts of ammonia to glutamine to generate glutamate under nitrogen-limited conditions. Therefore, the data suggest that P. mirabilis uses GdhA and the ammonia produced by urease activity to generate glutamate during infection67. This hypothesis has yet to be tested, but the portion of the tricarboxylic acid cycle that is required for α-ketoglutarate synthesis (involving the enzymes encoded by gltA, acnB and icd) is upregulated during UTIs67. Furthermore, P. mirabilis gdhA mutants exhibit less colonization of the bladder, kidneys and spleen than wild-type P. mirabilis, underscoring the importance of this system during UTIs67.
Metal acquisition
As the urinary tract is an iron-limited environment and iron is essential to the function of many proteins and enzymes, scavenging iron from the host is imperative for pathogenic bacteria. P. mirabilis was originally thought to lack siderophores81, leaving a large gap in our understanding of how this species persists in the urinary tract. However, iron is clearly necessary for P. mirabilis pathogenicity, as five genes found to be associated with iron acquisition result in attenuation of virulence when they are mutated21,23. One of these genes, hmuR2, encodes a haem receptor that contributes to bladder and kidney colonization82. Two other putative iron receptors encoded by PMI0842 and PMI2596 similarly contribute to P. mirabilis pathogenicity83.
Following annotation of the P. mirabilis str. HI4320 genome and microarray analysis of gene expression under iron-limited conditions, it is now known that P. mirabilis encodes at least 21 putative iron acquisition systems, two of which have been well characterized20,84. In keeping with its unique nature, P. mirabilis str. HI4320 encodes a previously undescribed non-ribosomal-peptide synthetase (NRPS)-independent siderophore (NIS) system, now known as proteobactin (encoded by the operon pbtABCDEFGHI)84. P. mirabilis also contains the nrp operon, encoded on the mosaic pathogenicity island ICEPm1 (integrative and conjugative element of P. mirabilis 1) and upregulated during iron limitation85,86. The nrp genes have homology to the yersiniabactin synthesis genes, but the nrp system of P. mirabilis is distinct from the yersiniabactin synthesis systems of other species84: although P. mirabilis can utilize enterobactins produced by other species, it cannot produce or utilize yersiniabactin, despite the high similarity between the yersiniabactin synthesis genes and the nrp locus84.
Proteobactin and the siderophore encoded by the nrp operon seem to be the primary mechanisms for iron chelation in P. mirabilis84. One study found these loci to be more prevalent in UTI isolates (present in all ten isolates tested) than in non-UTI isolates (present in only five of the ten tested isolates), suggesting that these loci contribute to fitness within the urinary tract84. Both siderophores are important for colonization of the bladder, although the yersiniabactin-related siderophore provides a greater contribution to overall fitness84. Furthermore, transcriptome profiling has verified that these iron uptake systems are upregulated in vivo67.
Zinc is another metal that is crucial for the function of numerous proteins and enzymes. P. mirabilis has a functional zinc uptake system (ZnuABC) that is required for growth under zinc limitation87. Mutation of znuC limits swimming and swarming motility, possibly owing to an impact on the flagellar master regulator FlhC, as in some species FlhC contains a zinc-binding site88. ZnuB is expressed during mouse UTIs and is recognized by the humoral response, and ZnuC is upregulated during mouse UTIs67,83. However, the contribution of the ZnuABC system to virulence remains unclear, as a znuC mutant colonizes the urinary tract to a similar level as the wild-type bacterium but is outcompeted during a challenge with both bacteria simultaneously87.
Toxins
Haemolysin was proposed as a virulence factor in P. mirabilis because strains with high haemolysin production are more lethal than strains with low haemolysin production, and the cytotoxicity of P. mirabilis to human renal epithelial cells is largely due to haemolysin89–91. Haemolysin is also thought to facilitate bacterial spread within the kidney and development of pyelo-nephritis during ascending UTIs19. However, mutation of hpmA, the gene encoding this toxin, does not appear to affect kidney colonization or tissue damage during infection74,92,93, indicating that either haemolysin is not as active during infection as the in vitro data suggest or the activity of other virulence factors masks its contribution.
Proteus toxic agglutinin (Pta), encoded by ICEPm1, is a bifunctional outer-membrane autotransporter that mediates cell–cell aggregation and also contains a catalytically active α-domain (a subtilisin-like alkaline protease domain) capable of lysing kidney and bladder cells86,94. This unusual adhesin–toxin was first identified as an outer-membrane surface-expressed protein that is recognized by the mouse immune system83, and loss of pta results in a significant colonization defect in the bladder, kidneys and spleen, as well as reduced pathology92,94. Notably, simultaneous inactivation of pta and hpmA results in a greater reduction in cytotoxicity than is caused by either mutation alone92, indicating that Pta and HpmA have an additive effect. Pta production by the hpmA mutant might therefore explain why no difference in pathogenicity is observed between the haemolysin mutant and the parent strain.
The genome of P. mirabilis str. HI4320 encodes three uncharacterized potential autotransporters that possess serine protease motifs and may also act as toxins (PMI0844, PMI2126 and PMI2341)20,67. This strain also encodes several other proteins that, on the basis of their similarity to known toxins, are themselves potential toxins, including PMI0004 and PMI2043 (which have homology to the cytotoxin RtxA), PMI1747 and PMI1748 (which together form a putative binary toxin, XaxAB), PMI0023 (a putative intimin–invasin), and PMI2028, PMI2029 and PMI2030 (which together form a putative type I secretion system)20. However, none of the genes encoding these proteins is significantly upregulated during an experimental UTI67.
P. mirabilis also encodes a type III secretion system (T3SS) for possible injection of effector proteins into target eukaryotic cells20. Although the P. mirabilis T3SS genes are expressed in liquid culture, mutation of P. mirabilis homologues of genes that are essential for T3SS function in other systems (spa47 and exsD) has no impact on the secreted protein profile of this bacterium or on its pathogenesis in a mouse model, indicating that the T3SS might not be a virulence determinant for P. mirabilis95. However, another putative T3SS gene, ipaD, is among the most highly upregulated genes during an experimental UTI, leaving it unclear exactly what this system contributes to virulence67.
Persistence and immune evasion
Once P. mirabilis gains access to the urinary tract, it has a remarkable ability to persist despite antibiotic treatment and catheter changes55. To persist within the host, bacteria must successfully evade innate and adaptive immune responses. One mechanism of immune evasion for many species is to vary the expression or composition of antigenic structures, such as outer-membrane proteins or fimbriae. In P. mirabilis, the MRP fimbriae are phase variable96. It is not yet known whether the 16 other types of fimbriae undergo phase variation, but any modulation of expression in this wide range of fimbriae would contribute to fitness. In addition, P. mirabilis str. HI4320 contains 13 putative orphan fimbrial genes that are not part of a complete operon and might contribute to further fimbrial diversity20,97. Flagellin is also thought to contribute to immune evasion via antigenic variation4,98,99.
P. mirabilis encodes a metalloproteinase, serralysin (ZapA), that cleaves serum and secretory immuno-globulin A1 (IgA1), IgA2 and IgG, thereby providing protection from the mucosal immune response100. ZapA might also cleave complement components C1q and C3, cell matrix components such as collagen, fibronectin and laminin, cytoskeletal proteins such as actin and tubulin, and certain antimicrobial peptides101. The importance of ZapA is underscored by the finding that mutation of zapA results in a dramatic decrease in the recovery of bacteria from the urine, bladder and kidneys102.
The formation of urinary stones by P. mirabilis generates a protective and nutrient-rich environment for the bacterium19. Stone formation and catheter encrustation together physically contribute to persistence by causing retention of urine, generating a reservoir of bacteria and preventing wash-out55,56. In addition, P. mirabilis bacteria have been visualized within urinary stones during a mouse UTI80. Urinary stones are thought to limit bacterial exposure to antibiotics and antibodies, and also, as a result of nutrient limitation, to possibly limit replication of the bacteria sequestered inside the stone, which would make these bacteria less sensitive to antimicrobials that target replication19,80.
P. mirabilis is also capable of invading urothelial cells to survive intracellularly89,90,103, probably representing another mechanism for immune evasion and persistence. Interestingly, one study reported that cell invasion provides protection against antibiotic treatment, although determining the exact contribution of invasion was complicated by the fact that P. mirabilis also caused the formation of crystals within the invaded cells, so these crystals might have been responsible for the protection103.
Multicellular interactions
In light of the finding that up to 77% of CAUTIs are polymicrobial and that P. mirabilis is generally the most common organism isolated from these infections13–18, it is important to identify ways in which P. mirabilis influences the pathogenicity of other organisms and vice versa. Although to date little work has focused on the interactions between uropathogens, several P. mirabilis virulence factors have the potential to enhance the pathogenicity of other species.
Swarming and interbacterial competition
P. mirabilis swarming represents a multicellular behaviour, and this unique type of motility facilitates the migration of non-motile species, such as K. pneumoniae and Staphylococcus aureus, on a catheter surface5,34. Therefore, swarming has the potential to influence polymicrobial UTIs by facilitating entry of other species into the urinary tract, although it is yet to be determined whether polymicrobial infection provides a direct benefit for P. mirabilis.
Another fascinating aspect of the interbacterial interactions during swarming is the Dienes phenomenon, in which two P. mirabilis swarming colonies of a single strain merge with each other, whereas swarms of different strains form a distinct boundary where they meet, known as a Dienes line104. Formation of the Dienes line requires direct cell–cell contact between living bacteria and is thought to involve killing of one strain at the boundary105. Interestingly, competitive killing is observed only during swarming, as strains that are sensitive to killing on swarm agar are not outcompeted in broth culture or on agar that is not swarming permissive105.
One explanation for the formation of the Dienes line involves the production of the bacteriocin proticine, which is capable of killing sensitive strains. Indeed, boundaries form between P. mirabilis strains that differ in proticine production and sensitivity106. However, some strains that are deficient in proticine production still form boundaries with other strains lacking proticine production, indicating that another mechanism mediates Dienes line formation107. In the search for this mechanism, a transposon mutant was identified that forms a boundary rather than merging with its parent strain, and the disrupted locus was named identification of self (Ids)107. Further work determined that idsABCDEF constitutes an operon, that idsD and idsE seem to encode strain-specific factors which are essential for self recognition, and that idsB, idsC and idsF encode factors which are essential for self recognition but can be complemented by ids genes from other strains107,108. As two swarm fronts merge, only a subset of cells in the advancing swarm expresses the ids genes and traverses the boundary of the other swarm, and this subset is sufficient to propagate the signal of self versus non-self108. Furthermore, ids expression decreases as an advancing swarm approaches another swarm of the same strain.
The ids system alone, however, does not fully explain the interstrain interactions that occur within the Dienes line, as certain mutations within this locus can result in boundary formation without any apparent competitive killing105. The ids locus encodes putative type VI secretion system (T6SS) effector proteins, so it was hypothesized that T6SS is involved in Dienes line formation107. In agreement with this hypothesis, our laboratory has identified additional loci encoding T6SS effector and structural proteins involved in Dienes line formation (C. J. Alteri and H.L.T.M., unpublished observations). The T6SS is thought to be involved in maintenance of a balanced relationship between the bacterium and the host as well as in mediating competitive interbacterial interactions, and the T6SS of Vibrio cholerae was recently shown to be involved in interspecies competition and killing109. The T6SS of P. mirabilis, along with proticine and the ids genes, may mediate a combination of inter-bacterial killing during swarming and Dienes line formation, competition on the catheter surface, and even possibly some form of interaction with the host during a UTI.
Urease, pH and nitrogen metabolism
Despite the fact that urease is produced by other common agents of CAUTIs, such as P. stuartii and M. morganii, P. mirabilis is the main species responsible for the formation of crystalline biofilms on catheters and the resulting blockage that causes urine retention, urine reflux and bacteriuria11,12. Therefore, P. mirabilis alone can substantially affect whether or not other species persist on the catheter and reach the urinary tract to cause infection. As the crystalline biofilms seem to provide protection for P. mirabilis19,80, these structures may provide similar protection to other species present during a polymicrobial infection, if these species can coexist within the biofilms.
The potent urease of P. mirabilis results in the urinary tract containing an abundance of ammonia that would not be present during a single-species infection by a urease-negative organism such as uropathogenic E. coli (UPEC), so other species might benefit from the generation of this preferred nitrogen source during a polymicrobial infection. For UPEC, this hypothesis is supported by the finding that expression of glnA, which is induced by nitrogen limitation, is upregulated fourfold in bacteria taken from the urinary tract110. Co-infection with P. mirabilis may alleviate the nitrogen-limited conditions for UPEC, thereby enhancing the growth or persistence of this urease-negative organism. Our laboratory has found that simultaneous co-infection with P. mirabilis and UPEC enhances colonization for both species, suggesting that these uropathogens readily coexist in the urinary tract (C. J. Alteri and H.L.T.M., unpublished observations). The reason for this enhanced colonization and the overall impact of this effect on pathology are active areas of research.
Conversely, the dramatic increase in pH as a result of urease activity might have a negative impact on other species. For instance, P. mirabilis outcompetes some urease-negative organisms (such as Enterobacter cloacae) and some less-potent urease-positive organisms (such as M. morganii, P. aeruginosa and K. pneumoniae) when co-cultured in a bladder model, even when P. mirabilis is introduced 72 hours after catheter colonization by the other organism12. This appears to be due, in part, to the urease activity of P. mirabilis, as the decrease in viability for these other species correlates with the rise in pH that occurs shortly after the introduction of P. mirabilis.
Horizontal gene transfer
The 94 kb ICEPm1 mobile pathogenicity island of P. mirabilis is present in several P. stuartii and M. morganii isolates, with up to 100% sequence identity for some genes, suggestive of DNA transfer between these species86. ICEPm1 is more prevalent in P. mirabilis clinical isolates taken from the urine of catheterized individuals than in clinical isolates taken from other body sites, suggesting that this mobile genetic element contributes to colonization and pathogenicity within the urinary tract. As CAUTIs are generally caused by self-inoculation with gut micro-biota9, the acquisition of ICEPm1 might be the reason why normally commensal microorganisms become able to colonize the urinary tract, or might explain why these commensals become pathogenic when they reach this site. Furthermore, it has been demonstrated that ICEPm1 can excise from the P. mirabilis chromosome and integrate into other ICEPm1-deficient P. mirabilis strains and at least one E. coli strain, as long as the integrase, chromosome-partitioning protein A (ParA) and type IV secretion system are intact111. These studies support the notion of horizontal gene transfer between potential uropathogens, a phenomenon that may also allow for transfer of antimicrobial resistance.
Cell–cell communication
N-acyl homoserine lactone (AHL) signalling molecules are utilized by several Gram-negative species to sense population density and coordinate gene expression112. P. mirabilis lacks a clear AHL synthase (LuxI) homologue and does not seem to produce this type of signalling molecule20,113. However, P. mirabilis encodes a LuxR family transcriptional regulator and seems to produce compounds with AHL-like activity; one study found that the addition of exogenous AHL to a P. mirabilis population has a strain-specific impact on virulence factor expression, swarming and biofilm formation20,114–117. AHLs secreted by other species on the catheter or during a polymicrobial UTI may therefore modulate P. mirabilis swarming or virulence. Fatty acids have also been proposed to influence swarming in a manner similar to AHLs, probably influencing swarm cell differentiation and flhDC expression, as well as biofilm formation, via the Rcs phosphorelay118.
The quorum sensing molecule autoinducer 2 (AI-2), encoded by luxS, can mediate both intra- and interspecies interactions. P. mirabilis possesses a luxS homologue and produces AI-2 (REF. 119). However, mutation of luxS in P. mirabilis str. BB2000 does not significantly affect swarming, virulence factor production, or survival in a mouse model, suggesting that AI-2 does not contribute to pathogenicity119. This lack of phenotype might indicate that P. mirabilis uses LuxS strictly as part of the activated methyl cycle, particularly as P. mirabilis str. HI4320 contains no clear homologue of the Lsr system for sensing and responding to AI-2. However, AI-2 produced by P. mirabilis might influence gene expression in other species that use this signalling molecule.
Putrescine has also been proposed as an extracellular signal that is capable of mediating cell–cell communication120. As putrescine is a component of the outer membrane for some P. mirabilis strains121, the signalling capabilities of this molecule remain unclear. If P. mirabilis utilizes putrescine for signalling, the bacteria may respond to putrescine produced by other species or scavenged from the host. Indeed, P. mirabilis upregulates a putrescine transporter during experimental infection67, although the importance of putrescine during UTIs is unknown.
Summary
Advances in our understanding of P. mirabilis pathogenicity have provided new insights into the regulation of swarm cell differentiation, the possible contribution of swarm cells during a UTI, unique aspects of P. mirabilis metabolism and iron acquisition, and previously unrecognized pathogenicity factors (summarized in FIG. 1 and TABLE 2). Despite these advances, many questions remain. There are many different fimbrial types encoded by P. mirabilis, and the binding specificity, role during infection and contribution to catheter colonization are unclear for many of these fimbriae. Investigations into P. mirabilis toxins will also be of interest, as only haemolysin and Pta have been well characterized to date, and there is ambiguity concerning the role of the T3SS. With respect to swarming, our knowledge of the regulatory network for sensing when conditions are favourable for swarming is incomplete, and the ability of P. mirabilis to coordinate swarming behaviour through cell–cell signalling remains an area of debate. What drives the rare event of swarm cell differentiation during a UTI and whether these isolated swarm cells contribute to infection are also unknown. Similarly, why P. mirabilis requires proticine, Ids factors and a T6SS to distinguish between strains and whether any of these systems are used for interspecies interactions are unclear.
Table 2.
Contribution of Proteus mirabilis virulence determinants to urinary tract infections
| Virulence determinants | Entry to the urinary tract
|
Ascending urinary tract infections
|
|||
|---|---|---|---|---|---|
| Catheter colonization | Swarming | Urine | Bladder | Kidneys | |
| Fimbriae | ATFs and/or MRK fimbriae? | MrpJ represses expression of fimbriae | MRP fimbriae, PMI3001 | MRP fimbriae and PMFs | MRK and MRP fimbriae |
|
| |||||
| Adhesins | ND | ND | ND | PMI2575 | AipA |
|
| |||||
| Motility | Not relevant | FlgE*, FliF*, FliL | CheW*, FlaD | CheW*, FliF*, FlaD | CheW*, FlgE*, FliF*, FlaD |
|
| |||||
| Regulators of gene expression | ND | DisA, HexA, Lrp, Rcs proteins, RppAB, RsmA, Umo proteins, WosA | AsnC*, HdfR*, HexA, NhaR*, UreR | AsnC*, HexA, HdfR*, UreR | AsnC*, HexA, NhaR*, UreR |
|
| |||||
| Urease | Promotes colonization | Coordinately expressed | UreF* | UreC, UreF* | UreC, UreF* |
|
| |||||
| Proteases and toxins | ND | Lon, HpmA, ZapA (coordinately expressed) | ZapA | Pta, ZapA | Pta, ZapA, U32 peptidase family protease*, HpmA? |
|
| |||||
| Metabolic pathways | ND | AceE*, CyaA*, SdhC* (upregulated) | CarA*,CbbC*, CyaA*, Edd*, GuaB*, SdaA*, SdhC* | AceE*, CarA*, CyaA*, GdhA, GuaB*, SdhC* | AceE*, CarA*, CbbC*, CyaA*, Edd*, GdhA, GuaB*, SdhC* |
|
| |||||
| Metal acquisition | ND | ZnuC | ZnuC | HmuR2, Nrp, Pbt, ZnuC, PMI0842 | HmuR2, ZnuC,PMI0842, PMI2596 |
|
| |||||
| Other | ND | CpsF*, CysJ, DppA, WaaL | DppA, DsbA*, ExbD*, HemY*, MetN*, MrcA*, NrpG*, ParE*, PpiA*, PstC*, PstS*, SerC*, SurA*, YidA*, PMI1000*, PMI1193*, PMI1448*, PMI3359*, PMI3705* | CpsF*, DppA, DsbA*, ExbD*, MrcA*, ParE*, PstC*, PstS*, SurA*, TaaP, PMI1193*, PMI2014* | CpsF*, CysJ, DppA, DsbA*, ExbD*, MetN*, MrcA*, NrpG*, ParE*, PpiA*, PstC*, PstS*, SufI*, SurA*, PMI0283*, PMI1184*, PMI1193*, PMI3705* |
ATFs, ambient-temperature fimbriae; GdhA, glutamate dehydrogenase; HpmA, haemolysin; HmuR2, haemin receptor; Lrp, leucine-responsive regulator; MRK, mannose-resistant Klebsiella-like; MRP, mannose-resistant Proteus-like; ND, no data; Pbt, proteobactin; PMFs, Proteus mirabilis fimbriae; Pta, Proteus toxic agglutinin; Rcs, regulator of colanic acid capsule synthesis; Umo, upregulator of flagellar master operon; Ure, urease operon; ZapA, serralysin; ZnuC, zinc uptake protein C.
Identified by signature-tagged mutagenesis.
The development of a vaccine for P. mirabilis UTIs is warranted owing to the increasing drug resistance among P. mirabilis isolates and the severe complications of infection. Vaccine development thus far has focused on using fimbrial subunits or the Pta autotransporter to reduce colonization and elicit a protective antibody response, but no vaccine strategy has yet provided complete protection against P. mirabilis UTIs in a mouse model92,122–126. The identification of novel virulence determinants and a better understanding of P. mirabilis pathogenicity will aid the design of effective vaccines. Furthermore, an understanding of the factors involved in catheter colonization, biofilm development and swarming will contribute to the development of therapeutics aimed at limiting the persistence of this troublesome species on catheters.
In light of the knowledge that many CAUTIs are poly-microbial, it is also important to consider how knowledge gained from single-species studies can be extended to polymicrobial infection. P. mirabilis clearly has the potential to facilitate entry of other species to the urinary tract and possibly to enhance their persistence, and it is therefore intriguing to speculate that therapeutics aimed at limiting P. mirabilis colonization may also impact the CAUTI burden caused by other species. Further investigation is necessary to determine how urinary tract pathogens interact during polymicrobial CAUTIs and to fully elucidate the impact of P. mirabilis on other uropathogens, as well as its contribution to the persistence and severity of polymicrobial CAUTIs.
Acknowledgments
Work in the H.L.T.M. laboratory is supported by the US National Institutes of Health (grants AI43363, AI59722 and DK94777). C.E.A. is supported by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (grant F32AI102552).
Glossary
- Pyelonephritis
An infection of the kidney parenchyma
- Urolithiasis
The formation of calculi (stones) in the kidney, bladder or urethra
- Prostatitis
Inflammation of the prostate gland
- Bacteriuria
The presence of bacteria in the urine
- Signature-tagged mutagenesis
A high-throughput method for generating pools of bacterial strains that contain transposon insertions to disrupt genes: in this method each transposon has a unique tag
- Nucleoid-associated protein
A protein that is associated with the region of a bacterial cell containing genetic material (the nucleoid) and that contributes to maintaining the supercoiled structure of the nucleic acid
- Autotransporter
A bacterial outer-membrane protein that is produced as a single polypeptide, consisting of a passenger domain which is transported through the outer membrane and a β-barrel domain that anchors the protein to the outer membrane and facilitates transport of the passenger domain
- Bowman’s capsule
A cup-shaped double membrane in the kidney that filters blood to remove organic waste, excess inorganic salts and water, which are then concentrated into urine
- O antigen
The bacterial cell wall antigen of lipopolysaccharide. O antigen is composed of repeating oligosaccharide subunits made up of 3–5 sugars
- Phase variation
Reversible alteration in the expression of antigenic proteins on the cell surface (generally ON versus OFF) to produce a clonal population of phenotypically heterogeneous bacteria
- GS–GOGAT system
A metabolic pathway that uses glutamine synthetase (GS), glutamine oxoglutarate aminotransferase (GOGAT) and ATP to assimilate ammonia into glutamate under nitrogen limitation. By contrast, when ammonia is abundant, this process is carried out by glutamate dehydrogenase in a one-step reaction that does not require ATP. The GS–GOGAT system is also known as the glutamate synthase cycle
- Siderophores
Low-molecular-mass organic compounds with a high affinity for chelating or binding iron. These compounds are produced by microorganisms to scavenge ferric iron
- Integrative and conjugative element
A self-transmissible mobile genetic element that resides within the chromosome of a host cell and can excise and transfer to a new host via conjugation
- Yersiniabactin
A siderophore produced by Yersinia spp. and many other bacterial species
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Hauser G. Über Fäulnissbacterien und deren Beziehungen zur Septicämie; ein Beitrag zur Morphologie der Spaltpilze. Vogel; 1885. [Google Scholar]
- 2.Hoeniger JFM. Development of flagella by Proteus mirabilis. J Gen Microbiol. 1965;40:29–42. [Google Scholar]
- 3.Wenner JJ, Rettger LFA. Systematic Study of the Proteus group of bacteria. J Bacteriol. 1919;4:331–353. doi: 10.1128/jb.4.4.331-353.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627–639. doi: 10.2165/00002512-200522080-00001. [DOI] [PubMed] [Google Scholar]
- 6.O’Hara CM, Brenner FW, Miller JM. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev. 2000;13:534–546. doi: 10.1128/cmr.13.4.534-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooton TM, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 8.Morris NS, Stickler DJ, McLean RJ. The development of bacterial biofilms on indwelling urethral catheters. World J Urol. 1999;17:345–350. doi: 10.1007/s003450050159. [DOI] [PubMed] [Google Scholar]
- 9.Mathur S, Sabbuba NA, Suller MT, Stickler DJ, Feneley RC. Genotyping of urinary and fecal Proteus mirabilis isolates from individuals with long-term urinary catheters. Eur J Clin Microbiol Infect Dis. 2005;24:643–644. doi: 10.1007/s10096-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 10.Wazait HD, et al. Catheter-associated urinary tract infections: prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital (1996–2001) BJU Int. 2003;91:806–809. doi: 10.1046/j.1464-410x.2003.04239.x. [DOI] [PubMed] [Google Scholar]
- 11.Stickler DJ, Feneley RC. The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord. 2010;48:784–790. doi: 10.1038/sc.2010.32. [DOI] [PubMed] [Google Scholar]
- 12.Macleod SM, Stickler DJ. Species interactions in mixed-community crystalline biofilms on urinary catheters. J Med Microbiol. 2007;56:1549–1557. doi: 10.1099/jmm.0.47395-0. [DOI] [PubMed] [Google Scholar]
- 13.Breitenbucher RB. Bacterial changes in the urine samples of patients with long-term indwelling catheters. Arch Intern Med. 1984;144:1585–1588. [PubMed] [Google Scholar]
- 14.Rahav G, Pinco E, Silbaq F, Bercovier H. Molecular epidemiology of catheter-associated bacteriuria in nursing home patients. J Clin Microbiol. 1994;32:1031–1034. doi: 10.1128/jcm.32.4.1031-1034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegman-Igra Y, Kulka T, Schwartz D, Konforti N. Polymicrobial and monomicrobial bacteraemic urinary tract infection. J Hospital Infect. 1994;28:49–56. doi: 10.1016/0195-6701(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 16.Dedeic-Ljubovic A, Hukic M. Catheter-related urinary tract infection in patients suffering from spinal cord injuries. Bosn J Basic Med Sci. 2009;9:2–9. doi: 10.17305/bjbms.2009.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon. 2003;49:71–82. doi: 10.1067/mda.2003.8. [DOI] [PubMed] [Google Scholar]
- 18.Kunin CM. Blockage of urinary catheters: role of microorganisms and constituents of the urine on formation of encrustations. J Clin Epidemiol. 1989;42:835–842. doi: 10.1016/0895-4356(89)90096-6. [DOI] [PubMed] [Google Scholar]
- 19.Coker C, Poore CA, Li X, Mobley HLT. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000;2:1497–1505. doi: 10.1016/s1286-4579(00)01304-6. [DOI] [PubMed] [Google Scholar]
- 20.Pearson MM, et al. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J Bacteriol. 2008;190:4027–4037. doi: 10.1128/JB.01981-07. This article describes many unusual and unique features of the genome of a P. mirabilis CAUTI isolate, including a vast number of fimbrial operons and a large portion of the genome devoted to motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himpsl SD, Lockatell CV, Hebel JR, Johnson DE, Mobley HLT. Identification of virulence determinants in uropathogenic Proteus mirabilis using signature-tagged mutagenesis. J Med Microbiol. 2008;57:1068–1078. doi: 10.1099/jmm.0.2008/002071-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Li X, Johnson DE, Mobley HLT. Identification of protease and rpoN-associated genes of uropathogenic Proteus mirabilis by negative selection in a mouse model of ascending urinary tract infection. Microbiology. 1999;145:185–195. doi: 10.1099/13500872-145-1-185. [DOI] [PubMed] [Google Scholar]
- 23.Burall LS, et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun. 2004;72:2922–2938. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams GJ, Stickler DJ. Some observations on the migration of Proteus mirabilis and other urinary tract pathogens over foley catheters. Infect Control Hosp Epidemiol. 2008;29:443–445. doi: 10.1086/529551. [DOI] [PubMed] [Google Scholar]
- 25.Stickler D, Ganderton L, King J, Nettleton J, Winters C. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res. 1993;21:407–411. doi: 10.1007/BF00300077. [DOI] [PubMed] [Google Scholar]
- 26.Morris NS, Stickler DJ, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58–63. doi: 10.1046/j.1464-410x.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 27.Stickler DJ, et al. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J Appl Microbiol. 2006;100:1028–1033. doi: 10.1111/j.1365-2672.2006.02840.x. [DOI] [PubMed] [Google Scholar]
- 28.Morris NS, Stickler DJ. Encrustation of indwelling urethral catheters by Proteus mirabilis biofilms growing in human urine. J Hosp Infect. 1998;39:227–234. doi: 10.1016/s0195-6701(98)90262-6. [DOI] [PubMed] [Google Scholar]
- 29.Rocha SPD, Pelayo JS, Elias WP. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol Med Microbiol. 2007;51:1–7. doi: 10.1111/j.1574-695X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 30.Mobley HL, et al. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J Infect Dis. 1988;157:264–271. doi: 10.1093/infdis/157.2.264. [DOI] [PubMed] [Google Scholar]
- 31.Yakubu DE, Old DC, Senior BW. The haemagglutinins and fimbriae of Proteus penneri. J Med Microbiol. 1989;30:279–284. doi: 10.1099/00222615-30-4-279. [DOI] [PubMed] [Google Scholar]
- 32.Massad G, Bahrani FK, Mobley HL. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect Immun. 1994;62:1989–1994. doi: 10.1128/iai.62.5.1989-1994.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zunino P, Geymonat L, Allen AG, Legnani-Fajardo C, Maskell DJ. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol Med Microbiol. 2000;29:137–143. doi: 10.1111/j.1574-695X.2000.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabbuba N, Hughes G, Stickler DJ. The migration of Proteus mirabilis and other urinary tract pathogens over Foley catheters. BJU Int. 2002;89:55–60. doi: 10.1046/j.1464-4096.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- 35.Stickler D, Hughes G. Ability of Proteus mirabilis to swarm over urethral catheters. Eur J Clin Microbiol Infect Dis. 1999;18:206–208. doi: 10.1007/s100960050260. [DOI] [PubMed] [Google Scholar]
- 36.Jones BV, Young R, Mahenthiralingam E, Stickler DJ. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect Immun. 2004;72:3941–3950. doi: 10.1128/IAI.72.7.3941-3950.2004. This report reveals the unique structure of swarm cell rafts and the helical connections formed by interwoven flagellar filaments between neighbouring swarm cells. It also determines the role of these structures in allowing P. mirabilis to migrate across catheter materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SM, Yerly J, Hu Y, Ceri H, Martinuzzi R. Structure of Proteus mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiol Lett. 2007;268:16–21. doi: 10.1111/j.1574-6968.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 38.Belas R, Suvanasuthi R. The ability of Proteus mirabilis to sense surfaces and regulate virulence gene expression involves FliL, a flagellar basal body protein. J Bacteriol. 2005;187:6789–6803. doi: 10.1128/JB.187.19.6789-6803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rather PN. Swarmer cell differentiation in Proteus mirabilis. Environ Microbiol. 2005;7:1065–1073. doi: 10.1111/j.1462-2920.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstein RM, Szostek B, Rather PN. Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis. FEMS Microbiol Rev. 2010;34:753–763. doi: 10.1111/j.1574-6976.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- 41.Calvo JM, Matthews RG. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart BR, Blumenthal RM. Unexpected coregulator range for the global regulator Lrp of Escherichia coli and Proteus mirabilis. J Bacteriol. 2011;193:1054–1064. doi: 10.1128/JB.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allison C, Lai HC, Gygi D, Hughes C. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol Microbiol. 1993;8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 44.Tan IK, Gajra B. Plasma and urine amino acid profiles in a healthy adult population of Singapore. Ann Acad Med Singap. 2006;35:468–475. [PubMed] [Google Scholar]
- 45.Thomas VJ, Collins CM. Identification of UreR binding sites in the Enterobacteriaceae plasmid-encoded and Proteus mirabilis urease gene operons. Mol Microbiol. 1999;31:1417–1428. doi: 10.1046/j.1365-2958.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 46.Coker C, Bakare OO, Mobley HL. H-NS is a repressor of the Proteus mirabilis urease transcriptional activator gene ureR. J Bacteriol. 2000;182:2649–2653. doi: 10.1128/jb.182.9.2649-2653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poore CA, Mobley HLT. Differential regulation of the Proteus mirabilis urease gene cluster by UreR and H-NS. Microbiology. 2003;149:3383–3394. doi: 10.1099/mic.0.26624-0. [DOI] [PubMed] [Google Scholar]
- 48.Jones BD, Mobley HL. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989;171:6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Orazio SE, Thomas V, Collins CM. Activation of transcription at divergent urea-dependent promoters by the urease gene regulator UreR. Mol Microbiol. 1996;21:643–655. doi: 10.1111/j.1365-2958.1996.tb02572.x. [DOI] [PubMed] [Google Scholar]
- 50.D’Orazio SE, Collins CM. The plasmid-encoded urease gene cluster of the family Enterobacteriaceae is positively regulated by UreR, a member of the AraC family of transcriptional activators. J Bacteriol. 1993;175:3459–3467. doi: 10.1128/jb.175.11.3459-3467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Island MD, Mobley HL. Proteus mirabilis urease: operon fusion and linker insertion analysis of ure gene organization, regulation, and function. J Bacteriol. 1995;177:5653–5660. doi: 10.1128/jb.177.19.5653-5660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholson EB, Concaugh EA, Foxall PA, Island MD, Mobley HL. Proteus mirabilis urease: transcriptional regulation by UreR. J Bacteriol. 1993;175:465–473. doi: 10.1128/jb.175.2.465-473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffith DP, Musher DM, Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 54.Nicholson EB, Concaugh EA, Mobley HL. Proteus mirabilis urease: use of a ureA-lacZ fusion demonstrates that induction is highly specific for urea. Infect Immun. 1991;59:3360–3365. doi: 10.1128/iai.59.10.3360-3365.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabbuba NA, Mahenthiralingam E, Stickler DJ. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol. 2003;41:4961–4965. doi: 10.1128/JCM.41.11.4961-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kunin CM. Urinary Tract Infections: Detection, Prevention, and Management. Williams & Wilkins; 1997. [Google Scholar]
- 57.Zhao H, Thompson RB, Lockatell V, Johnson DE, Mobley HLT. Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection. Infect Immun. 1998;66:330–335. doi: 10.1128/iai.66.1.330-335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson DE, et al. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect Immun. 1993;61:2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. This particular study clearly shows the impact of P. mirabilis urease activity on UTI severity and persistence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones BD, Lockatell CV, Johnson DE, Warren JW, Mobley HL. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990;58:1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dattelbaum JD, Lockatell CV, Johnson DE, Mobley HLT. UreR, the transcriptional activator of the Proteus mirabilis urease gene cluster, is required for urease activity and virulence in experimental urinary tract infections. Infect Immun. 2003;71:1026–1030. doi: 10.1128/IAI.71.2.1026-1030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alamuri P, et al. Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect Immun. 2010;78:4882–4894. doi: 10.1128/IAI.00718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zunino P, et al. Mannose-resistant proteus-like and P. mirabilis fimbriae have specific and additive roles in P. mirabilis urinary tract infections. FEMS Immunol Med Microbiol. 2007;51:125–133. doi: 10.1111/j.1574-695X.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 63.Sareneva T, Holthofer H, Korhonen TK. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990;58:3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee KK, Harrison BA, Latta R, Altman E. The binding of Proteus mirabilis nonagglutinating fimbriae to ganglio-series asialoglycolipids and lactosyl ceramide. Can J Microbiol. 2000;46:961–966. [PubMed] [Google Scholar]
- 65.Massad G, Lockatell CV, Johnson DE, Mobley HL. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect Immun. 1994;62:536–542. doi: 10.1128/iai.62.2.536-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zunino P, et al. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology. 2003;149:3231–3237. doi: 10.1099/mic.0.26534-0. [DOI] [PubMed] [Google Scholar]
- 67.Pearson MM, Yep A, Smith SN, Mobley HLT. Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect Immun. 2011;79:2619–2631. doi: 10.1128/IAI.05152-11. In addition to shedding new light on gene expression during infection, this work highlights the unique metabolic requirements of P. mirabilis during a UTI and the central role of nitrogen assimilation in pathogenicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zunino P, et al. New aspects of the role of MR/P fimbriae in Proteus mirabilis urinary tract infection. FEMS Immunol Med Microbiol. 2001;31:113–120. doi: 10.1111/j.1574-695X.2001.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 69.Bahrani FK, et al. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao H, Li X, Johnson DE, Blomfield I, Mobley HL. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Lockatell CV, Johnson DE, Mobley HLT. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol Microbiol. 2002;45:865–874. doi: 10.1046/j.1365-2958.2002.03067.x. [DOI] [PubMed] [Google Scholar]
- 72.Jansen AM, Lockatell V, Johnson DE, Mobley HLT. Mannose-resistant proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect Immun. 2004;72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latta RK, Grondin A, Jarrell HC, Nicholls GR, Berube LR. Differential expression of nonagglutinating fimbriae and MR/P Pili in swarming colonies of Proteus mirabilis. J Bacteriol. 1999;181:3220–3225. doi: 10.1128/jb.181.10.3220-3225.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mobley H, et al. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Rasko DA, Lockatell CV, Johnson DE, Mobley HLT. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 2001;20:4854–4862. doi: 10.1093/emboj/20.17.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearson MM, Mobley HLT. Repression of motility during fimbrial expression: identification of 14 mrpJ gene paralogues in Proteus mirabilis. Mol Microbiol. 2008;69:548–558. doi: 10.1111/j.1365-2958.2008.06307.x. This article bridges studies that revealed the unusually large number of distinct fimbrial types in P. mirabilis str. HI4320, and the link between reciprocal regulation of MRP fimbriae and expression of flagella. This article also describes the mechanism underlying repression of motility by MrpJ and its homologues, thus highlighting the exquisite dedication to reciprocal control of adherence and motility in P. mirabilis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane MC, Li X, Pearson MM, Simms AN, Mobley HLT. Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J Bacteriol. 2009;191:1382–1392. doi: 10.1128/JB.01550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jansen AM, Lockatell CV, Johnson DE, Mobley HLT. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect Immun. 2003;71:3607–3613. doi: 10.1128/IAI.71.6.3607-3613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allison C, Emödy L, Coleman N, Hughes C. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J Infect Dis. 1994;169:1155–1158. doi: 10.1093/infdis/169.5.1155. [DOI] [PubMed] [Google Scholar]
- 80.Li X, et al. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect Immun. 2002;70:389–394. doi: 10.1128/IAI.70.1.389-394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miles AA, Khimji PL. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975;8:477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- 82.Lima A, Zunino P, D’Alessandro B, Piccini C. An iron-regulated outer-membrane protein of Proteus mirabilis is a haem receptor that plays an important role in urinary tract infection and in in vivo growth. J Med Microbiol. 2007;56:1600–1607. doi: 10.1099/jmm.0.47320-0. [DOI] [PubMed] [Google Scholar]
- 83.Nielubowicz GR, Smith SN, Mobley HLT. Outer membrane antigens of the uropathogen Proteus mirabilis recognized by the humoral response during experimental murine urinary tract infection. Infect Immun. 2008;76:4222–4231. doi: 10.1128/IAI.00533-08. This is the first investigation to apply immunoproteomics to the study of P. mirabilis pathogenicity, identifying 37 antigens that are recognized by the humoral response and thus generating several leads for vaccine candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Himpsl SD, et al. Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis. Mol Microbiol. 2010;78:138–157. doi: 10.1111/j.1365-2958.2010.07317.x. This is the first study to identify siderophores produced by P. mirabilis, including a novel siderophore named proteobactin, and to describe the importance of siderophore production by P. mirabilis in the establishment of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaisser S, Hughes C. A locus coding for putative non-ribosomal peptide/polyketide synthase functions is mutated in a swarming-defective Proteus mirabilis strain. Mol Gen Genet. 1997;253:415–427. doi: 10.1007/s004380050339. [DOI] [PubMed] [Google Scholar]
- 86.Flannery EL, Mody L, Mobley HLT. Identification of a modular pathogenicity island that is widespread among urease-producing uropathogens and shares features with a diverse group of mobile elements. Infect Immun. 2009;77:4887–4894. doi: 10.1128/IAI.00705-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielubowicz GR, Smith SN, Mobley HLT. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect Immun. 2010;78:2823–2833. doi: 10.1128/IAI.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 89.Peerbooms PG, Verweij AM, MacLaren DM. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984;43:1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chippendale GR, Warren JW, Trifillis AL, Mobley HL. Internalization of Proteus mirabilis by human renal epithelial cells. Infect Immun. 1994;62:3115–3121. doi: 10.1128/iai.62.8.3115-3121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mobley HL, Chippendale GR, Swihart KG, Welch RA. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991;59:2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alamuri P, Eaton KA, Himpsl SD, Smith SN, Mobley HLT. Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect Immun. 2009;77:632–641. doi: 10.1128/IAI.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swihart KG, Welch RA. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990;58:1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alamuri P, Mobley HLT. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol Microbiol. 2008;68:997–1017. doi: 10.1111/j.1365-2958.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 95.Pearson MM, Mobley HLT. The type III secretion system of Proteus mirabilis HI4320 does not contribute to virulence in the mouse model of ascending urinary tract infection. J Med Microbiol. 2007;56:1277–1283. doi: 10.1099/jmm.0.47314-0. [DOI] [PubMed] [Google Scholar]
- 96.Bahrani FK, Mobley HL. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994;176:3412–3419. doi: 10.1128/jb.176.11.3412-3419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klemm P, Jorgensen BJ, Kreft B, Christiansen G. The export systems of type 1 and F1C fimbriae are interchangeable but work in parental pairs. J Bacteriol. 1995;177:621–627. doi: 10.1128/jb.177.3.621-627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belas R. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J Bacteriol. 1994;176:7169–7181. doi: 10.1128/jb.176.23.7169-7181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murphy CA, Belas R. Genomic rearrangements in the flagellin genes of Proteus mirabilis. Mol Microbiol. 1999;31:679–690. doi: 10.1046/j.1365-2958.1999.01209.x. [DOI] [PubMed] [Google Scholar]
- 100.Senior BW, Loomes LM, Kerr MA. The production and activity in vivo of Proteus mirabilis IgA protease in infections of the urinary tract. J Med Microbiol. 1991;35:203–207. doi: 10.1099/00222615-35-4-203. [DOI] [PubMed] [Google Scholar]
- 101.Belas R, Manos J, Suvanasuthi R. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect Immun. 2004;72:5159–5167. doi: 10.1128/IAI.72.9.5159-5167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker KE, Moghaddame-Jafari S, Lockatell CV, Johnson D, Belas R. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol Microbiol. 1999;32:825–836. doi: 10.1046/j.1365-2958.1999.01401.x. [DOI] [PubMed] [Google Scholar]
- 103.Mathoera RB, Kok DJ, Verduin CM, Nijman RJM. Pathological and therapeutic significance of cellular invasion by Proteus mirabilis in an enterocystoplasty infection stone model. Infect Immun. 2002;70:7022–7032. doi: 10.1128/IAI.70.12.7022-7032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dienes L. Reproductive processes in Proteus cultures. Proc Soc Exp Biol Med. 1946;63:265–270. doi: 10.3181/00379727-63-15570. [DOI] [PubMed] [Google Scholar]
- 105.Budding AE, Ingham CJ, Bitter W, Vandenbroucke-Grauls CM, Schneeberger PM. The dienes phenomenon: competition and territoriality in swarming Proteus mirabilis. J Bacteriol. 2009;191:3892–3900. doi: 10.1128/JB.00975-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Senior BW. Typing of Proteus strains by proticine production and sensitivity. J Med Microbiol. 1977;10:7–17. doi: 10.1099/00222615-10-1-7. [DOI] [PubMed] [Google Scholar]
- 107.Gibbs KA, Urbanowski ML, Greenberg EP. Genetic determinants of self identity and social recognition in bacteria. Science. 2008;321:256–259. doi: 10.1126/science.1160033. This investigation identifies a six-gene locus that is required for recognition of a parental P. mirabilis strain as self and which may be part of a T6SS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gibbs KA, Wenren LM, Greenberg EP. Identity gene expression in Proteus mirabilis. J Bacteriol. 2011;193:3286–3292. doi: 10.1128/JB.01167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci USA. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Snyder JA, et al. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72:6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flannery EL, Antczak SM, Mobley HLT. Self-transmissibility of the integrative and conjugative element ICEPm1 between clinical isolates requires a functional integrase, relaxase, and type IV secretion system. J Bacteriol. 2011;193:4104–4112. doi: 10.1128/JB.05119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boyer M, Wisniewski-Dye F. Cell–cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol Ecol. 2009;70:1–19. doi: 10.1111/j.1574-6941.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 113.Belas R, Schneider R, Melch M. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J Bacteriol. 1998;180:6126–6139. doi: 10.1128/jb.180.23.6126-6139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bainton NJ, et al. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- 115.Holden MTG, et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol Microbiol. 1999;33:1254–1266. doi: 10.1046/j.1365-2958.1999.01577.x. [DOI] [PubMed] [Google Scholar]
- 116.Stankowska D, et al. Influence of quorum sensing signal molecules on biofilm formation in Proteus mirabilis O18. Folia Microbiol (Praha) 2012;57:53–60. doi: 10.1007/s12223-011-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stankowska D, Kwinkowski M, Kaca W. Quantification of Proteus mirabilis virulence factors and modulation by acylated homoserine lactones. J Microbiol Immunol Infect. 2008;41:243–253. [PubMed] [Google Scholar]
- 118.Liaw SJ, Lai HC, Wang WB. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect Immun. 2004;72:6836–6845. doi: 10.1128/IAI.72.12.6836-6845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schneider R, Lockatell CV, Johnson D, Belas R. Detection and mutation of a luxS-encoded autoinducer in Proteus mirabilis. Microbiology. 2002;148:773–782. doi: 10.1099/00221287-148-3-773. [DOI] [PubMed] [Google Scholar]
- 120.Sturgill G, Rather PN. Evidence that putrescine acts as an extracellular signal required for swarming in Proteus mirabilis. Mol Microbiol. 2004;51:437–446. doi: 10.1046/j.1365-2958.2003.03835.x. [DOI] [PubMed] [Google Scholar]
- 121.Vinogradov E, Perry MB. Structural analysis of the core region of lipopolysaccharides from Proteus mirabilis serotypes O6, O48 and O57. Eur J Biochem. 2000;267:2439–2447. doi: 10.1046/j.1432-1327.2000.01262.x. [DOI] [PubMed] [Google Scholar]
- 122.Scavone P, Sosa V, Pellegrino R, Galvalisi U, Zunino P. Mucosal vaccination of mice with recombinant Proteus mirabilis structural fimbrial proteins. Microbes Infect. 2004;6:853–860. doi: 10.1016/j.micinf.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 123.Scavone P, et al. Intranasal immunisation with recombinant Lactococcus lactis displaying either anchored or secreted forms of Proteus mirabilis MrpA fimbrial protein confers specific immune response and induces a significant reduction of kidney bacterial colonisation in mice. Microbes Infect. 2007;9:821–828. doi: 10.1016/j.micinf.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 124.Pellegrino R, Galvalisi U, Scavone P, Sosa V, Zunino P. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol Med Microbiol. 2003;36:103–110. doi: 10.1016/S0928-8244(03)00103-2. [DOI] [PubMed] [Google Scholar]
- 125.Li X, et al. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect Immun. 2004;72:7306–7310. doi: 10.1128/IAI.72.12.7306-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X, et al. Development of an intranasal vaccine to prevent. urinary tract infection by Proteus mirabilis. Infect Immun. 2004;72:66–75. doi: 10.1128/IAI.72.1.66-75.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Armitage JP. Changes in metabolic activity of Proteus mirabilis during swarming. J Gen Microbiol. 1981;125:445–450. doi: 10.1099/00221287-125-2-445. [DOI] [PubMed] [Google Scholar]
- 128.Pearson MM, Rasko DA, Smith SN, Mobley HLT. Transcriptome of swarming Proteus mirabilis. Infect Immun. 2010;78:2834–2845. doi: 10.1128/IAI.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Majdalani N, Heck M, Stout V, Gottesman S. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J Bacteriol. 2005;187:6770–6778. doi: 10.1128/JB.187.19.6770-6778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clemmer KM, Rather PN. Regulation of flhDC expression in Proteus mirabilis. Res Microbiol. 2007;158:295–302. doi: 10.1016/j.resmic.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 131.Cusick K, Lee YY, Youchak B, Belas R. Perturbation of FliL interferes with Proteus mirabilis swarmer cell gene expression and differentiation. J Bacteriol. 2011;194:437–447. doi: 10.1128/JB.05998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morgenstein RM, Rather PN. Role of the Umo proteins and the Rcs phosphorelay in the swarming motility of wild-type and an O-antigen (waaL) mutant of Proteus mirabilis. J Bacteriol. 2012;194:669–676. doi: 10.1128/JB.06047-11. The work presented in this article shows how several pathways involved in swarm cell differentiation and swarming motility converge to control this unique type of motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hatt JK, Rather PN. Characterization of a novel gene, wosA, regulating FlhDC expression in Proteus mirabilis. J Bacteriol. 2008;190:1946–1955. doi: 10.1128/JB.01010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dufour A, Furness RB, Hughes C. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol Microbiol. 1998;29:741–751. doi: 10.1046/j.1365-2958.1998.00967.x. [DOI] [PubMed] [Google Scholar]
- 135.Clemmer KM, Rather PN. The Lon protease regulates swarming motility and virulence gene expression in Proteus mirabilis. J Med Microbiol. 2008;57:931–937. doi: 10.1099/jmm.0.47778-0. [DOI] [PubMed] [Google Scholar]
- 136.Jiang SS, et al. Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob Agents Chemother. 2010;54:2000–2009. doi: 10.1128/AAC.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang WB, et al. Role of RppA in the regulation of polymyxin B susceptibility, swarming, and virulence factor expression in Proteus mirabilis. Infect Immun. 2008;76:2051–2062. doi: 10.1128/IAI.01557-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stevenson LG, Rather PNA. Novel gene involved in regulating the flagellar gene cascade in Proteus mirabilis. J Bacteriol. 2006;188:7830–7839. doi: 10.1128/JB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J Med Microbiol. 2003;52:19–28. doi: 10.1099/jmm.0.05024-0. [DOI] [PubMed] [Google Scholar]