Abstract
Parkinson disease (PD) is a debilitating neurodegenerative motor disorder, with its motor symptoms largely attributable to loss of dopaminergic neurons in the substantia nigra. The causes of PD remain poorly understood, although environmental toxicants may play etiologic roles. Solvents are widespread neurotoxicants present in the workplace and ambient environment. Case reports of parkinsonism, including PD, have been associated with exposures to various solvents, most notably trichloroethylene (TCE). Animal toxicology studies have been conducted on various organic solvents, with some, including TCE, demonstrating potential for inducing nigral system damage. However, a confirmed animal model of solvent-induced PD has not been developed. Numerous epidemiologic studies have investigated potential links between solvents and PD, yielding mostly null or weak associations. An exception is a recent study of twins indicating possible etiologic relations with TCE and other chlorinated solvents, although findings were based on small numbers, and dose–response gradients were not observed. At present, there is no consistent evidence from either the toxicological or epidemiologic perspective that any specific solvent or class of solvents is a cause of PD. Future toxicological research that addresses mechanisms of nigral damage from TCE and its metabolites, with exposure routes and doses relevant to human exposures, is recommended. Improvements in epidemiologic research, especially with regard to quantitative characterization of long-term exposures to specific solvents, are needed to advance scientific knowledge on this topic.
Keywords: Parkinson's disease, Parkinsonism, Solvents, Trichloroethylene, n-Hexane, Toluene, Neurotoxicology, Epidemiology
Introduction
Parkinson's disease (PD) is a debilitating degenerative disorder that affects up to 2% of persons over age 65 (Wright Willis et al., 2010). PD is a disease involving multiple systems and brain regions; but most clinically appreciable features are related to motor symptoms, including rest tremor, bradykinesia, postural instability, and gait disturbance (Postuma et al., 2012; Samii et al., 2004). PD is the predominant form of parkinsonism, which also includes motor disorders secondary to stroke affecting basal ganglia, medications, and a few toxicants, e.g. solvents and carbon monoxide poisoning. The underlying cause of motor symptoms associated with PD is dopamine (DA) deficiency due to loss of dopaminergic neurons, primarily in the substantia nigra pars compacta (SNpc). Proteinaceous inclusions, known as Lewy bodies and neurites, in surviving neurons are the pathologic hallmarks of PD (Agid, 1991). Pathogenesis mechanisms involve the interplay between oxidative stress, inflammation, mitochondrial damage, abnormal protein aggregation and deficient removal, especially of α-synuclein, a major component of Lewy bodies (Betarbet et al., 2005; Cannon and Greenamyre, 2011). These reactions, which may be due to endogenous (e.g., dopamine metabolites) or exogenous toxicants, lead to compromised mitochondrial function, diminished energy output, and accelerated cell death by necrosis and or apoptosis (Cannon and Greenamyre, 2011; Dawson and Dawson, 2003).
Although some causal genetic loci have been identified, Mendelian inheritance accounts for a small fraction (~5–10%) of PD (Martin et al., 2011). Consequently, environmental factors, including workplace and community toxicants, diet, medications, and lifestyle factors, such as tobacco use, and gene/environment interactions are generally thought to account for the majority of cases, either increasing or decreasing risk. Cigarette smoking has consistently been found to be associated with reduced risk (Ritz et al., 2007), and seemingly protective effects, although not as consistently observed, have been related to caffeine intake and non-steroidal anti-inflammatory medications (Wirdefeldt et al., 2011).
Identification of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a street drug contaminant, as a cause of irreversible human parkinsonism (Langston et al., 1983), and its experimental verification in various species (Langston et al., 1987), triggered a substantial body of research into potential environmental risk factors. Pesticides have received most attention in this regard. The focus on pesticides was initially prompted by recognition that the active metabolite of MPTP (MPP+) is structurally similar to the herbicide paraquat. Epidemiological findings are suggestive of associations with insecticides and herbicides, although consistent evidence implicating any specific pesticides is lacking (Berry et al., 2010; van der Mark et al., 2012). Metals, especially manganese, and industrial solvents have also been the focus of experimental and human epidemiological research (Cannon and Greenamyre, 2011; Caudle et al., 2012). Toxicological and epidemiologic support for an etiologic role for manganese in PD is mixed and controversial (Mortimer et al., 2012), the clinical features of manganism and PD have considerable overlap, whereas deposition of manganese as well as associated brain damage occurs preferentially in brain regions other than the SNpc, at least in the cases exposed to high concentrations of manganese (Santamaria et al., 2007).
Although long considered as possible PD risk factors, solvents have received somewhat less attention than pesticides or metals despite widespread solvent use in many workplaces (Caudle et al., 2012). Solvents represent a class of industrial chemicals with many commercial applications, including metal degreasing, dry cleaning, and as ingredients of paint thinners and detergents. Solvents are classified by their chemical properties, organic or inorganic, and by the chemical composition, such as chlorine substitution. Exposure can occur from inhalation of vapors, dermal uptake, or ingestion, depending on exposure source and chemical composition. Exposure sources are multiple and variable in concentration. Occupational exposures are common in dry cleaning, metal degreasing, and paint stripping occupations. Contamination of drinking water supplies, such as from Superfund waste sites, is a potential exposure source to the general population. Air emissions from industrial facilities can also create widespread community exposures. Many solvents are lipophilic, and can thus enter central and peripheral nervous system tissue. Some solvents have well-established neurotoxic effects, including dizziness, loss of consciousness, behavioral abnormalities, and peripheral neuropathy (Bale et al., 2011; Spencer et al., 1980). As we will discuss in detail, it has been suggested that certain solvents have potential to produce motor system abnormalities, including parkinsonism that is similar to idiopathic PD.
In this paper, we will review evidence from toxicological and human studies regarding possible roles of solvents, as a class, and where data permit, as individual chemicals, in causing PD and related parkinsonian disorders. The review will begin with a brief summary of clinical case reports which prompted much of the concern about solvents in this context, followed, respectively, by systematic reviews of toxicological and epidemiologic research findings. In the latter, we will distinguish findings relevant to PD specifically from those associated with the broader designation of parkinsonian syndrome features.
Clinical case reports
Concerns about solvents possibly inducing PD have arisen from case reports of PD or clinical signs of parkinsonism during the past 20 years, mainly among exposed workers (Gralewicz and Dyzma, 2005). These reports span a range of isolated cases of parkinsonism associated with various solvents, including n-hexane, carbon disulfide, toluene, methanol, trichloroethylene (TCE), and mixed solvents. Interpreting these reports has been complicated because of small numbers, and because the clinical features described were seldom characteristic of PD (Goldman, 2010). Moreover, exposure information was frequently limited or ambiguous; many of the reported cases were exposed to multiple solvents, or held jobs where solvent exposures were inferred, such as painters. The largest and most extensive clinical case series investigation, conducted by Pezzoli et al. (2000) in Italy, indicated that PD patients exposed to hydrocarbon solvents (not specified by class or chemical) had earlier disease onset, more severe disease, and reduced response to treatment than did non-exposed PD patients. In humans, n-hexane is known to cause narcosis at high doses and peripheral neuropathy at lower levels (Spencer et al., 1980). Motor effects are less well substantiated, although there has been a reported case of parkinsonian-like symptoms in an exposed worker (Pezzoli et al., 1989).
Methanol is used as a fuel additive and as a precursor for the production of plastics, formaldehyde, acetic acid and explosives. Exposure can occur via inhalation of methanol vapor, dermal exposure to aqueous solutions containing methanol or deliberate or accidental ingestion. Methanol has been reported to produce parkinsonian-like symptoms in humans following recovery from a large overdose (Davis and Adair, 1999; Reddy et al., 2010) or chronic exposure (Finkelstein and Vardi, 2002). Methanol intoxication resembles that of ethanol initially causing lethargy, confusion, headache, nausea, ataxia and visual impairment. Clinical manifestation of the parkinsonian-like symptoms tends to appear soon after recovery from the acute effects of methanol and progresses much more rapidly than in PD. However, the pathological lesions are located in the palladium and putamen (Albin, 2000; Chen et al., 1991; Feany et al., 2001; Rubinstein et al., 1995) whose damage is certainly capable of producing clinical parkinsonism. Methanol toxicity is caused by its metabolite formic acid accumulating in the eye, the major target organ for acute toxicity in humans, where it has been shown to selectively inhibit the activity of mitochondrial cytochrome oxidase leading to ATP depletion and the demise of retinal and optic nerve neurons (see Eells in Wallace et al., 1997). Humans and monkeys are sensitive to formic acid as there is a deficiency in formate metabolism related in part to low hepatic tetrahydrofolate levels, while non-primates are insensitive to methanol toxicity (Tephly, 1991). Thus methanol does not cause PD but parkinsonism which encompasses multiple different movement disorders, that all seem to share deficits such as rigidity, tremor, slowness of movement and postural instability.
Toluene (methylbenzene) is a solvent which is commonly used in many industrial products such as paint, paint thinner, glue, or ink, and is frequently abused or misused by inhalation for its euphoric effects. Acute intoxication following inhalation is clinically characterized by reversible behavioral changes, euphoria, headache, and ataxia. In the acute stage, no alterations are seen on structural imaging. Chronic abusers, however, can develop irreversible neurological damage with structural alterations (Geibprasert et al., 2010; King, 1982; Lazar et al., 1983). Findings include toluene-induced chronic toxic encephalopathy with changes in cerebellar and cerebral white matter, demyelination and gliosis, leading to cerebellar dysfunction; psychiatric disorders; spasticity and cognitive changes. Symptoms of attention deficit; decline in visual-spatial skills, frontal lobe function, and memory retrieval, and hearing and visual loss; and cranial nerve abnormalities may also occur. Long-term exposure will result in brain atrophy, predominantly in the cerebellum (Filley et al., 2004; Lolin, 1989; Yucel et al., 2008). There is one case study report of a 22 year-old man who persistently sniffed paint thinners (mainly toluene) over a period of 10 years and developed bilateral intention hand tremor, followed gradually by ataxic gait, speech deterioration and hand tremor at rest. An MRI brain scan revealed extensive damage to the basal ganglia; while single photon emission computed tomography (SPECT) with 123I Ioflupane or DaT-scan (dopamine transporter scan) revealed a bilateral decrease in presynaptic dopamine reuptake (Papageorgiou et al., 2009). Thus, toluene like methanol would appear to cause parkinsonism features, but not necessarily PD.
The first case report of a possible link between chronic exposure to TCE and parkinsonian symptoms was in a worker who had primarily been exposed to TCE, but also had been exposed to other volatile components while working in the plastics industry (Guehl et al., 1999). Since this initial report, the onset of PD has been reported in 3 workers chronically exposed to TCE (Kochen et al., 2003). Gash et al. (2008) subsequently reported an additional 3 PD cases exposed to TCE at the same small instrument plant. A follow-up survey of self-reported parkinsonian signs conducted among former workers (n=65) suggested only a modest effect of TCE exposure. From the clinical case report literature described above, there are indications that some solvents may be associated with PD or related motor effects. However, it should be appreciated that, although case reports provide signals of potential human toxicity related to environmental agents, they remain anecdotal evidence that ultimately requires corroboration by formal toxicological, epidemiological and structural as well as functional neuroimaging studies, which we review next.
Animal models of solvent exposure
To date, there is no animal model that can faithfully recapitulate all aspects of human PD. The most widely used models, are MPTP (Langston et al., 1987) and 6-hydroxydopamine (6-OHDA) (Blandini et al., 2008), which reproducibly yield nigral neuronal loss. Because rodents do not have pigments in nigral neurons, neuronal loss is typically assessed after tyrosine hydroxylase immunoreactivity (TH+). As in human PD (Fearnley and Lees, 1991), 50–60% loss of dopaminergic neurons is required to produce behavioral signs (Dauer and Przedborski, 2003; Zigmond et al., 2002). In addition, dopaminergic degeneration can be assessed by measuring the concentration of DA and its metabolites, homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in the striatum. DA concentration in the striatal portion of the nigrostriatal system is a fairly reliable predictor of damage, although a deficit of typically 70–80% is required before signs of toxicity are observed (Wang et al., 2012; Zigmond et al., 2002).
To address relevant biochemical and behavioral effects of solvents, it is important to summarize what is known about MPTP and 6-OHDA animal models of PD. For MPTP, here is the prevalent hypothesis: MPTP crosses the blood–brain barrier into astrocytes where it undergoes reduction via the enzyme monoamine oxidase B to form MPP+. It is then extruded from astrocytes by the organic cation transporter 3 (Cui et al., 2009). Once in the extracellular space MPP+ enters dopaminergic neurons via the dopamine transporter located on the presynaptic terminal of dopaminergic neurons, accounting at least in part for the selectivity for neurons of the SNpc. Once inside these neurons, how MPP+ precisely leads to neuronal death remains to be investigated. The proposed mechanisms include inhibition of mitochondrial respiration at complex I (Tipton and Singer, 1993), increased oxidative damage (Chen et al., 2009; Williamson et al., 2012), secondary microglial activation (Lee et al., 2012); α-synuclein accumulation and aggregation with the formation of Lewy body-like inclusions, especially in those treated with a chronic MPTP regimen (Gibrat et al., 2009), impairment of ubiquitin–proteosome function (Betarbet et al., 2005; Fornai, et al., 2006) as well as mitochondrial translocation of DJ-1 (Andres-Mateos et al., 2007).
The behavioral effects in mice exposed to MPTP can be assessed in a number of ways, motor function using the grid performance test (Tillerson and Miller, 2003) or the more recent modification of this method, the vertical grid test (Kim et al, 2010). Other methods used include rearing behavior (Schwarting et al., 1999), open field test (Crocker et al., 2003) and rearing and foot fault behavior (Goldberg et al., 2011). To determine if the observed behavioral deficits are dopamine-dependent, then levodopa (L-DOPA) can be administered and the behavioral tests repeated. Depending on the dose of MPTP, signs of motor deficit may take many days or weeks or extend to months to be detected.
The other chemically-induced PD model is using 6-OHDA which is not able to cross the blood–brain barrier and is typically injected into the SNpc, striatum or medial forebrain bundle (MFB) where it produces a massive, virtually complete, lesion of nigral dopaminergic cell bodies. The injection is commonly carried out unilaterally, with the contralateral hemisphere serving as control. The neuronal damage induced by 6-OHDA is mainly due to massive oxidative stress caused by the toxin. 6-OHDA is a structural analog of DA and shows high affinity for the dopamine transporter, and thereby concentrates in DA neurons. Once inside, 6-OHDA undergoes prompt auto-oxidation, promoting a high rate of free radical formation (mostly hydrogen peroxide). 6-OHDA can also accumulate in mitochondria, where it inhibits the activity of the electron transport chain by blocking complex I.
Neuronal cell loss can be evaluated by apomorphine or amphetamine stimulated rotations. The 6-OHDA model is quite versatile, and depending on the injection site, you can examine rapid and complete neuronal cell loss or partial cell loss which is progressive, the later having value for examining therapeutic interventions (Blandini et al., 2008).
In the next section, we will divide the toxicology review into findings for the major classes of solvents: a) non-chlorinated and b) chlorinated solvents, focusing on selected chemicals for which clinical case reports suggest possible nigral system toxicity, and relevant biochemical or behavioral effects have been investigated explicitly, with MPTP and 6-OHDA models as references.
Non-chlorinated solvents
Pezzoli et al. (1990) examined the effect of n-hexane and its metabolite 2,5-hexanedione on the concentration of DA and its metabolite HVA in the striatum of mice, following 400 mg/kg/day i.p. for 5 days/week for 6 weeks. They found that both chemicals reduced striatal DA concentration by 38% and 33% and HVA concentration by 33% and 17% for n-hexane and 2,5-hexanedione respectively, without affecting 5-hydroxyindole acetic acid (5-HIAA) or noradrenaline (NE) concentrations. Thus these hydrocarbons appear to have a selective effect on DA and its metabolites in the striatum, however no histological studies were reported to confirm neuronal cell loss. These authors also studied rats, where they depleted nigrostriatal DA content by about 23% by direct administration of 6-OHDA. Two weeks later they infused 5 mg of n-hexane or 2,5-hexanedione into the striatum, and measured apomorphine rotation 20 and 30 days after treatment. 2,5-Hexanedione administration increased rotation compared to the controls, ipsilateral to the lesion, suggesting that 2,5-hexanedione was stimulating pre-synaptic release of DA from the non-lesioned side of the brain. A few days after the last behavioral measurement the concentration of striatal DA and HVA was determined on either side of the brain. Under these conditions, DA was reduced by 69% and 71% and HVA by 34% and 42% on the lesioned side compared to the non-lesioned side for n-hexane and 2,5-hexanedione respectively. This study supports the finding in mice, namely that n-hexane or 2,5-hexanedione caused a marked loss of DA and its metabolite HVA, following direct injection into the rat striatum. In addition, in rats behavioral changes indicative of DA depletion were observed following 2,5-hexanedione.
In summary, this one study indicates the potential for n-hexane and 2,5-hexanedione to lower DA levels in the striatum of mice and rats when given as large i.p. doses or direct injection into the striatum. Unfortunately, pathology of the SN was not examined to confirm the loss of TH+ neurons. It should be noted that the route of administration of these solvents is not relevant to human exposure. Further studies with these solvents characterizing the nigrostriatal damage, are needed to confirm these findings and examine the dose–response relationship at routes relevant to exposure in the workplace.
The primary target for n-hexane and its metabolite 2,5-hexanedione is axons in the peripheral and central nervous system where it causes atrophy (Spencer et al., 1980). Mechanistically 2,5-hexanedione forms 2,5-dimethyl pyrrole adducts with ε-amino residues of the amino acid lysine in neurofilamentous and microtubule-associated proteins leading to reduction in axonal transport, and axonal degeneration (DeCaprio et al., 2009; Zhang et al., 2010). Whether this mechanism is relevant to the injury reported in the striatum in mice and rats given these hydrocarbons is not known. It is however relevant that cytochrome P450 2E1 responsible for the metabolism of n-hexane to 2,5-hexanedione is present in dopaminergic neurons in the SNpc (Watts et al., 1998) and hence adduct formation in dopaminergic neurons is a possibility. Thus, these hydrocarbons primarily cause axonal atrophy, with one paper reporting DA depletion in the striatum of mice and rats. Clearly further work is required to substantiate this later finding.
The organic solvent toluene is widely used in industry. Few studies with toluene in experimental animals have examined the nigrostriatal region of the brain. Recently, Hester et al. (2011) examined gene changes in rat striatum following 6 h and 18 h exposure to 1000 ppm toluene, and found no gene changes related to dopaminergic pathways. Acute exposure of rats to toluene by inhalation at 100, 300 or 1000 ppm for 8 h produced a small increase in DA in the striatum after 1000 ppm (Rea et al., 1984). Microdialysis studies in free-moving rats exposed to 500, 1000 or 2000 ppm toluene for 2 h also increased the concentration of extracellular striatal DA at 1000 and 2000 ppm (Stengård et al., 1994). In contrast, when toluene was given i.p. to rats at doses of 80, 250 or 800 mg/kg, which had an in-dwelling cannula in the striatum, no changes were seen in striatal DA or its metabolites over a 4 h period (Kondo et al., 1995). Thus after high dose acute exposure to toluene there is no evidence of a decrease in striatal DA. von Euler et al. (1989, 1991, 1993, 2000) examined the effect of toluene exposure at 40 or 80 ppm for 6 h/day from days to many months on brain neurochemistry and behavior. Overall these studies showed a small effect on memory, an increase in DA-mediated locomotor activity after repeated exposure and a 30–40% increase in the number of D2 receptors in the rat neostriatum. Such an increase of DA receptors may explain the attenuation of the apomorphine-induced behavior observed after toluene exposure. Cintra et al. (1999) exposed rats to toluene via inhalation for four weeks at 40 or 80 ppm and found no changes in DA or DOPAC concentration or in DA turnover in the neostriatum or SNpc. Stereology counts of TH+ neurons of the SNpc and dorsomedial neostriatum showed no neuronal cell loss following exposure to 80 ppm toluene. Glial fibrillary acidic protein (GFAP), a glial protein which usually increases after tissue damage was not altered in the neostriatum. Thus, neurochemical and neuropathological findings show that acute or subchronic exposure to toluene does not cause degeneration of the nigrostriatal DA system in rats. Toluene does however affect other brain regions especially the parietal cortex, as shown by magnetic imaging studies in living rats and by autoradiograms of frozen brain sections (von Euler et al., 2000). The mechanism whereby toluene causes toxicity to selected brain regions is currently not understood.
Methanol toxicity, as discussed earlier, in human cases of intentional ingestion, is due to the metabolite of methanol formic acid which accumulates in the eye leading to blindness. However, rodents do not develop ocular effects (Tephly, 1991). Eels and colleagues have developed a rat model of methanol intoxication in which folate-dependent formate oxidation was selectively inhibited, allowing formate to accumulate to toxic concentrations. Under these conditions they observed visual toxicity with histological evidence of mitochondrial disruption analogous to the human poisoning syndrome (Eells et al., 2000). We have found no published reports looking at the SNpc with regard to DA concentration or dopaminergic neuronal cell loss, using this rat model of methanol intoxication. But it should be noted that the toxic metabolite of methanol, formic acid is an inhibitor of mitochondrial complex I, a common finding for some chemicals that produce a parkinsonian-like syndrome.
Chlorinated solvents
Only three major chlorinated solvents will be discussed, trichloroethylene (TCE), perchloroethylene (PCE) and dichloromethane (DCM), as these have been widely used in industry. TCE and PCE given acutely to rodents, and following high exposure levels in humans are central nervous system (CNS) depressants (Bale et al., 2011; Evans and Balster, 1991). TCE was used for many years as an anesthetic in humans (Hewer, 1975). It is known that anesthetics act on lipoprotein membranes. Early studies of cellular toxicity with a variety of anesthetics, halothane, methoxyflurane, chloroform and TCE, showed inhibition of rat liver mitochondrial respiration selectively at complex I, with a mean inhibitory range of 0.8–2.6 mM (Hall et al., 1973). TCE, PCE and DCM are lipid soluble and can readily enter the brain at sufficiently high concentrations, and can induce reversible inhibition of mitochondrial complex I. Recently, Bale et al. (2011) reviewed these three solvents with respect to their effect on the CNS and potential neurotoxic mechanisms in experimental animals. They concluded that there were commonalities in the neurobehavioral and neurophysiological effects reported in vivo, which suggested a common mechanism of action. These common findings included changes in spontaneous activity, cognition, sleep cycle, visual and auditory function and impaired motor incoordination. Both PCE and DCM showed a biphasic locomotor activity which initially increased and then decreased, while TCE only seemed to decrease locomotor activity. Based on available data, they hypothesized that GABAergic and glutamatergic systems may be involved in the general CNS depression and motor incoordination events (Bale et al., 2011).
Trichloroethylene
The first reported effect of TCE on the SNpc was by Guehl et al. (1999). They exposed 28 week old OF1 male mice to neat TCE at 400 mg/kg/day, 5 days/week for 4 weeks by i.p. injection, and then left the mice for a week before termination. They reported a 50% reduction in TH+ neurons in the SNpc in TCE-treated mice compared to the controls, indicating a marked effect of TCE on the SNpc.
More recently Gash et al. (2008) exposed 5 month old male Fischer rats to 1 g/kg TCE in olive oil for 5 days/week for 6 weeks by oral gavage. They measured the concentration of DA and its metabolites in the SNpc and striatum after TCE exposure, and reported a 20% decrease in DA concentration and a decrease in DOPAC and HVA in the SNpc (Table 1) while striatal DA concentration was maintained in the surviving neurons, the major effect being a 40% reduction in DOPAC and HVA concentration (Table 1). Analysis of the DOPAC/DA ratio in the striatum declined from 0.176 in control to 0.113 in TCE-treated rats, indicating a decrease in DA metabolism. The extent of the deficit of DA at 20% control after 6 weeks of exposure would not be sufficient to produce motor effects. In rats similarly exposed to TCE the number of TH+ positive neurons in the SNpc was decreased by 45% which was mirrored by the loss of total neuronal cell number (Gash et al., 2008) (Table 1). These authors also reported prominent cytoplasmic α-synuclein-positive inclusions in the SNpc and dorsal motor nucleus of the vagus nerve in the TCE-treated animals, which were absent or rare in the vehicle-treated animals. Pathological changes in PD include α-synuclein inclusions in the SNpc and the dorsal motor nucleus of the vagus nerve (Braak et al., 2004). The authors also reported inhibition of mitochondrial complex I activity in the SNpc of TCE-treated rats, a common finding with chemicals that produce a parkinsonian-like syndrome.
Table 1.
The effect of TCE on the concentration of DA and its metabolites in the substantia nigra and striatum, and the number of tyrosine hydroxylase positive cells in the substantia nigra pars compacta in Fischer 344 rats.
| Measurement | Substantia nigra (% control) | Striatum (% control) | ||||
|---|---|---|---|---|---|---|
| Dopamine & metabolites | DA | DOPAC | HVA | DA | DOPAC | HVA |
| TCE 1 g/kg | 80±10* | 72±4** | 80±5 | 102±2 | 62 ±3*** | 82±3** |
| 6 weeks | (8) | (8) | (8) | (8) | (8) | (8) |
| Substantia nigra pars compacta (% control) | ||||||
| Neuronal cell counts | TH+ neurons | Fluorogold neurons | ||||
| TCE 1 g/kg | 55±3** | Reduced | ||||
| 6 weeks | (6) | (3) | ||||
The data is from Gash et al. (2008). Control values for dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the SN and striatum were not given. Control numbers for TH+ neurons in the SN were 42,000 ±1000. Fluorogold was given by injection into the striatum retrogradely labeling the neurons in the SN.
Results are Mean ± S.E.M. with the number of animals shown in brackets; values for DA and its metabolites have been read off bar charts, but the statistical analysis is as reported by the authors.
p<0.05 compared to control.
p<0.01 compared to control.
p<0.001 compared to control.
This is the first report in rats exposed to high doses of TCE over a 6 week period, showing a small but variable reduction in DA content in the SNpc, which is associated with a 45% loss of dopaminergic neurons in the SNpc. These findings plus an increase in α-synuclein inclusions in the SNpc, strongly resemble the findings with MPTP suggesting a parkinsonian response.
In a subsequent study Liu et al. (2010) using the same dosing protocol examined the effects of TCE-treatment on the concentration of DA and its metabolites in the striatum of TCE-treated rats after 2 and 6 weeks of exposure to 1 g/kg/day. The authors did not report the values for DA or HVA after 2 weeks of exposure but did report a 30% decrease in DOPAC (Table 2). Following 6 weeks of exposure to TCE the concentration of DA was unchanged while the metabolites were reduced suggesting a decrease in DA metabolism in the striatum (Table 2) similar to that reported by Gash et al. (2008). No changes were observed in striatal 5-HT or 5-HIAA, indicating selectivity for dopaminergic neurons after 2 weeks of exposure. Liu et al. (2010) also investigated the dose–response relationship for the loss of TH+ neurons and total loss of neurons in the SNpc after 6 weeks of exposure to TCE, and detected a statistically significant reduction after 1 and 0.5 g/kg/day but not after 0.2 g/kg/day (Table 2). These findings were supported by a number of other important measurements in rats given 1 g/kg/day TCE for 6 weeks (Liu et al., 2010), and are summarized as follows: 1) no loss of dopaminergic neurons in the ventral tegmental area; 2) absence of effects on: cholinergic or GABAergic neurons in the striatum, GABAergic Purkinje cells in the cerebellum or myelin in the brainstem; 3) no effect on spontaneous motor activity; 4) a small effect on the duration to stay on a rotarod after 5 and 6 weeks of exposure; 5) the presence of intracellular α-synuclein accumulation in the SNpc and in the dorsal motor nucleus of the vagus nerve; 6) some evidence for an increase in protein carbonyls, lipid peroxidation and protein tyrosine nitration in the SNpc indicating oxidative stress; 7) evidence of apoptosis as judged by an increase in caspase 3-stained neurons and evidence of microglia activation primarily after 2 weeks of exposure; 8) inhibition of mitochondrial complex I in SNpc after 2 weeks of exposure.
Table 2.
The effect of TCE on the concentration of DA and its metabolites in the substantia nigra and striatum, and the number of tyrosine hydroxylase positive cells in the substantia nigra pars compacta in Fischer 344 rats.
| Measurement | Substantia nigra (% control) | Striatum (% control) | ||||
|---|---|---|---|---|---|---|
| Dopamine & metabolites | DA | DOPAC | HVA | DA | DOPAC | HVA |
| TCE 1 g/kg | ND | ND | ND | NR | 69.8 | NR |
| 2 weeks | 104±2 | 65 ± 3** | 87 ± 4* | |||
| 6 weeks | (6) | (6) | (6) | |||
| Substantia nigra pars compacta (% control) | ||||||
| Neuronal cell counts | TH+ neurons | Nissl stained neurons | ||||
| TCE 1 g/kg | 59.4±2** | 65.7±3.4** | ||||
| 6 weeks | (6) | (6) | ||||
| TCE 0.5 g/kg | 74.4±3* | |||||
| 6 weeks | (6) | |||||
| TCE 0.2 g/kg | 79.9±4.5 | |||||
| 6 weeks | (6) | |||||
The data is from Liu et al. (2010). Control values for dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the SN and striatum are not given in the paper, the values for DA and its metabolites have been read off bar charts, but the statistical analysis is as reported by the authors. The total number of TH+ neurons in the SN of vehicle control animals was 45,000 ± 2500, and for Nissl stained neurons 57,260±3211.
Results are Mean ± S.E.M. with the number of animals shown in brackets; ND, not determined; NR, not reported.
p<0.05 compared to control.
p<0.01 compared to control.
This study builds on the work of Gash et al. (2008) confirming their findings and extending the work to lower doses, where a dose–response relationship is observed for the effect of TCE on dopaminergic neurons in the SNpc. These authors have also provided evidence of inhibition of mitochondrial complex I, oxidative stress, apoptosis and a microglial cell reaction in the SNpc after TCE administration and for selectivity to dopaminergic neurons. Some behavioral changes were reported after 5 and 6 weeks of exposure to 1 g/kg/day TCE using the rotarod, however this is not a good measure of behavior associated with DA depletion. Assuming DA loss in the SNpc was the same as in that reported by Gash et al. (2008) using the same protocol, dose and time, then a loss of 20% of DA, would not be expected to have caused behavioral changes.
In a third study, involving some of the same research group Sauerbeck et al. (2012) exposed rats to 1 g/kg TCE for 2 weeks and determined the number of TH+ neurons and total neuronal cell number in the SNpc and found no effect on either parameter (Table 3). It was noted that the total number for TH+ cells and total neurons in the SNpc in control animals reported in this paper (Table 3) is low compared to the other two papers (Tables 1 & 2) using the same methodology. Liu et al. (2010) reported a ratio of TH+ neurons to total neurons of about 79%, while Sauerbeck et al. report a ratio of 41%. The reason for the difference is not clear, but as controls were compared to treated under the same conditions, the lack of effect is real, but this does highlight some of the issues associated with stereology. These authors also reported no reduction in mitochondrial state 3 respiration (complex I activity) in the SNpc after 2 weeks of exposure to TCE, while there was 75% inhibition of complex I activity in the striatum, suggesting that cells in this brain region must have been struggling to survive. The main thrust of the Sauerbeck et al. (2012) work was to examine the interaction between brain injury and chemically-induced injury using TCE exposure and they clearly showed that a combination of mild or moderate brain injury and TCE exposure had a more marked effect than either treatment alone on the loss of dopaminergic neurons in the SNpc. Thus brain injury needs to be considered as part of a multifactorial model of PD.
Table 3.
The effect of TCE on the number of tyrosine hydroxylase positive cells in the substantia nigra pars compacta in Fischer 344 rats.
| Measurement | Substantia nigra pars compacta (% control) | |
|---|---|---|
| Neuronal cell counts TCE 1 g/kg | TH+ neurons | Nissl stained neurons |
| 1 week | ND | ND |
| 2 weeks | 94± 3 (6) | 100±2 (6) |
The data is from Sauerbeck et al. (2012). The total number of TH+ neurons in the SN of vehicle control animals was 9800 ± 4000, and for Nissl stained neurons 24,000 ± 2400, the values for TCE treated rats were read off a bar chart.
Results are Mean ± S.E.M. with the number of animals shown in brackets; ND, not determined.
In summary, these three studies show that repeated oral administration of TCE to rats at high doses (>0.2 g/kg/day for 6 weeks) causes depletion of dopaminergic neurons in the SNpc. While a single study in mice given high doses of TCE i.p. also resulted in loss of dopaminergic neurons in the SNpc. Clearly high doses of TCE have the ability to damage dopaminergic neurons; further dose–response studies, especially at concentration(s) that are meaningful to typical human exposures, e.g. via inhalation, are needed to help judge the potential risk of humans exposed to TCE.
Perchloroethylene and dichloromethane
We have found no information on the effect of PCE or DCM on the SNpc in animal studies; although the effects on the CNS following acute and chronic exposure have been reviewed (Bale et al., 2011). DCM undergoes metabolism to produce carbon monoxide, which can build up in the blood stream and interact with hemoglobin (Andersen et al., 1991), carbon monoxide poisoning results in hypoxic brain damage and the major brain regions affected are the cerebral cortex, cerebral white matter, basal ganglia, and especially the globus pallidus (Prockop and Chichkova, 2007). Recovery from carbon monoxide poisoning has resulted in parkinsonian-like symptoms in some people (Rissanen et al., 2010; Tanner, 1992). However, carbon monoxide is not specific for the SNpc, as the primary effects occur in the white matter and to neurons in the globus pallidus, putting carbon monoxide with other chemicals that do not cause PD, but parkinsonism due to disruption of basal ganglia circuitry.
Mode of action of TCE and relevance to humans
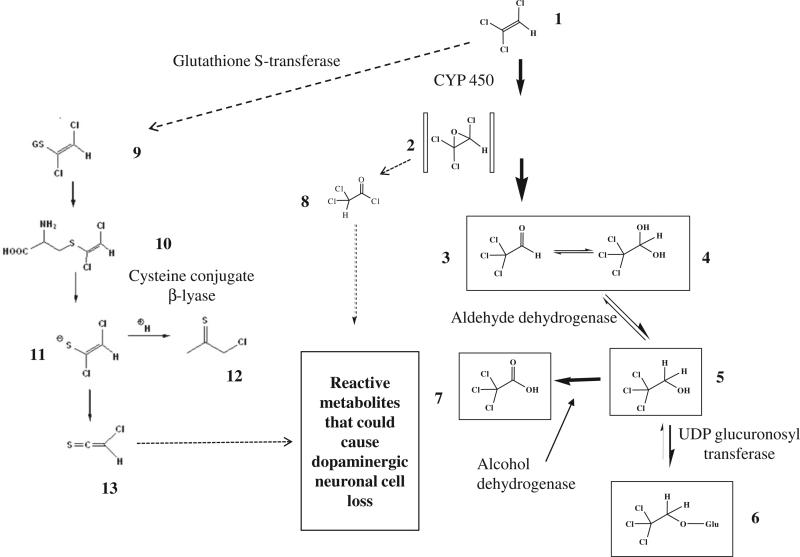
TCE of all the solvents evaluated would appear to cause damage to dopaminergic neurons in rats and mice after high exposure levels, so is it TCE per se or a metabolite that is causing the selective toxicity to the SNpc? TCE we know is readily absorbed via inhalation, through the skin or by oral ingestion in experimental animals and humans. TCE undergoes metabolism by cytochrome P450(s), primarily CYP2E1 and to a lesser extent by CYP1A1/2, CYP2B1/2, CYP2C11/6 to form trichloroacetaldehyde (chloral, Fig. 1, metabolite 3), the major metabolites of which are trichloroethanol (TCE-OH) (Fig. 1, metabolite 5) and its glucuronide (Fig. 1, metabolite 6) and trichloroacetic acid (TCA) (Fig. 1 metabolite 7) (see review by Lash et al., 2000). During the oxidative metabolism of TCE to chloral, TCE oxide is formed (Fig. 1, metabolite 2) which is a reactive electrophile that rearranges to acylating intermediates (Cai and Guengerich, 2000), which could interfere with essential pathways in dopaminergic neurons. CYP2E1 has been localized in the SNpc of rat brain, and co-localizes with TH+ neurons (Watts et al., 1998). This raises the question of whether local metabolism of TCE in dopaminergic neurons by CYP 2E1 could account for the selective injury. It is also known that CYP2E1 is a potent generator of free radicals and dopaminergic neurons may be more vulnerable to oxidative stress and free radical injury (Liu et al., 2010; Shahabi et al., 2008). At this stage we cannot rule out other major metabolites of TCE such as TCE-OH and TCA (Fig. 1), which could easily be tested by exposing rats to these metabolites. It is know that in rats once cytochrome P450 metabolism of TCE is saturated, then biotransformation can occur via glutathione conjugation (Fig. 1, metabolite 9), leading to the cysteine conjugate S-(1,2-dichlorovinyl)-l-cysteine (DCVC) (Fig. 1, metabolite 10). DCVC is known to undergo metabolic activation by the enzyme cysteine conjugate β-lyase to generate a toxic metabolite (Fig. 1, metabolite 13) (Anders, 2004), this enzyme is present in the brain, known as kynurenine amino-transferase for which DCVC is a substrate. However, metabolism via glutathione conjugation would seem unlikely to account for damage to the SNpc as the doses of TCE used in mice 400 mg/kg/day (Guehl et al., 1999) and rats 500 mg/kg (Liu et al., 2010), are below the concentration for saturation of the cytochrome P450 pathway.
Fig. 1.
Metabolism of trichloroethylene and its possible relevance to dopaminergic neuronal cell loss in the substantia nigra pars compacta. Trichloroethylene (1); trichloroethylene oxide (2); chloral (3); chloral hydrate(4); trichloroethanol (5); trichloroethanol glucuronide (6); trichloroacetic acid (7); trichloroacetyl chloride (8); S-(1,2-dichlorovinyl)glutathione (9); S-(1,2-dichlorovinyl)-l-cysteine (10); 1,2-dichloroethenethiolate (11); chlorothionoacetyl chloride (12); chlorothioketene (13). TCE is primarily metabolized by CYP450 to TCE oxide which has been shown to rearrange to form a reactive chloroacyl chloride intermediate that can react with proteins and cause enzyme inhibition (Cai and Guengerich, 2000). This reactive metabolite could be formed in dopaminergic neurons in the SNpc leading to cell death. At this stage we cannot rule out metabolism via GSH conjugation followed by activation by cysteine conjugate β-lyase to form chlorothioketene which could lead to toxicity to dopaminergic neurons. This later reaction would seem less likely.
A group in Germany has shown that a metabolite of TCE, chloral (Fig. 1, metabolite 3) can react with the endogenous biogenic amine, tryptamine to form 1-trichloromethyl-1,2,3,4-tetrahydro-β-carboline (TaClo). The structural similarity of TaClo to MPTP has led to extensive research for a possible link between TaClo, dopaminergic neurons and PD (Riederer et al., 2002). However, tryptamine is associated with the neurotransmitter 5-hydroxytryptamine (5-HT) and Gerlach et al. (1998) conducted studies in rats with a microdialysis probe in the striatum, to measure extracellular neurotransmitter concentrations, and then administered TaClo. They reported an immediate increase in extracellular 5-HT with a 2-fold increase in the first hour after TaClo administration and about a 4-fold increase in the second and third hours, while changes in DA only showed a 0.3-fold increase and DOPAC a 0.6-fold increase over the same time period. Thus in vivo TaClo seems to act on 5-HT neurons with a very much smaller effect on dopaminergic neurons. Despite this observation, extensive research has continued looking for an effect of TaClo on the DA system and although TaClo inhibits mitochondrial activity at complex I, similar to MPTP, and when injected into the SNpc of rats produced an ~15% decline in neuron number (Bringmann et al., 1995), any link between TaClo formation from TCE and damage to the SNpc seems tenuous and probably misguided.
In summary, we currently do not know in rodents whether it is TCE per se or its metabolites that are responsible for damage to dopaminergic neurons in the SNpc, following large oral doses. Hence it is not possible at this stage to give a clear view on the risk to humans as inhalation a major route of absorption for humans has not been examined. The TaClo hypothesis does not seem plausible and a role for DCVC unlikely. Regarding the major metabolites of oxidative metabolism, chloral, TCE-OH and TCA, extensive toxicology has been conducted with these chemicals in experimental animals (Bull, 2000; Green et al., 2003; NTP, 2002) and there have been no reports of any effect on the SNpc, although the major focus of these studies has not been on the CNS. Thus, further research is required to identify if it is TCE itself or a metabolite responsible for damage to the SNpc and then to try to understand the biochemical basis for the selective toxicity.
Epidemiological evidence
Associations of solvents with PD have been investigated largely in the context of clinic- or population-based case–control studies. There are also several cohort and proportionate mortality studies. The epidemiologic findings are summarized in Table 4. In the vast majority of studies, solvent exposures were treated as a single entity, without distinguishing between classes or specific chemicals. The notable exception is the study of twins by Goldman et al. (2012) which provided findings for specific solvents. Also, few studies provided quantitative data on exposure intensity of duration to enable dose–response estimation.
Table 4.
Epidemiology studies of solvents and PD.
| Study | Location | Design | “Solvents” RRa (95% CI) | Specific solvents | Comments |
|---|---|---|---|---|---|
| Ohlson and Hogstedt (1981) | Sweden | Case–control; 91 cases, 75 controls | 1.1 (0.4–2.9) | CS2, 3 cases vs. 0 controls exposed | Hospital-based study; controls with subarachnoid hemorrhage; all subjects 35–69 yrs. |
| Seidler et al. (1996) | Germany | Case–control; 380 cases, 379 neighborhood controls, 376 regional controls | Neighborhood controls: In free time: 2.6 (1.2–5.4) At work: 1.6 (1.1–2.4) Regional controls: In free time: 3.4 (1.5–7.5) At work: 1.8 (1.2–2.7) |
– | Clinic-based study; exposures based on self-report and job/exposure matrix |
| Smargiassi et al. (1998) | Italy | Case–control; 86 cases, 86 controls | 2.78 (1.23–6.26) | – | Exposure (occupational or residential) ≥ 10 yrs, based on questionnaire response and industrial hygiene review |
| DePalma et al. (1988) | Italy | Case–control; 100 cases, 200 controls | 1.15 (0.65–2.03) | – | Controls from nephrology, hemostasis specialty clinics; interaction of solvents and CYP2D6 poor metabolizer status (14.47 [1.16–185.23]) |
| Pezzoli et al. (2000) | Italy | Case series; 188 exposed, 802 not exposed | – | Disease severity, reduced Rx response, and early onset assoc. with ‘hydrocarbon solvents’ | |
| McDonnell et al. (2003) | UK | Nested case–control; 182 cases, 423 controls | 1.53 (0.81–2.87) | – | Dose–response with duration exposure: OR=3.59 (1.26–10.26) >30 yrs exposure; mentions TCE, CCL4 |
| Park et al. (2005) | USA | Proportionate mortality (1982–91) 33,678 PD deaths | 1.07 (1.00–1.13)a | Benzene: 1.05 (0.98–1.12)a | Jobs based on ‘usual occupation’ on death certificate; exposures based on job/exposure matrix |
| Ascherio et al. (2006) | USA | Cohort; 413 cases Cohort (143,325) | ~0.8 (0.5–1.2) | – | Only graphical data for ‘Chemicals/Acid solvents’ |
| Charles et al. (2006) | Hawaii | Cohort; 1049 men aged 71–93 yrs | Clinical signsa: Hand movements: 0.98 (0.56–1.69), p-trend 0.77; Posture: 1.18 (0.68–2.06), p-trend 0.84; Gait: 1.50 (0.86–2.59), p-trend 0.28; Facial expression: 1.57 (0.89–2.79), p-trend 0.19 |
– | Japanese American men; exposures determined 1965–74, clinical exams 1991–99 |
| Dick et al. (2007) | Scotland, Italy, Sweden, Romania, Malta | Case–control; 767 cases, 1989 controls | Low vs. none 1.21 (0.93–1.57) High vs. none 0.94 (0.72–1.21) |
– | Exposures rated by industrial hygiene judgment; interaction between GSTM1 null and solvents (2.34 [1.08–8.77]) |
| Petersen et al. (2008) | Faroe Islands | Case–control; 79 cases, 154 controls | 1.68 (0.80–3.50) | – | Exposure based on questionnaire responses (ever/never) |
| Tanner et al. (2009) | USA, Canada | Case–control; 519 cases, 511 controls | Gluing: 1.31 (0.85–2.02); Cleaning w/ solvents: 1.01 (0.74–1.38); Stripping wood, paint: 1.30 (0.59–2.85) House painting: 1.11 (0.61–2.00) Industrial painting: 1.18 (0.80–1.74) |
– | Clinic-based study; controls non-blood relatives (excluding spouses), acquaintances; mention that no associations seen with solvents, TCE, CCL4 (data not shown) |
| Firestone et al. (2010) | USA | Case–control; 404 cases, 526 controls | Men: 1.0 (0.7–1.3); Women: 1.7 (1.0–3.0) |
– | Unpublished data: no associations for any solvents, except for toluene in women: OR 2.3 (0.8–7.1) [4 cases vs. 1 control] |
| Feldman et al. (2011) | Sweden | Cohort (Swedish twin registry) Cohort 14,169 men; 204 PD cases | Any exposure: 0.9 (0.7–1.3) Highest exposure: 1.4 (0.6–2.9) | – | Exposures based on self-report and job/exposure matrix |
| Goldman et al. (2012) | USA | Nested case–control study in twins cohort; 99 cases, 99 unaffected twin controls | 1.7 (0.8–3.7) | TCE: 6.1 (1.2–33) PERC: 10.5 (0.97–113) TCE or PERC: 8.9 (1.7–47) Toluene: 1.3 (0.5–3.3) Xylene: 2.2 (0.4–12) n-Hexane: 1.3 (0.4–4.1) CCL4: 2.3 (0.9–6.1) |
Industrial hygiene classification of exposures (incl. hobbies); trends for duration and cumulative index similar to ever/never |
– Not reported.
Relative risk (95% confidence interval).
In general, observed associations of PD with solvents were modest, with most relative risk estimates in the 1.0–1.5 range. The results most suggestive of possible etiologic relations were detected for TCE, PCE, and carbon tetrachloride in the US twins study (Goldman et al., 2012), although risk estimates were based on very small numbers of exposed subjects, and thus are statistically imprecise. A possible dose–response gradient with years of exposure was observed in the UK Rolls Royce cohort (McDonnell et al., 2003), but there has been little corroborative evidence from other studies.
Discussion
Some solvents have established neurotoxic properties, and also pose relatively widespread exposures in the workplace and ambient environment. Consequently, there is good rationale for investigating solvents as potential PD risk factors. Toxicological evidence for the involvement of non-chlorinated solvents causing selective damage to dopaminergic neurons in the SNpc is largely non-existent. Solvents, including n-hexane, methanol and toluene, primarily cause injury to the peripheral nervous system, injury to the eyes and CNS-cerebellar atrophy respectively. However, there is evidence in humans of parkinsonism following methanol or toluene overdose or chronic exposure where damage is seen in other brain regions. Another known trigger of parkinsonism includes chronic exposure to manganese, which causes degeneration in the sub-thalamic nucleus and the pallidum. In addition, altered copper homeostasis, for example in Wilson's disease, can lead to damage to basal ganglia nuclei and the SNpc causing a parkinsonism that is moderately responsive to L-DOPA (Caudle et al., 2012). Carbon disulphide can also cause neurobehavioral problems associated with parkinsonian-like symptoms in relatively young subjects. However, all of these chemicals also cause other neurological problems and can hardly be considered specific dopaminergic toxins. Thus there does appear to be a number of chemicals including solvents that cause parkinsonism, by damaging the basal ganglia (methanol, manganese, toluene and copper), demyelination in the cerebellum (toluene) and spinal cord (n-hexane). The mechanisms whereby these chemicals cause selective toxicity to these brain regions are not fully understood. It is also clear that interaction between solvent exposure and brain injury can exacerbate dopaminergic neuronal cell damage as shown by Sauerbeck et al. (2012) with TCE. Toxicological research has identified TCE as demonstrating selective nigral system damage in rats and mice exposed to large oral or i.p. doses. However, the relevance of these findings to exposure in the workplace, or in the general environment, where exposure levels are usually very much lower, is not known. Toxicological studies by a relevant route to human exposure, especially inhalation, are necessary before any definitive conclusions can be drawn.
At present, it is not known if it is TCE itself, or one of its major or minor metabolites that is responsible for the neuronal cell loss in the SNpc observed in experimental animals. Further work is needed to establish the critical steps underlying nigral system toxicity to enable more accurate risk characterization. Based on available evidence, the loss of dopaminergic neurons in the SNpc could merely be a high-dose rodent phenomenon, which may not be relevant to humans environmentally exposed or exposed in the workplace. Dose–response studies across a range of doses, some at or below permissible workplace standards would be helpful.
There is incomplete evidence for PCE and DCM with regard to injury to the SNpc. There are, however, some common findings for TCE and PCE, such as formation of the metabolite TCA and minor metabolites via conjugation with glutathione. Assuming one of these common metabolites is responsible for the neuronal injury, then PCE may produce a similar effect in rodents to that of TCE. If this were the case, then the aforementioned issues regarding relevance to human exposures in the workplace would need to be taken into account.
The epidemiologic evidence for etiologic relations of solvents and PD is mixed and, at most, weakly supportive of associations. This may be due to the non-specific nature of data obtained in most epidemiologic studies, which are mostly population-based case–control studies relying on self-reported jobs and exposures. The designation of exposure as ‘solvents’ or ‘hydrocarbon solvents’ is far too nebulous to enable identification of potential associations with specific solvents. In other words, much of the epidemiologic data may suffer from exposure misclassification, which may mask true associations. This problem is by no means unique to solvents. In fact, it might be noted that exposure data for other agents investigated in many epidemiologic studies of PD, notably pesticides and metals, often represent aggregated, non-specific designations, which limits causal interpretations.
The recent report of seemingly strong associations (albeit statistically imprecise) for TCE and other chlorinated solvents observed in the US twins study (Goldman et al., 2012), stands out in that there was a considerable effort undertaken to characterize and report results for specific exposures. Several features of this study deserve comment. Rigorous efforts were made to ensure diagnostic accuracy of PD. The solvent exposure assessment was undertaken in a standardized, thorough manner suitable for the case–control design. Although a substantially higher percentage of proxy respondents provided interview for cases (46%), than for controls (18%), the findings were not materially different when data for proxy respondents were excluded from the analysis. The design comparing affected to non-affected twin controls has the advantage of controlling for potential confounding due to genetic and shared environmental factors, such as early childhood exposures. However, there are limitations to this study. An obvious limitation is the small number of exposed subjects, and the resultant statistical imprecision of relative risk estimates based on fewer than ten TCE-only exposed subjects who had PD. There was also no indication of dose–response gradients, the presence of which ordinarily strengthens causal arguments. This may be due to inaccuracies of the exposure data, despite intensive assessment efforts, or may reflect the role of chance in producing the observed risk elevations. As the authors appropriately note, conformation of these findings from other epidemiologic studies is clearly needed before conclusions can be reached regarding causation and prevention of PD.
On balance, the convergence of toxicological and epidemiologic research suggests a plausible association between TCE exposure and PD. However, there remain ambiguities in the evidence, and in particular, epidemiologic support is derived largely from a single, albeit scientifically sound, study that has important limitations.
Suggestions for future research
We can offer some suggestions for future toxicological and epidemiologic research on solvents and PD. Thus far, as the toxicology findings in rats with TCE have all come from one group in the same institution (Gash et al., 2008; Liu et al., 2010; Sauerbeck et al., 2012); thus, confirmation by other research groups is needed. Studies relating blood and tissue levels of TCE and its metabolites in the SNpc and striatum, in experimental animals, over time would be valuable to help eliminate the involvement of certain metabolites, provide data for PK-PB modeling, and help with human risk assessment. Confirmation as to whether it is TCE per se or one of its metabolites that is responsible for injury to the SNpc, could be obtained by administering the major or minor metabolites. Once the proximate metabolite causing injury to SNpc neurons or striatal dopaminergic projects is known then studies investigating biochemical mechanisms and the basis for the site-selective toxicity should be conducted.
To date, most epidemiologic research has consisted of population-based case–control studies, for well-known reasons: relative ease of enumerating large case numbers with accurate clinical information; and, ample opportunities to investigate solvents in the context of multiple environmental and genetic factors. However, despite the convenience of this approach, we argue that more such studies or even substantially larger population-based case–control studies are unlikely to add much etiologic insight. Such studies inevitably suffer from ambiguities in exposure assessment. Moreover, the contribution to PD risk in the population-at-large of any specific environmental agent is likely to be small, based on past research for very plausible candidates, notably pesticides and metals. Thus, statistical power in case–control studies to characterize genuinely causal relations with solvents will be limited.
Instead, we recommend that future epidemiological research should focus on occupational and community groups with the greatest exposures to solvents for which there is some coherent epidemiologic and toxicological support. TCE would appear to be a reasonable candidate, although there may be good reasons to consider other solvents, especially as solvent exposures in human populations are typically mixed and variable over time. Investigations of PD in workplaces where specific solvent exposures can be identified and, ideally, quantified over time, would be highly desirable. PD incidence, rather than mortality, should be ascertained. This could be accomplished in industries that provide medical care coverage to employees. Cohort and nested case–control studies of PD could then be undertaken. A complementary approach that might be adopted would be systematic cross-sectional and longitudinal studies of parkinsonian signs and symptoms among actively employed and retired workers whose exposures are adequately assessed. Community studies of PD or parkinsonian features pose daunting logistical challenges when efforts are made to obtain personal level health and exposure data. A feasible, yet still informative, option may be ecological correlational analyses examining associations between air and/or water supply levels of solvents and PD incidence or mortality.
One advantage that the toxicology field could take is rapid progression of imaging markers that become readily available to most major medical centers. Despite various caveats associated with structural and functional neuroimaging, these methods are “gold standard” of today to verify clinical diagnosis of PD (Wang et al., 2012).
Conclusions
At present, there is no clear cut evidence from either the toxicological or epidemiologic perspective that any specific solvent or class of solvents is an established cause of PD. There are, however, indications that TCE may have potential etiologic relevance. Further research is required to understand the underlying mechanism of toxicity of TCE to the SNpc in experimental animals. Improved epidemiologic research that offers extensive, valid assessments of clinical outcomes and exposures to specific solvents may provide much needed answers to important public health and scientific questions.
Acknowledgments
The authors thank the Halogenated Solvent Industry Alliance Inc. for support in preparing this review.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Agid Y. Parkinson's disease: pathophysiology. Lancet. 1991;337:1321–1327. doi: 10.1016/0140-6736(91)92989-f. [DOI] [PubMed] [Google Scholar]
- Albin RL. Basal ganglia neurotoxins. Neurol. Clin. 2000;18:665–680. doi: 10.1016/s0733-8619(05)70217-6. [DOI] [PubMed] [Google Scholar]
- Anders MW. Glutathione-dependent bioactivation of haloalkanes and haloalkenes. Drug Metab. Rev. 2004;36:583–594. doi: 10.1081/dmr-200033451. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Clewell HJ, III, Gargas ML, MacNaughton MG, Reitz RH, Nolan RJ, McKenna MJ. Physiologically based pharmacokinetic modeling with dichloromethane, its metabolite, carbon monoxide, and blood carboxyhemoglobin in rats and humans. Toxicol. Appl. Pharmacol. 1991;108:14–27. doi: 10.1016/0041-008x(91)90264-f. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson's disease. Ann. Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Bale AS, Barone S, Jr., Scott CS, Cooper GS. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol. Appl. Pharmacol. 2011;255:113–126. doi: 10.1016/j.taap.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson's disease. Cell Death Differ. 2010;17:1115–1125. doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Ubiquitin–proteasome system and Parkinson's diseases. Exp. Neurol. 2005;191(Suppl. 1):S17–S27. doi: 10.1016/j.expneurol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Martignoni E. The 6-hyroxydopamine model: news from the past. Parkinsonism Relat. Disord. 2008;14:S124–S129. doi: 10.1016/j.parkreldis.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bringmann G, God R, Feineis D, Wesemann W, Riederer P, Rausch WD, Reichmann H, Sontag KH. The TaClo concept: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline (TaClo), a new toxin for dopaminergic neurons. J. Neural Transm. Suppl. 1995;46:235–244. [PubMed] [Google Scholar]
- Bull RJ. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ. Health Perspect. 2000;108(Suppl. 2):241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Guengerich FP. Acylation of protein lysine's by trichloroethylene oxide. Chem. Res. Toxicol. 2000;13:327–335. doi: 10.1021/tx000003p. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. 2011;124:225–250. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson's disease. Neurotoxicology. 2012;33:178–188. doi: 10.1016/j.neuro.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles LE, Burchfiel CM, Fekedulegn D, Kashon ML, Ross GW, Sanderson WT, Petrovitch H. Occupational exposures and movement abnormalities among Japanese-American men: the Honolulu–Asia Aging Study. Neuroepidemiology. 2006;26:130–139. doi: 10.1159/000091178. [DOI] [PubMed] [Google Scholar]
- Chen JC, Schneiderman JF, Wortzman G. Methanol poisoning: bilateral putaminal and cerebellar cortical lesions on CT and MR. J. Comput. Assist. Tomogr. 1991;15:522–524. [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: critical role for the astrocyte. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Aguirre JA, Andbjer B, Finnman UB, Hagman M, Agnati LF, Höglund C, Möller A, Fuxe K. Subchronic toluene exposure in low concentrations produces signs of reduced dysfunction in the 6-hydroxydopamine lesioned nigrostriatal dopaminergic system of the rat. Neurosci. Lett. 1999;274:5–8. doi: 10.1016/s0304-3940(99)00112-3. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis LE, Adair JC. Parkinsonism from methanol poisoning: benefit from treatment with anti-Parkinsonian drugs. Mov. Disord. 1999;14:520–522. doi: 10.1002/1531-8257(199905)14:3<520::aid-mds1026>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Dawson T, Dawson V. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, Kinney EA, LoPachin RM. Comparative covalent protein binding of 2,5-hexanedione and 3-acetyl-2,5-hexanedione in the rat. J. Toxicol. Environ. Health A. 2009;72:861–869. doi: 10.1080/15287390902959508. [DOI] [PubMed] [Google Scholar]
- DePalma G, Mozzoni P, Mutti A, Calzetti S, Negrotti A. Case–control study of interactions between genetic and environmental factors in Parkinson's disease. Lancet. 1988;352:1986–1987. doi: 10.1016/s0140-6736(05)61332-3. [DOI] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Söderkvist P, Felice A, Geoparkinson Study Group Environmental risk factors for Parkinson's disease and parkinsonism: the Geoparkinson study. Occup. Environ. Med. 2007;64(673-80):666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG. Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology. 2000;21:321–330. [PubMed] [Google Scholar]
- Evans EB, Balster RL. CNS depressant effects of volatile organic solvents. Neurosci. Biobehav. Rev. 1991;15:233–241. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- Feany MB, Anthony DC, Frosch MP, Zane W, De Girolami U. Two cases with necrosis and hemorrhage in the putamen and white matter. Brain Pathol. 2001;11:121–122. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Feldman AL, Johansson AL, Nise G, Gatz M, Pedersen NL, Wirdefeldt K. A 43-year prospective cohort study in men. Parkinsonism Relat. Disord. 2011;17:677–682. doi: 10.1016/j.parkreldis.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Halliday W, Kleinschmidt-DeMasters BK. The effect of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004;63:1–12. doi: 10.1093/jnen/63.1.1. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Vardi J. Progressive parkinsonism in a young experimental physicist following long term exposure to methanol. Neurotoxicology. 2002;23:521–525. doi: 10.1016/s0161-813x(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, Longstreth WT, Jr., Checkoway H. Occupational factors and risk of Parkinson's disease: a population-based case–control study. Am. J. Ind. Med. 2010;53:217–223. doi: 10.1002/ajim.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Lazzeri G, Bandettini Di Poggio A, Soldani P, De Blasi A, Nicoletti F, Ruggieri S, Paparelli A. Convergent roles of alpha-synuclein, DA metabolism, and the ubiquitin–proteasome system in nigrostriatal toxicity. Ann. N. Y. Acad. Sci. 2006;1074:84–89. doi: 10.1196/annals.1369.007. [DOI] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson ML, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi D-Y, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. Trichloroethylene: parkinsonism and complex I mitochondrial neurotoxicity. Ann. Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Geibprasert S, Gallucci M, Krings T. Addictive illegal drugs: structural neuroimaging. Am. J. Neuroradiol. 2010;31:803–808. doi: 10.3174/ajnr.A1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach M, Xiao AY, Heim C, Lan J, God R, Feineis D, Bringmann G, Riederer P, Sontag KH. 1-Trichloromethyl-1,2,3,4-tetrahydro-beta-carboline increases extracellular serotonin and stimulates hydroxyl radical production in rats. Neurosci. Lett. 1998;257:17–20. doi: 10.1016/s0304-3940(98)00791-5. [DOI] [PubMed] [Google Scholar]
- Gibrat C, Saint-Pierre M, Bousquet M, Lévesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J. Neurochem. 2009;109:1469–1482. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- Goldberg NR, Hampton T, McCue S, Kale A, Meshul CK. Profiling changes in gait dynamics resulting from progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal lesioning. J. Neurosci. Res. 2011;89:1698–1706. doi: 10.1002/jnr.22699. [DOI] [PubMed] [Google Scholar]
- Goldman SM. Trichloroethylene and Parkinson's disease: dissolving the puzzle. Expert Rev. Neurother. 2010;10:835–837. doi: 10.1586/ern.10.61. [DOI] [PubMed] [Google Scholar]
- Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korel M, Chade AR, Kasten M, Priestley B, Chou KL, Fernandez HH, Cambi F, Langston JW, Tanner CM. Solvent exposures and parkinson disease risk in twins. Ann. Neurol. 2012;71:776–784. doi: 10.1002/ana.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralewicz S, Dyzma M. Organic solvents and the dopaminergic system. Int. J. Occup. Med. Environ. Health. 2005;18:103–113. [PubMed] [Google Scholar]
- Green T, Dow J, Foster JR. Increased formic acid excretion and the development of kidney toxicity in rats following chronic dosing with trichloroethanol, a major metabolite of trichloroethylene. Toxicology. 2003;191:109–119. doi: 10.1016/s0300-483x(03)00206-3. [DOI] [PubMed] [Google Scholar]
- Guehl D, Bezaed E, Dovero S, Boraud T, Bioulac B, Gross C. Trichloroethylene and parkinsonism: a human and experimental observation. Eur. J. Neurol. 1999;6:609–611. doi: 10.1046/j.1468-1331.1999.650609.x. [DOI] [PubMed] [Google Scholar]
- Hall GM, Kirtland SJ, Baum H. The inhibition of mitochondrial respiration by inhalational anaesthetic agents. Br. J. Anaesth. 1973;45:1005–1009. doi: 10.1093/bja/45.10.1005. [DOI] [PubMed] [Google Scholar]
- Hester SD, Johnstone AF, Boyes WK, Bushnell PJ, Shafer TJ. Acute toluene exposure alters expression of genes in the central nervous system associated with synaptic structure and function. Neurotoxicol. Teratol. 2011;33:521–529. doi: 10.1016/j.ntt.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hewer CL. Trichlorethylene. Anaesthesia. 1975;30:483–487. [PubMed] [Google Scholar]
- Kim ST, Son HJ, Choi JH, Ji IJ, Hwang O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson's disease. Brain Res. 2010;1306:176–183. doi: 10.1016/j.brainres.2009.09.103. [DOI] [PubMed] [Google Scholar]
- King MD. Neurological sequelae of toluene abuse. Hum. Toxicol. 1982;1:281–287. doi: 10.1177/096032718200100311. [DOI] [PubMed] [Google Scholar]
- Kochen W, Kohlmüller D, De Biasi P, Ramsay R. The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson's disease. Adv. Exp. Med. Biol. 2003;527:253–263. doi: 10.1007/978-1-4615-0135-0_29. [DOI] [PubMed] [Google Scholar]
- Kondo H, Huang J, Ichihara G, Kamijima M, Saito I, Shibata E, Ono Y, Hisanaga N, Takeuchi Y, Nakahara D. Toluene induces behavioral activation without affecting striatal dopamine metabolism in the rat: behavioral and microdialysis studies. Pharmacol. Biochem. Behav. 1995;51:97–101. doi: 10.1016/0091-3057(94)00365-p. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston J, Irwin I, Ricaurte G. Neurotoxins, parkinsonism, and Parkinson's disease. Pharmacol. Ther. 1987;32:19–49. doi: 10.1016/0163-7258(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Lash LH, Fisher JW, Lipscomb JC, Parker JC. Metabolism of trichloroethylene. Environ. Health Perspect. 2000;108(Suppl. 2):177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar RB, Ho SU, Melen O, Daghestani AN. Multifocal central nervous system damage caused by toluene abuse. Neurology. 1983;33:1337–1340. doi: 10.1212/wnl.33.10.1337. [DOI] [PubMed] [Google Scholar]
- Lee KW, Zhao X, Im JY, Grosso H, Jang WH, Chan TW, Sonsalla PK, German DC, Ichijo H, Junn E, Mouradian MM. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS One. 2012;7:e29935. doi: 10.1371/journal.pone.0029935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Choi D-Y, Hunter R, Pandya JD, Cass WA, Sullivan PG, Kim H-C, Gash DM, Bing G. Trichloroethylene induces dopaminergic neurodegeneration in Fischer 344 rats. J. Neurochem. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolin Y. Chronic neurological toxicity associated with exposure to volatile substances. Hum. Toxicol. 1989;8:293–300. doi: 10.1177/096032718900800407. [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson's disease. Annu. Rev. Genomics Hum. Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell L, Maginnis C, Lewis S, Pickering N, Antoniak M, Hubbard R, Lawson I, Britton J. Occupational exposure to solvents and metals and Parkinson's disease. Neurology. 2003;61:716–717. doi: 10.1212/wnl.61.5.716. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Borenstein AR, Nelson LM. Associations of welding and manganese exposure with Parkinson disease: review and meta-analysis. Neurology. 2012;79:1174–1180. doi: 10.1212/WNL.0b013e3182698ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP Technical report on the toxicology and carcinogenesis studies of chloral hydrate (CAS no. 302-17-0) in B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2002;502:1–197. [PubMed] [Google Scholar]
- Ohlson CG, Hogstedt C. Parkinson's disease and occupational exposure to organic solvents, agricultural chemicals and mercury—a case-referent study. Scand. J. Work Environ. Health. 1981;7:252–256. doi: 10.5271/sjweh.2549. [DOI] [PubMed] [Google Scholar]
- Papageorgiou SG, Karantoni E, Pandis D, Kouzoupis AV, Kalfakis N, Limouris GS. Severe dopaminergic pathways damage in a case of chronic toluene abuse. Clin. Neurol. Neurosurg. 2009;111:864–867. doi: 10.1016/j.clineuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am. J. Ind. Med. 2005;48:63–67. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, Jørgensen PJ, Budtz-Jørgensen E, Grandjean P. Impact of dietary exposure to food contaminants on the risk of Parkinson's disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Barbieri S, Ferrante C, Zecchinelli A, Fao V. Parkinsonism due to n-hexane exposure. Lancet. 1989;2:874. doi: 10.1016/s0140-6736(89)93050-x. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Ricciardi S, Masotto C, Mariani CB, Carenzi A. n-Hexane induces Parkinsonism in rodents. Brain Res. 1990;531:355–357. doi: 10.1016/0006-8993(90)90801-h. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Canesi M, Antonini A, Righini A, Perbellini L, Barichella M, Mariani CB, Tenconi F, Tesei S, Zecchinelli A, Leenders KL. Hydrocarbon exposure and Parkinson's disease. Neurology. 2000;55:667–673. doi: 10.1212/wnl.55.5.667. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov. Disord. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J. Neurol. Sci. 2007;262:122–130. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Rea TM, Nash JF, Zabik JE, Born GS, Kessler WV. Effects of toluene inhalation on brain biogenic amines in the rat. Toxicology. 1984;31:143–150. doi: 10.1016/0300-483x(84)90006-4. [DOI] [PubMed] [Google Scholar]
- Reddy NJ, Sudini M, Lewis LD. Delayed neurological sequelae from ethylene glycol, diethylene glycol and methanol poisonings. Clin. Toxicol. (Phila.) 2010;48:967–973. doi: 10.3109/15563650.2010.532803. [DOI] [PubMed] [Google Scholar]
- Riederer P, Foley P, Bringmann G, Feineis D, Bruckner R, Gerlach M. Biochemical and pharmacological characterization of 1-trichloro-1,2,3,4-tetrahydro-β-carboline: a biologically relevant neurotoxin? Eur. J. Pharmacol. 2002;442:1–16. doi: 10.1016/s0014-2999(02)01308-0. [DOI] [PubMed] [Google Scholar]
- Rissanen E, Paavilainen T, Virta J, Marttila RJ, Rinne JO, Airas L. Carbon monoxide poisoning-induced nigrostriatal dopaminergic dysfunction detected using positron emission tomography (PET). Neurotoxicology. 2010;31:403–407. doi: 10.1016/j.neuro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch. Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Rubinstein D, Escott E, Kelly JP. Methanol intoxication with putaminal and white matter necrosis: MR and CT findings. Am. J. Neuroradiol. 1995;16:1492–1494. [PMC free article] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-science review: does manganese exposure during welding pose a neurological risk? J. Toxicol. Environ. Health B Crit. Rev. 2007;10:417–465. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Hunter R, Bing G, Sullivan PG. Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits. Exp. Neurol. 2012;234:85–94. doi: 10.1016/j.expneurol.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RK, Sedelis M, Hofele K, Auburger GW, Huston JP. Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson's disease. Neurotox. Res. 1999;1:41–56. doi: 10.1007/BF03033338. [DOI] [PubMed] [Google Scholar]
- Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, Oertel WH, Ulm G, Schneider E. Possible environmental, occupational, and other etio-logic factors for Parkinson's disease: a case–control study in Germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- Shahabi HN, Andersson DR, Nissbrandt H. Cytochrome P450 2E1 in the substantia nigra: relevance for dopaminergic neurotransmission and free radical production. Synapse. 2008;62:379–388. doi: 10.1002/syn.20505. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case–control study of occupational and environmental risk factors for Parkinson's disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998;19:709–712. [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH, Sabri MI, Veronesi B. The enlarging view of hexacarbon neurotoxicity. Crit. Rev. Toxicol. 1980;7:279–356. doi: 10.3109/10408448009037489. [DOI] [PubMed] [Google Scholar]
- Stengård K, Höglund G, Ungerstedt U. Extracellular dopamine levels within the striatum increase during inhalation exposure to toluene: a microdialysis study in awake, freely moving rats. Toxicol. Lett. 1994;71:245–255. doi: 10.1016/0378-4274(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Tanner CM. Occupational and environmental causes of parkinsonism. Occup. Med. 1992;7:503–513. [PubMed] [Google Scholar]
- Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, Bressman S, Deligtisch A, Marrasm C, Lyons KE, Bhudhikanok GS, Roucoux DF, Meng C, Abbott RD, Langston JW. Occupation and risk of parkinsonism: a multicenter case–control study. Arch. Neurol. 2009;66:1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- Tephly TR. Toxicity of methanol. Life Sci. 1991;48:1031–1041. doi: 10.1016/0024-3205(91)90504-5. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J. Neurosci. Methods. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- van der Mark MBM, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ. Health Perspect. 2012;120:340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler G, Fuxe K, Benfenati F, Hansson T, Agnati LF, Gustafsson JA. Neurotensin modulates the binding characteristics of dopamine D2 receptors in rat striatal membranes also following treatment with toluene. Acta Physiol. Scand. 1989;135:443–448. doi: 10.1111/j.1748-1716.1989.tb08602.x. [DOI] [PubMed] [Google Scholar]
- von Euler G, Ogren SO, Bondy SC, McKee M, Warner M, Gustafsson JA, Eneroth P, Fuxe K. Subacute exposure to low concentrations of toluene affects dopamine-mediated locomotor activity in the rat. Toxicology. 1991;67:333–349. doi: 10.1016/0300-483x(91)90032-v. [DOI] [PubMed] [Google Scholar]
- von Euler G, Ogren SO, Li XM, Fuxe K, Gustafsson JA. Persistent effects of subchronic toluene exposure on spatial learning and memory, dopamine-mediated locomotor activity and dopamine D2 agonist binding in the rat. Toxicology. 1993;77:223–232. doi: 10.1016/0300-483x(93)90162-l. [DOI] [PubMed] [Google Scholar]
- von Euler M, Pham TM, Hillefors M, Bjelke B, Henriksson B, von Euler G. Inhalation of low concentrations of toluene induces persistent effects on a learning retention task, beam-walk performance, and cerebrocortical size in the rat. Exp. Neurol. 2000;163:1–8. doi: 10.1006/exnr.1999.7288. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Eells JT, Madeira VM, Cortopassi G, Jones DP. Mitochondria-mediated cell injury: symposium overview. Fundam. Appl. Toxicol. 1997;38:23–37. doi: 10.1006/faat.1997.2320. [DOI] [PubMed] [Google Scholar]
- Wang J, Hokestra JG, Zuo C, Cook TJ, Zhang J. Biomarkers of Parkinson's disease: current status and future. Drug Discov. Today. 2012 doi: 10.1016/j.drudis.2012.09.001. http://dx.doi.org/10.1016/jdrudis.2012. 09.001. pii.S1359-6446(12) 00304-2. [DOI] [PMC free article] [PubMed]
- Watts PM, Riedl AG, Douek DC, Edwards RJ, Boobis AR, Jenner P, Marsden CD. Co-localization of P450 enzymes in the rat substantia nigra with tyrosine hydroxylase. Neuroscience. 1998;86:511–519. doi: 10.1016/s0306-4522(97)00649-0. [DOI] [PubMed] [Google Scholar]
- Williamson TP, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology. 2012;33:272–279. doi: 10.1016/j.neuro.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur. J. Epidemiol. 2011;26(Suppl. 1):S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Takagi M, Walterfang M, Lubman DI. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci. Biobehav. Rev. 2008;32:910–926. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gavin T, DeCaprio AP, LoPachin RM. Gamma-diketone axonopathy: analyses of cytoskeletal motors and highways in CNS myelinated axons. Toxicol. Sci. 2010;117:180–189. doi: 10.1093/toxsci/kfq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Hastings TG, Perez RG. Increased dopamine turnover after partial loss of dopaminergic neurons: compensation or toxicity? Parkinsonism Relat. Disord. 2002;8:389–393. doi: 10.1016/s1353-8020(02)00019-6. [DOI] [PubMed] [Google Scholar]