Abstract
Primary progressive aphasia (PPA) is a language predominant neurodegenerative disorder that has three recognized variants: nonfluent/agrammatic, semantic, and logopenic. This report describes a 60-year-old man who presented with a progressive decline in verbal output that does not fit the currently accepted PPA subtypes. The patient exhibited a paucity of verbal output and impaired phonemic fluency with minimal associated language, cognitive, or behavioral deficits. Focal cortical thinning/hypometabolism of the left superior frontal region and a cerebrospinal fluid profile not consistent with Alzheimer’s disease pathology were identified. This case of isolated progressive dynamic aphasia extends the current boundaries of PPA diagnostic variants.
Keywords: Cerebrospinal fluid, magnetic resonance imaging, neurodegenerative disease, PET scan, primary progressive aphasia
INTRODUCTION
A progressive language disorder associated with regional, left-hemispheric frontotemporal atrophy at autopsy was first recognized by Pick [1] and Serieux [2] in the 1890 s. Patients with insidious onset, progressive language deterioration and the relative absence of non-linguistic cognitive, behavioral, and affective symptoms early in the course have since been characterized as having primary progressive aphasia (PPA) [3, 4]. PPA was initially divided into two subtypes: progressive nonfluent and fluent variants, the latter characterized by prominent anomia and single-word comprehension deficits originally termed semantic dementia [5]. A third subgroup, logopenic variant PPA, has been more recently characterized [6].
Clinical-radiographic-pathologic comparative studies have delineated three PPA variants, formalized in 2011 in new international consensus diagnostic criteria [7]. The nonfluent/agrammatic PPA variant is characterized by agrammatism and effortful, halting speech and associated with left posterior fronto-insular atrophy/hypoactivity usually due to tau or less often TAR DNA-binding protein (TDP-43) pathology. The semantic PPA variant is characterized by impaired naming and single-word comprehension and associated with anterior temporal atrophy/hypoactivity usually due to TDP-43 pathology. The logopenic PPA variant is characterized by impaired single-word retrieval and repetition and associated with left temporoparietal atrophy/hypofunction and commonly Alzheimer’s disease (AD) pathology.
Here, we report a case of dynamic aphasia in an individual with insidious onset, progressively diminished verbal output, with minimal associated language, cognitive, behavioral, or motor deficits, and demonstrate left superior frontal atrophy/hypometabolism. This case extends the boundaries of the currently accepted PPA classification schemes.
MATERIALS AND METHODS
Neuropsychological evaluation
The patient underwent periodic neuropsychological assessment of language and non-language domains over approximately a four-year period. Tests performed included: Western Aphasia Battery-Revised (WAB-R) [8]; Cambridge Semantic Battery (CSB) [9, 10]; Boston Naming Test (BNT), modified version [11]; phonemic and categorical fluency [12]; Word list test of memory from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [13]; Montreal Cognitive Assessment (MOCA) [14]; Dementia Rating Scale (DRS) [15]; Geriatric Depression Scale (GDS) [16].
Neuroimaging studies
Axial T1-weighted and 3-D SPGR magnetic resonance images (MRI) were acquired on a 1.5T scanner. In addition, quantitative cortical thickness analysis was performed by comparing this patient to age-matched controls (n = 20) using a cortical surface-based reconstruction as described by Dickerson and colleagues [17]. Positron emission tomography (PET) scans were acquired following an intravenous injection of 18-fluorodeoxyglucose (18-FDG).
Cerebrospinal fluid sampling
Cerebrospinal fluid (CSF) analysis for proteins associated with AD was performed through Athena Diagnostics (ADmark®).
RESULTS
Case report
A60-year-old, right-handed, Caucasian retired electrical engineer presented with two years of insidious onset, progressive decline in verbal output. He complained of word-finding difficulties and reported “trouble keeping thoughts in [his] mind. . . (and) losing words in [his] head.” Over this period, he displayed otherwise normal language and cognitive abilities. He continued to drive, play golf, and manage the house-hold finances without difficulty. There was no history of apathy, depression, or comportmental changes. The patient acknowledged frustration about his predicament and had been previously started on Escitalopram. His medical history was notable for hypertension treated with Olmesartan. There was no known family history of neurodegenerative disease.
Prior to our cognitive-behavioral neurology evaluation, the patient had undergone neuropsychological and neurologic evaluations within the first year of symptom onset. Neuropsychological testing showed high-average to average performance on tasks of orientation, attention, psychomotor speed, visual perception/construction, and abstraction. DRS was within normal limits (141/144). Grammar, comprehension, repetition, reading, writing, and prosody were normal. However, he exhibited markedly reduced phonemic fluency (FAS = 14, z = −2.3) with less severe categorical word list generation impairments (animals = 13, z= −1.2). He correctly named all 60 BNT items. Brain MRI was reportedly normal, but unavailable for review.
On initial presentation to our group, neurologic exam was unremarkable, including preserved eye movements and no Parkinsonism. There was a striking paucity of spontaneous verbal output, with associated word-finding pauses; however, utterances in response to questions included grammatical, well-articulated phrase lengths of greater than 6 words. He did not stutter and his speech was without dysarthria, phonetic distortions, or phonemic paraphasias. His performance on portions of the WAB-R and CSB demonstrated: Auditory Comprehension: appropriate verbal exchange; preserved ability to follow task directions except for lengthy commands, particularly those in which the agent completing the action appeared at the end of the sentence (e.g. “point to the pen with the book”). Verbal Expression: in constrained tasks, such as picture descriptions and generation of definitions, speech was grammatical, articulate, and meaningful though sparse. He had difficulty forming a cohesive narrative that related characters and actions to each other. Naming: spontaneously named 29/30 (modified BNT) and 63/64 (CSB) items. Repetition: intact for single words, phrases, and sentences. He repeated all but one word in a lengthy sentence (“Pack my box with five dozen jugs of liquid detergent”). Word Generation: phonemic fluency: FAS = 11, z = −2.3. Categorical fluency: animals = 15, z = −0.8. Written Language: preserved spontaneous and cued writing. Reading: accurately read short paragraphs and commands. Praxis: normal limb, oral, and buccofacial praxis to complex commands. Calculations: quick, accurate arithmetic performance. Orientation: preserved. Attention: digit span: 5 forward, 4 backwards (9th percentile). Executive Function: accurate and well-organized clock. Spatial span backwards, 4 (50th percentile). Memory (CERAD word list): encoded 17/30 (3,7,7 over 3 trials: #10th percentile), freely recalled 8/10 after 5 minute delay (#66th percentile), recognized 100% with one false positive (#21st percentile). Visual-spatial: accurate 3-dimensional cube drawing. MOCA: 28/30, losing points on word fluency and repetition. DRS: 132/144, with initiation scale deficits. GDS: 4 (normal).
The patient has been followed longitudinally for over four years during which time his symptoms have become more severe, but he has maintained this distinctive language phenotype (Table 1). He has become less engaged socially, which could reflect apathy; however, according to the patient and his wife, the major source of his withdrawal is his reduced ability to quickly respond verbally, which he finds embarrassing.
Table 1.
Longitudinal language test scores. Note: all scores unless specified detail Western Aphasia Battery-Revised scores. CSB indicates Cambridge Semantic Battery
| Language Test Scores | Year 1 | Year 3 | Year 5 |
|---|---|---|---|
| Verbal Expression | fluent, grammatical | grammatical, yet sparse | grammatical, poor language initiation |
| Repetition | intact | 96/100 | 90/100 |
| Boston Naming Test | 60/60 | 29/30 | 27/30 |
| Sentence Comprehension | intact | 40/40 | 34/40 |
| Sequential Commands | intact | 62/80 | 72/80 |
| Writing | intact | intact | grammatical, shortened phrase length |
| Reading Commands | intact | 20/20 | 20/20 |
| Lexical/Picture Semantics | not obtained | CSB: 63/64 | CSB: 61/64 |
| Phonemic fluency: FAS | 14 | 11 | 9* |
| Semantic fluency: Animals | 13 | 15 | 5 |
Denotes score acquired in year 4.
Neuroimaging studies
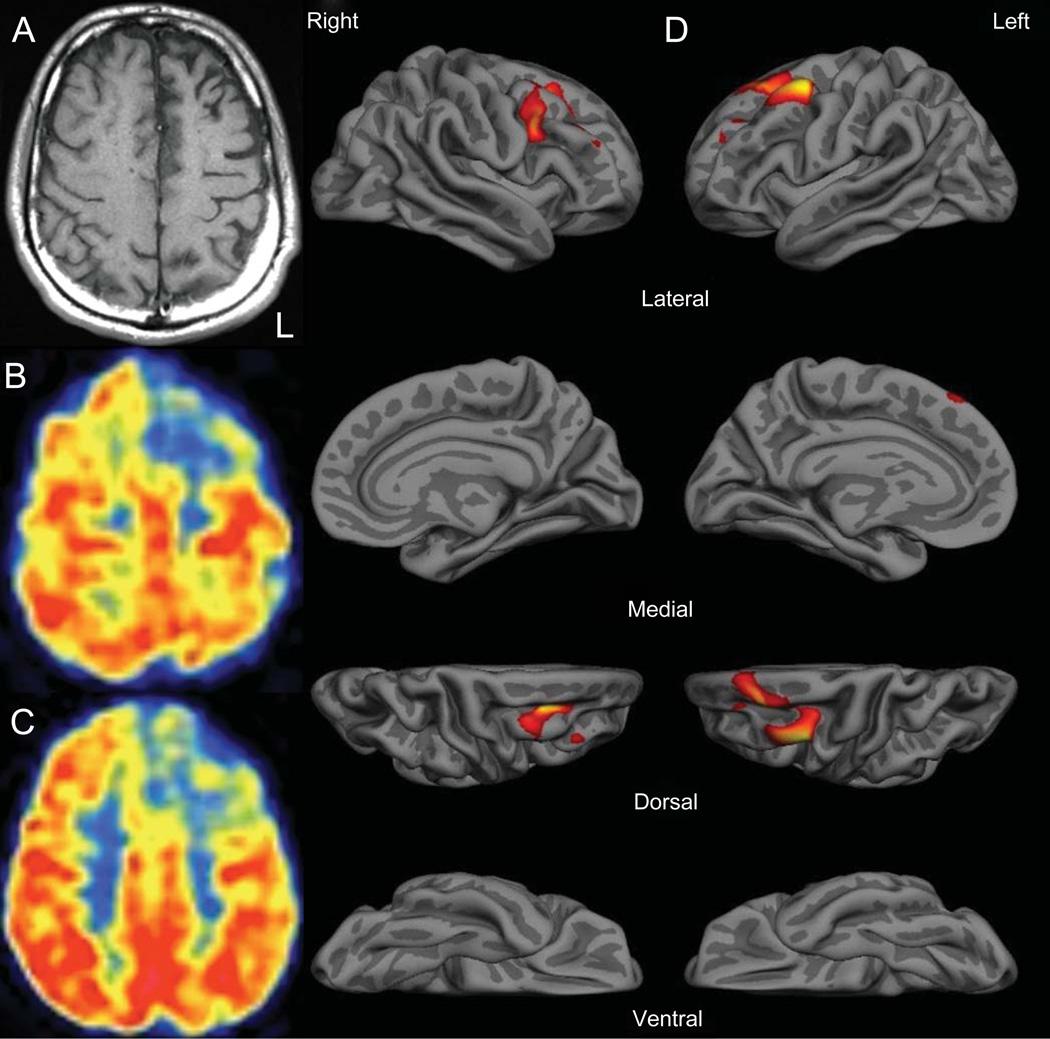
T1-weighted MRI showed gyral atrophy in the left frontal region (Fig. 1). 18-FDG PET revealed focal hypometabolism in the left superior and middle frontal gyri. Cortical thickness was reduced in the mid-caudal superior frontal and caudal middle frontal gyri (left greater than right) compared to healthy aged-matched controls (n = 20).
Fig. 1.
Left lateralized frontal atrophy and hypometabolism. A) T1-weighted MRI demonstrating mild left anterior frontal atrophy. B, C) FDG-PET showing left anterior frontal hypometabolism. D) Quantitative cortical thickness analysis. Focal cortical thickness reductions (red on color map) occurred in left > right superior and middle frontal gyri. L indicates left.
Cerebrospinal fluid sampling
CSF protein analysis was not consistent with AD pathology: Aβ42: 980.2 pg/ml; total-tau: 201.1 pg/ml; phosphorylated-tau: 45.8 pg/ml; Aβ42/total-tau ratio was 2.05 (non-AD pattern: >1.0).
DISCUSSION
A language-based syndrome characterized by marked reduction in spontaneous propositional speech despite preserved naming, comprehension, and the ability to produce speech was first characterized by Lichtheim in 1885 and termed dynamic aphasia; Luria subsequently classified dynamic aphasia as a subtype of transcortical motor aphasia [18]. Luria described this phenomenon as “aphasia without aphasia” and theorized a disturbance in the “transition from the initial thought to the linear scheme of the phrase” as an explanation for this clinical phenotype. Patients with dynamic aphasia have been infrequently detailed in the literature as a result of axial and extra-axial left frontal tumors [19, 20], progressive supranuclear palsy (PSP) [21, 22], left prefrontal infarctions [23], and bilateral striatocapsular infarctions [24]. Several prior cases [25–27] have been reported of “primary progressive dynamic aphasia” [26] attributed to a frontotemporal neurodegenerative process, but these reports have not included detailed modern neuroimaging and/or CSF biomarkers.
This patient’s productive speech deficit—insidiously progressive over more than four years and the major source of functional impairment—is consistent with PPA [28]. The salient features of his aphasia include a paucity of verbal output, particularly in situations requiring spontaneous narrative generation, deficits in phonemic fluency out of proportion to categorical fluency deficits, and modest verbal working memory impairments. Speech was grammatical with minimal to no deficits in naming, repetition, comprehension, and written language. He also lacked signs of oral/buccofacial apraxia and his speech was not poorly articulated, phonetically distorted, or aprosodic to suggest an apraxia of speech [29]. Thus, the clinical features are not consistent with the three major PPA variants, or with a premotor speech impairment as has been described in neurodegenerative cases with posterior frontal hypoperfusion [30], but rather with a dynamic aphasia variant of PPA. Neuroimaging studies identified left-lateralized cortical thinning in the caudal superior and middle frontal gyri, with prominent left superior frontal hypometabolism, providing further support for localization consistent with some prior studies of dynamic aphasia and distinct from that of the three major PPA variants.
The patient’s CSF showed an Aβ/tau pattern suggestive of non-AD pathology [31]. Although this patient’s clinical syndrome could be due to a new proteinopathy, we suspect a tauopathy with an atypical anatomic distribution. The tauopathies have overlapping pathological features and include corticobasal degeneration (CBD), PSP, and forms of frontotemporal dementia [32]; Kertesz and colleagues have categorized the resulting clinical syndromes under the term “Pick complex” [33]. Both CBD and PSP are tauopathies that can display language disturbances, with CBD associated with progressive non-fluent aphasia [33–35] and PSP with dynamic aphasia [21, 22]. However, over a four-plus-year period, our patient has not demonstrated the cardinal features associated with CBD (asymmetric rigidity, apraxia, cortical sensory loss, alien-limb), or PSP (axial dystonia, bradykinesia, falls, vertical gaze palsy), making these clinical entities unlikely. Furthermore, although many patients with behavioral variant frontotemporal dementia (bvFTD) develop sparse spontaneous speech, the absence of salient personality and comportmental changes in this case is inconsistent with a diagnosis of bvFTD.
Several overlapping hypotheses have been proposed to account for dynamic aphasia, including reduced ability to select between competing verbal responses [20], disruption of the executive aspects of language output [36], or impairments in lexical search strategies [24] and verbal planning [19]. The neuroanatomically focal aspects of this case strongly suggest that the left superior and middle frontal gyri are critical for complex discourse and verbal activation-retrieval functions [37], with disruption leading to dynamic aphasia. This notion is consistent with other functions associated with left prefrontal cortex including the central executive component of working memory and the multi-faceted processes involved in organization and planning [38]. This and related cases in the literature highlight the need to continue to refine diagnostic subtypes within the spectrum of PPA.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1556).
REFERENCES
- 1.Pick A. On the relationship between sensile cerebral atrophy and aphasia. Prager Medicinische Wochenschrift. 1892;17:165–167. [Google Scholar]
- 2.Serieux P. On a case of pure verbal deafness. Revue Medicale. 1893;13:733–750. [Google Scholar]
- 3.Mesulam MM. Primary progressive aphasia–a language-based dementia. N Engl J Med. 2003;349:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- 4.Grossman M. Primary progressive aphasia: Clinicopathological correlations. Nat Rev Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snowden JSPK, Oxbury S, Funnell E. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioral Neurology. 1989;2:167–182. [Google Scholar]
- 6.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kertesz A. Western aphasia battery-revised. Austin, TX: Pro-Ed; 2006. [Google Scholar]
- 9.Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, Hodges JR. Semantic dementia and fluent primary progressive aphasia: Two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- 10.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 11.Saxton J, Ratcliff G,Munro CA, Coffey EC, Becker JT, Fried L, Kuller L. Normative data on the Boston Naming Test and two equivalent 30-item short forms. Clin Neuropsychol. 2000;14:526–534. doi: 10.1076/clin.14.4.526.7204. [DOI] [PubMed] [Google Scholar]
- 12.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. New York: Oxford University Press; 1991. [Google Scholar]
- 13.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 15.Mattis S. Dementia rating scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 16.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luria AR, Tsvetkova LS. The mechanism of ‘dynamic aphasia’. Found Lang. 1968;4:296–307. [Google Scholar]
- 19.Costello AL, Warrington EK. Dynamic aphasia: The selective impairment of verbal planning. Cortex. 1989;25:103–114. doi: 10.1016/s0010-9452(89)80010-3. [DOI] [PubMed] [Google Scholar]
- 20.Robinson G, Blair J, Cipolotti L. Dynamic aphasia: An inability to select between competing verbal responses? Brain. 1998;121(Pt 1):77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Esmonde T, Giles E, Xuereb J, Hodges J. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatry. 1996;60:403–410. doi: 10.1136/jnnp.60.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson G, Shallice T, Cipolotti L. Dynamic aphasia in progressive supranuclear palsy: A deficit in generating a fluent sequence of novel thought. Neuropsychologia. 2006;44:1344–1360. doi: 10.1016/j.neuropsychologia.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Freedman M, Alexander MP, Naeser MA. Anatomic basis of transcortical motor aphasia. Neurology. 1984;34:409–417. doi: 10.1212/wnl.34.4.409. [DOI] [PubMed] [Google Scholar]
- 24.Gold M, Nadeau SE, Jacobs DH, Adair JC, Rothi LJ, Heilman KM. Adynamic aphasia: A transcortical motor aphasia with defective semantic strategy formation. Brain Lang. 1997;57:374–393. doi: 10.1006/brln.1997.1750. [DOI] [PubMed] [Google Scholar]
- 25.Snowden JS, Griffiths HL, Neary D. Progressive language disorder associated with frontal lobe degeneration. Neurocase. 1996;2:429–440. [Google Scholar]
- 26.Warren JD, Warren JE, Fox NC, Warrington EK. Nothing to say, something to sing: Primary progressive dynamic aphasia. Neurocase. 2003;9:140–155. doi: 10.1076/neur.9.2.140.15068. [DOI] [PubMed] [Google Scholar]
- 27.Kartsounis LD, Crellin RF, Crewes H, Toone BK. Primary progressive non-fluent aphasia: A case study. Cortex. 1991;27:121–129. doi: 10.1016/s0010-9452(13)80275-4. [DOI] [PubMed] [Google Scholar]
- 28.Mesulam M, Weintraub S. Primary progressive aphasia and kindred disorders. Handb Clin Neurol. 2008;89:573–587. doi: 10.1016/S0072-9752(07)01254-7. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler W. Apraxia of speech. Handb Clin Neurol. 2008;88:269–285. doi: 10.1016/S0072-9752(07)88013-4. [DOI] [PubMed] [Google Scholar]
- 30.Didic M, Ceccaldi M, Poncet M. Progressive loss of speech: A neuropsychological profile of premotor dysfunction. Eur Neurol. 1998;39:90–96. doi: 10.1159/000007914. [DOI] [PubMed] [Google Scholar]
- 31.Bian H, Van Swieten JC, Leight S, Massimo L, Wood E, Forman M, Moore P, de Koning I, Clark CM, Rosso S, Trojanowski J, Lee VM, Grossman M. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kertesz A, McMonagle P, Jesso S. Extrapyramidal syndromes in frontotemporal degeneration. J Mol Neurosci. 2011;45:336–342. doi: 10.1007/s12031-011-9616-1. [DOI] [PubMed] [Google Scholar]
- 33.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 34.Lippa CF, Cohen R, Smith TW, Drachman DA. Primary progressive aphasia with focal neuronal achromasia. Neurology. 1991;41:882–886. doi: 10.1212/wnl.41.6.882. [DOI] [PubMed] [Google Scholar]
- 35.Mimura M, Oda T, Tsuchiya K, Kato M, Ikeda K, Hori K, Kashima H. Corticobasal degeneration presenting with nonfluent primary progressive aphasia: A clinicopathological study. J Neurol Sci. 2001;183:19–26. doi: 10.1016/s0022-510x(00)00470-6. [DOI] [PubMed] [Google Scholar]
- 36.Alexander MP. Impairments of procedures for implementing complex language are due to disruption of frontal attention processes. J Int Neuropsychol Soc. 2006;12:236–247. doi: 10.1017/S1355617706060309. [DOI] [PubMed] [Google Scholar]
- 37.Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ. Speech production:Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter PA, Just MA, Reichle ED. Working memory and executive function: Evidence from neuroimaging. Curr Opin Neurobiol. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]