Summary
An endovascular model for producing and studying middle cerebral artery acute ischemic strokes was developed to avoid the need for an open surgical approach to the middle cerebral, anterior cerebral, posterior cerebral and internal carotid arteries. Endovascular occlusion of these vessels followed by Xenon-CT cerebral blood flow study confirmed the production of a middle cerebral artery distribution infarct in two primates. The methodology, advantages and drawbacks of this model are discussed.
Key words: stroke model, endovascular, primate
Introduction
A variety of models have been described for the production of a permanent ischemic stroke in non-human primates. These models typically involve an open surgical approach. In 1975, Symon described a number of stroke models in baboons including the intracranial sphenoidal wing, retroorbital, and trans-orbital approaches1. Other models include the subtemporal middle cerebral occlusion approach and the implanted balloon model 2,3. Each of these involves exo-vascular exposure of the cranial contents with its attendant risks of infection and spinal fluid leak. In addition to these risks are the post-surgical animal care issues which can be difficult in an animal that wants to explore its wounds.
We sought to develop a rapid, minimally invasive primate acute stroke model which could be used to assess devices and protocols aimed at managing an acute middle cerebral artery (MCA) distribution ischemic event. Our endovascular approach will be described below along with correlating CT, Xenon blood flow, and angiographic findings.
Methods
Two Rhesus monkeys weighing 8.0 kg and 8.1 kg, respectively, were used for this feasability study. Each animal was anesthetized with ketamine (10 mg/kg IM) and intubated at which point anesthesia was maintained using fentanyl (25 micrograms/kg/hr) and diazepam (2.5 mg/hr titrated to blood pressure with systolic maximum 150 mm Hg). A small femoral cut down incision was made to isolate the femoral vein and artery. Using a 5F micropuncture set, a 5F femoral sheath was inserted into the femoral artery. This sheath was then continuously perfused with heparinized saline. Throngh the sheath a 5F angiographic catheter was advanced over a hydrophilic wire and selective catheterization was carried out of the right common carotid artery. A continuous flushing system was attached to the catheter and a 0.018 inch microcatheter (Tracker Turbo, Target Therapeutics, Inc, Fremont, CA) over a 0.014 inch microwire (Transcend EX, Target Therapeutics, Inc, Fremont, CA ) was advanced through the angiography catheter and into the internal carotid artery. Using the road mapping technique the microcatheter was then advanced into the right MCA's Ml segment. After confirming the catheter's position, the Ml and occasionally M2 segment were occluded using 5 mm straight, fibered coils (Target Therapeutics, Inc, Fremont, CA). The catheter was then withdrawn to the internal carotid artery (ICA) bifurcation where additional 5 mm straight and/or complex coils were deposited to reduce contralateral crossfill from the anterior cerebral artery (ACA). The catheter was next withdrawn into the ICA and the ICA was occluded using 2.5 mm fibered coils (figure 1). The microcatheter was now removed and the vertebral artery was catheterized with the diagnosticcatheter. Using road mapping techniques the microcatheter over a microwire was advanced into the right posterior cerebral artery's (PCA) P2 segment. This vessel was then occluded using 5 mm straight, fibered coils (figure 2). Control arteriography at the end of the procedure confirmed right P2 and right Ml occlusion with patency of all other vessels.
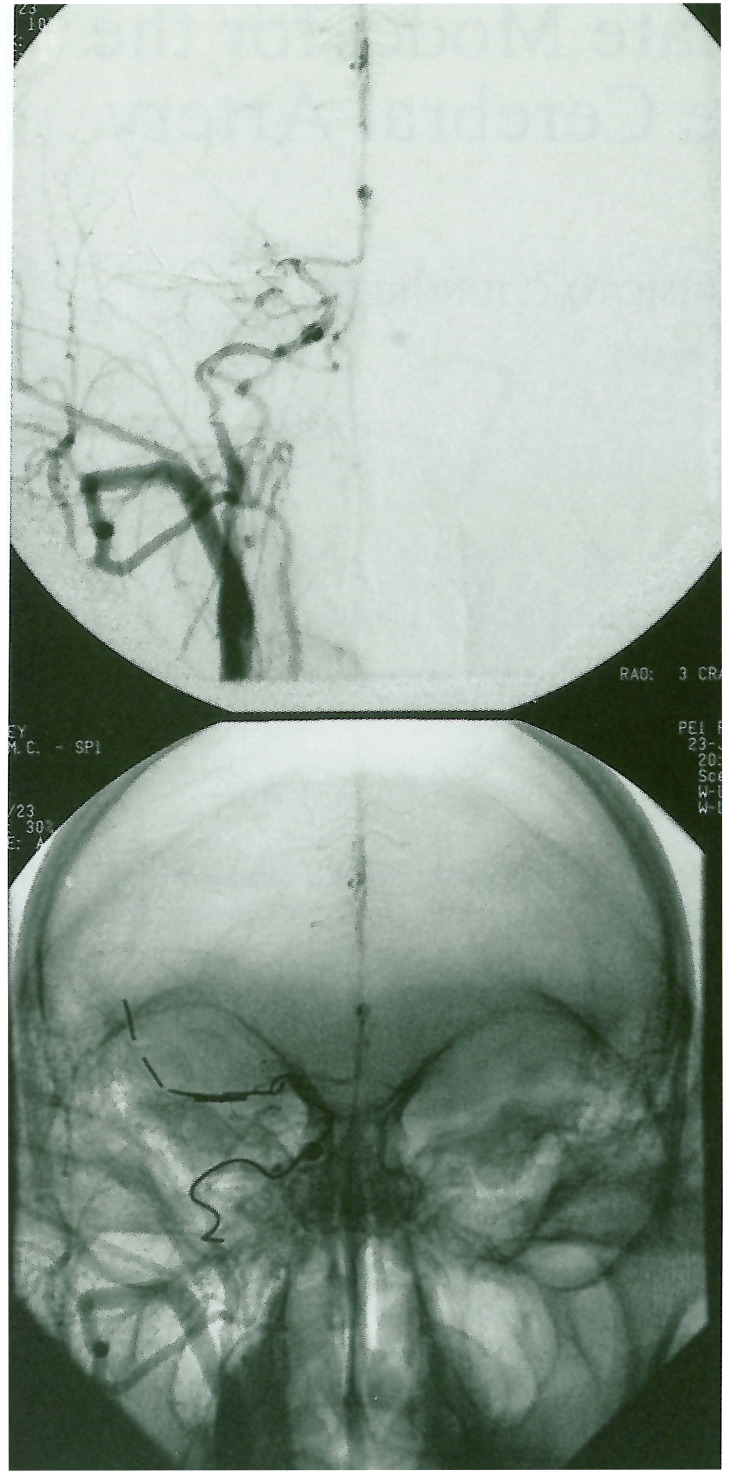
Figure 1.
Right common carotid arteriogram (AP projection) following right MCA and ICAocclusion with fibered coils demonstrates absent opacification of the right MCA distal to the lenticulostriate vessels.
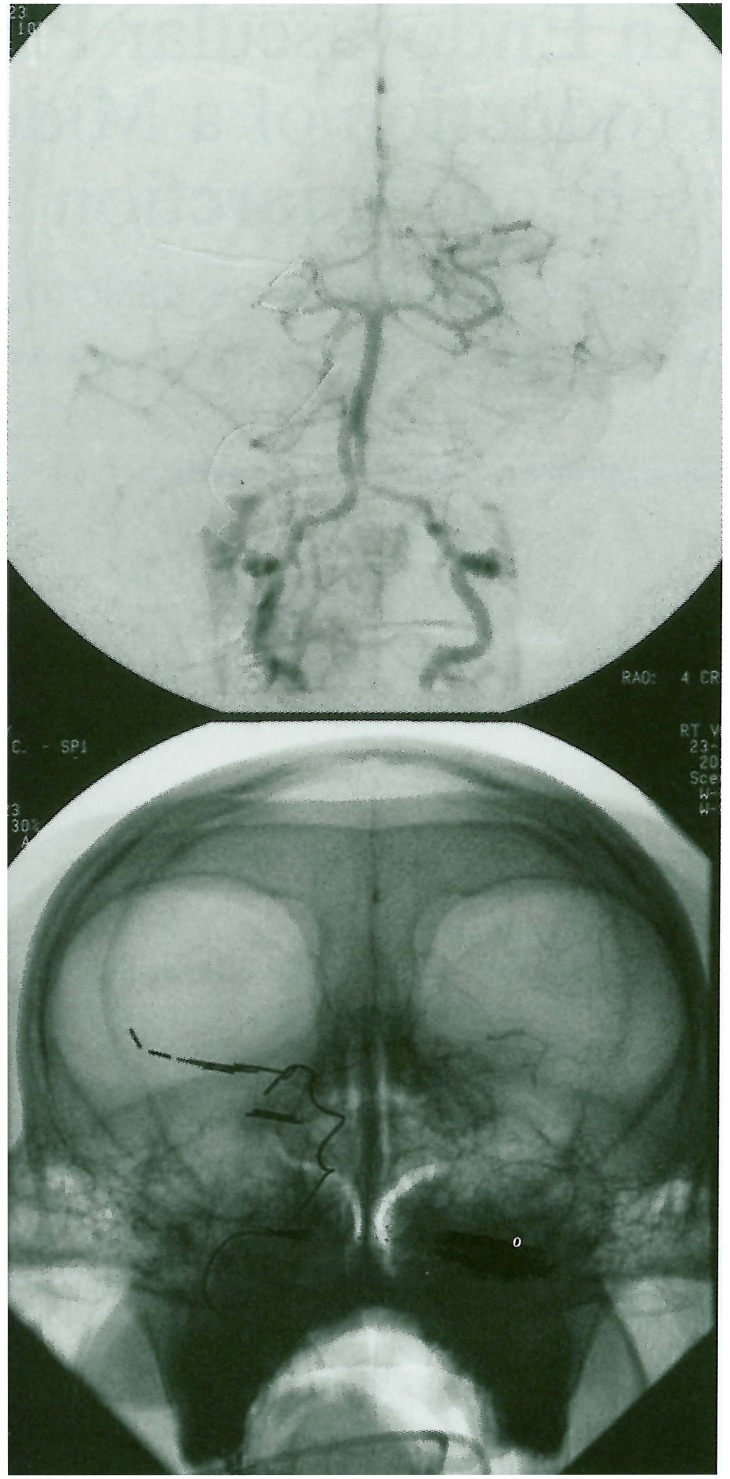
Figure 2.
Right vertebral artery arteriogram following right P2 occlusion using fibered coils. No distal PCA or MCA opacification (via the Pcomm) is seen.
Following vessel occlusion the animal was immediately transferred to the CT scanner where a head CT scan and Xenon-CT blood flow study (CBF) were completed. The animal was then transferred to the surgical laboratory for further evaluation of a device which remains unmentioned at this time.
Results
Using the above described endovascular stroke model we were able to rapidly produce reliable, well demarcated MCA distribution ischemic strokes in two non-human primates. CBF and CT hypodensity correlated well with the distribution of occluded vessels (figure 3). All animals survived the acute post-procedural period for completion of this non-survival study which allowed for experimental device implantation and study over four hours. The animal was then perfused and fixed with 2% paraformaldehyde during deep pentobarbital anesthesia. Histological study demonstrated MCA distribution infarction (figure 4).
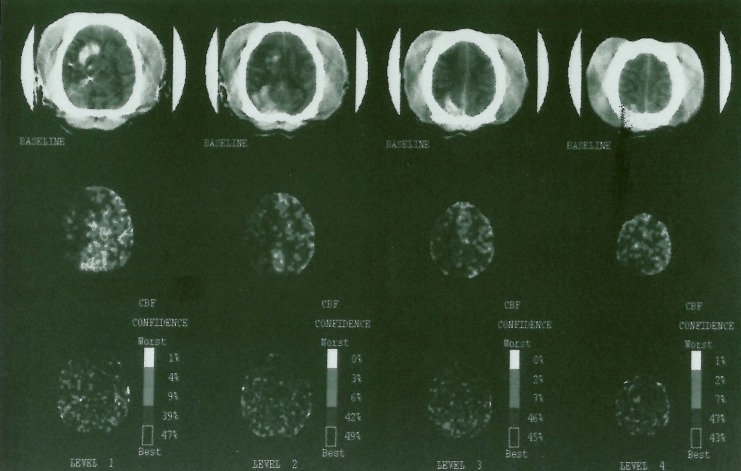
Figure 3.
Stable Xenon-CT scan showing axial images with corresponding blood flow maps. Right MCA distribution hypodensity can be seen to correlate with low to absent flows on the blood flow maps in the MCA regions. The high density areas in the right hemisphere represent contrast extravasation in a hyperacute stroke. These are as are not hemorrhagic as was confirmed at autopsy.
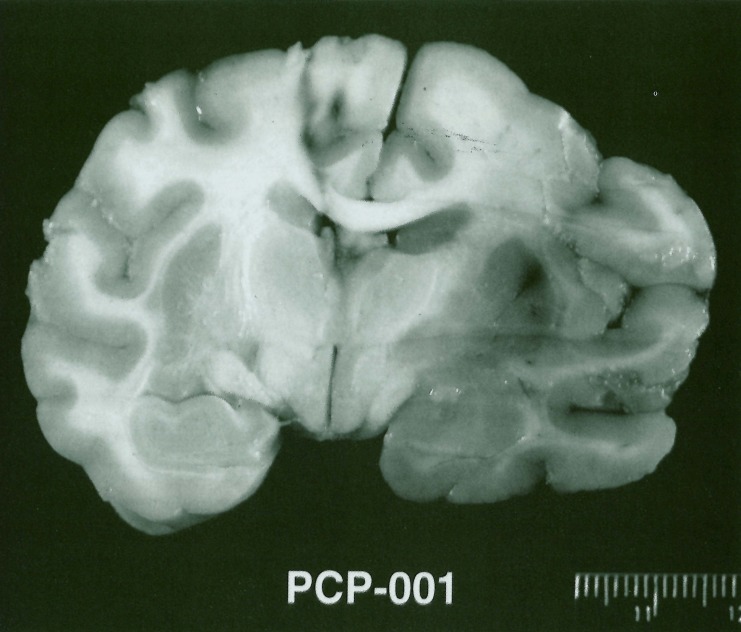
Figure 4.
Coronal section of fixed brain demonstrating experimental right MCA distribution stroke.
Discussion
An endovascular protocol for inducing an acute ischemic infarct is attractive for a number of reasons. First, the procedure requires minimal surgical manipulation thus making post-stroke care easier in terms of wound and pain management. Second, unlike exovascular models which require pre-surgical angiography to assess Circle of Willis anatomy prior to vessel ligation, the endovascular route allows forsimultaneous evaluation of the circulatory anatomy and collateral status before, during, and after vessel occlusions. Knowledge of circulatory variations and collaterals at the time of vessel sacrifice and after vessel sacrifice should provide for more consistent infarctions. Third, an endovascular approach allows for easier occlusion of anterior and posterior circulation vessels. When doing prior trans-orbital ligations we found that mesial PCA temporal lobe collateral blood supply often limited the degree of resultant infarction. By endovascularly occluding the Ml, ICA bifurcation, and P2 the only available collateral flow to the right hemisphere is the contralateral anterior cerebral artery's (ACA) contribution via a patent anterior communicating artery (Acomm) and the PI supply via a patent posterior communicating artery (Pcomm). In cases where such collateral flow is exorbitant, the PI origin of the Pcomm as well as the ipsilateral A2 segment can also be covered and occluded, respectively, to eliminate alternative sources of blood supply.
Our model is not without its drawbacks. First, the procedure requires the expertise of an endovascular surgeon, a commodity that may not be readily available at all centers. In our environment, neurosurgical and interventional radiologic cross-training of neurosurgeons avoided this pitfall. Second, the procedure requires high quality angiographic equipment preferably with biplane road mapping capabilities. A low weight primate's extra-and intracranial vasculature issmall in caliber and prone to vasospasm. This necessitates high resolution imaging so as to avoid inadvertent vessel perforations or inaccurate deposition of embolic materials. Third, the femoral artery in such primates is quite small and exceedingly friable. Access is at times difficult and unsuccessful initial attempts at placement of the guide wire and sheath are not uncommon with frequent side and back wall punctures. Despite these drawbacks, most centers carrying out primate stroke research have all of the necessary prerequisites in place to successfully carry out the model.
Post-occlusion CT scanning and CT-Xenon CBF study help confirm the production of the desired infarction. While we were successful in producing a profound MCA ischemic event in our first two primates we may encounter cases where this does not occur due to collateral supply as described above. In such cases, return to the angiography suite for additional embolization will help investigators avoid studying animals with sub-optimal ischemic regions. This will in turn reduce animal wastage.
Conclusion
Endovascular production of selective arterial infarctions provides investigators with an alternative to the usual surgical methods. While our technique requires particular expertise as well as specialized equipment we feel it also allows for an elegant minimally invasive, rapid, and reproducible means of creating a primate stroke model. The use of concomitant CT-Xenon CBF studies confirms the development of the desired ischemic event thus assuring the investigator that the model has been completed satisfactorily prior to its use to investigate further procedures, devices, or tissue characteristics.
Acknowledgment
This work was carried out under a protocol approved by the University of Pittsburgh Animal Care and Use Committee. This work was funded by a grant from Seacoast Technologies, Inc, Hampton Falls, New Hampshire and Boston Scientific Corporation-Target Therapeutics Inc, Fremont, California.
References
- 1.Symon L. Experimental model of stroke in the baboon. In: Meldrum BS, Marsden CD, editors. In Advances in Neurology. Vol. 10. New York: Raven Press; 1975. pp. 199–212. [PubMed] [Google Scholar]
- 2.Symon L, Pasztor E, Branston NM. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: An experimental study of the technique of hydrogen clearance in baboons. Stroke. 1974;5:355–363. doi: 10.1161/01.str.5.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Del Zoppo GJ, Copeland BR, et al. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]