Summary
We report a case of mirror aneurysms at the middle cerebral artery bifurcation with rupture on the left side. After six years, the patient had subarachnoid haemorrhage from a de novo aneurysm which developed separate from but adjacent to the already present aneurysm on the right side. The mechanism of development of multiple aneurysms, especially of the mirror-image type cannot be explained based only on haemodynamic factors and congenital segmental arterial vulnerability which is generalised than focal is highly likely. The sequence of development of aneurysms in this patient along with existing knowledge regarding rupture of aneurysms in conditions like polycystic kidney disease raise questions about the current trend of treating all patients with coincidental, unruptured aneurysms as they may never bleed from such aneurysms but could still be at risk of SAH from newly developing aneurysms.
Key Words: cerebral arterial aneurysm, aetiology, congenital origin, unruptured aneurysms
Introduction
The development of aneurysms (AA) at various sites in the intracranial vasculature cannot be explained purely on the basis of haemodynamic forces alone. With the help of this case report, we try to illustrate that genetically determined segmental arterial susceptibility may play an important role. Such evidence is vital in redefining our understanding of the aetiopathogenesis of AA and in predicting the natural history and risk of rupture of incidentally discovered AA.
Case Summary
A female patient, 39 years old, was first admitted to Bicetre in January 1994 with a clinical diagnosis of subarachnoid haemorrhage (SAH). She was not hypertensive and there was no significant medical illness in the past. Cranial CT showed presence of blood along the left Sylvian fissure and a cerebral angiogram confirmed a 8mm sized saccular aneurysm at the left middle cerebral artery (MCA) bifurcation. A 2mm saccular aneurysm was incidentally noted at the right MCA division (figure 1).
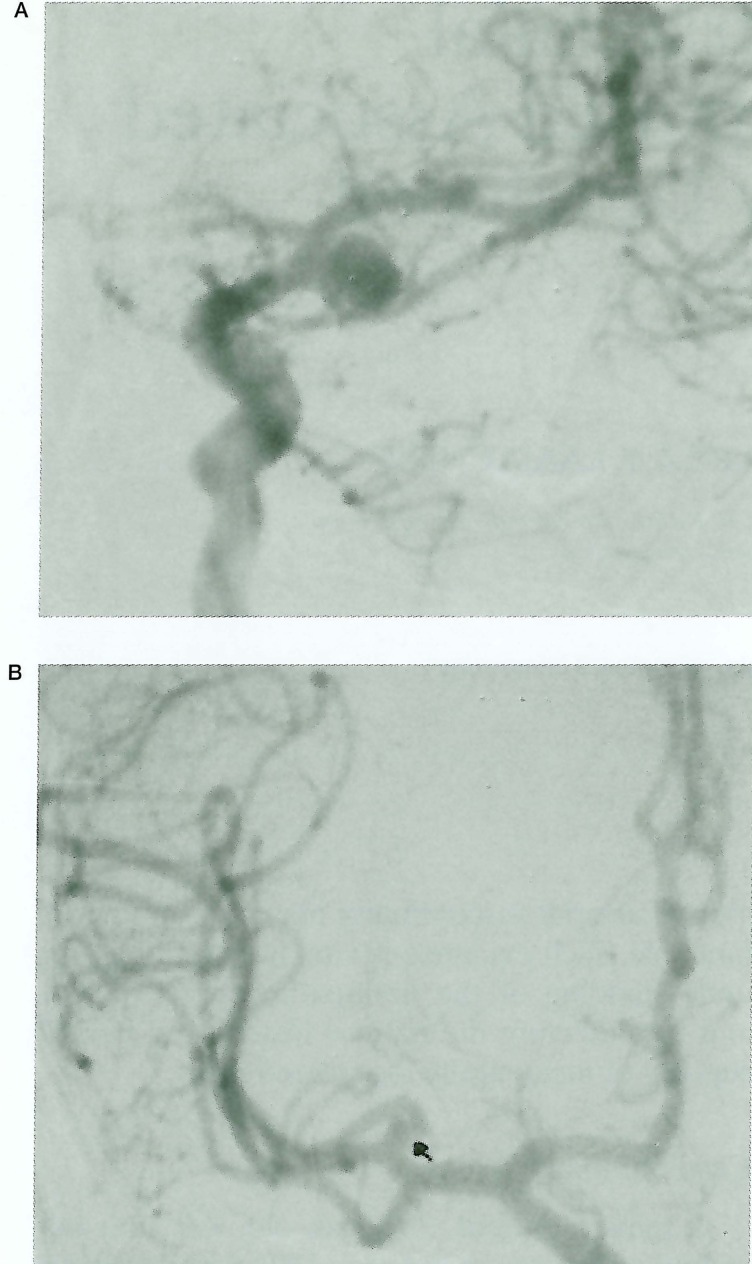
Figure 1.
A) Left carotid angiogram showing the saccular aneurysm at MCA bifurcation B) Right carotid angiogram showing the 2mm aneurysm at MCA division.
The patient underwent craniotomy and clipping of the left MCA aneurysm and made an uneventful recovery. Although she was advised about the need to treat the right MCA aneurysm, she was lost for follow-up. In November 2000, she had another emergency admission with suspected SAH. CT this time showed a blood clot along the right Sylvian fissure. In the initial angiograms, a 7mm saccular aneurysm was shown at the right MCA division and it was thought that the previously known small aneurysm has grown in size and ruptured. 3D rotational angiograms, were obtained and these clearly showed two separate aneurysms at the right MCA division (figure 2). The previously known 2mm aneurysm was unchanged and a new aneurysm (which has ruptured) has developed immediately adjacent to and medial to it. Another small saccular aneurysm also was noted at the internal carotid bifurcation.
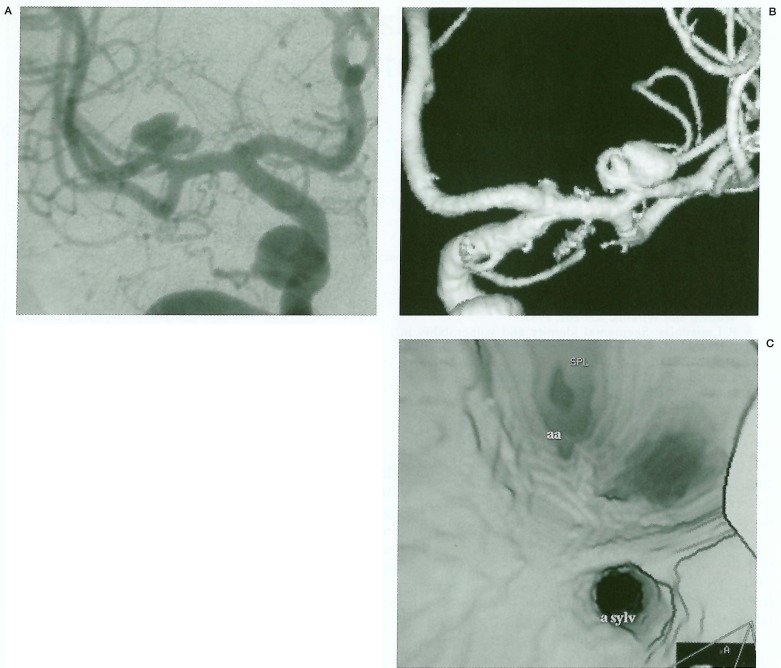
Figure 2.
A) Right carotid angiogram - a lobulated saccular aneurysm at the MCA trifurcation is seen. B) 3D angio shows the previously existing small aneurysm separate from the newly formed larger aneurysm. C) Internal view from the 3D angio showing two separate aneurysm openings.
At craniotomy, the neurosurgeons confirmed the presence of two separate aneurysms at the right MCA bifurcation and both were clipped. The patient made a good recovery.
Discussion
With significant improvements being made in the treatment modalities available for intracranial AA, there is increasing debate about their aetiopathogenesis. Structural weakness of intracranial arteries along with haemodynamic forces is widely believed as the major underlying cause 1-4 and the currently available treatment modalities (surgical and endovascular) are also based on such assumptions.
However, haemodynamic shear stresses alone cannot explain the occurrence of AA at various locations within the intracranial vasculature especially at locations like the MCA bifurcation, origin of posterior inferior cerebellar artery, etc, where such forces would be expected to be small. Moreover, there is no satisfactory explanation for the development of multiple aneurysms especially in a mirror-image location based on haemodynamic principles.
In the case of this patient, if haemodynamic forces were mainly responsible, they should target the already existing 2mm aneurysm, which represents an area of established weakness in the vessel wall. The fact that a second aneurysm has developed in an immediately adjacent location strongly raises the possibility of this arterial segment being weak congenitally. An initial triggering mechanism (hormonal, infective, shear stress etc.) has lead to the development of the small aneurysm from a focal area of the weakened arterial segment. This then surprisingly has remained quiescent probably with the aid of some kind of vessel wall repairing mechanism. Subsequently with the second trigger, an adjacent focus of the arterial segment was chosen.
A multifactorial origin for intracranial AA has been put forward by some authors 5-7. The segmental identity or vulnerability of arteries to certain diseases is established during development8. Secondary mutations or triggers can then act upon such a constitutional vasculopathic state and stimulate the overproduction of arterial wall without remodelling, resulting in aneurysm formation. It is an accepted fact that genetically based diseases can express themselves phenotypically or clinically at a later time during life9. A good example is that of Adult Polycystic Kidney Disease (APKD) Type 1, where intracranial AA do not occur at birth but usually develop nearly ten years earlier than the non APKD forms 10. Why someaneurysms occur in mirror-image locations can be explained based on a congenital metameric principle as was postulated by Campos et al in a recent analysis of 113 patients with multiple aneurysms 11.
The incidence of multiple aneurysms quoted in the literature varies from 15 to 45% 11 and for most teams involved in the management of SAH, coincidental unruptured aneurysms is a frequent occurrence. The natural history of such aneurysms is not clearly understood and the current trend is to treat them12. In our patient, the aneurysm that bled was not the one that was existing but a new one that grew in the meantime. In patients with unruptured aneurysms on follow-up such as those with a family history of SAH and APKD, rupture most commonly occurs from aneurysms not demonstrated previously13. If our patient had treatment for her incidental aneurysm that would not have protected her from future SAH and moreover if she had undergone endovascular treatment, it will be considered as failure of therapy with the second SAH.
This case also suggests the possibility of natural repair mechanisms operating in the vessel wall after the first insult or injury which should be responsible for the small aneurysm remaining without increasing in size. Such natural healing processes may account for the difference in behaviour between unruptured and ruptured aneurysms and if scientific research can prove their presence, major changes will ensue in our approach to treating unruptured aneurysms.
We believe that cases like this raise important issues regarding the development and behaviour of AA which should make us question established concepts and stimulate new thought and research aimed at achieving a longer lasting cure for a condition which has significant ethical, therapeutic and economic implications.
References
- 1.Schievink WI. Intracranial aneurysms. New England J of Med. 1997;336(1):28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 2.Yong-Zhong G, van Alphen HA. Pathogenesis and histopathology of saccular aneurysms: review of the literature. Neurol Res. 1990;12(4):249–255. doi: 10.1080/01616412.1990.11739952. [DOI] [PubMed] [Google Scholar]
- 3.Sekhar LN, Heros RC. Origin, growth and rupture of saccular aneurysms: a review. Neurosurgery. 1981;8(2):248–260. doi: 10.1227/00006123-198102000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Stebhens WE. Etiology of intracranial berry aneurysms. J Neurosurg. 1989;70(6):823–831. doi: 10.3171/jns.1989.70.6.0823. [DOI] [PubMed] [Google Scholar]
- 5.Pia HW, Langmaid C, Zierski J. Cerebral aneurysms. Advances in diagnosis and therapy. Springer Verlag; 1979. [Google Scholar]
- 6.Oostergard JR, Voldby B. Intracranial arterial aneurysms in children and adolescents. J Neurosurgery. 1983;48:832–837. doi: 10.3171/jns.1983.58.6.0832. [DOI] [PubMed] [Google Scholar]
- 7.Weir B. Aneurysms affecting the nervous system. William and Wilkins; 1987. pp. 209–261. [Google Scholar]
- 8.Lasjaunias P. Segmental identity and vulnerability in cerebral arteries. Interventional Neuroradiology. 2000;6:113–124. doi: 10.1177/159101990000600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 10.Pirson Y, Chauveau D. Intracranial aneurysms in autosomal polycystic kidney disease. In: Watson ML, Torres VE, editors. Polycystic Kidney Disease. Oxford: Oxford University Press; 1996. pp. 530–547. [Google Scholar]
- 11.Campos C, Churojana A, et al. Multiple intracranial arterial aneurysms: a congenital metameric disease? Interventional Neuroradiology. 1998;4:293–299. doi: 10.1177/159101999800400405. [DOI] [PubMed] [Google Scholar]
- 12.Wiebers O. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Eng J Med. 1998;339:17–25. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 13.Chauveau D, Pirson Y, et al. Intracranial aneurysms in autosomal dominant polycystic disease. Kidney International. 1994;45:1140–1146. doi: 10.1038/ki.1994.151. [DOI] [PubMed] [Google Scholar]