Abstract
Hypoxia has been implicated in the pathogenesis of preeclampsia, a hypertensive disorder of pregnancy. However, in vivo evidence and mechanistic understanding remain elusive. Preeclampsia is associated with impaired placental angiogenesis. We have recently shown that interleukin (IL)-10 can support trophoblast-driven endovascular cross-talk. Accordingly, we hypothesize that pathologic levels of oxygen coupled with IL-10 deficiency induce severe preeclampsia-like features coupled with elevated production of anti-angiogenic factors, apoptotic pathways, and placental injury. Exposure of pregnant wild type and IL-10−/− mice to 9.5% oxygen resulted in graded placental injury and systemic symptoms of renal pathology, proteinurea (wild type 645.15±115.73 versus 198.09±93.45; IL-10−/− 819.31±127.85 versus 221.45±82.73 µg/mg/24 hours) and hypertension (wild type 118.37±14.45 versus 78.67±14.07; IL-10−/− 136.03±22.59 versus 83.97±18.25 mmHg). Recombinant IL-10 reversed hypoxia-induced features in pregnant IL-10−/− mice confirming the protective role of IL-10 in preeclampsia. Hypoxic exposure caused marked elevation of soluble fms-like tyrosine kinase 1 (110.8±20.1 versus 44.7±11.9 ng/ml) in IL-10−/− mice compared to their wild type counterparts (81.6±13.1 versus 41.2±8.9 ng/ml), whereas soluble endoglin was induced to the similar levels in both strains (~380±50 versus 180±31 ng/ml). Hypoxia-induced elevation of p53 was associated with marked induction of pro-apoptotic protein Bax, down-regulation of Bcl-2, and trophoblast-specific apoptosis in utero-placental tissue. Collectively, we conclude that severe preeclampsia pathology could be triggered under certain threshold oxygen levels coupled with intrinsic IL-10 deficiency which lead to excessive activation of anti-angiogenic and apoptotic pathways.
Keywords: Hypoxia, Hypertension, Interleukins, Apoptosis, Mouse, Angiogenesis
Preeclampsia is one of the most common complications of pregnancy. This condition, associated with hypertension, edema and proteinurea, affects 5–10% of pregnancies worldwide and entails severe consequences for both the mother and the fetus.1–3 Preeclampsia presents as either a mild or severe condition, distinguished most often by severity of hypertension and proteinuria symptoms. Although long-term postpartum effects are not well investigated in children borne to preeclamptic mothers, intrauterine growth restriction (IUGR) is a relatively common co-morbidity.4 Both preeclampsia and IUGR are thought to be associated with increased apoptosis in the placenta, altered trophoblast proliferation and differentiation, syncytial knots, and circulating placental debris as well as random production of anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng).5–11 Importantly, placental bed biopsies from preeclampsia show signs of poor perfusion and ischemia at least in part due to defects in remodeling of spiral arteries and shallow trophoblast invasion.4,12–14 It has been hypothesized that hypoxic/ischemic injury is the trigger for excessive placental cell death and expression of anti-angiogenic factors.15–17 However, in vivo evidence for hypoxia to induce preeclampsia-like syndrome in animals has not yet been reported.
During human gestation, early placentation develops in a relatively low oxygen (O2) tension environment (17.9 mmHg or 2.5% O2).18 The developmental switch of trophoblasts from a proliferative to an invasive phenotype is controlled by the placental oxygen levels.16 Further, disruption of regulatory genes controlling responses to hypoxia leads to failure in placentation and fetal death.19 However, studies on hypoxia have not yet resolved the controversial in vitro findings. Studies with trophoblast cell lines, first trimester cytotrophoblasts, and placental explants suggest that low oxygen tension may induce trophoblast proliferation and/or apoptosis and thus impairs trophoblast invasion.16,19–21 On the other hand, hypoxia-induced enhanced trophoblast invasion and differentiation to invasive phenotype have been suggested.22,23 To date it has not been possible to discern what oxygen levels impart in vivo pathologic effects at the maternal-fetal interface and their role in the onset of preeclampsia and IUGR. Although maternal hypoxia (11%) was shown to increase vascularity and potentiate trophoblast invasion in pregnant rats,24 it is possible that this level of oxygen tension is not sufficient to cause pathologic effects leading to preeclampsia-like symptoms. It is also plausible that intrauterine cytokine milieu and balance of vascular factors may modulate the effects of hypoxic levels of oxygen. Hypoxia affects the cytokine balance by reducing interleukin (IL) 10 production and inducing IL-6 and IL-8 in placental explants and trophoblasts.25 Thus, IL-10 deficiency coupled with hypoxia-induced inflammatory milieu may affect trophoblast functions and perturb the expression of apoptosis-associated pathways at the maternal-fetal interface. We hypothesize that hypoxia under certain threshold levels could lead to placental injury, apoptosis and preeclampsia-like symptoms that are likely to be exacerbated by IL-10 deficiency.
Our studies demonstrate that exposure to 9.5% oxygen levels during gestational days (gd) 7.5 through 17 in IL-10−/− mice induces a full spectrum of features that closely mirror severe preeclampsia-like disease. Our results provide evidence for pregnancy specific constellation of events including production of anti-angiogenic factors, sFlt-1 and sEng, and activation of placental apoptotic pathways. Importantly, exogenous IL-10 was found to reverse hypoxia-induced preeclampsia-like features.
Materials and Methods
Syngeneic matings involving C57BL/6 (wild type or IL-10−/−) mice were used for timed pregnancies. The day when the vaginal plug was detected was considered as gestational day (gd) 0. All protocols were approved by the Lifespan Animal Welfare Committee and conducted according to its guidelines. Mice (n=4–6 per group) were placed in the hypoxia chamber calibrated for varying (9.5%, or 11%) levels of O2, 5% CO2, and 85.5% N2 from either gd 7.5, 10, 15 through gd 17 (see online Data Supplement for details http://hyper.ahajournals.org). To evaluate the protective role of IL-10 in hypoxia-induced preeclampsia, a subset of IL-10−/− mice were injected (i.p.) with recombinant mouse IL-10 26,27 (R&D Systems, MN, USA 500 ng/mouse, n=6) or saline daily from gd 8 through gd 16 and exposed to 9.5% hypoxia from gd 7.5 to gd 17. On gd 17, animals were sacrificed, fetus separated from placenta and weighed for monitoring IUGR. Before sacrificing the animals, blood pressure was monitored using a noninvasive computerized tail-cuff system (Digi-med, Inc., Louisville, KY). On gd 16, mice were transferred to the metabolic cage placed in the hypoxia chamber throughout the period of experiment, for 24-hour urine collection. The metabolic chamber is designed to collect urine and fecal matter separately and minimizes their mixing and animals had access to food and water ad libitum. To assess proteinuria, urinary albumin and creatinine were measured using the Albuwell and Creatinine Companion kits. For assessing the renal pathology, fixed kidney tissue were stained with hematoxylin/eosin and periodic acid Schiff reagent as described.10 Morphological changes were recorded using SPOT™ Advanced software at 10× and 100× magnification. For measuring circulating levels of mouse sFlt-1, sEng and VEGF by ELISA, mice were sacrificed on gd 17, and blood was collected by cardiac puncture. After 3–5 minutes at room temperature, blood was centrifuged at 500 g for 5 minutes and supernatant collected and stored at −80°C until further use (details are available in the online Data Supplement http://hyper.ahajournals.org). Similarly, non-pregnant mice (n=6) were subjected to normoxia or hypoxia treatment for 9.5 days and monitored for blood pressure, proteinuria, kidney pathology, serum sFlt-1 and sEng levels as described. Local hypoxia was detected using the marker EF-5, a gift from Dr. Cameron Koch (University of Pennsylvania). EF-5 (10 µM) was administered intraperitoneally four hours before harvesting the utero-placental tissues on gd 13. The tissue was snap-frozen and 10-µm sections were processed and probed for EF-5-positive signal using ELK3–51 antibody (1:30) as described.28 HIF-1α, p53, p21, Bax, Bcl-2, caspase 3, VEGF A, VEGF C, phospho-Stat3, total Stat3, and actin were monitored by Western blot in placental tissue collected from gd 17 (details are provided in the online Data Supplement http://hyper.ahajournals.org). Placental expression of sFt-1 and sEng transcripts was monitored after normalizing to β2-microglobulin (β2M) expression by real-time quantitative PCR. For detection of apoptotic cells, placental tissue was fixed in 10% paraformaldehyde, sectioned and subjected to TUNEL staining. Utero-placental tissue structural orientation was demarcated by H&E staining. Sections from snap frozen placental tissue were double stained with florescent tagged anti-pancytokeratin antibody and TUNEL. Data were compared using the Student’s t-test if the data were normally distributed. p< 0.05 was considered significant. Data are presented as the mean ± SEM from at least three independent experiments. Further methodological details are available in the online Data Supplement (http://hyper.ahajournals.org)
Results
Hypoxia induces a full spectrum of preeclampsia-like features in pregnant IL-10−/− mice
We first assessed the effects of graded levels of hypoxia and duration of exposure on induction of preeclampsia-like features in pregnant wild type and IL-10−/− mice. As shown in Table S1 (available in the online Supplement Data), exposure to 11% or 9.5% O2 from gd 10, or 15 through gd 17 did not induce any preeclampsia-like symptoms in wild type mice. Similarly, no major changes were observed in IL-10−/− mice, except for trend towards hypertension and proteinuria with 9.5% O2 from gd 10 to gd 17(Table S1). Exposure to 8.5% O2 levels from gd 7.5 or gd 10 through gd 17 proved lethal to pregnant mice. However, pregnant mice, particularly IL-10−/− mice, when exposed to 9.5% O2 from gd 7.5 through gd 17 experienced full spectrum of preeclampsia-like symptoms (Figure 1) and these conditions were used for further studying hypoxia-induced pathologic effects.
Figure 1. Exposure to 9.5%, not 11%, O2 induces preeclampsia-like symptoms in pregnant mice.
Pregnant mice were exposed to 21% O2 (normoxia), 11%, or 9.5% O2 from gestational day (gd) 7.5 to gd 17. The term hypoxia is assigned to 9.5% O2. Comparative data on gd 17 are shown for (A) systolic blood pressure (n=6); (B) albumin/creatinine (proteinuria) levels of 24-hour urine samples (n=6); (C) representative fetal units harvested on gd 17 after exposure to 9.5% and 21% O2 (n=6) are depicted. Panel (D) compares the average fetal weight on gd 17 (n=58) from multiple independent experiments exposed to 21%, 11% or 9.5% O2. All values are expressed as Mean ± SEM. ** indicates p<0.05 as compared to respective wild type or IL-10−/− mice exposed to 21% O2, and aa indicate p<0.05 between wild type and IL-10−/− mice exposed to 9.5% O2.
As demonstrated in Figure 1A, exposure to 9.5% but not 11% O2 from gd 7.5 to gd 17 led to hypertension in wild type and IL-10−/− mice, albeit much higher readings in IL-10−/− mice. Urinary albumin excretion (normalized to creatinine) was also significantly increased in 9.5% O2-exposed wild type and IL-10−/− mice (Figure 1B). Surprisingly, the excreted protein content was significantly higher in IL-10−/− mice. In addition to hypertension and proteinuria, the other prominent clinical features of preeclampsia are fetal IUGR and maternal kidney pathology. Figure 1C demonstrates comparative size of fetuses on gd 17 from animals exposed to hypoxic (9.5% O2) conditions and those kept under normoxia (21% O2) conditions. Under 9.5% O2 conditions, we did not observe fetal resorption or preterm birth in pregnant animals as assessed by inspection of uterine horns at different stages of pregnancy or by allowing animals to deliver. Unlike exposure to 11% or 21% O2, it is evident that 9.5% O2 caused significant IUGR in both wild type and IL-10−/− fetuses with the latter showing significantly more enhanced effects as indicated by the fetal weights from multiple experiments as shown in Figure 1D. Thus, these data indicate that preeclampsia-like symptoms induced by hypoxia were exacerbated by IL-10 deficiency.
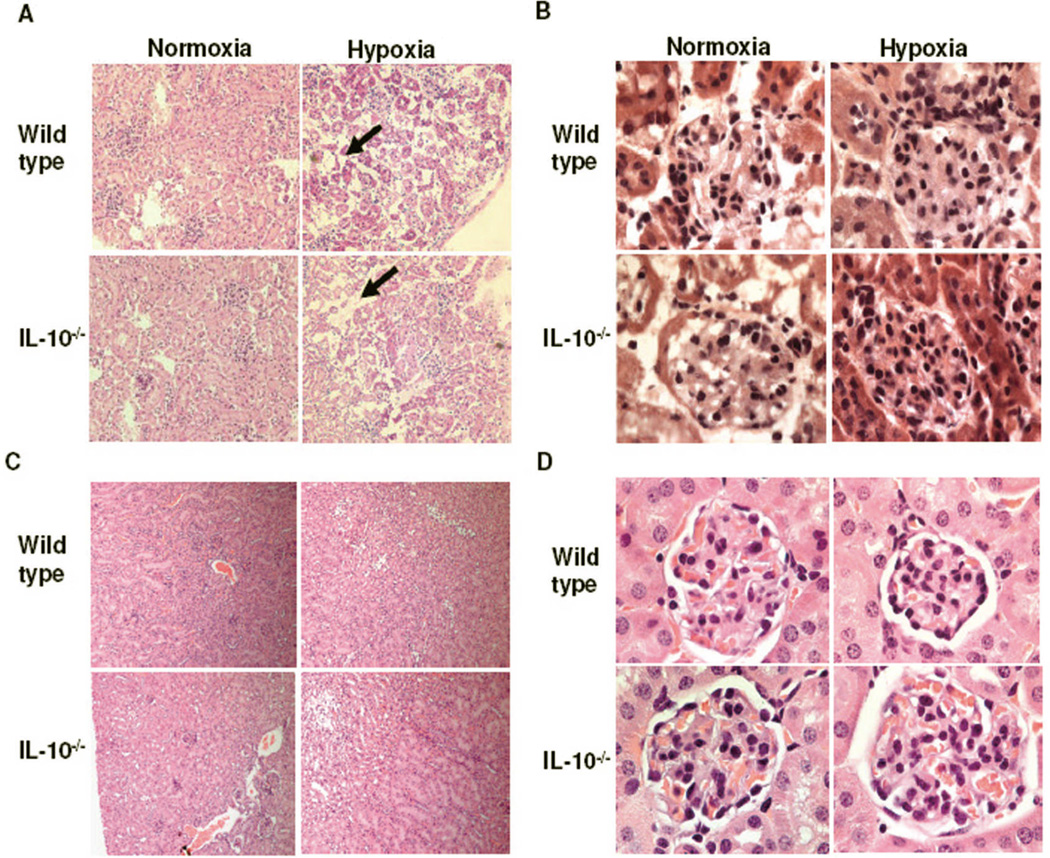
Figure 2 shows the hypoxia-induced renal pathologic changes in both wild type and IL-10−/− mice. Assessment of the renal tissue (10× magnification, Figure 2A) clearly shows disorganized kidney tissue. Atrophic tubules and interstitial edema with dispersed glomerular enlargement were observed. Extensive capillary occlusion with swollen cytoplasm was particularly evident at higher magnification (100× magnification, Figure 2B). Similar effects have been observed in sFlt-1-treated pregnant rats.29 Importantly, in contrast to pregnant mice, identical hypoxia (9.5% O2) treatment for 9.5 days in non-pregnant mice did not exhibit renal pathology. A representative histology of kidney section from non-pregnant mice subjected to hypoxia is shown in Figure 2C (10× magnification) and Figure 2D (100× magnification, a single glomeruli), suggesting pregnancy-specific impact of hypoxia on renal pathology. Interestingly, the murine preeclampsia models involving sFlt-1 or autoantibodies against angiotensin 1 receptor (AT1-AAs) have shown some pathologic effects in non-pregnant animals.29–31 However in the current model of preeclampsia, our data demonstrate that hypoxic conditions (9.5% O2) employed did not cause hypertension (Figure S1 A), proteinurea (Figure S1 B) or excess production of sFlt-1 (Figure S1 C) in non-pregnant animals, suggesting that the model established here can be used to study pregnancy specific onset of hypoxia-induced preeclampsia-like disease.
Figure 2. Maternal hypoxia induces renal pathology in pregnant mice.
On gd 17, kidneys were harvested and renal tissue processed for H&E staining from at least three animals per group. A representative image of renal tissue section from pregnant mice exposed to 21% (normoxia) or 9.5% (hypoxia) is shown: (A) Lower magnification (10×) shows atrophic tubules and interstitial edema in hypoxia-exposed groups. Bold arrows show the increased interstitial space due to atrophy and (B) Higher magnification (100×) shows glomerulus that is diffusely enlarged and hypertrophy of intracapillary cells upon hypoxia exposure. A representative histology of kidney section from non-pregnant mice subjected to identical conditions of hypoxia is shown at low magnification (10×, Panel C) and at higher magnification (100×, Panel D). Unlike pregnant mice, identical hypoxia treatment in non-pregnant mice did not exhibit renal pathology.
Hypoxia induces higher production of sFlt-1 in pregnant IL-10−/− mice
The anti-angiogenic factors sFlt-1 and sEng can cause peeclampsia-like symptoms in a rat model when administered at high doses.10,29 Here, we show that in response to 9.5% O2, serum levels of sFlt-1 were significantly elevated in pregnant wild type and IL-10−/− mice (Figure 3A). sFlt-1 production was comparatively much higher in IL-10−/− mice. On the other hand, although sEng was also induced in response to hypoxic conditions, no significant differences were observed between wild type and IL-10−/− mice (Figure 3B), suggesting specific effects of IL-10 deficiency only on sFlt-1 production. We also assessed sFlt-1 and sEng at the mRNA level (Figure S2) and the data corroborated those shown in Figure 3. Next, we assessed the serum and placental levels of pro-angiogenic factors VEGF A and VEGF C by ELISA and Western blotting and found no significant effect of 9.5% O2 on overall levels of these growth factors (Figure S3 A–C). Overall, these results point to pregnancy-specific response to hypoxia.
Figure 3. Serum levels of sFlt-1 and sEng in pregnant wild type and IL-10−/− mice exposed to normoxia or hypoxia.
(A) A significant increase was observed in the serum levels of sFlt-1 in response to hypoxia in pregnant mice, with significantly higher levels in IL-10−/− mice (n=6). (B) sEng levels were also elevated in response to hypoxia, although no differences were observed between wild type and IL-10−/− mice (n=6). All values are shown as Mean ± SEM. ** indicates p<0.05 as compared to normoxia. aa indicates p<0.05 between wild type and IL-10−/− mice in response to hypoxia.
Recombinant IL-10 rescues hypoxia-induced preeclampsia-like symptoms in mice
To support our earlier observations that IL-10−/− mice are hypersensitive to hypoxia-induced effects, we hypothesized that their reconstitution with recombinant IL-10 should protect against hypoxia-induced preeclampsia-like features. Thus, daily administration of recombinant IL-10 from gd 8 to gd 16 was evaluated to reverse the hypoxia-induced effects. As shown in Figure 4, IUGR (Figure 4A) and fetal weight (Figure 4B), elevated systolic blood pressure (Figure 4C), and proteinuria (Figure 4D) were restored to normal values as compared to those under normoxia conditions. Further, recombinant IL-10 significantly reduced hypoxia-induced excess production of sFlt-1 (Figure 4E). The dose and duration of administration of IL-10 were established based on pilot experiments. These data strongly point to the protective role of IL-10 against hypoxia-induced preeclampsia.
Figure 4. Recombinant IL-10 (rIL-10) rescues hypoxia-induced preeclampsia like features.
Pregnant IL-10−/− mice were injected (i.p.) recombinant IL-10 daily from gd 8 to gd 16 with concomitant exposure to 9.5% O2 in hypoxia chamber. On gd 17 the animals were removed from the hypoxia chamber and evaluated. Panel A shows a representative image of recovery of fetal size in response to IL-10 treatment. Panel B shows the average weight of fetus on gd 17 from different treatment groups (n=6 each). Panel C shows comparative systolic blood pressure measurements in response to different treatments (n=6 in each group). IL-10 treatment prevented the onset of hypoxia-induced hypertension. (D) Comparative proteinuria data from different treatment groups show that rIL-10 reverses the hypoxia-induced proteinuria. (E) Analysis of circulating levels of sFlt-1 in response to different treatments shows that rIL-10 prevents hypoxia-induced excess production of sFlt-1. All values are shown as Mean ± SEM. ** indicates p<0.05 as compared to respective experimental groups.
Maternal hypoxia causes utero-placental injury, particularly in the placental junctional zone
To directly correlate the data presented so far to hypoxia-induced placental injury, we next attempted to map hypoxic regions in the utero-placental tissue in vivo, using the hypoxia sensor EF5. A representative experiment is shown in Figure 5 from tissue harvested on gd13 from both wild type and IL-10−/− mice. In Figure 5A, EF5-specific staining was observed only in tissues from hypoxia exposed mice, with prominent effects in IL-10−/− mice. To map the hypoxic injury in the utero-placental tissue, we oriented EF5 fluorescence with H & E staining of the corresponding tissue to denote different regions (Figure 5A). Although some EF5 signals could be seen in the mesometrial and decidua basalis regions, a careful alignment of hypoxic regions suggested predominant EF5 signal in the placental junctional zone compared with normoxia controls (4× and 20× magnification). No significant EF5 signal was observed in the inner labyrinth layer. Wild type mice exposed to hypoxia showed weaker EF5 staining. These observations are further supported by higher placental HIF-1α protein expression in response to hypoxia (Figure 5B). Unlike the enhanced preeclampsia pathology observed in IL-10−/− mice, no IL-10-dependent differences were observed for the induction of HIF-1α protein levels, suggesting that the extent of hypoxic injury is not dependent on the HIF-1α-mediated effects.
Figure 5. Maternal hypoxia induces injury in the placenta.
(A) EF5 staining for normoxia- and hypoxia-exposed placenta in wild type and IL-10−/− mice. Upper panel shows H&E sections mapped for mesometrium (M), decidua basalis (DB), junctional zone (JZ) and labyrinthine (LB) of utero-placental units from animals from normoxia or hypoxia (4×). Middle panel shows EF5 staining of the utero-placental section under low magnification (4×). Hypoxic injury was found localized to the junctional zone (JZ, indicated by the box). The lower panel shows the higher magnification (20×) of the junctional zone. IL-10−/− mice exhibits more severe hypoxic injury in JZ. (B) Immunoblot of HIF-1α protein in gd 13 utero-placental tissue. Hypoxia induced HIF-1α protein in both wild type and IL-10−/− tissue. A representative of three independent experiments is shown.
IL-10 deficiency promotes hypoxia-induced trophoblast apoptosis in the placenta
As shown above, chronic hypoxic conditions of 9.5% O2 induced severe preeclampsia-like pathology in IL-10−/− mice with milder effects in wild type mice. Next, we performed experiments to delineate whether maternal hypoxic exposure caused apoptosis in vivo. Although some TUNEL-positive areas were observed in utero-placental tissue from normoxia exposed mice or hypoxia-exposed wild type mice, a large amount of TUNEL-positive cells were found in the gd 17 IL-10−/− placenta after exposure to hypoxia (data not shown).
In order to determine whether trophoblasts were undergoing apoptosis, the IL-10−/− placental sections from normoxic and hypoxic conditions were double-stained with TUNEL and an antibody for pancytokeratin for immunofluorescence analysis. As shown in Figure 6, no significant TUNEL positive signal was detectable in tissue from mice housed under normoxic conditions. However, exposure to hypoxia resulted in TUNEL positive staining and the overlay with the pancytokeratin staining demonstrated that TUNEL immunofluorescence was mainly present in cytokeratin positive cells in the junctional zone.
Figure 6. Maternal hypoxia induces apoptosis in trophoblasts at the maternal-fetal interface.
A representative immunofluorescence staining with pancytokeratin antibody (left panel) and TUNEL (middle panel) in IL-10−/− placenta harvested on gd 17 is shown. Overlay (right panel) for both markers indicated trophoblasts undergoing apoptosis upon exposure to hypoxia. The apoptotic region coincides with the junctional zone.
Hypoxia induces robust activation of p53-Bax-Bcl2-caspase 3 signaling pathways in IL-10−/− mice
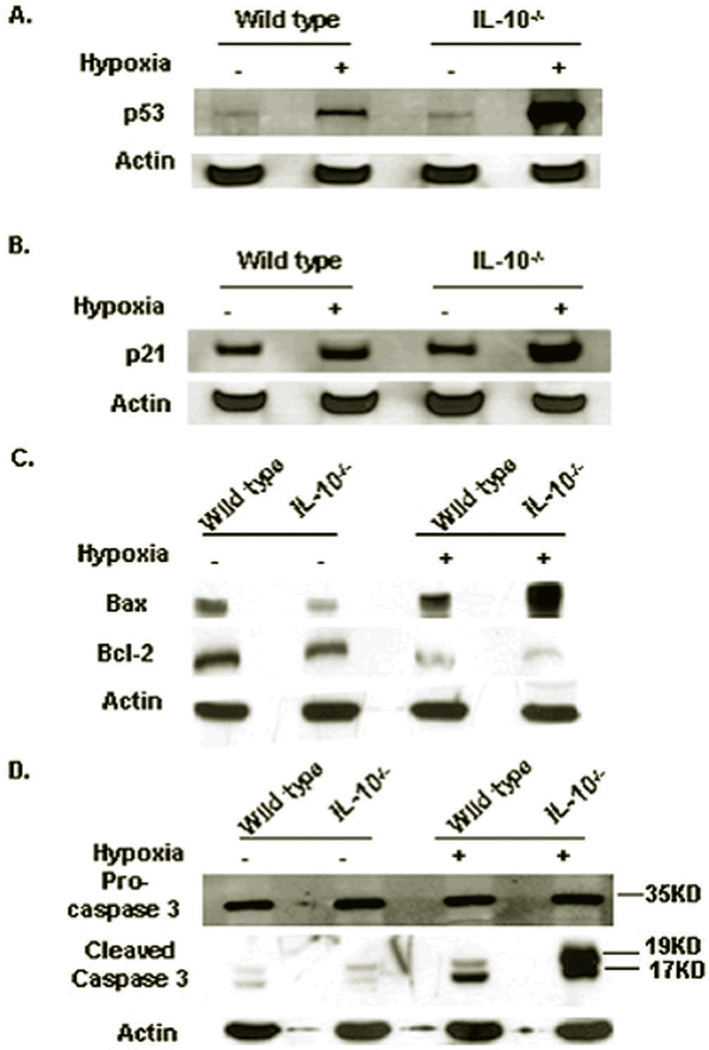
Since hypoxia was shown to induce cell death in trophoblasts, we next sought to investigate mechanistic pathways for hypoxia-induced apoptosis in the placenta. Hypoxia induces two major signaling pathways: p53 and Stat3 phosphorylation.32,33 First, Stat3 protein levels and phosphorylation in wild type and IL-10−/− utero-placental tissue were examined. No differences were observed for Stat3 phosphorylation and total protein levels after exposure to hypoxia (Figure S4). In contrast, p53 was induced in response to hypoxia and its induction was especially prominent in the IL-10−/− tissue (Figure 7A). Consistent with this, the p53-dependent kinase inhibitor p21 was induced in a similar manner (Figure 7B).
Figure 7. Maternal hypoxia induces p53-Bax-caspase3 pathway for placental cell death.
(A) p53 protein expression in tissue harvested on gd 17. Hypoxia induces significant induction of p53 compared to normoxia tissue, and the induction was more robust in IL-10−/− tissue when compared to wild type mice. (B) A representative immunoblot of p21 protein. p21 exhibits a parallel expression profile as that seen for p53. (C) Bax and Bcl-2 expression in normoxic and hypoxic tissue. Bax is induced significantly, particularly in IL-10−/− tissue, whereas Bcl-2 expression decreased significantly. (D) Procaspase 3 and cleaved caspase 3 expression in gd 17 utero-placental tissue. Active caspase 3 bands are uniquely prominent in hypoxic tissue. Data represent at least three independent experiments for each protein.
To further dissect the p53-mediated apoptosis signaling pathways, modulation of its downstream targets, pro-apoptotic Bax and anti-apoptotic Bcl-2, was investigated. Bax was robustly increased by hypoxia in IL-10−/− placental tissues compared to tissue from wild type mice (Figure 7C). In contrast, Bcl-2 was concurrently decreased by hypoxia (Figure 7C). To induce active cell death, production of active caspase 3 is critical. The cleaved caspase 3 was elevated in response to hypoxic conditions. Significantly, the amount of cleaved caspase 3 in IL-10−/− placental tissue was drastically higher compared to that seen in wild type tissue (Figure 7D). These data corroborate enhanced apoptosis observed in the placenta of hypoxia-exposed IL-10−/− mice. The blunting of apoptotic signaling proteins in wild type placenta resulting in milder phenotype clearly suggest the protective role of IL-10 in limiting the preeclampsia pathology.
Discussion
Although several reports have discussed the role of low oxygen tension in modulating trophoblast invasion and cell death,16,19 direct in vivo evidence linking pathologic association of hypoxia with preeclampsia and mechanistic understanding of this pathology are still unsatisfactory. Here we provide compelling evidence for hypoxia-induced preeclampsia-like features in pregnant mice. As observed from the differential effects in wild type and IL-10−/− mice in this study, the severity of the disease can be programmed by predispositions such as that resulting from IL-10 deficiency. In this regard, several studies have reported that IL-10 deficiency is associated with adverse pregnancy outcomes such as recurrent spontaneous abortion (RSA), preterm birth, and preeclampsia. 34–37 To buttress this argument, we show here that use of recombinant IL-10 efficiently rescues hypoxia-induced hypertension, proteinuria, IUGR and excess production of sFlt-1 in IL-10−/− mice. This corroborates with the notion that IL-10 is a critical cytokine for maintenance of normal pregnancy, particularly in response to inflammatory triggers. 38–41 Moreover, we have demonstrated that IL-10 rescues endovascular cross-talk between trophoblasts and endothelial cells and suppresses inflammation coupled with endothelial cell dysfunction.26,42
Other published reports describing in vitro effects of low oxygen tension on placental development and trophoblast health have pointed to disparate consequences.19,20 These could very well be explained by non-pathologic and pathologic levels of O2. Our in vivo results confirm that 9.5%, not 11%, O2 from gd 7.5-gd 17, imparts pathologic consequences and induces preeclampsia-like features, including hypertension, proteinuria, IUGR, production of sFlt-1 and sEng, and renal pathology. As our results demonstrate, the gestational age of initial hypoxia exposure is critical to the onset of preeclampsia symptoms since exposure from either gd 10 or gd 15 to gd 17 did not result in preeclampisia-like features. Importantly, these features occurred strictly in a pregnancy-specific manner as no pathology was seen in non-pregnant mice. In addition, hypoxia-exposed mice, when allowed to recover, experienced a normal second pregnancy cycle, with the exception of mild IUGR (data not shown). Our data concur with the notion that low oxygen levels under certain threshold can adversely affect pregnancy and can contribute to the onset of the preeclampsia disease.43
It is well documented that O2 levels fluctuate during the early stages of pregnancy and regulate trophoblast differentiation and invasion.44,45 This process could also be significantly affected by maternal hypoxic conditions and has been shown to be defective in preeclampsia, suggesting that the O2 levels might have altered and probably reached the severe hypoxia levels,20 at least in a significant proportion of preeclampsia patients. Recently, attempts have been made in the mouse and rat models to map the in vivo effects of hypoxia.24,46 In the rat model, exposure to 11% O2 levels between gd 6.5 and 13.5 caused more extensive opening of uterine blood vessels and increased invasion by trophoblasts. 24 In this report, it was not clear if there was a localized hypoxic injury in the placenta. Schaffer et al 47 have reported on the hypoxic injury in the mouse placenta exposed to acute hypoxia (6–7% O2) for 24 hrs. Under these conditions, HIF-1α was robustly induced in the periphery but not the labyrinth of the placenta. We report a similar pattern of hypoxic injury as detected by intense EF5 staining particularly in IL-10−/− mice. Our data are intriguing in that exposure to 9.5% O2 between gd 7.5 and gd17 caused placental hypoxic injury which could be mapped primarily to the junctional zone bordering the decidua. Hypoxia in wild type mice resulted only in a weaker EF5 signal that corroborates with the milder activation of apoptotic pathways and systemic effects, implying the moderating role of IL-10. It is noteworthy that hypoxia is reported to inhibit IL-10 production in placental tissues25 which could possibly explain the onset of milder preeclampsia-like symptoms in wild type mice exposed to 9.5% O2. Recent studies in mice that are deficient in natural killer cells suggest that impaired spiral artery remodeling may not cause hypoxic injury and hypertension.48,49 Since these mice are IL-10 proficient, we speculate that IL-10 minimizes placental hypoxic injury and cell death pathways as suggested by our current work.
Our data for the first time demonstrate that maternal hypoxia induces placenta specific overproduction of sFlt-1 and sEng as non-pregnant mice under identical conditions did not exacerbate sFlt-1 or sEng levels. Although the trigger for increased production of these anti-angiogenic factors is not clear, studies clearly demonstrate that reduced uterine placental perfusion (RUPP)46,50, placental hypoxia15,51,52 and other upstream factors 53 can lead to excess production of sFlt-1 and sEng. However, it is not known whether maternal hypoxia can cause placental injury and cause excessive production of sFlt-1 and sEng. In this regard, our results are noteworthy in that maternal hypoxia induced significant production of these anti-angiogenic factors, albeit at much higher rate in IL-10−/− mice. Moreover, IL-10 deficiency seems to regulate the severity of hypertension, proteinuria, IUGR, hypoxic injury, and placental apoptosis as observed in IL-10−/− mice. Since sFlt-1 and sEng are randomly elevated in preeclampsia patients, it is plausible that other concurrent pathways contribute equally to hypoxia-induced pathology.
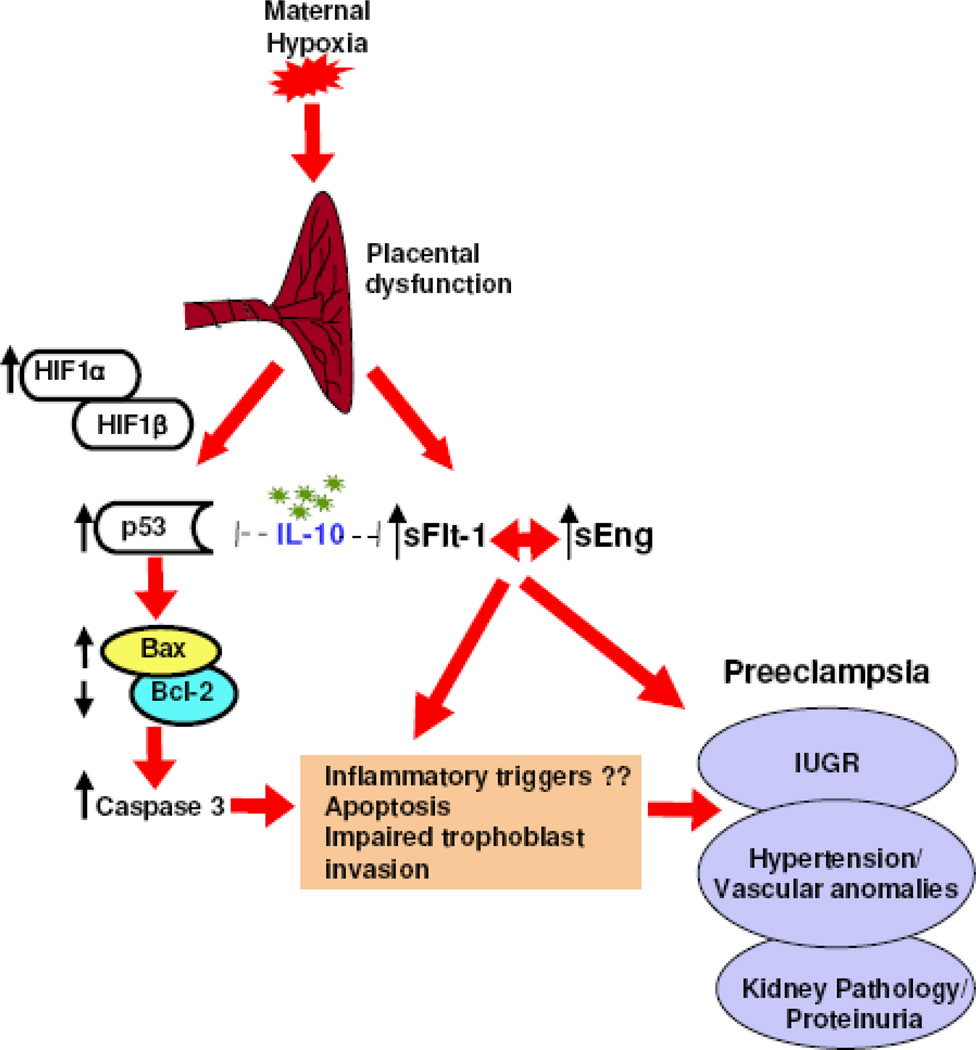
Several in vitro studies have suggested that hypoxic conditions cause apoptosis in a subpopulation of trophoblasts and that the p53 and Bcl-2 pathways may be involved.16,45,54 In agreement with these findings, our studies provide direct in vivo evidence that exposure to hypoxia beyond early pregnancy stages induces apoptotic machinery in the placenta with pronounced effects in IL-10−/− mice. Our data support a model (Figure 8) whereby hypoxia activates the p53 signaling pathway resulting in excessive production of Bax protein, down-regulation of Bcl-2, and hyperactivation of caspase 3. Concurrently, hypoxia also triggers increased placental expression and release of sFlt-1 and sEng. These proteins have been postulated to contribute to systemic complications, including hypertension and proteinuria. These signaling pathways are partially blocked by endogenous IL-10 in wild type mice and by administration of recombinant IL-10 in IL-10−/− mice, suggesting that deficiency in this cytokine may trigger severe pathology.
Figure 8. Schematic model for maternal hypoxia-induced p53 signaling and anti-angiogenic pathways and preeclampsia-like disease in pregnant mice.
IL-10 is depicted as a modulator of preeclampsia through partial inhibition of p53 and sFlt-1 induction.
Using the IL-10−/− mouse model described here, we were able to investigate the precise role of hypoxia in the pathogenesis of preeclampsia. Our studies provide evidence that hypoxia caused localized injury in the utero-placental tissue, disrupted the placental and systemic angiogenic balance, and contributed to the preeclampsia pathology. Absence of IL-10 further exacerbated this imbalance and contributed to severe preeclampsia. Thus, our model provides potential opportunities to study the influence of IL-10, hypoxia and immune cell interactions, particularly uterine NK cells and regulatory T cells, and their contribution to preeclampsia.
Perspectives
Here, we provide in vivo evidence for hypoxia-induced preeclampsia-like features. We demonstrate that O2 beyond a threshold is capable of inducing severe preeclampsia-like features in pregnant IL-10−/− mice. The pathogenesis in this in vivo model for preeclampsia implicates placental injury, elevation of soluble anti-angiogenic factors and activation of p53 signaling cascade resulting in placental apoptosis. Wild type mice exhibit mild features of preeclampsia, and rescue of preeclampsia-like features by recombinant IL-10 suggests a regulatory role of IL-10 in programming the severity of preeclampsia. The in vivo model described here can be used to characterize trophoblast functions, immune regulation and signaling pathways leading to preeclampsia-like disease in response to graded O2 levels.
Supplementary Material
Acknowledgements
We thank Paula Weston for her help in histochemistry. We thank Drs. James Padbury and Jared Robins for critical reading of the manuscript.
Sources of Funding
This work was supported in part by the NIH grant P20RR018728 and by the Rhode Island Research Alliance Collaborative Research Award 2009-28.
Footnotes
Disclosures
None
References
- 1.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 2.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia - A pressing problem: An executive summary of a National Institute of Child Health and Human Developmental Workshop. Reproductive Sciences. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 4.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 5.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 6.Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28:S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Huppertz B, Kingdom JC. Apoptosis in the trophoblast-role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004;11:353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Redline RW, Paterson P. Pre-eclampsia is associated with an excess of proliferative, immature intermidiate trophoblasts. Human Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 10.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive trophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 14.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 15.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 inin vivoandin vitromodels of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasionin vitroand models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia reoxygenetion. A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Cir Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 18.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 19.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulatedin vivoby HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 21.Crocker IP, Wareing M, Ferris GR, Jones CJ, Cartwright JE, Baker PN, Aplin JD. The effect of vascular origin, oxygen, and tumor necrosis factor alpha on trophoblast invasion of maternal arteriesin vitro. J Path. 2005;206:476–485. doi: 10.1002/path.1801. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Chiu K, Brescia RJ, Combs CA, Katz MA, Kitzmiller JL, Heilbron DC, Fisher SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 23.Graham CH, Postovit LM, Park H, Canning MT, Fitzpatrick TE. Adriana and Luisa Castellucci award lecture 1999: role of oxygen in the regulation of trophoblast gene expression and invasion. Placenta. 2000;21:443–450. doi: 10.1053/plac.2000.0543. [DOI] [PubMed] [Google Scholar]
- 24.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Tewari N, Kalkunte S, Murray DW, Sharma S. The water channel aquaporin 1 is a novel molecular target of polychlorinated biphenyls forin uteroanomalies. J Biol Chem. 2009;284:15224–232. doi: 10.1074/jbc.M808892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 28.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] : analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer. 1995;72:869–874. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat Med. 2008;14:810–812. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 31.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 33.Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 34.Plevyak M, Hanna N, Mayer S, Murphy S, Pinar H, Fast L, Ekerfelt C, Ernerudh J, Berg G, Matthiesen L, Sharma S. Deficiency of decidual IL-10 in first trimester missed abortion: a lack of correlation with the decidual immune cell profile. Am J Reprod Immunol. 2002;47:242–250. doi: 10.1034/j.1600-0897.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- 35.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, Sharma S. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 37.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL-10 promoter polymorphism. Placenta. 2006;27:445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 in human placental tissues and isolated cytotorophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 39.White CA, Johansson M, Roberts CT, Ramsay AJ, Robertson SA. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol Reprod. 2004;70:123–131. doi: 10.1095/biolreprod.103.018754. [DOI] [PubMed] [Google Scholar]
- 40.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J. Immunol. 183:1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaxton JE, Sharma S. Interleukin-10: A Multi-Faceted Agent of Pregnancy. Am. J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R713–R719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Norris WE, Kalkunte S. Beyond the threshold: An etiological bridge between hypoxia and immunity in preeclampsia. J Reprod. Immunol. 2010;85:112–116. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingdom JC, Kaufmann P. Oxygen and placental vascular development. Adv Exp Med Biol. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- 45.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schäffer L, Vogel J, Breymann C, Gassmann M, Marti HH. Preserved placental oxygenation and development during severe systemic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2006;290:R844–R851. doi: 10.1152/ajpregu.00237.2005. [DOI] [PubMed] [Google Scholar]
- 48.Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC, Adams MA, Croy BA. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension. 2010;55:729–737. doi: 10.1161/HYPERTENSIONAHA.109.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leno-Durán E, Hatta K, Bianco J, Yamada AT, Ruiz-Ruiz C, Olivares EG, Croy BA. Fetal-placental hypoxia does not result from failure of spiral arterial modification in mice. Placenta. 2010;31:731–737. doi: 10.1016/j.placenta.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 51.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Utero-placental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 52.Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, Post M, Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalkunte S, Norris W, Tewari N, Hansson S, Padbury J, Sharma S. Elevated heme and IL-10 deficiency trigger pre-eclampsia like symptoms. Am J Reprod Immunol. 2008;59:485. [Google Scholar]
- 54.Ratts VS, Tao XJ, Webster CB, Swanson PE, Smith SD, Brownbill P, Krajewski S, Reed JC, Tilly JL, Nelson DM. Expression of BCL-2, BAX, and BAK in the trophoblast layer of the human term placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–366. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.