Abstract
While putative disease-preventing lycopene metabolites are found in both tomato (Solanum lycopersicum) products and in their consumers, mammalian lycopene metabolism is poorly understood. Advances in tomato cell culturing techniques offer an economical tool for generation of highly-enriched 13C-lycopene for human bioavailability and metabolism studies. To enhance the 13C-enrichment and yields of labeled lycopene from the hp-1 tomato cell line, cultures were first grown in 13C-glucose media for three serial batches and produced increasing proportions of uniformly labeled lycopene (14.3 +/− 1.2 %, 39.6 +/− 0.5 %, and 48.9 +/− 1.5% with consistent yields (from 5.8 to 9 mg/L). An optimized 9-day-long 13C-loading and 18-day-long labeling strategy developed based on glucose utilization and lycopene yields, yielded 13C-lycopene with 93% 13C isotopic purity, and 55% of isotopomers were uniformly labeled. Furthermore, an optimized acetone and hexane extraction led to a four-fold increase in lycopene recovery from cultures compared to a standard extraction.
Keywords: carotenoids, tracers, lycopene, tomato, nutrition, plant cell culture
1. Introduction
Epidemiological and laboratory studies suggest that tomato (Solanum lycopersicum) consumption may reduce the risk for a number of chronic diseases including cardiovascular disease and cancers, notably prostate cancer (Giovannucci, Ascherio, Rimm, Stampfer, Colditz & Willett, 1995, Rissanen, Voutilainen, Nyyssonen & Salonen, 2002, WCRF & AICR, 2007). Tomatoes are a dietary source of a variety of phytochemicals including the red carotenoid lycopene, a linear tetraterpene. Lycopene along with its carotenoid precursors, phytoene and phytofluene, derived from tomatoes, have numerous hypothesized bioactivities that may mediate health promotion, including participation in antioxidant defense systems (Canene-Adams, Campbell, Zaripheh, Jeffery & Erdman, 2005, Engelmann, Clinton & Erdman, 2011). Moreover, an array of lycopene metabolites have been identified in human plasma and tissues (Khachik, Carvalho, Bernstein, Muir, Zhao & Katz, 2002, Kopec et al., 2010), but the origin of these potentially bioactive metabolites is unknown. Plants have many carotenoid cleavage dioxygenase (CCD) enzymes responsible for carotenoid metabolism, (Auldridge, McCarty & Klee, 2006) while in humans only two cleavage enzymes are known to impact carotenoid metabolism. B-carotene monooxygenase 1 (BCMO-1) is responsible for central cleavage of some pro-vitamin A carotenoids, and carotenoid dioxygenase 2 (BCDO-2) primarily acts to eccentrically cleave carotenoids (von Lintig, 2012). The role of these mammalian enzymes in the metabolism of lycopene is only beginning to be elucidated (Ford, Clinton, von Lintig, Wyss & Erdman, 2010). Therefore, lycopene metabolism occurs in both plant and animal tissues, yet it is currently unknown whether the series of chain-shortened lycopene metabolites previously detected in human plasma are endogenously produced following consumption of lycopene from tomato products and/or if the pre-formed metabolites are absorbed directly from tomato-containing foods (Kopec et al., 2010). A source of inexpensive, isotopically-labeled lycopene will greatly enhance our ability to better discriminate between endogenous pools of lycopene and newly ingested molecules, while also allowing us to identify metabolites produced in humans (Yeum & Russell, 2002).
Stable and radioactive hydrogen and carbon isotopes have been successfully incorporated into carotenoids for clinical tracer investigations of carotenoid absorption and metabolism (Kelm, Flanagan, Pawlosky, Novotny, Clevidence & Britz, 2001, Novotny, Kurilich, Britz & Clevidence, 2005, Tang et al., 2005, Tang, Qin, Dolnikowski, Russell & Grusak, 2005, Tang, Qin, Dolnikowski, Russell & Grusak, 2009). Carotenoids can be either extrinsically labeled by chemical incorporation of isotopes, or intrinsically labeled by the biological incorporation of isotopes, resulting in the labeled carotenoid housed within a labeled plant-food matrix (van Lieshout, West & van Breemen, 2003). A hybrid of the two methods is achieved when a biolabeled carotenoid is isolated from a biological matrix and utilized as an extrinsic dose. Intrinsically-labeled plant material allows for investigations related to bioavailability of a carotenoid from within the plant food matrix, but because plant-produced labeled carotenoid metabolites are also already present in that tissue, one cannot use the tracer to characterize the production of or to identify human carotenoid metabolic products. Purified, extrinsic dosing experiments can provide insight into the metabolism of a tracer compound over time, allowing for the identification of novel metabolites.
Currently very few, if any, chemically-synthesized, extrinsically labeled carotenoids are commercially-available, so the majority of recent clinical trials have used intrinsically labeled plant material (Kelm, Flanagan, Pawlosky, Novotny, Clevidence & Britz, 2001, Novotny, Kurilich, Britz & Clevidence, 2005, Tang et al., 2005, Tang, Qin, Dolnikowski, Russell & Grusak, 2005, Tang, Qin, Dolnikowski, Russell & Grusak, 2009). Advances in D2O-hydroponic and 13CO2-growth chamber methodologies have generated intrinsically labeled kale, miniature tomatoes, golden rice, and maize for absorption and metabolism studies of lutein, beta-carotene, and lycopene. These tools have advanced our understanding of carotenoid bioavailability and human conversion of pro-vitamin A carotenoids to vitamin A, but relatively low isotopic enrichment (Tang et al., 2005, Tang, Qin, Dolnikowski, Russell & Grusak, 2009) and long exposure times necessary to intrinsically label plant reproductive tissues (i.e. tomato) (van Lieshout, West & van Breemen, 2003) make these impractical for production of highly enriched lycopene tracers for the discovery and pharmacokinetic characterization of low-concentration lycopene metabolites.
Plant cell culture offers an option for producing isotopically labeled plant secondary metabolites for nutritional studies. Plant cell culture lends itself to labeled molecule production by allowing for intense environmental control for optimal metabolite production, as well as labeled precursor feeding for isotope incorporation (Lila, 2004). In vitro 14C and 13C labeling strategies have been applied for production of labeled isoflavones, anthocyanins, and stilbenes (Engelmann, Reppert, Yousef, Rogers & Lila, 2009, Grusak, Rogers, Yousef, Erdman & Lila, 2004, Krisa, Teguo, Decendit, Deffieux, Vercauteren & Merillon, 1999, Reppert, Yousef, Rogers & Lila, 2008, Vitrac et al., 2002, Yousef et al., 2004), with 13C-resveratrol production from grape cultures reaching semi-commercial yields (Yue, Zhang & Deng, 2011).
A number of recent advances have been made in the area of isotopically-labeled tomato carotenoid production from tomato cell cultures, with utilization of different herbicide treatment protocols for desired carotenoid accumulation profiles (Campbell, Rogers, Lila & Erdman, 2006, Engelmann, Rogers, Lila & Erdman, 2009, Engelmann et al., 2010). The identification of a high-lycopene yielding tomato cell line for 13C-lycopene production has significant potential for producing highly enriched 13C-lycopene due to the ability to grow a small amount of unlabeled inoculum in a carotenoid production (CP) medium containing 100% uniformly labeled 13C-glucose as the carbon source (Engelmann et al., 2010). In a previous report, we observed a distribution of 13C-lycopene isotopomers with a yield of ~ 3.5 mg 13C-lycopene/L culture, with the uniformly labeled isotopomer being most prominent of all isotopomers (Engelmann et al., 2010). These cultures were single-batch cultured such that cells were grown for one CP cycle with labeled glucose and then harvested. However, for human clinical investigations of lycopene absorption and metabolism using 13C tracers from plant cell culture to be economically and temporally feasible, greater yields of more highly enriched material is needed.
In the current study we demonstrate that the previously-described in vitro hp-1 tomato cell lycopene production system (Engelmann et al., 2010) can be repeatedly batch cultured for up to three growth cycles in CP media containing the lycopene cyclase inhibitor, CPTA, for consistent lycopene yields. This serial culturing strategy was then utilized for 13C-lycopene labeling, using uniformly labeled 13C-glucose as the labeled precursor material. This approach resulted in substantially increased proportions of uniformly labeled lycopene (13C40H56) with each repeated culturing cycle, as confirmed by LC-MS. Based on the observation that using cells previously grown in labeled media as inoculum for a subsequent labeling phase significantly increases 13C40H56 isotopomeric purity, we conducted a two phase time-course study, identifying the optimal duration for a 13C-loading phase for generation of a maximal amount of 13C-labeled inoculum, and the optimal duration of a 13C-lycopene labeling phase, based on lycopene yield and labeled glucose utilization. The most favorable conditions were then tested in a two phase loading and labeling trial, resulting in 13C-lycopene consisting of 55% uniformly labeled lycopene and a mixture of other highly enriched isotopomers. Further tracer production efficiency was achieved by developing and testing a more thorough lycopene extraction protocol. In this study, we demonstrate novel approaches for the efficient production of highly enriched 13C-lycopene.
2. Materials and Methods
2.1 PLANT MATERIAL AND CELL CULTURING METHODS
Tomato (Solanum lycopersicum) cell suspension cultures were previously established from the high lycopene mutant ‘Ailsa Craig’ hp-1 tomato line (obtained from the Tomato Genetics Resource Center, University of California, Davis, CA), as previously described (Engelmann et al., 2010). Tomato cell suspension cultures were continuously maintained in 40 mL of a previously described liquid maintenance media (Robertson, Mahoney, Goodman & Pavlath, 1995), and were subcultured every 7 days by transferring 2 mL of packed cells and 4 mL of spent media. For CP growth cycles, 4 mL of packed cells with 8 mL of spent media from maintenance cultures were transferred to 80 mL of CP media formulated as previously described (Robertson, Mahoney, Goodman & Pavlath, 1995) and the lycopene cyclase inhibitor, 2-(4-chlorophenyl-thio)triethylamine (CPTA; provided by Betty Ishida, USDA) was filter-sterilized and aseptically added to cultures (0.0745 g/L media) as previously described (Engelmann et al., 2010) on day 1 of a 12 day-long growth period (unless otherwise specified) to stimulate lycopene accumulation. Cultures were wrapped in foil to exclude light and maintained on rotary shakers (Innova 2300, New Brunswick Scientific, New Brunswick, NJ) set to 150 RPM at 25 °C. Culture fresh mass accumulation was measured for all experiments as previously described (Engelmann et al., 2010), and cells and media were reserved and stored at −80 °C for biochemical analyses as needed.
2.2 SERIAL SUBCULTURING
2.2.1 SERIAL CAROTENOID PRODUCTION BATCH CULTURING TRIAL WITH UNLABELED MEDIA
CP media (80 mL) containing glucose (30 g/L) was inoculated with cells and spent media from maintenance cultures, and cells were grown for a 7-day adaptation period. Stock cultures, continually maintained in sucrose maintenance media, were used to inoculate glucose-containing CP media for a 7-day adaptation period. To initiate the first batch of CP cultures batches, 80 mL of fresh glucose-containing CP media were inoculated and CPTA was added. Seven-day-old cultures from the first CP batch growth cycle were used to inoculate the second batch, which in turn provided inoculum for batch 3, and this process was repeated for four CP batch growth cycles. Each batch of cultures (n = 2–3 flasks) was harvested 12 days after inoculation.
2.2.2 EFFECT OF CULTURE ENCLOSURE CONDITIONS ON ISOTOPIC ENRICHMENT OF LYCOPENE
Six cultures were initiated, treated with CPTA, and grown in either a previously-described labeling chamber (Grusak, Rogers, Yousef, Erdman & Lila, 2004) or on an open-platform shaker (n=3/growth condition treatment) for one 13C-CP cycle.
2.2.3 IMPLEMENTATION OF SERIAL BATCH CULTURING FOR 13C-LYCOPENE PRODUCTION
The effect of serially culturing cells in 13C-glucose-containing media on the 13C lycopene isotopomers distribution was tested in five trials of 3 serial 13C-CP batches to produce sufficient 13C-lycopene for an ongoing human clinical trial investigating tomato carotenoid absorption and metabolism. The first serial batch of cultures was inoculated with cells and spent media from maintenance cultures. All serial batches were grown with 13C-glucose-containing CP media containing CPTA as described in the unlabeled trial. The proportion of each isotopomer was determined by duplicate analyses for one sample from each batch.
2.3. 13C-LOADING AND LABELING
2.3.1 LOADING AND LABELING TIME COURSE STUDIES
To promote efficient glucose utilization during the production of 13C-lycopene, two time course experiments were performed (3 trials/time course study, 14 flasks/trial). First, a 21-d study of the loading phase (n = 14 flasks/trial; 3 trials; two flasks harvested every 3 d) was conducted to determine the optimal growth period duration based on maximal media glucose depletion and identification of the end of the log phase of growth. Next, the same study design was repeated for labeling phase cultures that were initiated from loading phase cultures grown for the optimal number of days based on the findings of the loading phase study. Optimal duration of the labeling phase was determined by maximal media glucose depletion and lycopene yield. These time course studies were carried out using unlabeled glucose-containing media.
2.3.2 MEDIA GLUCOSE ANALYSIS
Media was formulated with a glucose concentration of 30 g/L, and thus this concentration was assumed for day 0 of the growth cycle. At each harvest time point, media was reserved and stored at −80 °C until subsequent glucose analysis by high pressure liquid chromatography (HPLC)-refractive index detector (RID) as previously described (Ha et al., 2011, Lu, Choi, Engelmann Moran, Jin & Erdman, 2011). Samples were prepared for duplicate analysis by brief centrifugation to eliminate cell debris, dilution (1:10) with HPLC-grade water, and were injected onto an Agilent 1260 Infinity HPLC-RID system equipped with an autosampler.
2.3.3 13C-LOADING AND LABELING TRIAL
The 2-stage 13C-loading and labeling batch strategy was tested in a small trial by initiating 1 flask of 13C-CP media for the loading cycle. This culture was grown for 9 d and then used to inoculate 6 culture flasks, with either 4 mL PCV and 4 mL (4 + 4) of spent media (n = 3) or with 4 mL PCV and 8 mL (4 + 8) of spent media (n = 3). The reduced volume of spent media was tested because this particular culture had a high cell-to-media ratio. Cell mass was recorded. Carotenoid concentrations were measured in one sample from each rep (n = 3/inoculation treatment) and 13C-lycopene enrichments were measured in one pooled sample for each inoculation treatment.
2.4 COMPARISON OF SEMI-PREPARATIVE CAROTENOID EXTRACTION METHODS
To compare the semi-preparative extraction efficiency of a previously described acetone and hexane (AH) method (Lu, Choi, Engelmann Moran, Jin & Erdman, 2011) versus a previously described biphasic hexane:ethanol:water (HEW) tomato cell carotenoid extraction method (Lu, Engelmann, Lila & Erdman, 2008), a homogenous sample of tomato cells was divided into 10-3 g replicates (n = 5/extraction method). In brief, for the previously described biphasic method (Lu, Engelmann, Lila & Erdman, 2008), 3 g of cells were first homogenized in 0.1% butylated hydroxytoluene (BHT) in ethanol, cells were saponified by aqueous KOH at 60 °C, water was added and then hexane was used to extract carotenoids from the polar fraction. The hexanes wash was repeated three times, these layers were pooled and concentrated to dryness under reduced pressure, and stored under argon for <24 hr before analysis. The AH method consisted of soniporation of 3 g of tomato cells with 20 mL of acetone for 15 min in an ultrasonic water bath (sonication), centrifugation for 5 min at 4 °C and 2400 RPM, and acetone collection. The acetone extraction step was repeated twice more with 20 mL of acetone and longer sonication of 30 min. Acetone fractions were gravity-filtered through Whatman #1 filter paper and then vacuum-filtered with a 0.2 um nylon filter to remove and cell debris. Acetone extracts were concentrated under reduced pressure using a Speedvac (Savant AS160, Savant, Thermo-Scientific). When the majority of acetone was removed, a small amount of water from the plant cells remained, which was washed with 1 mL of hexane 3 times, or until the hexane wash was colorless. The two methods were also compared for analytical scale extraction (5-0.25 g samples/extraction method) efficiency. Extraction efficiency was determined of the basis of the mass of major tomato carotenoids, lycopene, phytoene, and phytofluene extracted per gram of cells (FW).
2.5 ANALYTICAL CAROTENOID EXTRACTION AND QUANTITATION BY HPLC-PDA
Carotenoids were extracted from the unlabeled serial subculturing trial for analysis using the HEW method. For lycopene analysis of samples from the unlabeled, serial subculturing experiment, an HPLC system equipped with Dynamax Model SD-200 pumps (Agilent, Santa Clara, CA), Galaxie Chromatography Data System (Agilent), a Varian Prostar dual wavelength detector (Agilent), column cooler set to 18 °C, YMC C30 column (150 × 4.6 mm, 3 μm) (Waters, Milford, MA) using a solvent gradient method previously described (Campbell, Rogers, Lila & Erdman, 2006).
For subsequent 13C-labeling experiments and the time-course experiments, carotenoids were extracted using the AH extraction protocol for 0.25 g cell material and were quantified using an HPLC-PDA system that was described previously (Lu, Choi, Engelmann Moran, Jin & Erdman, 2011). For all carotenoid analyses, external standard curves were used for identification (by retention time and spectra) and quantitation of phytoene, phytofluene, and lycopene. Phytoene and phytofluene standards (donated by Hansgeorg Ernst, BASF, Ludwigshafen, Germany) and lycopene standards (isolated from Redivivo 10% Lycopene beadlets donated from DSM, Basel, Switzerland) were prepared as previously described (Lu, Choi, Engelmann Moran, Jin & Erdman, 2011) using published extinction coefficients (Rodriguez-Amaya, 2001). All carotenoid analyses were performed within 24 hr of extraction and general precautions were taken for working with carotenoids (Schiedt & Liaaen-Jensen, 1995).
2.6 LYCOPENE CRYSTALLIZATION FROM CAROTENOID EXTRACT
Lycopene was isolated from carotenoid-rich extracts utilizing a modification of a previously-published crystallization method (Kopec et al., 2010). Carotenoid-rich AH extracts were suspended in 1:1 methanol/chloroform solution and heated in a 60 °C shaking water bath for 5 min or until the extract was completely dissolved. Dissolved carotenoids were allowed to cool at room temperature for 5 minutes and then transferred to a −20 °C freezer overnight to facilitate crystal formation. The next day, fine lycopene crystals were isolated by vacuum filtration on a 0.2 um nylon filter and were rinsed 3 x with ice-cold methanol. The uncrystallized carotenoids and methanol rinse were reserved and a second and then third crystallization was attempted to capture any previously uncrystallized lycopene. Crystallized lycopene was then eluted through the filter into a clean collection flask using dichloromethane. The dichloromethane was completely removed under a stream of argon gas and lycopene was stored at −80 °C until further use.
2.7 DETERMINATION OF 13C-CAROTENOID ENRICHMENT BY HPLC-MS
For carotenoid mass isotopomer distribution analysis, lycopene was purified from the other components of the carotenoid-rich extract by crystallization as described above. The lycopene was dissolved in methyl-tert-butyl ether and injected onto an Agilent 1100 LC/MSD Trap XCt Plus system (Agilent). The gradient method and column were previously described (Lu, Choi, Engelmann Moran, Jin & Erdman, 2011) but the flow rate was decreased to 0.6 mL/min and the column temperature was maintained at 25 °C. The MS system was operated, as previously reported, in positive ion mode using atmospheric pressure chemical ionization (APCI) at 2877 V, 400 °C, a nebulizer pressure of 60 psi, and dry temperature of 350 °C (Engelmann et al., 2010). The m/z was analyzed for a range of biolabeled isotopomers and native lycopene in 13C lycopene samples. The mass (M) of unlabeled lycopene is m/z 536.5 and in positive ion mode it is detected as m/z 537.5 (M + H+). Since lycopene is a 40-carbon molecule (C40H56), the mass of uniformly labeled lycopene molecule is detected as (M+H++40) m/z 577.5. Previously, we determined that in vitro labeling yielded a range of mass isotopomers (Engelmann et al., 2010), and thus each possible 13C-isotopomer was quantitated (M + H+ to M+H++40) between m/z 537.5 to 577.5 by extracting ion chromatograms for each putative mass and comparing the relative all-trans and 5-cis lycopene peak areas to calculate relative proportions of each isotopomer for each 13C-labeled lycopene sample. Relative percentages (PM+ i) of each isotopomer [M + i; (M+H++0 to M+H++40)] were calculated as follows:
| Equation 1 |
Where AM+i = is the peak area of the isotopomer of interest. It is also of interest to calculate the relative percentages of 13C and 12C in recovered lycopene molecules to evaluate the efficiency of incorporation of 13C from media glucose, versus the unlabeled carbon load that persists from unlabeled inoculum. The percent of carbon occurring as the 13C isotope in labeled lycopene was calculated to be:
| Equation 2 |
In this calculation, the proportion of 13C in each mass isotopomer (M+H++0 to M+H++40) was calculated by dividing the number of 13C atoms present in a specific lycopene isotopomer by the total number of carbons in lycopene (i/40). This value was multiplied by the observed abundance of that isotopomer in the sample (PM+i). The products for each isotopomer were summed and multiplied by 100 to obtain the percent total 13C occurring in the lycopene sample.
2.8 STATISTICS
Group differences were identified by analysis of variance within the proc glm program of SAS 9.3 (SAS Institute, Cary, North Carolina) for groups where the assumptions of normality and homogeneity of variance were met. When a significant overall F-value was detected, then orthogonal contrasts were made to identify specific significant (α = 0.05) differences between groupings. When normality and/or homogeneity of variance assumptions for parametric ANOVA were violated, then the non-parametric Kruskal-Wallis test was used and pr>chi-squared values were evaluated for significance (α=0.05). Linear relationship equations and R2-values were calculated using Excel, Microsoft Office Professional Plus 2010.
3. Results and Discussion
3.1 SERIAL CAROTENOID PRODUCTION CYCLES
Tomato cell cultures have not been previously grown serially in CP batches. To determine the impact of using cells from a CP batch as inoculum on lycopene yield, a trial consisting of four serial carotenoid-production batches was conducted. Culture fresh cell mass decreased linearly with an increasing number of serial growth cycles (y = −45.5x + 243.2; R2=0.998; y = g FW/L culture and X = number of serial growth cycles; 200, 148, 108, and 61 g FW/L over the four serial growth cycles, respectively; each value represents the average of 2–3 flask repetitions). Mass accumulation was reduced by 70% between cycle 1 and cycle 4. Alternatively, the concentration of lycopene in the cells increased linearly (y = 9.3x + 13.2, R2=0.975) over the four serial growth cycles (23, 29, 43, 49 ug LYC/g cells). Consequently, total lycopene yields from cells remained largely stationary over the first three growth cycles (4.1, 3.5, and 3.8 mg/L for cycles 1, 2, and 3, respectively) and dropped to 2.5 mg/L by the fourth serial CP batch. Since overall lycopene yields were not negatively impacted by three serial batch culturing cycles, this approach was utilized for 13C-lycopene labeling. However, upon discovering that serial culturing in CP media negatively impacts culture fresh mass accumulation, a small trial (n = 3 flasks/treatment) of 4 serial batches was performed to compare batch 1 cultures that were either directly inoculated by maintenance cultures or by cultures that had been adapted to CP media for 7 days. Omission of a CP media adaptation growth cycle did not negatively impact lycopene yields in this trial (data not shown). Based on these results, subsequent serial 13C-labeling trials were performed in 3 serial CP batches, without a 7-day CP media adaptation growth cycle.
3.2 SERIAL BATCH CULTURING WITH 13C-GLUCOSE MEDIA
3.2.1 Effect of Culture Enclosure Conditions on 13C-Enrichment of Lycopene
Batch size is an important criterion for feasibility when using cell cultures for biolabeling milligram amounts of stable isotope-tracers needed for human nutritional studies (van Lieshout, West & van Breemen, 2003). The use of traditionally-used labeling chambers required for trapping radioactive CO2 expired by cells grown with 14C-sucrose can limit batch size (Grusak, Rogers, Yousef, Erdman & Lila, 2004), and thus elimination of a labeling chamber for stable isotope labeling is desirable. Biolabeling by plant cell culture methods results in a range of isotopomers, even when the sole carbohydrate source in the media is uniformly labeled glucose, thus raising concerns about environmental carbon exposure with an open platform shaker. Therefore, the effect of an enclosed chamber shaker versus open shaker on carotenoid enrichment was tested. Cultures grown on the open-platform shaker (n = 3 flasks) accumulated greater cell mass (262 g/L) than cultures grown in the chamber (n = 3 flasks) (192 g/L), reached 20% greater total carotenoid concentrations (25 vs 21 ug/g, respectively), and had an overall 60% greater carotenoid yield (6.5 vs. 4.0 mg/L). The isotopic enrichment profiles, however, were identical with 11% of lycopene being uniformly labeled, 18% as the M + 39 isotopomer, 19% as the M + 38 isotopomer, and overall 81–82% of lycopene molecules in both enclosure conditions had enrichments between M + 35 – M + 40. This result largely eliminates the effect of exposure to 12CO2 as a factor in isotopic enrichment, which follows with the fact that the cultures are foil-wrapped for light exclusion and provided carbohydrate in the media, therefore are unable and do not need to perform photosynthetic carbon fixation. This further suggests that any small amount of 12C incorporated into lycopene likely derives from unlabeled inoculum. Based on these results, subsequent experiments sought to reduce the amount of unlabeled carbon occurring in inoculum by serially increasing the proportion of 13C in cells. As growth on the open-platform shaker did not negatively impact lycopene yield or 13C-lycopene enrichment, all subsequent labeling experiments were conducted on the open-platform shaker. This advance permitted marked scaling-up of 13C-carotenoid production from serial batches of tomato cells.
3.2.2 Serial 13C-batch culturing growth and carotenoid measures
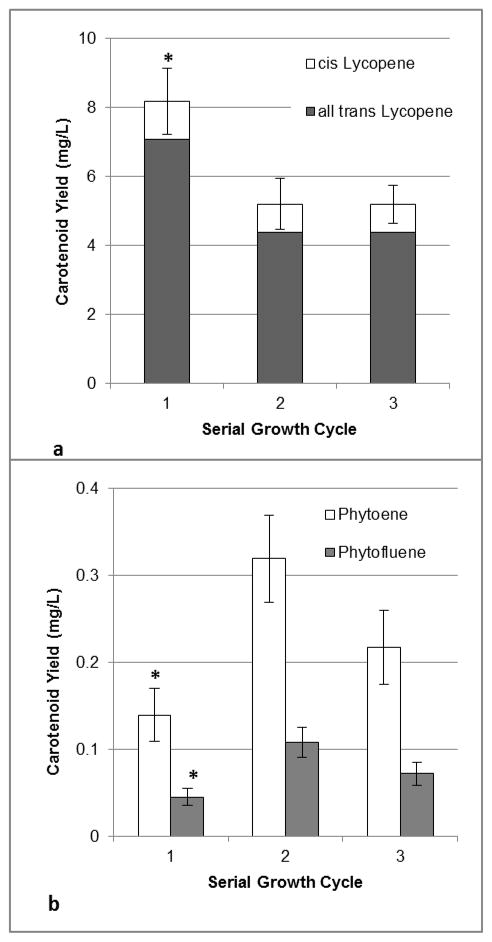
A total of five serial 13C-labeling trials were performed to generate sufficient 13C-lycopene for a human clinical trial, which is currently underway. Culture fresh mass decreased linearly (y=−21.2 x + 230.1; R2=0.96) over the three serial cycles (211 +/− 7, 183 +/− 7, and 169 +/− 5 g FW/L culture, for cycles 1, 2, and 3, respectively), as observed in the aforementioned unlabeled pilot trial. Total carotenoid concentrations (sum of lycopene cis isomers, all-trans lycopene isomer, phytoene, and phytofluene) from cultured cells grown in serial batches 1, 2, or 3 were not significantly different (Table 1). Nor was there a significant effect of serial batch culturing on individual carotenoid concentrations (Table 1). The relative proportions of each carotenoid across batches were also similar with 90% total lycopene, 8% phytoene, and 2% phytofluene. Overall carotenoid yields were significantly greater from cultures grown during the first serial cycle (9.0 +/− 1.0 mg total carotenoids/L culture) than from subsequent cycles (5.8 +/− 0.8 and 5.8 +/− 0.6 mg/L from the second and third cycles, respectively). Individual carotenoid yields also varied by number of serial CP run (Figure 1), with cultures grown in the first labeling batch producing greater amounts of total carotenoids than cultures from the second and third runs.
Table 1.
Carotenoid Concentrations (ug carotenoid/g cells; mean ± SEM; n = 5 trials, duplicate analyses/trial) in cells grown in 13C-glucose-containing carotenoid production media with CPTA for 1, 2, or 3 serial labeling cycles.
| Serial Labeling Cycle | Total Lycopene | Phytoene | Phytofluene | Total Carotenoids |
|---|---|---|---|---|
| 1 | 38.4 ± 3.4 | 2.9 ± 0.1 | 1.0 ± 0.1 | 42.2 ± 3.5 |
| 2 | 28.9 ± 4.6 | 2.6 ± 0.3 | 0.8 ± 0.1 | 32.3 ± 5.0 |
| 3 | 31.0± 3.7 | 2.7 ± 0.2 | 0.8 ± 0.1 | 34.4 ± 3.9 |
Figure 1.
All-trans and cis-lycopene (A) and phytoene and phytofluene (B) yields from tomato cell cultures serially transferred and grown in 13C-glucose-containing carotenoid production media for 1, 2, or 3 growth cycles. Each bar represents the average of 5 trials, with duplicate analyses performed for each trial. Error bars represent the standard error of the mean. Significant differences (α= 0.05) between serial growth cycles, within a carotenoid type (lycopene, phytoene, and phytofluene) are denoted by an asterisk (*).
3.2.3 Increasing enrichment of 13C-lycopene with serial 13C-labeling batches
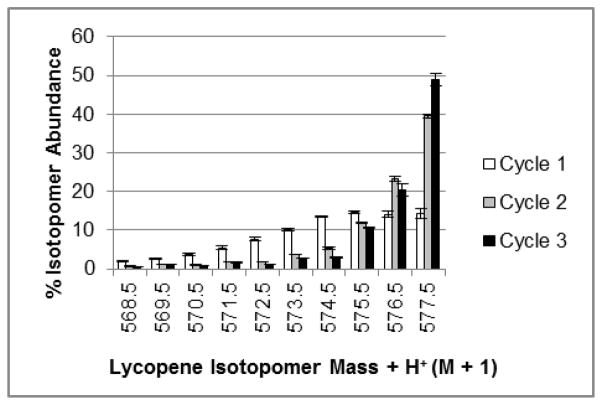
Utilizing small masses of cells from 13C-CP batches as inoculum for subsequent 13C-CP batches increased the proportion of highly enriched 13C-lycopene over three serial labeling cycles. Each subsequent growth cycle on 13C-glucose shifted the isotopomeric distribution toward the more uniformly labeled isotopomers (Figure 2), with 67% of lycopene in the first growth cycle having enrichments between M + 35 and M + 40, and increasing to 84% and 86% with two and three growth cycles, respectively. Specifically, the proportion of uniformly labeled lycopene significantly increased with each repeated culturing cycle with 13C-glucose, with 14.3 +/− 1.2 % in cycle 1, 39.6 +/− 0.5 % in cycle 2 and 48.9 +/− 1.5% in cycle 3 (Figure 2). The enrichment of lycopene from the first batch differs from our previous report of ~ 45% uniformly labeled lycopene being produced from tomato cells grown in a single labeling batch (Engelmann et al., 2010). This difference is likely attributable to that work being based on one trial that was analyzed with a less sensitive LC-MS 13C-isotopomer quantitation method than the method used in the current investigation. Recent advances in our lab have allowed the adaptation of more sensitive separation and detection methods (Lu, Choi, Engelmann Moran, Jin & Erdman, 2011). The natural abundance of 13C is 1.1%, and in these trials, serially culturing cells with 13C-glucose media increased the percent of 13C in lycopene from 87.8% in batch 1, to 92.2% in batch 2, and 93.1 % in the third serial batch. Previously, one of the drawbacks of intrinsically labeled carotenoids was that there is too broad of a distribution of isotopomers, weakening detection capabilities by LC-MS of ingested carotenoids. This is attributed to insufficient duration of exposure of plant material to isotopically labeled precursor compounds (van Lieshout, West & van Breemen, 2003). Levels of enrichment here are much greater than previous reports in other in vitro 13C-labeling systems [21–36% in resveratrol (Yue, Zhang & Deng, 2011)], and are more similar to those obtained with 13CO2 labeling of whole kale with 98% enrichment in beta-carotene and lutein) (Kelm, Flanagan, Pawlosky, Novotny, Clevidence & Britz, 2001), and with similar growth period duration. Using the serial culturing strategy, this hurdle can be overcome to yield a very highly enriched tracer with a compact isotopomer profile for enhanced detection of ingested lycopene and resultant metabolites.
Figure 2.
Isotopomeric distribution of 13C-enriched lycopene over three serial growth cycles. Bars represent the percent of enriched lycopene present as a specific isotopomer, out of all possible 13C isotopomers detected in positive ion mode [m/z 537.5 (M+H++0) – m/z 577.5 (M+H++40)]. Here the M+H++30 – M+H++40 isotopomers are represented. Data represent the average of five trials with one analysis/trial, and error bars represent the standard error of the mean.
3.3 TWO-PHASE 13C-LOADING AND LABELING BATCH STRATEGY FOR EFFICIENT 13C-BIOLABELING
Serially culturing tomato cells with 13C-glucose was effective for increasing the proportion of uniformly labeled lycopene over three growth periods. This approach led to a very marked increase in the proportion of uniformly labeled 13C-lycopene, occurring after just two serial growth cycles with 13C-glucose media and a minimal shift in isotopomeric profile with a third serial subculturing cycle. Since just two serial batches substantially increase isotopic enrichment and isotopic purity of uniformly labeled lycopene, a system where batch 1 cultures serve exclusively as labeled inoculum (loading phase) for a batch 2 labeling phase was characterized, first with unlabeled glucose media by which growth durations were determined based on glucose utilization, mass yield and carotenoid yields.
3.3.1 Determination of loading cycle duration by fresh mass yield and media glucose utilization
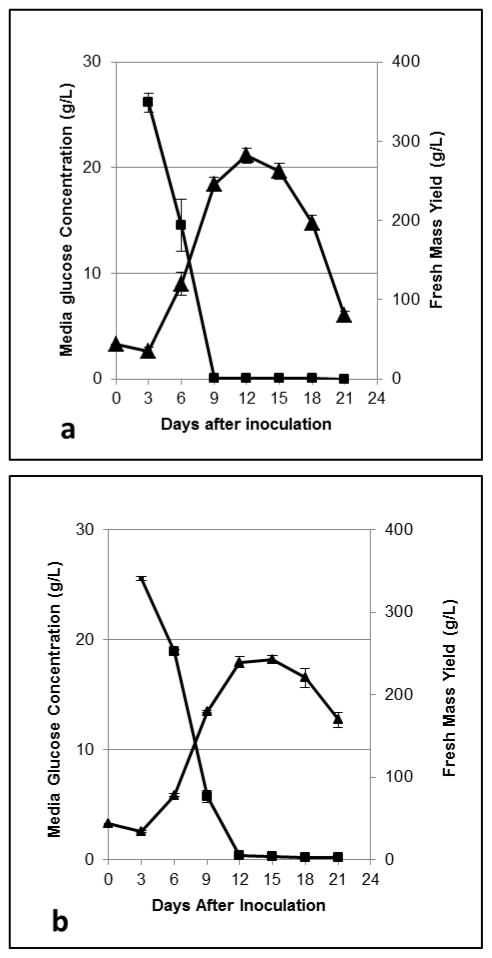
Plant cell cultures were grown in CP media containing unlabeled glucose and were harvested every three days for a 21-day-long time course study to identify the peak mass accumulation and time of complete utilization of media glucose. A typical sigmoidal growth curve was observed, and of the experimental data, the greatest mass accumulation occurred on day 12 (282 +/− 10 g FW/L culture), which appeared to be during the stationary phase of growth (Figure 3 A). The observed end of the logarithmic phase occurred on day 9 (245 +/− 9 g FW/L culture), which coincided with observed exhaustion of media glucose (Figure 3A). This suggests that media glucose in this system is likely the limiting factor in media, determining the time course of culture growth. The day 9 time point was chosen for future transfers to the labeling phase because media glucose is fully utilized at this point and cells at the end of the log phase of growth tend to be more viable than those in stationary phase (Mustafa, de Winter, van Iren & Verpoorte, 2011).
Figure 3.
Culture fresh mass accumulation (triangles) and media glucose utilization (squares) during the loading phase time course experiment (A) and the labeling phase time course experiment (B). Markers represent the average of three trials analyzed in duplicate. Error bars represent SEM.
3.3.2 Determination of labeling cycle duration
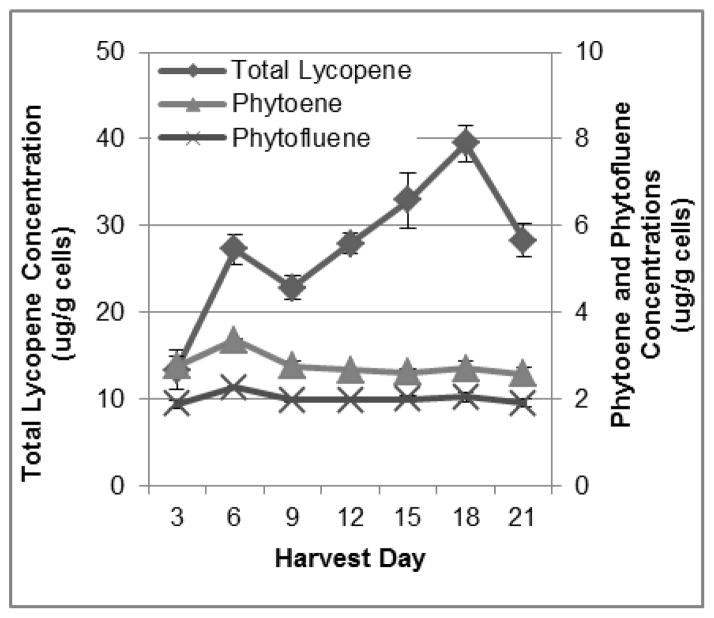
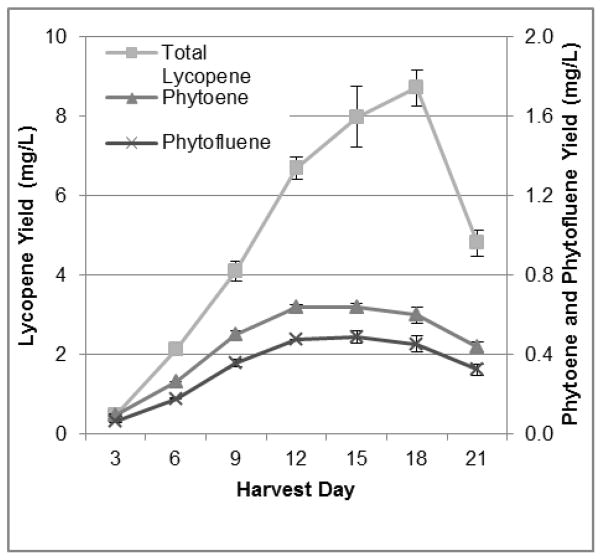
Labeling phase time course experiments were carried out in unlabeled glucose-containing CP media with CPTA, using cells that were grown for a 9-day-long loading phase in CPTA-free glucose-containing CP media. Cultures reached similar peak fresh mass accumulation on days 12 and 15 of the growth cycle (239 +/− 7 and 242 +/− 5 g FW/L culture, respectively), and media glucose was exhausted by day 12 (Figure 3B). This is shifted by 3 d from the loading phase, suggesting a slightly longer lag in cell growth in the labeling phase. Cell lycopene concentrations changed markedly by nearly 3-fold, over the time course from 13.4 +/− 2.4 ug/g on day 3 to 39.5 +/− 2.1 ug/g on day 18, and then dropped by 28% between day 18 and day 21 to 28.3 +/− 1.9 ug/g (Figure 4). Lycopene concentrations continued to increase for three days after peak mass was reached, indicating that while primary metabolism associated with cell growth and division was slowed, secondary metabolite formation continued. This observation could be due to a commonly seen phenomenon in plants in situations where stress, in this case potentially lowered glucose concentrations, leading to changes in osmolarity and/or energy pools, may stimulate further secondary metabolite accumulation (Lila, 2006, Ramachandra Rao & Ravishankar, 2002). Alternatively, concentrations of the colorless precursors of lycopene did not change greatly over the 21-day period (Figure 4) suggesting that these are transient intermediates that need not accumulate before lycopene accumulation. Total lycopene yield increased by 18-fold from 0.45 +/− 0.08 mg/L culture on day 3 to 8.71 +/− 0.46 mg/L culture on day 18, and yield dropped by 45%, or 3.9 mg, between day 18 and day 21 (Figure 5). Yields of phytoene and phytofluene did increase over the growth period, with logarithmic accretion from day 3 to day 12 and then a plateau from day 12 to day 18.
Figure 4.
Cellular carotenoid concentrations in the loading phase experiments. Each data point represents the average of three trials, with duplicate analyses for each trial. Error bars represent the standard error of the mean.
Figure 5.
Carotenoid yields from labeling phase time course trials; lycopene (square), phytoene (triangle), phytofluene (X). Each data point represents the average of three trials, analyzed in duplicate. Error bars represent the standard error of the mean.
3.3.3 13C-Loading and Labeling Trial
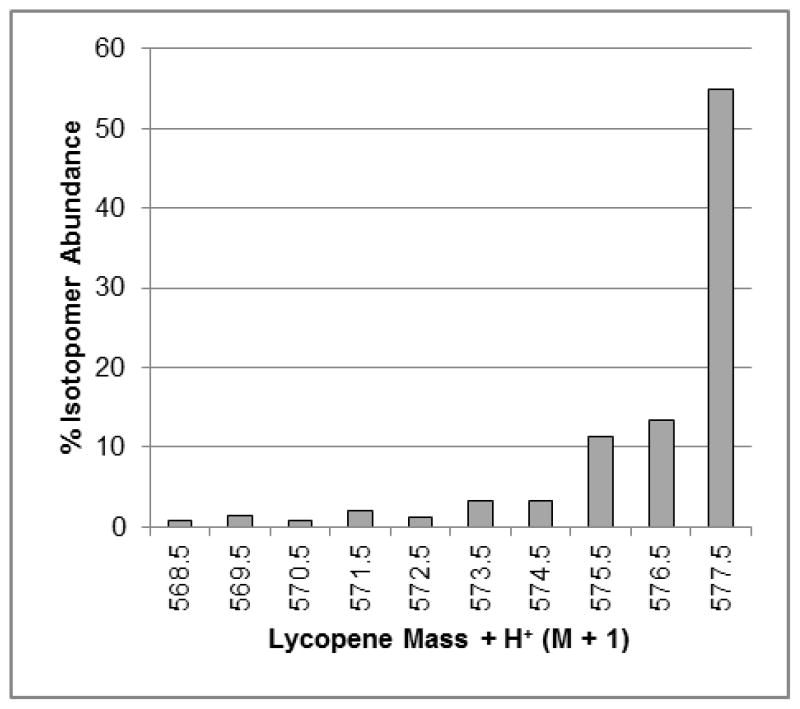
Labeling cultures were harvested on day 18 and culture mass yield was 185 ± 7 g/L culture, and lycopene, phytoene, and phytofluene yields were 5.2 ± 0.6, 0.47 ± 0.03, and 0.18 ± 0.01 mg/L culture. These lycopene and cell mass yields were lower than predicted for a day 18 harvest, and cultures had started to darken in color suggesting that cultures had exited the stationary phase and entered death phase. As fluctuations in carotenoid yields and cell culture growth can occur, in the future carotenoid concentrations and mass yields can be monitored real-time by using unlabeled sentinel cultures during the time points around 18 days, allowing identification of peak carotenoid yields. The proportions of 13C-lycopene isotopomers between M + H+ + 31 – M + H+ + 40 are presented in Figure 6. The proportion of uniformly labeled lycopene was similar between the cultures inoculated with differing volumes of spent media (4 and 8 mL), with 55% and 61% of the enriched lycopene isotopomers being uniformly labeled, respectively. These observed levels of enrichment are greater than the anticipated 40% uniformly labeled isotopomeric purity based on the serial subculturing trials. Ninety-two percent of the lycopene isotopomers were present in the M + H++ 30 – M + H++ 40 masses (Figure 6) and 94% of the carbon in the lycopene mixture was present as 13C in both sets of samples. This trial demonstrates that a 13C-loading and labeling strategy can be effectively used to produce very highly enriched 13C-lycopene, and that spent media volume in inoculum can be as low as 4 mL with no adverse effects on carotenoid yield or enrichment.
Figure 6.
Isotopomeric distribution of 13C-enriched lycopene from labeling cultures grown using the 13C-loading and labeling strategy. Bars represent the percent of enriched lycopene present as a specific isotopomer, out of all possible 13C isotopomers detected in positive ion mode [m/z 537.5 (M+H++0) – m/z 577.5 (M+ H++40)]. Here the M+ H++31 – M+ H++40 isotopomers are represented. Data represent one analysis of one lycopene sample pooled from three individual extraction replicates.
3.4. Extraction and Purification Methods
A key concern of phytochemical production from plant cell culture is the development of efficient extraction methods. The previously established hexane:ethanol:water (HEW) extraction approach (Lu, Engelmann, Lila & Erdman, 2008) was compared with an acetone:hexane (AH) semi-preparative scale extraction. The AH extraction method captured 4.1-fold more total lycopene (36.2 +/− 5.4 ug/g cells) than the HEW method (7.12 +/− 1.31 ug/g), and this same significant relationship was also present when analyzed for all-trans lycopene and for cis-lycopene extraction (Table 2). This translated to significantly greater recovery of total carotenoids as well by the AH method, although phytoene and phytofluene extraction was non-significantly increased by the AH method, compared to the HEW method. Both extraction methods were repeated until the final solvent wash was colorless, however it was noted that the cells that underwent the HEW extraction were generally darker in color than the AH extracted cells, indicating incomplete pigment extraction from the cells. The 4-fold increase in lycopene extracted from cell cultures is a marked advance, allowing tomato cell culture to serve as an efficient source for stable-isotope tracer lycopene from human studies.
Table 2.
Carotenoid extraction yields (ug carotenoid/g; mean ± SEM; n = 1 trial with 5 replicate analyses/trial) from 3 g of tomato cells by different solvent extraction methods.
| Extraction method | ||||
|---|---|---|---|---|
| Carotenoid (ug/g cells) | Hexane:Ethanol: Water | Acetone:Hexane | p-value | Fold-difference |
| Total Lycopene* | 7.1 ± 1.3 | 36.2 ± 5.4 | 0.009 | 5.1 |
| all trans lycopene* | 5.7 ± 1.1 | 31.8 ± 4.9 | 0.009 | 5.6 |
| cis lycopene | 1.1 ± 0.2 | 3.7 ± 0.4 | 0.0005 | 3.4 |
| Phytoene | 3.1 ± 0.6 | 4.2 ± 0.5 | 0.2 | 1.4 |
| Phytofluene | 1.9 ± 0.3 | 2.5 ± 0.4 | 0.2788 | 1.3 |
| Total Carotenoids * | 11.7 ± 2.2 | 42.1 ± 6.1 | 0.009 | 3.6 |
Analyzed by non-parametric ANOVA, Kruskal-Wallis test.
4. CONCLUSIONS
Isotopically labeled tracers are needed for human nutritional research, however advances in studying the absorption and metabolism of dietary bioactive compounds such as carotenoids has been hampered by inconsistent or prohibitively expensive commercial availability. Plant cell culture offers a unique method to customize production of labeled phytochemical tracers. In this paper we described a series of advances that can be applied for the production of highly-enriched carotenoids and can be translated for the production of other phytochemical tracers for nutritional studies. Repeated batch culturing with 13C-glucose as well as utilization of a 2-stage 13C-loading and 13C-labeling strategy substantially shifts isotopomeric enrichment profiles and resultant isotopomeric purity of uniformly-labeled lycopene, offering a robust tool for the quantitative evaluation of lycopene and its metabolites in humans. For the first time, intrinsically-labeled lycopene derived from plant cell cultures has been isolated for extrinsic dosing studies in humans that will provide much needed insight regarding lycopene absorption kinetics, tissue biodistribution, as well as the determination of which metabolites are produced. These studies will enhance our understanding of mechanisms whereby lycopene may mediate health promoting bioactivities.
Highlights.
Plant cell culturing strategies to yield highly enriched 13C-lycopene are proposed.
3 repeated growth cycles with 13C-glucose media increased % of [U]-13C-lycopene from 14% to 49%.
2-phase 13C-loading and labeling strategy yields 55% [U]-13C-lycopene.
2-phase 13C-loading and labeling strategy yields 93% 13C-atom% pure lycopene.
Acetone extraction recovers 4-fold more lycopene than hexane-ethanol-water.
Acknowledgments
This work was funded by the National Center for Complementary and Alternative Medicines of the (NIH/NCCAM-5R21AT005166-01; 5R21AT005166-02). The authors would like to thank The Ohio State University James Cancer Center’s Pelotonia Postdoctoral Fellowship Program for funding Nancy E. Moran. Dr. Lucas (Zhong) Li, Director of the University of Illinois Metabolomics Center funded by the Roy J. Carver Charitable Trust and the National Center for Research Resources, assisted in LC-MS analysis of 13C-lycopene. Rachel Kopec of Steven Schwartz’s lab, The Ohio State University, assisted in method development for lycopene crystallization from tomato cell culture. The HPLC-RID analysis was conducted in the lab of Dr. Yong-Su Jin of the Department of Food Science and Human Nutrition at the University of Illinois with the assistance of Panchalee Pathanibul.
ABBREVIATIONS USED
- APCI
atmospheric pressure chemical ionization
- CP
carotenoid production
- CPTA
2-(4-chlorophenyl-thio)triethylamine
- HPLC-PDA
high pressure liquid chromatography-photodiode array
- HPLC-RID
high-pressure liquid chromatography refractive index detector
- LC-MS
tandem liquid chromatography and mass spectrometer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nancy E. Moran, Email: nancy.moran@osumc.edu.
Randy B. Rogers, Email: rbrogers@illinois.edu.
Chi-Hua Lu, Email: luchi@missouri.edu.
Lauren E. Conlon, Email: lconlon2@illinois.edu.
Mary Ann Lila, Email: maryann_lila@ncsu.edu.
Steven K. Clinton, Email: steven.clinton@osumc.edu.
John W. Erdman, Jr., Email: jwerdman@illinois.edu.
References
- Auldridge ME, McCarty DR, Klee HJ. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Current opinion in plant biology. 2006;9(3):315–321. doi: 10.1016/j.pbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. Journal of Agricultural and Food Chemistry. 2006;54(3):747–755. doi: 10.1021/jf0581269. [DOI] [PubMed] [Google Scholar]
- Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. The Journal of nutrition. 2005;135(5):1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- Engelmann NJ, Clinton SK, Erdman JW., Jr Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Advances in nutrition (Bethesda, Md) 2011;2(1):51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann NJ, Campbell JK, Rogers RB, Rupassara SI, Garlick PJ, Lila MA, Erdman JW., Jr Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [(13)C]-carotenoid production. Journal of Agricultural and Food Chemistry. 2010;58(18):9979–9987. doi: 10.1021/jf101942x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann NJ, Reppert A, Yousef G, Rogers RB, Lila MA. In vitro production of radiolabeled red clover (Trifolium pratense) isoflavones. Plant cell, tissue and organ culture. 2009;98(2):147–156. doi: 10.1007/s11240-009-9547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann NJ, Rogers RB, Lila MA, Erdman JW., Jr Herbicide treatments alter carotenoid profiles for 14C tracer production from tomato (Solanum lycopersicum cv. VFNT cherry) cell cultures. Journal of Agricultural and Food Chemistry. 2009;57(11):4614–4619. doi: 10.1021/jf803905d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. The Journal of nutrition. 2010;140(12):2134. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. Journal of the National Cancer Institute. 1995;87(23):1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- Grusak MA, Rogers RB, Yousef GG, Erdman JW, Jr, Lila MA. An enclosed-chamber labeling system for the safe 14C-enrichment of phytochemicals in plant cell suspension cultures. In Vitro Cellular and Developmental Biology Plant. 2004;40(1):80–85. [Google Scholar]
- Ha SJ, Galazka JM, Kim SR, Choi JH, Yang X, Seo JH, Glass NL, Cate JH, Jin YS. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):504–509. doi: 10.1073/pnas.1010456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MA, Flanagan VP, Pawlosky RJ, Novotny JA, Clevidence BA, Britz SJ. Quantitative determination of 13C-labeled and endogenous beta-carotene, lutein, and vitamin A in human plasma. Lipids. 2001;36(11):1277–1282. doi: 10.1007/s11745-001-0842-1. [DOI] [PubMed] [Google Scholar]
- Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Experimental biology and medicine (Maywood, NJ) 2002;227(10):845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. Journal of Agricultural and Food Chemistry. 2010;58(6):3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisa S, Teguo PW, Decendit A, Deffieux G, Vercauteren J, Merillon JM. Production of 13C-labelled anthocyanins by Vitis vinifera cell suspension cultures. Phytochemistry. 1999;51(5):651–656. doi: 10.1016/s0031-9422(99)00068-0. [DOI] [PubMed] [Google Scholar]
- Lila MA. Anthocyanins and human health: an in vitro investigative approach. 2004;5:306. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila MA. The nature-versus-nurture debate on bioactive phytochemicals: the genome versus terroir. Journal of the science of food and agriculture. 2006;86(15):2510–2515. [Google Scholar]
- Lu CH, Engelmann NJ, Lila MA, Erdman JW., Jr Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. Journal of Agricultural and Food Chemistry. 2008;56(17):7710–7714. doi: 10.1021/jf801029k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Choi J, Engelmann Moran N, Jin Y, Erdman JW., Jr Laboratory-scale production of 13C-labeled lycopene and phytoene by bioengineered. Escherichia coli. 2011;59(18):9996. doi: 10.1021/jf202599z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa NR, de Winter W, van Iren F, Verpoorte R. Initiation, growth and cryopreservation of plant cell suspension cultures. Nature protocols. 2011;6(6):715–742. doi: 10.1038/nprot.2010.144. [DOI] [PubMed] [Google Scholar]
- Novotny JA, Kurilich AC, Britz SJ, Clevidence BA. Plasma appearance of labeled beta-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. Journal of lipid research. 2005;46(9):1896–1903. doi: 10.1194/jlr.M400504-JLR200. [DOI] [PubMed] [Google Scholar]
- Ramachandra Rao S, Ravishankar GA. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnology Advances. 2002;20(2):101–153. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Reppert A, Yousef GG, Rogers RB, Lila MA. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. Journal of Agricultural and Food Chemistry. 2008;56(17):7860–7865. doi: 10.1021/jf801413z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Salonen JT. Lycopene, atherosclerosis, and coronary heart disease. Experimental biology and medicine (Maywood, NJ) 2002;227(10):900–907. doi: 10.1177/153537020222701010. [DOI] [PubMed] [Google Scholar]
- Robertson GH, Mahoney NE, Goodman N, Pavlath AE. Regulation of lycopene formation in cell suspension culture of VFNT tomato (Lycopersicon esculentum) by CPTA, growth regulators, sucrose, and temperature. Journal of Experimental Botany. 1995;46(287):667–73. [Google Scholar]
- Rodriguez-Amaya DB. A Guide to Carotenoid Analysis in Foods. Washington, D. C: OMNI Research ILSI Human Nutrition Institute; 2001. [Google Scholar]
- Schiedt K, Liaaen-Jensen S. Isolation and Analysis. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: Isolation and Analysis. 1A. Basel: Birkhauser Verlag; 1995. p. 81. [Google Scholar]
- Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden Rice is an effective source of vitamin A. The American Journal of Clinical Nutrition. 2009;89(6):1776–1783. doi: 10.3945/ajcn.2008.27119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Ferreira AL, Grusak MA, Qin J, Dolnikowski GG, Russell RM, Krinsky NI. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. The Journal of nutritional biochemistry. 2005;16(4):229–235. doi: 10.1016/j.jnutbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. The American Journal of Clinical Nutrition. 2005;82(4):821–828. doi: 10.1093/ajcn/82.4.821. [DOI] [PubMed] [Google Scholar]
- van Lieshout M, West CE, van Breemen RB. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: a review. The American Journal of Clinical Nutrition. 2003;77(1):12–28. doi: 10.1093/ajcn/77.1.12. [DOI] [PubMed] [Google Scholar]
- Vitrac X, Krisa S, Decendit A, Vercauteren J, Nuhrich A, Monti JP, Deffieux G, Merillon JM. Carbon-14 biolabelling of wine polyphenols in Vitis vinifera cell suspension cultures. Journal of Biotechnology. 2002;95(1):49–56. doi: 10.1016/s0168-1656(01)00441-2. [DOI] [PubMed] [Google Scholar]
- von Lintig J. Metabolism of carotenoids and retinoids related to vision. Journal of Biological Chemistry. 2012;287(3):1627–1634. doi: 10.1074/jbc.R111.303990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WCRF, & AICR. Summary: food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, D.C: American Institute for Cancer Research; 2007. [Google Scholar]
- Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annual Review of Nutrition. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- Yousef G, Seigler D, Grusak M, Rogers R, Knight C, Kraft T, Erdman J, Jr, Lila M. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. Journal of Agricultural and Food Chemistry. 2004;52(5):1138–1145. doi: 10.1021/jf035371o. [DOI] [PubMed] [Google Scholar]
- Yue X, Zhang W, Deng M. Hyper-production of C-13-labeled trans-resveratrol in Vitis vinifera suspension cell culture by elicitation and in situ adsorption. Biochemical engineering journal. 2011;53(3):292–296. [Google Scholar]