INTRODUCTION
Superior canal dehiscence syndrome (SCDS) is caused by a dehiscence, or opening, in the bone overlying the superior semicircular canal (1). Patients with SCDS often have vestibular and auditory signs and symptoms such as pressure- and/or sound-induced vertigo or oscillopsia, enhanced sensitivity to bone-conducted sound, and autophony (2). Diagnosis is made when there is evidence of dehiscence on computed tomography (CT) scan as well as evidence of pressure transmission through the dehiscence. The pressure transmission may be manifested in auditory signs such as conductive hyperacusis or conductive hearing loss with preserved stapedial reflexes, or in vestibular signs such as nystagmus provoked by sound or pressure changes (2,3). However, in some cases evidence of pressure transmission may not be found in audiological signs or in the vestibular examination. In such cases, vestibular evoked myogenic potential (VEMP) testing can play a key role in determining whether or not an apparent dehiscence on CT is actually causing pathologic pressure transmission between the otic and intracranial spaces. Recent evidence underlines the importance of this adjunctive information in that CT scan errors can lead to the appearance of dehiscence when thin bone still remains intact over the superior canal (4,5).
During the cVEMP test, sound stimuli delivered to the ear activates the saccule and produces inhibition of the ipsilateral sternocleidomastoid muscle via the vestibulo-collic pathway (6,7). In SCDS, the dehiscence acts as a “third mobile window,” creating a path of lower impedence for the transmission of pressure and acoustic energy to the vestibule (2,8,9). Therefore, individuals with SCDS have lower cVEMP thresholds and elevated amplitudes (10–12).
Investigations have recently focused on the ocular VEMP (oVEMP) in patients with SCDS (12–17). During oVEMP testing, sound stimuli activate the otolith organs, leading to excitation of the extraocular muscles via the vestibulo-ocular pathways (14–16). Unlike the cVEMP, which is an inhibitory response primarily mediated by an uncrossed (ipsilateral) pathway, the oVEMP is an excitatory response mediated by a crossed (contralateral) pathway, such that stimulation of one ear activates the inferior oblique muscle of the contralateral eye (18,19). The measured response has been shown to be electromyographic and not electro-oculographic because the “n10” peak latency of the response precedes the actual eye movement (12). Increased oVEMP amplitudes and lowered thresholds have also been found in SCDS (12,13). However, the percentage of amplitude enlargement is more pronounced for the oVEMP than the cVEMP (12,13). This results in a smaller amount of overlap in amplitude between healthy individuals and patients with SCDS, suggesting that the oVEMP may be of greater utility when diagnosing SCDS (12,13).
In the present study, we examined the pre-operative o- and cVEMP responses of patients with surgically-confirmed SCDS in response to air (clicks and tone bursts) and bone (taps with a reflex-hammer and Mini-Shaker device) conduction stimulation at the midline skull (Fz). Previous reports have been limited to cases where SCDS was diagnosed by HRCT, but not surgically confirmed (12,13). However, recent evidence suggests that HRCT overestimates the size of anatomical superior canal dehiscence (SCD) and, in extreme cases, may suggest dehiscence when bone actually remains intact over the SC (5). Furthermore, this study used age-matched controls to address the known decrement in VEMP responses with age (20–24) Our aims were twofold: first, to define the best single-step suprathreshold VEMP screening test for SCDS; second, to obtain further insight into the relative sensitivity of vestibular afferents to sound and skull taps in the presence of a superior canal dehiscence.
METHODS
Subjects
Eleven, age-matched, healthy subjects (right ear only) with no hearing or vestibular deficits (mean: 50 years, range 33–66 years) and 11 patients with surgically-confirmed SCDS (mean: 50 years, range 32–66 years), were enrolled in this study. All patients with SCDS were tested pre-operatively. VEMPs were also measured in seven patients following surgery to plug the dehiscent SC via middle cranial fossa approach (mean 14 weeks, range 12–15 weeks). All subjects gave informed consent for o- and cVEMP testing through a protocol approved by the Institutional Review Board at the Johns Hopkins University School of Medicine.
Stimuli and recording techniques
A commercial electromyographic (EMG) system (Medelec Synergy, Care Fusion, software version 14.1, Dublin, OH) was used for VEMP testing. Air conducted stimuli (ACS) were delivered monaurally via intra-auricular speakers from VIASYS Healthcare (Madison, WI) with foam eartips (Aearo Company Auditory Systems, Indianapolis, IN). Two types of ACS were delivered: (1) 0.1-ms, 105 dB nHL (140 dB peak SPL) clicks of positive polarity at a repetition rate of 5 per second; and (2) 500 Hz, 125 dB SPL tone bursts of positive polarity, with a linear envelope (1 ms rise/fall time, 2 ms plateau), at a repetition rate of 5 per second. One hundred sweeps were averaged for each ACS test. Two types of midline taps were delivered at Fz (midline at the hairline, 30% of the distance between inion and nasion): (1) manual taps delivered with an Aesculap model ACO12C reflex-hammer fitted with an inertial microswitch trigger; and (2) “mini taps,” as described by Curthoys (25) were delivered with a Brüel and Kjær Mini-Shaker Type 4810 (1-ms clicks of positive polarity, with a repetition rate of 5 per second, at 115 dB (7 Newtons)). EMG signals were amplified (2500 μV) and band-pass filtered (20 – Hz 2000 Hz). Fifty sweeps were averaged for each bone conducted test.
Protocol for cVEMP Testing
Subjects lay semi-recumbent with their upper bodies elevated at a 30 degree angle from horizontal. They were instructed to lift their heads up from the headrest by flexing their necks to provide tonic background muscle activity during auditory stimulation and recording. The electrode montage consisted of a non-inverting electrode placed at the midpoint of the sternocleidomastoid muscle belly, an inverting electrode placed on the sternoclavicular junction, and a ground electrode placed on the manubrium sterni.
Protocol for oVEMP Testing
Subjects lay semi-recumbent with their upper bodies elevated at a 30 degree angle from horizontal. They were instructed to maintain maximum upgaze during auditory stimulation and recording. The electrode montage consisted of a non-inverting electrode placed on the cheek approximately 3 mm below the margin of the lower eyelid and centered beneath the pupil, an inverting electrode centered 2 cm below the non-inverting electrode, and a ground electrode placed on the manubrium sterni.
Before oVEMP testing, 20° vertical saccades were performed to ensure that symmetrical signals were recorded from both eyes. If the signal change showed > 25% asymmetry, the electrodes were removed and new ones applied.
cVEMP Response Parameters
The p13 potential was identified as the first distinctive positive peak in the waveform, occurring 10–14 ms after stimulus onset, and the n23 potential was identified as the first distinctive negative peak, occurring 19–23 ms after stimulus onset.
The raw peak-to-peak amplitude was calculated as the sum of the p13 and n23 amplitudes. The corrected peak-to-peak amplitude was calculated by dividing the raw peak-to-peak amplitude by the mean rectified background EMG activity recorded during the 10-ms interval prior to stimulus onset. This correction factor accounted for the varying tonic muscle contraction that affected cVEMP amplitudes.
oVEMP Response Parameters
The n10 potential was identified as the first distinctive negative peak in the waveform, occurring 7–11 ms after stimulus onset, and the p16 potential was identified as the first distinctive positive peak in the waveform, occurring 12–16 ms after stimulus onset. The n10 amplitude was calculated as the amplitude from baseline to the peak of the n10 response.
Dizziness Handicap Index (DHI)
Each subject completed the Dizziness Handicap Inventory (DHI) prior to surgical repair. The DHI was designed to quantify the impact of dizziness on everyday life (26). The DHI is a 25-item questionnaire which evaluates patient perceptions of the handicap imposed by vestibular dysfunction. The questions and scoring of the 25 item DHI was done as previously described (26). The total DHI score ranged from 0 to 100, with higher scores representing greater disability. Previous work by our group demonstrated significant reductions in DHI scores comparing post- to pre-operative queries in patients with SCDS (27).
Dehiscence Size
Dehiscence length and width were measured intra-operatively using a small section of millimeter-scored sterile plastic measuring tape placed adjacent to the dehiscence and viewed under the operating microscope at 10–20X magnification. Accuracy of the measurement was estimated to be ± 0.5 mm. Area was calculated by multiplying length times width.
Tone-Evoked Nystagmus
Binocular infrared goggles (Micromedical Technologies, Inc., Chatham, Illinois) were used to search for nystagmus while pure tones were presented monaurally via TDH39 headphones with a calibrated audiometer (Micromedical Technology - Inview). Tones were presented for approximately 0.5–1 second at intensities up to 110 dB HL from 125 Hz through 6 kHz. Nystagmus was considered to represent SC excitation if it consisted of a combination of vertical and torsional eye movements with slow phases directed upward and rolling the superior poles of the eyes to the contralateral side (i.e., excitatory in the plane of the affected canal).
Statistical Analysis
The Mann Whitney U test was used to compare VEMP responses between SCDS patients and control subjects. The Wilcoxon’s Signed Ranks test was used to compare VEMP responses within SCDS patients. Spearman’s Rank Order Correlation was used to analyze the relationship between VEMP amplitudes and clinical features of SCDS including DHI scores, presence of tone-evoked eye nystagmus, dehiscence area, and average air-bone gap, using one-tailed test (assuming elevated VEMP amplitudes would correlate with these clinical features of SCDS). A significance level of 0.05 was used for all analyses. A Bonferroni correction was applied to the correlations because of multiple comparisons. All statistical analysis was performed using SPSS 18.0.
RESULTS
Ocular VEMPs
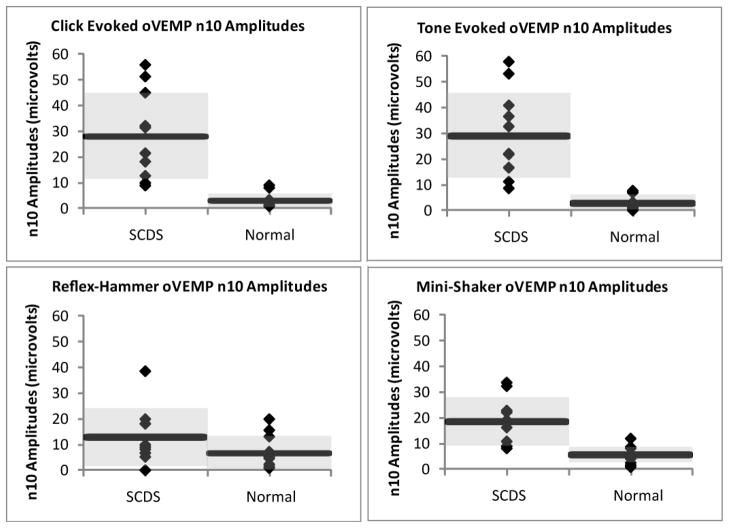
Figure 1 displays the n10 oVEMP amplitudes in 11 healthy ears and 11 patients with surgically-confirmed SCDS, in response to clicks, tone bursts, reflex-hammer taps, and Mini-shaker taps. The affected SCDS ears demonstrated significantly larger oVEMP n10 amplitudes compared to the healthy ears in response to clicks (U = 1.00, p < 0.001), tone bursts (U = 0.00, p < 0.001), and Mini-shaker (U = 5.5, p < 0.001) stimuli; however, there was no significant difference between groups for reflex-hammer stimuli (U = 30, p = 0.152). The affected ears of SCDS had, on average, a 9-fold increase in n10 amplitude in response to clicks, a 10-fold increase in response to tone bursts, a 2-fold increase in response reflex-hammer taps, and a 3-fold increase in response Mini-shaker taps, when compared with healthy subjects. Table 1 shows the mean ± 1 SD for these oVEMP n10 amplitudes.
Figure 1. oVEMP n10 Amplitudes in SCDS Patients and Normal Subjects in Response to Clicks, Tones, Reflex-Hammer Taps, and Mini-shaker Taps.
Each diamond represents the oVEMP n10 amplitude of an individual subject, each horizontal bar represents the mean values within a group, and the shaded gray area encompasses one standard deviation above and below the mean.
Table 1.
oVEMP n10 Amplitudes in Normals and SCDS Affected Ears (Mean ± 1 SD).
| Stimulus | Normal | SCDS (Affected Ear) |
|---|---|---|
| Click | 3.00 ± 2.81 | 27.98 ± 16.62 |
| Tone | 2.90 ± 3.02 | 29.04 ± 16.65 |
| Reflex Hammer | 6.64 ± 6.63 | 12.83 ± 11.44 |
| Mini-Shaker | 5.62 ± 3.26 | 18.50 ± 9.48 |
ACS (especially tone bursts) produced a greater increase in amplitude and a lesser degree of overlap between patients and healthy subjects, than did midline taps (see Figure 1). Receiver Operating Characteristic (ROC) Curves were generated for each stimulus, shown in Figure 2. The area under the ROC curve (standard error) for each stimulus was: 0.989 (0.018) for clicks, 1.000 (0.000) for tone bursts, 0.665 (0.135) for reflex-hammer taps, and 0.938 (0.054) for Mini-shaker taps. Tone bursts demonstrated 100% sensitivity (and 100% specificity) with n10 amplitudes of 8.25 μV and above being considered positive for SCDS.
Figure 2. Receiver Operating Characteristic (ROC) Curves n10 oVEMP Amplitude.
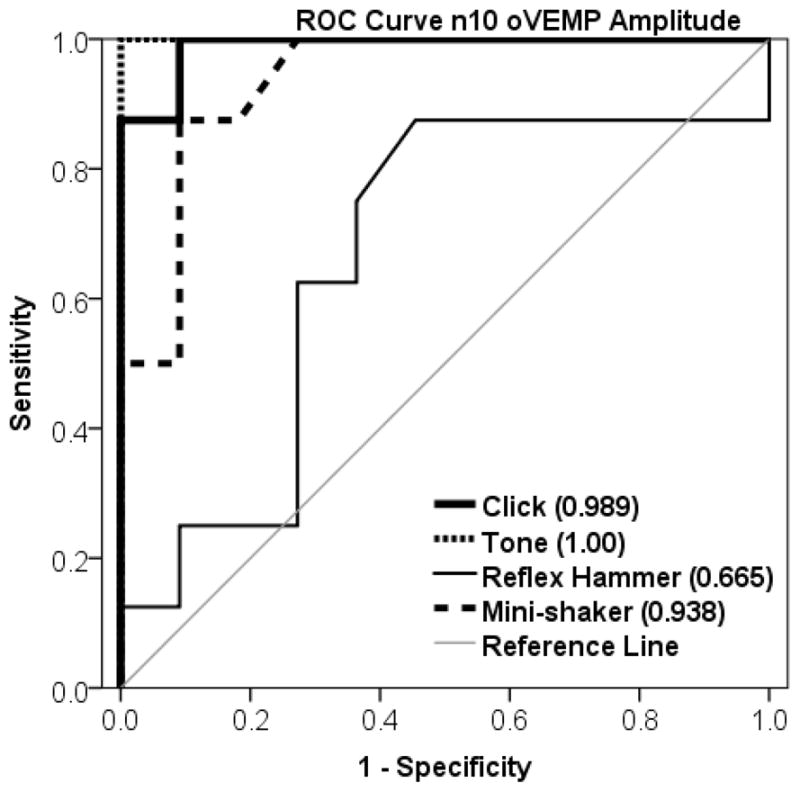
Each stimulus is depicted with a different weighted line. The reference line represents a useless test (an area of 0.5).
Cervical VEMPs
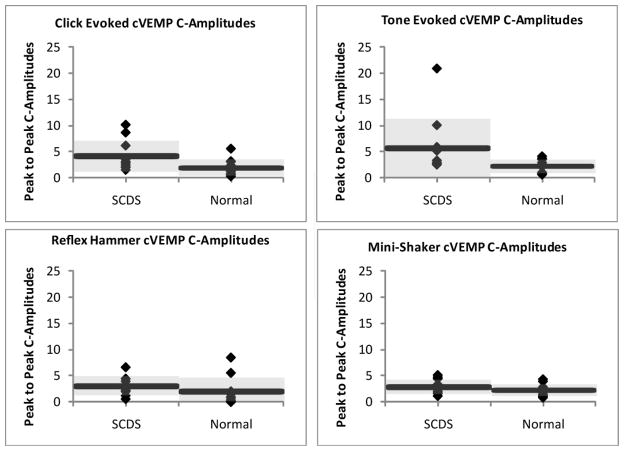
Corrected peak-to-peak cVEMP amplitudes in response to clicks, tone bursts, reflex-hammer taps, and Mini-shaker taps are displayed in Figure 3 for the 11 healthy ears and 11 SCDS patients. The affected SCDS ears demonstrated significantly larger cVEMP corrected peak-to-peak amplitudes compared to the healthy ears in response to click (U = 26.5, p = 0.026) and tone burst stimuli (U = 26.5, p = 0.025); however, no significant difference was noted in response to reflex-hammer taps (U = 26, p = 0.074) or Mini-shaker taps (U = 40, p = 0.291). The affected ears of SCDS had, on average, a 2-fold increase in corrected peak-to-peak amplitude in response to clicks and tones when compared with healthy subjects. Table 2 shows the mean (± 1 SD) cVEMP corrected peak-to-peak amplitudes for healthy subjects and SCDS affected ears.
Figure 3. Corrected peak-to-peak cVEMP Amplitudesin Response to Clicks, Tones, Reflex-Hammer Taps, and Mini-shaker Taps.
Each diamond represents the corrected peak-to-peak cVEMP amplitude of an individual subject, each horizontal bar represents the mean values within a group, and the shaded gray area encompasses one standard deviationabove and below the mean.
Table 2.
cVEMP Corrected Peak-to-Peak Amplitudes in Normals and SCDS Affected Ears (Mean ± 1 SD).
| Stimulus | Normal | SCDS – Affected Ear |
|---|---|---|
| Click | 1.9 ± 1.6 | 4.1 ± 3.0 |
| Tone | 2.2 ± 1.3 | 5.7 ± 5.6 |
| Hammer | 2.0 ± 2.6 | 3.0 ± 1.8 |
| Shaker | 2.2 ± 1.1 | 2.8 ± 1.4 |
Receiver Operating Characteristic (ROC) curves were generated for each stimulus, shown in Figure 4. The area under the ROC curve (standard area) for each stimulus was: 0.784 (0.107) for clicks, 0.744 (0.116) for tone bursts, 0.727 (0.121) for reflex-hammer taps, and 0.648 (0.134) for Mini-shaker taps.
Figure 4. Receiver Operating Characteristic (ROC) Curves cVEMP Corrected Peak-to-Peak Amplitude.
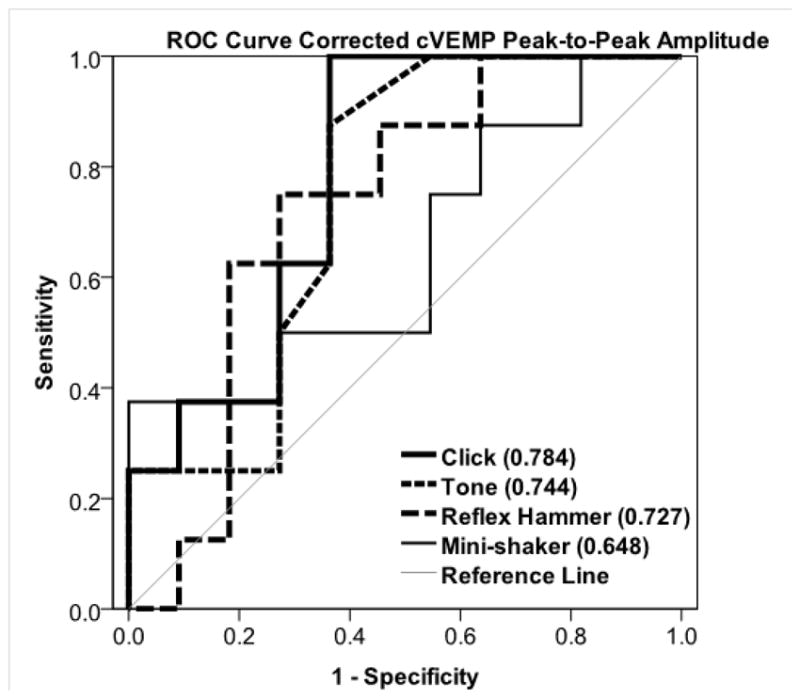
Each stimulus is depicted with a different weighted line. The reference line represents a useless test (an area of 0.5).
Post Operative VEMPs
Both o- and cVEMPs were measured in seven patients following surgical repair of the dehiscent SC, via middle cranial fossa approach. A Wilcoxon’s Signed Ranks test was completed comparing pre- and post surgical repair VEMP amplitudes. OVEMP n10 amplitudes were significantly lower in the post surgical period for click (Z = −2.366, p = 0.018), tone burst (Z = −2.366, p = 0.018), and Mini-shaker tap stimuli (Z = −2.366, p = 0.018); however, no significant difference between groups was found for reflex-hammer taps (Z = −1.826, p = 0.018). Likewise, corrected cVEMP peak-to-peak amplitudes were significantly lower in the post-surgical period in response to all stimuli: clicks (Z = −2.201, p = 0.028), tone bursts (Z = −2.366, p = 0.018), reflex-hammer taps (Z = −2.201, p = 0.028) and Mini-shaker taps (Z = −2.366, p = 0.018).
VEMP Relationship to Clinical Findings
Table 3 displays the n10 oVEMP amplitudes, presence or absence of tone-evoked nystagmus, DHI scores, surgically-measured dehiscence area and average air-bone gap (average 250, 500, 1000, and 2000 Hz) for the 11 surgically repaired ears. Both oVEMP n10 amplitudes and cVEMP corrected peak-to-peak amplitudes for all stimuli were plotted as a function of DHI score, presence of tone-evoked eye nystagmus, dehiscence area, and average air-bone gap. The only significant correlation found after correcting for multiple comparisons was between average air-bone gap and corrected cVEMP amplitude in response to 500 Hz tone bursts (r = 0.768, p = 0.003).
Table 3.
n10 oVEMP Amplitudes, Tone-evoked Eye Movements, DHI score, Dehiscence Area, and Average Air-bone gap for the 11 surgically repaired ears
| Patient | oVEMP n10 Amplitude (microvolts) | Tone-evoked Eye Movements | DHI Score | Dehiscence Area (mm2) | Average Air-bone gap (dB) | |||
|---|---|---|---|---|---|---|---|---|
| Clicks | Tones | Reflex Hammer | Mini-Shaker | |||||
| 1 | 55.9 | 58.0 | N/A | 22.3 | Yes | 34 | 2.0 | 12.5 |
| 2 | 51.3 | 53.3 | 18.1 | 32.5 | Yes | 48 | 2.5 | 25 |
| 3 | 45.0 | 41.0 | 38.6 | 33.9 | Yes | 40 | 2.5 | 13.75 |
| 4 | 32.1 | 36.7 | N/A | 23.0 | No | 68 | 4.0 | 26.25 |
| 5 | 21.4 | 21.9 | 5.2 | 11.0 | Yes | 54 | 1.0 | 16.25 |
| 6 | 21.4 | 22.2 | N/A | 16.4 | No | 28 | 1.3 | 16.25 |
| 7 | 9.8 | 16.8 | 9.9 | 19.3 | No | 48 | 4.0 | 32.5 |
| 8 | 12.6 | 11.3 | 6.7 | 9.2 | Yes | 46 | 4.0 | 21.25 |
| 9 | 8.8 | 8.7 | 9.0 | 9.1 | Yes | 26 | 2.0 | 18.75 |
| 10 | 31.4 | 32.8 | 19.9 | N/A | Yes | 70 | 2.1 | 16.25 |
| 11 | 18.1 | 16.7 | 8.1 | 8.3 | Yes | N/A | 4.0 | 3 |
DISCUSSION
In the present study, we examined suprathreshold sound and tap-evoked o- and cVEMPs in surgically-confirmed SCDS patients to: 1) determine the best single-step suprathreshold screening test for SCDS and 2) obtain further insight into whether SCDS equally enhances responses to ACS and midline taps. We found that both ACS (clicks and tone bursts) and midline taps (Mini-Shaker taps) produced oVEMP n10 amplitudes that were significantly elevated in ears with surgically-confirmed SCDS compared with control subjects. ACS produced greater increases in oVEMP amplitudes than did midline taps. Similarly for cVEMPs, ACS (clicks and tone bursts) produced higher corrected peak-to-peak amplitudes in ears with surgically-confirmed SCDS as compared to control subjects; however, midline taps (reflex-hammer and Mini-Shaker taps) failed to produce significantly different cVEMP amplitudes between the groups. Overall, ACS proved to have the highest diagnostic yield, producing the greatest relative increase in amplitude, and the least overlap in amplitude when comparing surgically-confirmed SCDS patients and control subjects.
Best Single-Step Suprathreshold Screening Test
SCDS has typically been characterized by lower cVEMP thresholds (13,28). However, thresholds require numerous VEMP trials and can therefore be time intensive for the operator and effortful for the patient. Our findings demonstrate that suprathreshold oVEMP n10 amplitudes in response to ACS are an effective “single-step” means of diagnosing SCDS. In response to 500 Hz tone bursts, ROC analysis suggested an n10 amplitude cut off of ≥8.25 μv is 100% sensitive (and 100% specific) in diagnosing SCDS. Likewise, ROC analysis indicated that click-evoked oVEMP n10 amplitudes were nearly equally effective (approached 100% sensitivity) for identifying SCDS. Because oVEMP amplitudes have been documented to decrease with increasing age (20,24), an age-matched control group was used. We found no overlap between control subjects and those with surgically confirmed SCDS for ACS oVEMP responses using this age-matched group. Thus, although the sample size in this study was small (n = 11), a robust performance of oVEMP amplitudes in separating cases of SCDS from age-matched controls was demonstrated. This has recently been confirmed in a larger sample (n = 29) of surgically confirmed SCDS patients (29). Conversely, corrected cVEMP suprathreshold amplitudes do not perform as well as oVEMP suprathreshold n10 amplitudes in separating SCDS from control ears, regardless of stimulus type (ACS verus midline taps). This is in agreement with the observations of previous investigators (12,13). For ACS, significant mean differences in corrected cVEMP amplitudes were present between SCDS and control groups, but a large degree of overlap still existed (Table 2).
Sensitivity of Vestibular Afferents to ACS and Midline Taps
Preliminary findings suggest that oVEMPs evoked by taps applied at Fz are a reflection of a crossed pathway from the utricle and superior vestibular nerve to the contralateral eye, activating the inferior oblique muscle (30,31). Similar to the cVEMP, enhancement of the oVEMP response to midline bone taps in SCDS is thought to occur as the result of the dehiscence acting as a “third mobile window,” creating a path of lower impedence for the transmission of pressure (2). Our findings demonstrate that midline taps are not as effective as ACS for SCDS diagnosis, for both o- and cVEMPs. In response to midline taps, cVEMP amplitudes were not significantly differerent between SCDS and normal ears (Table 2). For oVEMP, n10 amplitudes were significantly larger in ears with SCDS in response to Mini-shaker stimuli, which is in agreement with other findings (17); however, the amount of overlap between groups was larger with Mini-shaker taps than that with ACS (Table 1).
The present study does not contradict previous findings of enhanced oVEMP responses to midline taps in SCDS. Rather, it simply demonstrates that the third window effect in SCDS seems to magnify responses to ACS more so than to this form of midline bone taps. This indicates that SCDS can be diagnosed using already available equipment that is familiar to most otology and neurotology clinics. This is not to say that midline taps do not have a valuable role in vestibular assessment. Indeed, oVEMPs evoked by Fz taps appear to be useful for assessing the integrity of otolith ocular pathways, and particularly of interest for their apparent ability to characterize the utriculo-ocular reflex (32).
Correlations with Clinical Presentation
In the current study, we found no significant correlations between o- and cVEMP amplitudes and tone-evoked nystagmus, DHI score, or dehiscence size as measured surgically, not just radiographically. We did note a significant correlation between average low frequency air-bone gap and corrected cVEMP amplitudes in response to 500 Hz tone burst stimuli, even though cVEMP suprathreshold amplitudes are not sensitive for diagnosing SCDS. Click-evoked cVEMP thresholds and DHI scores have previously been compared, with no significant association found (33). Pfammatter et al. (34) reported that dehiscence size was associated with lower cVEMP thresholds and a greater number of clinical signs and symptoms. However, thir measures of dehiscence size were not obtained at surgery but estimated from CT, and the radiographic measures may be significantly erroneous (5).
A correlation between oVEMP amplitude (i.e., transient eye movement in response to brief, repeated acoustic or midline tap stimuli) and tone evoked nystagmus was anticipated; however, no relationship was found. One explanation may be the clinical heterogeneity of SCDS in that patients with similar dehiscences can have very different presentations, even asymptomatic. A second explanation may be anatomical. In order to generate nystagmus, endolymph presumably must move within the membranous canal. This might happen only when the dehiscence allows contact between the dura and the membranous duct, allowing the dura to push on the duct wall. This dural contact would act analogous to the “micropusher” technique used by vestibular researchers to displace endolymph in order to study vestibular nerve responses (35,36). Third, the lower impedance of the dehiscent labyrinth via the third window phenomenon, may be sufficient to increase delivery of sound and midline tap stimuli to the utricle and saccule to enhance VEMP responses in nearly all cases of SCDS. Measurements of eye movements in three dimensions might help to resolve this dilemma, as the transient eye movements evoked in the oVEMP responses may be different in patients with nystagmus in response to sustained sound compared to those without inducible nystagmus. Finally, the dissociation between tone-evoked nystagmus and oVEMP amplitude could reflect a difference in the receptors contributing to each response. If the enhanced oVEMPs in SCDS are predominantly of otolith origin whereas the sound-evoked nystagmus represents a nearly pure superior canal-mediated response (37) then a dissociation would be expected. The three patients who did not have tone-evoked nystagmus had tone-evoked oVEMP amplitudes that were neither very low nor very high compared with the other SCDS patients.
CONCLUSION
Of the VEMP amplitudes examined (oVEMP vs cVEMP), oVEMPs yield significantly higher reflex amplitudes in surgically confirmed SCDS and less overlap when compared with normal controls. Of the stimulus types (air-conduction vs midline taps), air-conducted, suprathreshold, oVEMP amplitudes provide the best separation between intact and dehiscent labyrinths. It is therefore recommended that suprathreshold oVEMPs in response to ACS are the best single-step screening test for SCDS, particularly when compared to an age-matched control sample.
Acknowledgments
The authors wish to acknowledge Karin Eibenberger for her assistance in calibration of the MiniShaker device.
Funding: Doris Duke Charitable Foundation Clinical Research Fellowship
Garnett Passe and Rodney Williams Memorial Foundation Australia
NIH/NIDCD R01 DC005040: Evaluation of vestibular function in Ménière’s disease; PI: John Carey, M.D.
NIH Training Grant T32DC000023; PI: Eric Young, Ph.D
Contributor Information
Kristen L. Janky, Email: kristen.janky@boystown.org, Boys Town National Research Hospital, 555 N. 30th St., Omaha, NE 68131, Phone: 402-498-6535, Fax: 402-452-5015.
Kimanh D. Nguyen, Email: KimanhNguyen@mednet.ucla.edu, David Geffen School of Medicine at UCLA, Department of Head and Neck Surgery, 10833 Le Conte Ave., CHS 62-132, Los Angeles, CA 90095-1624, Phone: 410-955-7381, Fax: 410-955-0035.
Miriam Welgampola, Email: miriam@icn.usyd.edu.au, Royal Prince Alfred Hospital, Central Clinical School, University of Sydney, Australia, Phone: 61295158830, Fax: 61295158347.
M. Geraldine Zuniga, Email: mzuniga2@jhmi.edu, Johns Hopkins University, 601 N. Caroline St. 6th Floor, Baltimore, MD 21287-0910, Phone: 410-955-7381, Fax: 410-955-0035.
John P Carey, Email: jcarey@jhmi.edu, Johns Hopkins University, 601 N. Caroline St. 6th Floor, Baltimore, MD 21287-0910, Phone: 410-955-7381, Fax: 410-955-0035.
References
- 1.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124(3):249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115(10):1717–27. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 3.Minor LB, Cremer PD, Carey JP, Della Santina CC, Streubel SO, Weg N. Symptoms and signs in superior canal dehiscence syndrome. Ann NY Acad Sci. 2001;942(410):259–73. doi: 10.1111/j.1749-6632.2001.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 4.Penninger RT, Tavassolie TS, Carey JP. Cone-beam volumetric tomography for applications in the temporal bone. Otol Neurotol. 2011;32(3):453–60. doi: 10.1097/MAO.0b013e31820d962c. [DOI] [PubMed] [Google Scholar]
- 5.Tavassolie TS, Penninger RT, Zuñiga MG, Minor LB, Carey JP. Multislice computed tomography in the diagnosis of superior canal dehiscence: how much error, and how to minimize it? Otol Neurotol. 2012;33(2):215–22. doi: 10.1097/MAO.0b013e318241c23b. [DOI] [PubMed] [Google Scholar]
- 6.Colebatch JG. Vestibular evoked potentials. Curr Opin Neurol. 2001;14(1):21–6. doi: 10.1097/00019052-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42(8):1635–6. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- 8.Chien W, Ravicz ME, Rosowski JJ, Merchant SN. Measurements of human middle- and inner-ear mechanics with dehiscence of the superior semicircular canal. Otol Neurotol. 2007;28(2):250–7. doi: 10.1097/01.mao.0000244370.47320.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Songer J, Rosowksi J. A mechano-acoustic model of the effect of superior canal dehiscence on hearing in chinchilla. J Acoust Soc Am. 2007;122(2):943–51. doi: 10.1121/1.2747158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brantberg K, Bergenius J, Tribukait A. Vestibular-evoked myogenic potentials in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 1999;119(6):633–40. doi: 10.1080/00016489950180559. [DOI] [PubMed] [Google Scholar]
- 11.Streubel SO, Cremer PD, Carey JP, Weg N, Minor LB. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Oto-laryngol Suppl. 2001;545(11):41–9. doi: 10.1080/000164801750388090. [DOI] [PubMed] [Google Scholar]
- 12.Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70(6):464–72. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren SM, Aw ST, Halmagyi GM, Todd NPM, Colebatch JG. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J Neurol, Neurosurg Psychiatry. 2008;79(5):559–68. doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- 14.Todd NPM, Rosengren SM, Colebatch JG. Tuning and sensitivity of the human vestibular system to low-frequency vibration. Neurosci Lett. 2008;444(1):36–41. doi: 10.1016/j.neulet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Todd NPM, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118(2):381–90. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119(9):2135–47. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Manzari L, Burgess AM, McGarvie LA, Curthoys IS. Ocular and Cervical Vestibular-Evoked Myogenic Potentials to 500 Hz Fz Bone-Conducted Vibration in Superior Semicircular Canal Dehiscence. Ear Hear. 2012 doi: 10.1097/AUD.0b013e3182498c09. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116(8):1938–48. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Weber KP, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol. 2012 doi: 10.1113/jphysiol.2011.226225. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basta D, Todt I, Ernst A. Characterization of age-related changes in vestibular evoked myogenic potentials. J Vestib Res. 2007;17(2–3):93–8. [PubMed] [Google Scholar]
- 22.Brantberg K, Granath K, Schart N. Age-related changes in vestibular evoked myogenic potentials. Audiol Neurootol. 2007;12(4):247–53. doi: 10.1159/000101332. [DOI] [PubMed] [Google Scholar]
- 23.Janky KL, Shepard N. Vestibular Evoked Myogenic Potential (VEMP) Testing: Normative Threshold Response Curves and Effects of Age. J Am Acad Audiol. 2009;20(8):514–22. doi: 10.3766/jaaa.20.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piker EG, Jacobson GP, McCaslin DL, Hood LJ. Normal characteristics of the ocular vestibular evoked myogenic potential. J Am Acad Audiol. 2011;22(4):222–30. doi: 10.3766/jaaa.22.4.5. [DOI] [PubMed] [Google Scholar]
- 25.Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Ex Brain Res. 2006;175(2):256–67. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424–7. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 27.Crane BT, Minor LB, Carey JP. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118(10):1809–13. doi: 10.1097/MLG.0b013e31817f18fa. [DOI] [PubMed] [Google Scholar]
- 28.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–7. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuniga MG, Janky KL, Nguyen KD, Welgampola MSCJ. Ocular vs. cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol. doi: 10.1097/MAO.0b013e31827136b0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzari L, Tedesco A, Burgess AM, Curthoys IS. Ocular vestibular-evoked myogenic potentials to bone-conducted vibration in superior vestibular neuritis show utricular function. Otolaryngol Head Neck Surg. 2010;143(2):274–80. doi: 10.1016/j.otohns.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Curthoys IS, Iwasaki S, Chihara Y, Ushio M, McGarvie L a, Burgess AM. The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol. 2011;122(3):611–6. doi: 10.1016/j.clinph.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, Macdougall HG, et al. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann N Y Acad Sci. 2011;1233:231–41. doi: 10.1111/j.1749-6632.2011.06147.x. [DOI] [PubMed] [Google Scholar]
- 33.Crane BT, Minor LB, Carey JP. Three-dimensional computed tomography of superior canal dehiscence syndrome. Otol Neurotol. 2008;29(5):699–705. doi: 10.1097/MAO.0b013e3181776726. [DOI] [PubMed] [Google Scholar]
- 34.Pfammatter A, Darrouzet V, Gärtner M, Somers T, Van Dinther J, Trabalzini F, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010;31(3):447–54. doi: 10.1097/MAO.0b013e3181d27740. [DOI] [PubMed] [Google Scholar]
- 35.Dickman JD, Reder PA, Correia MJ. A method for controlled mechanical stimulation of single semicircular canals. J Neurosci Methods. 1988;25(2):111–9. doi: 10.1016/0165-0270(88)90147-1. [DOI] [PubMed] [Google Scholar]
- 36.Rabbitt RD, Boyle R, Highstein SM. Mechanical indentation of the vestibular labyrinth and its relationship to head rotation in the toadfish, Opsanus tau. J Neurophysiol. 1995;73(6):2237–60. doi: 10.1152/jn.1995.73.6.2237. [DOI] [PubMed] [Google Scholar]
- 37.Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55(12):1833–41. doi: 10.1212/wnl.55.12.1833. [DOI] [PubMed] [Google Scholar]