Summary
When performing tasks, humans are thought to adopt task sets that configure moment-to-moment data processing. Recently developed mixed blocked/event-related designs allow task set-related signals to be extracted in fMRI experiments, including activity related to cues that signal the beginning of a task block, “set-maintenance” activity sustained for the duration of a task block, and event-related signals for different trial types. Data were conjointly analyzed from mixed design experiments using ten different tasks and 183 subjects. Dorsal anterior cingulate cortex/medial superior frontal cortex (dACC/msFC) and bilateral anterior insula/frontal operculum (aI/fO) showed reliable start-cue and sustained activations across all or nearly all tasks. These regions also carried the most reliable error-related signals in a subset of tasks, suggesting that the regions form a “core” task-set system. Prefrontal regions commonly related to task control carried task-set signals in a smaller subset of tasks and lacked convergence across signal types.
Introduction
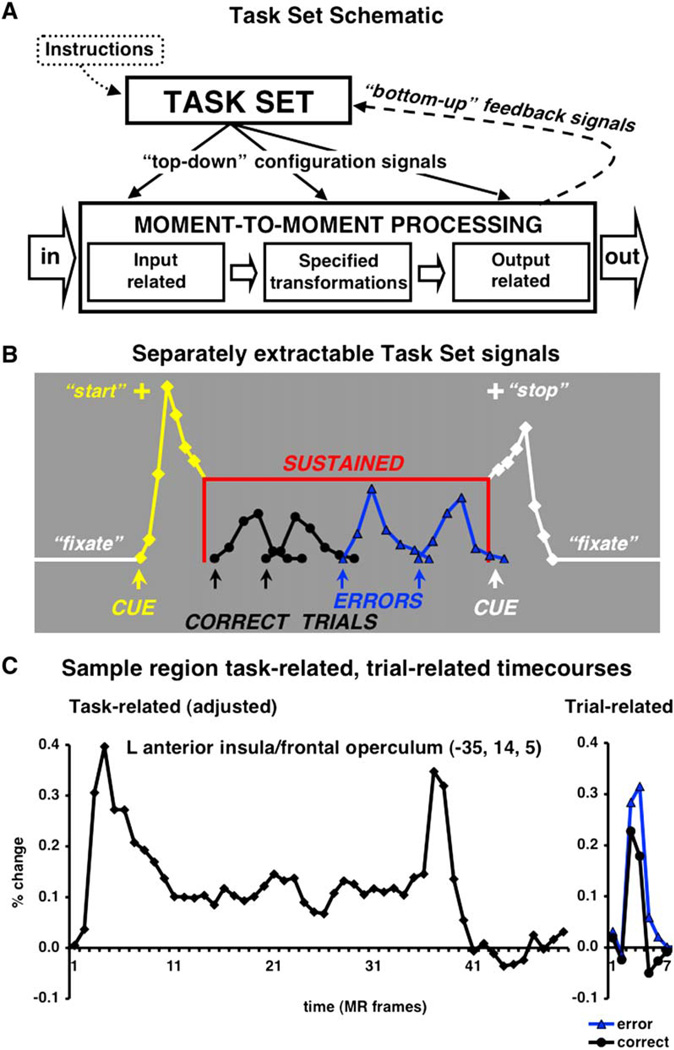
When specified task demands are present, moment-to-moment processing pathways for sensory inputs, cognitive categorizations and motor outputs must be configured (Meiran, 1996). To perform a specific task, humans are thought to enter a task-dependent cognitive state, mode, or set that is maintained for the duration of the task (Logan and Gordon, 2001; Figure 1A). Models of task set postulate executive processes that select and modulate downstream moment-to-moment processes relevant to the specific task at hand (Baddeley, 1996; Desimone and Duncan, 1995; Logan and Gordon, 2001; Meyer and Kieras, 1997; Miller and Cohen, 2001; Norman and Shallice, 1986; Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977).
Figure 1. Task Set-Related Signals.
(A) In response to the task instructions, a task-set system should instantiate and maintain task sets that aid in the configuration of downstream moment-to-moment processes. Such a task-set system should also receive “bottom-up” feedback about ongoing performance.
(B) Only the mixed blocked/event-related fMRI design allows the separate extraction of three different types of task set-related signals. Activity time-locked to the start of a task block (yellow) is likely important for the instantiation of task sets. To ensure continued performance success, task sets should be sustained for the length of the task period (red). Error-related signals (blue) can provide performance feedback to the task-set system.
(C) The adjusted activity (left) remaining after the extraction of trial-related activity (right) closely resembles our model of cue-related and sustained activity. The start cue occurred on the first MR frame (time to repeat [TR] of EPIBOLD acquisition), the stop cue appeared on MR frame 35. MR frames 36 to 50 were fixation-only baseline.
Behaviorally task-level control has been addressed in several different ways, including dual task, task switching, and task context effects on processing. It is not surprising then that these paradigms have been the major way that task control has been addressed in the imaging literature as well. For example, task-switching paradigms designed to identify activation differences between repeat and switch trials have served as a valuable tool for the study of task-set processes (Sohn et al., 2000; Yeung et al., 2006) Important previous single-unit and neuroimaging studies of executive systems have also measured activity during the delay between a cue and the following stimulus at the trial level (MacDonald et al., 2000; Saito et al., 2005; Sakai and Passingham, 2003). Nonetheless, in many cases, these studies provide indirect measures of task set-related activity.
The advent of mixed blocked/event-related fMRI (Donaldson, 2004; Donaldson et al., 2001; Visscher et al., 2003; Wenger et al., 2004) designs, first described by Chawla et al., (1999) allow the direct and separate extraction of three types of task set-related signals: (1) signals tied to the start of a specific task condition, which should be, at least in part, related to the instantiation of task parameters; (2) activity sustained at a constant level across the task period, some of which likely reflects task set maintenance; and (3) error-related feedback activity (Figures 1B and 1C), to monitor processing and encourage adjustment of top-down signals. The use of mixed blocked/event-related designs is crucial for our enquiries into the nature of task-set processes, because of the need to disambiguate sustained (set-maintenance) signals and stimulus bound or trial-related signals. This requires the jittered presentation of trials under a particular task set so that the enduring set-related signals and transient start-cue and trial-related signals are not confounded and can be estimated separately.
In dual-task situations, when subjects must simultaneously instantiate and maintain two different task sets, performance often worsens (Pashler, 1994). Therefore, some task-set processes may constitute a core resource of limited capacity that is shared across different complex tasks (Broadbent, 1982; Posner and Snyder, 1975). The present study is directed at this possibility by measuring potential “top-down” signals consistent across a large number of tasks (Duncan and Owen, 2000) in order to directly identify these core, or “central processing resources.” Our analyses test the hypothesis that a limited set of brain regions will show each of the three types of task set-related signals described above across a wide range of tasks. Such regions would form a core task-set system that is anatomically separate from domain-specific task-set functions and the brain’s data-processing systems (Posner and Petersen, 1990). Data from 183 subjects and ten different task conditions were included in this cross-studies analysis. The included tasks used a mix of visual and auditory inputs, spoken and button-press responses, and several kinds of stimuli, such as pictures, words, abstract symbols, letters, Gabor patches, and tones (Table 1). For taskset instantiation and maintenance signals, data from all subjects and tasks were used; only two of the ten task conditions provided enough errors for the study of error-related signals.
Table 1.
Tasks Included in Analyses
| Task Condition | Stimuli | Input | Output |
|---|---|---|---|
| 1. Letter identification (ID) | Letters | Visual | Speech |
| 2. Verb generation and reading | Nouns | Visual | Speech |
| 3. Object naming | Images | Visual | Speech |
| 4. Reading aloud | Nonwords/words | Visual | Speech |
| 5. Matching | Symbols/letters | Visual | Button |
| 6. Living/nonliving judgment | Images | Visual | Button |
| 7. Physical and semantic judgments | Nouns | Visual | Button |
| 8. Visual search | Gabor patches | Visual | Button |
| 9. Motor timing | Tones | Auditory | Button |
| 10. Abstract/concrete judgment | Nouns | Auditory | Button |
Sorted by input and output modality. Lines demarcate input/output combinations.
Results
Two complementary analyses were performed on the start-cue and sustained signals (see Experimental Procedures). A conjunction analysis was used to map the number of task conditions during which any given voxel was significantly activated. We chose to supplement our conjunction analysis with a fixed-effects analysis in which the statistical reliability of signals in each condition contributed to the overall statistical strength of the final map to show that the same areas that were activated strongly, on average over tasks, were also activated consistently. The conjunction analysis required that all the task-related effects survive a statistical criterion. The fixed-effects analysis provides a measure of statistical strength of effect across studies, making it amenable to standard region of interest (ROI) definition procedures. The fixed-effects map could potentially be dominated by only a few of the task conditions; therefore, the fixed-effects statistical map needed to show strong similarity to the conjunction map. Strong overlap between the conjunction and fixed-effects analyses would also indicate that the obtained results are robust to differences in the analysis stream.
Start-Cue Activity
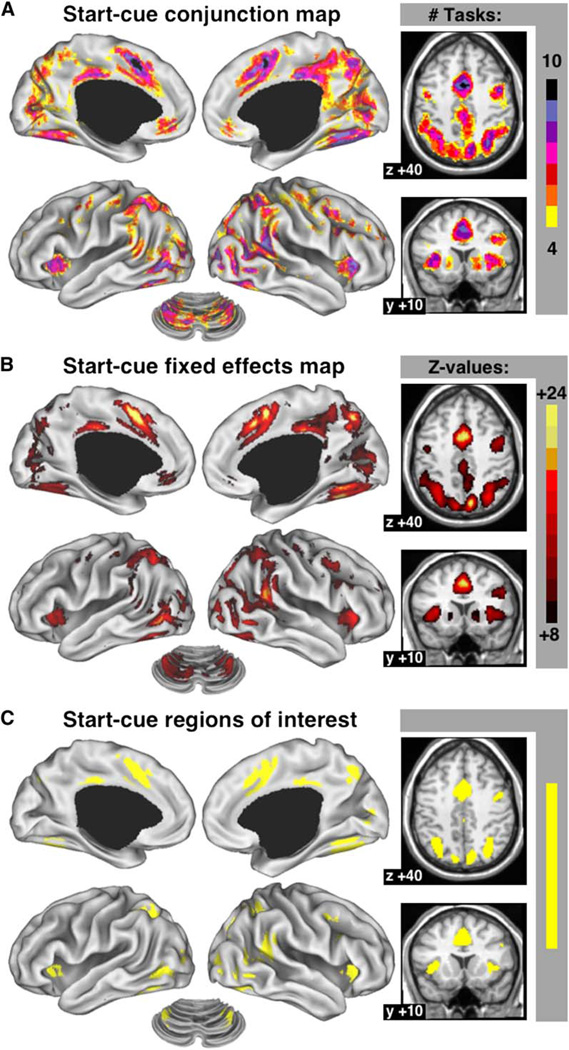
Figure 2A maps the number of task conditions for which start cue-related activity passed the statistical threshold at each voxel. Several regions showed start-cue activity across many tasks. Figure 2B is a fixed-effects map of the main effect of time for start-cue activity at a voxel. This statistical map highlights very similar regions as the conjunction map (compare to Figure 2A), making it highly unlikely that the observed effects were driven by only a few of the conditions. A region on the border of the dorsal anterior cingulate/medial superior frontal cortex (dACC/msFC) showed start-cue activity for all task conditions (Table 2). The left and right anterior insula/frontal operculum (aI/fO) contained voxels that showed start cue-related activity for nine of ten conditions. The right temporoparietal junction (TPJ) contained a few voxels with significant start-cue activity in all ten tasks. Voxels in the intraparietal sulcus (IPS) bilaterally, right lateral frontal cortex, left and right extrastriate visual cortex, left and right fusiform cortex, and the precuneus all showed start-cue activity in nine of ten tasks. Anterior prefrontal cortex (aPFC) showed start-cue activity in six of ten tasks on the left and seven of ten tasks on the right.
Figure 2. Cross-Study Analyses of Start Cue-Related Activity.
(A) Conjunction image showing the number of studies for which a voxel carried significant start-cue activity. Only voxels with significant start-cue activity in at least four conditions are shown. Activations were displayed on an inflated surface rendering of the human brain, using the CARET program (Van Essen et al., 2001).
(B) Fixed-effects analysis map of start-cue activity.
(C) ROI derived from the fixed-effects map. All time courses were significant (p < 0.001).
Table 2.
Start-Cue Activity Regions of Interest
| Coordinates |
Peak z Value |
Peak # Studies |
|||
|---|---|---|---|---|---|
| Brain Region | x | y | z | ||
| R Fusiform | 35 | −65 | −9 | 24.3 | 9 |
| dACC/msFC | 0 | 7 | 44 | 24.0 | 10 |
| R Precuneus | 10 | −69 | 39 | 23.5 | 9 |
| posterior cingulate | 0 | −29 | 30 | 22.9 | 9 |
| R TPJ | 53 | −46 | 17 | 22.1 | 10 |
| L Fusiform | −34 | −62 | −15 | 22.0 | 9 |
| R IPS | 30 | −61 | 39 | 20.9 | 9 |
| R aI/fO | 35 | 17 | 5 | 20.9 | 9 |
| R Thalamus | 8 | −18 | 8 | 20.7 | 9 |
| L Precuneus | −9 | −72 | 37 | 20.1 | 8 |
| L IPS | −31 | −59 | 42 | 19.9 | 9 |
| L aI/fO | −35 | 13 | 5 | 19.8 | 9 |
| R Frontal cortex | 41 | 3 | 36 | 19.4 | 9 |
| L Middle occipital | −30 | −86 | 3 | 18.8 | 9 |
| R Lingual | 8 | −82 | 4 | 18.2 | 8 |
dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; TPJ, temporoparietal junction; IPS, intraparietal sulcus; aI/fO, anterior insula/frontal operculum.
Separate analysis of the statistical maps for each of the individual studies demonstrated the cross-study activations not to be artifacts of averaging across groups of subjects, because each of the subject groups showed very similar patterns of joint activity (Duncan and Owen, 2000). To describe regional start-cue signals, we identified the top 15 most reliably activated ROI from the fixed-effects statistical map (Figure 2C; Table 2; see Experimental Procedures) and plotted the responses averaged over voxels within these regions.
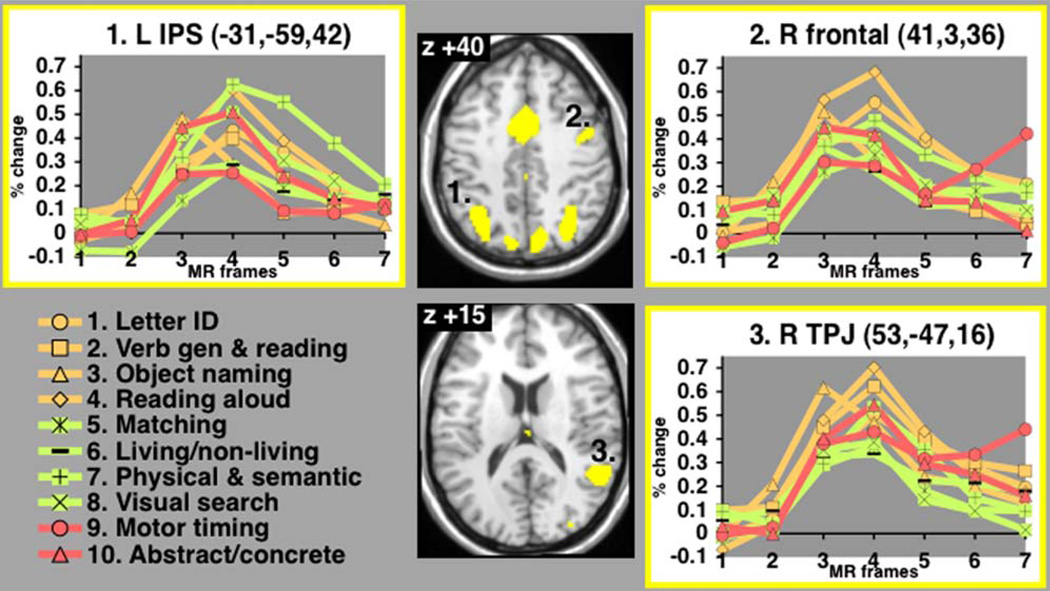
Figure 3 shows time courses of start cue-related activity for ten tasks in representative regions of interest. The dACC/msFC and aI/fO are explored separately in a later section. The right TPJ (Talairach coordinates—53, −47, 16), right frontal cortex (41, 3, 36) and left IPS (−31, −59, 42) showed significant time courses of start-cue activity for all ten tasks (Talairach and Tournoux, 1988). For several of the task conditions in each of these regions, blood oxygenation level-dependent (BOLD) activity does not return to baseline by the seventh time point, because transient start-cue activity “transitions onto” positive sustained activity (also see Figure 1C).
Figure 3. Regionwise Start-Cue Time Courses.
Start cue-related activity in representative regions (responses averaged across voxels). Percent BOLD signal change displayed on y axis; MR frames (TR) displayed on×axis. Since the TR differed from 2.5 s for two of the conditions (see Table 6) the hemodynamic responses (finite impulse responses) may not be directly comparable.
Sustained Activity
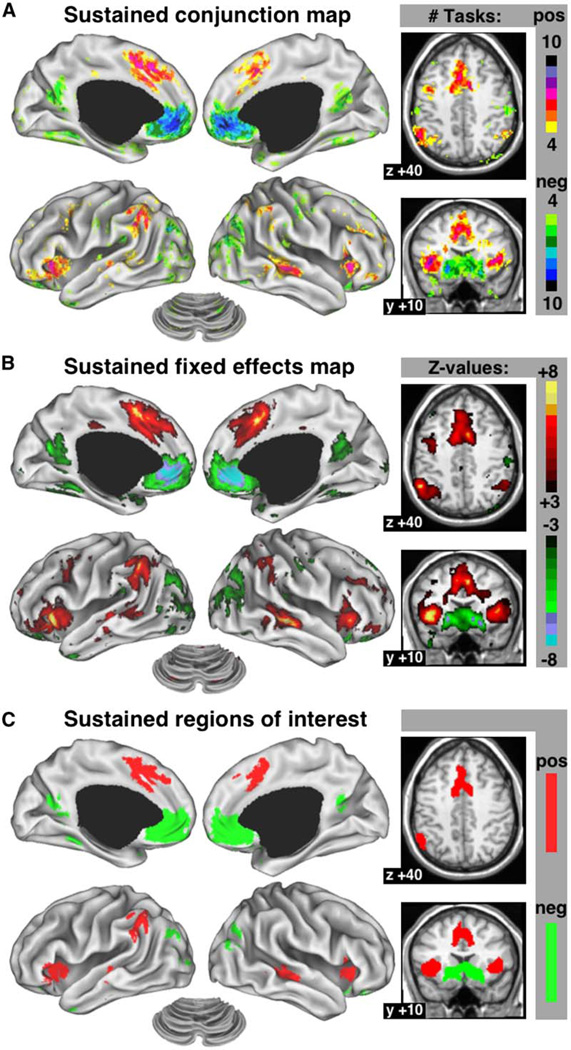
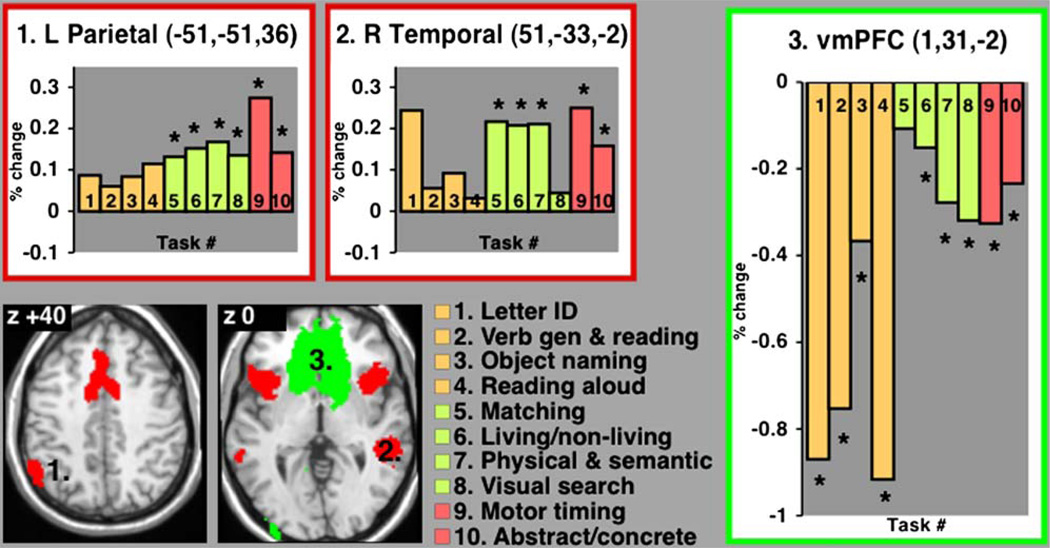
Brain regions with significant sustained activity across many different tasks may be generally important for task-set maintenance. Figure 4A shows the number of conditions for which sustained activity was significant at any given voxel (see Experimental Procedures). Both the dACC/msFC and bilateral aI/fO contained some voxels with significant positive sustained activity in eight of ten task conditions, as did the left inferior parietal lobule (IPL) and right middle temporal cortex. The fixed-effects statistical map for sustained activity (Figure 4B) again showed great similarity to the conjunction map. Several regions showed positive sustained activity in seven tasks, among them right IPL and left middle temporal cortex. Right aPFC showed positive sustained activity in up to six tasks, while left a PFC carried sustained signals in four tasks. Several regions consistently showed negative sustained activity, decreased below baseline, across tasks. The vmPFC showed the most reliable negative sustained activity. Extrastriate visual cortex and the precuneus also carried negative sustained activity, but less consistently so. From the fixed-effects statistical map, we derived the top 15 ROI for sustained activity (Figure 4C; Table 3) and plotted the responses averaged over voxels within these regions.
Figure 4. Cross-Study Analyses of Sustained Activity.
(A) Conjunction image showing voxels with significant sustained activity across conditions. The yellow-to-red scale shows voxels with positive sustained activity in four or more conditions, while the green-to-blue scale shows voxels with negative sustained activity in four or more conditions.
(B) Fixed-effects analysis map of sustained signals.
(C) ROI derived from the fixed-effects map.
Table 3.
Sustained Activity Regions of Interest
| Coordinates |
Peak z Value |
Peak # Studies |
|||
|---|---|---|---|---|---|
| Brain Regions | x | y | z | ||
| L aI/fO | −39 | 15 | 2 | 8.5 | 8 |
| dACC/msFC | −2 | 13 | 44 | 7.8 | 8 |
| R Middle temporal | 51 | −33 | −2 | 7.7 | 8 |
| R aI/fO | 40 | 16 | 4 | 7.3 | 8 |
| L IPL | −51 | −51 | 36 | 7.2 | 8 |
| L Middle temporal | −53 | −31 | −5 | 6.6 | 7 |
| vmPFC | 1 | 31 | −2 | −9.9 | 10 |
| L Posterior cingulate | −11 | −57 | 13 | −7.0 | 7 |
| R Posterior temporal | 44 | −74 | 26 | −6.4 | 7 |
| R Posterior cingulate | 10 | −56 | 16 | −5.8 | 6 |
| L Posterior temporal | −40 | −78 | 24 | −6.3 | 9 |
| L Anterior fusiform | −25 | −44 | −12 | −6.2 | 7 |
| L Temporal pole | −33 | 4 | −29 | −6.3 | 7 |
| R Temporal pole | 34 | 2 | −29 | −6.2 | 7 |
| L Middle occipital | −34 | −92 | 2 | −5.7 | 6 |
aI/fO, anterior insula/frontal operculum; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; IPL, inferior parietal lobule; vmPFC, ventromedial prefrontal cortex.
Figure 5 shows the profiles of sustained activity across ten tasks in three representative ROI. A region in left parietal cortex (−51, −51, 36) showed positive sustained activity for all ten tasks, but interestingly the activity was only individually statistically significant (p < 0.05) for the six task conditions in which subjects responded by pressing a button instead of speaking. Regionwise, negative sustained activity in vmPFC (1, 31, −2) reached statistical significance in nine of ten tasks.
Figure 5. Regionwise Sustained Activity.
Percent BOLD signal change of sustained activity for ten task conditions in representative regions (responses averaged across voxels). Asterisk indicates statistically significant sustained activity (p < 0.05) for individual tasks.
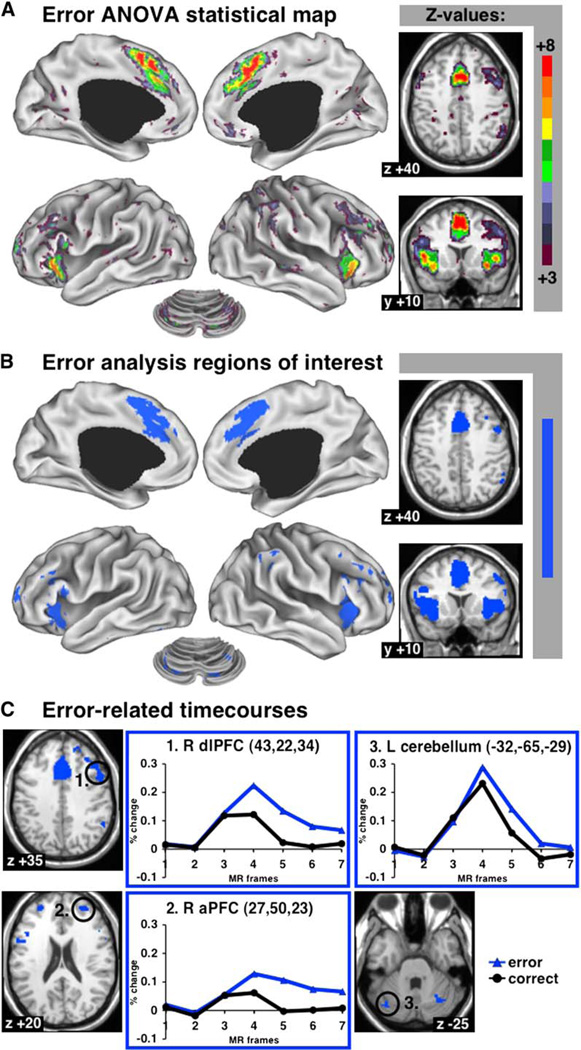
Error-Related Activity
Brain regions forming part of a core task-set system likely also receive trial-by-trial performance feedback, such as error feedback. Data for two of the ten task conditions (#8, #10; same subjects) contained sufficient errors to perform an error analysis (see Experimental Procedures). Figure 6A shows brain regions with significant differences between correct and error trials. The most reliable error-related activity was found in the dACC/msFC and bilateral aI/fO, followed by bilateral cerebellar regions and left and right aPFC, right dlPFC, and right IPL. The top 13 ROI were derived from the ANOVA map for error-related activity (Figure 6B; Table 4), and the responses were averaged over the voxels within these regions.
Figure 6. Error-Related Activity.
(A) Statistical map showing regions with differences in trial-related activity between correct and error trials (ANOVA).
(B) Regions of interest for error-related activity.
(C) Regionwise error-related data in representative regions of interest (responses averaged across voxels). Percent BOLD signal change displayed on y axis; MR frames (TR) displayed on×axis.
Table 4.
Error-Related Regions of Interest
| Coordinates |
Peak z Value |
|||
|---|---|---|---|---|
| Brain Regions | x | y | z | |
| dACC/msFC | 0 | 19 | 41 | 9.1 |
| L aI/fO | −39 | 16 | 7 | 8.1 |
| R aI/fO | 39 | 17 | 3 | 77 |
| R cerebellar Pyramis | 18 | −80 | −33 | 6.5 |
| R anterior PFC | 27 | 50 | 23 | 6.4 |
| L cerebellar Pyramis | −19 | −78 | −33 | 6.2 |
| R dorsolateral PFC | 43 | 22 | 34 | 6.1 |
| L anterior PFC | −28 | 51 | 15 | 5.8 |
| R Thalamus | 11 | −12 | 8 | 5.7 |
| L cerebellar Tuber | −32 | −65 | −29 | 5.4 |
| R IPL | 51 | −47 | 42 | 5.2 |
| L Thalamus | −12 | −15 | 7 | 5.0 |
| R cerebellar Tuber | 31 | −61 | −29 | 5.0 |
dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; aI/fO, anterior insula/frontal operculum; IPL, inferior parietal lobule.
In Figure 6C, correct and error time courses from three regions, other than the dACC/msFC and aI/fO, are displayed. The regions in right dlPFC (43, 22, 34), right aPFC (27, 50, 23), and the left cerebellum (−32, −65, −29) all showed greater trial-related activity for the error trials than for the correct trials, as did all other error-related regions of interest.
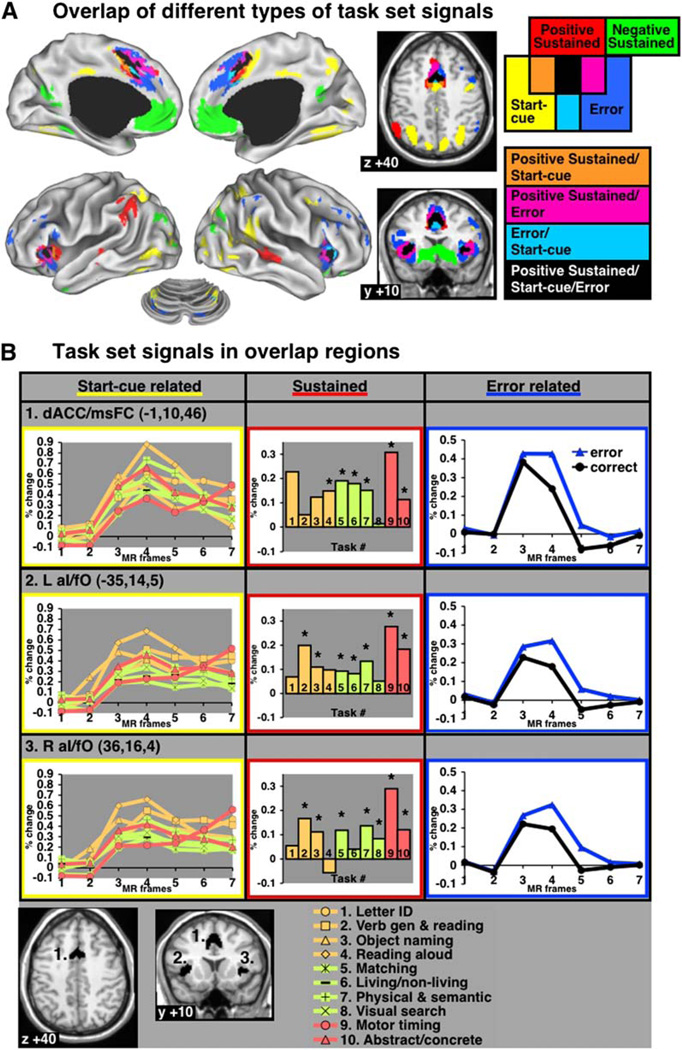
Conjunction of Start-Cue, Sustained, and Error-Related Activity
One reasonable configuration of a task-set system (Figure 1A) would have regions carrying out core executive functions for the configuration of task sets active during both the instantiation and maintenance of task sets, while also receiving performance feedback. Hence, to identify core regions of such a putative human task-set system, we conjoined the regions of interest for start-cue, sustained, and error-related activity.
Figure 7A shows the overlap of thresholded statistical maps for start-cue, sustained, and error-related activations. Only three regions of the brain, dACC/msFC (−1, 10, 46), left aI/fO (−35, 14, 5), and right aI/fO (36, 16, 4), met the statistical thresholds set for all three types of potentially task set-related activity (Figure 7B; Table 5). Bilateral aPFC (R—27, 50, 23; L—−28, 51, 15) also showed three task-set signal types, but for a smaller subset of tasks, and hence did not meet criterion for core task-set regions.
Figure 7. Conjunction of the Fixed-Effects Maps for Start-Cue, Sustained, and Error-Related Activity.
(A) Conjunction map of task-set signals.
(B) Regionwise data from overlap regions (ROI shown on bottom). For start-cue and error-related time courses, percent BOLD signal change is displayed on the y axis and MR frames (TR) are displayed on the×axis. Asterisk indicates significant (p < 0.05) sustained activity. All start-cue activity was significant (p < 0.001).
Table 5.
Overlap Regions: Three Task-Set Signal Types
| Coordinates |
|||
|---|---|---|---|
| Brain Regions | x | y | z |
| dACC/msFC | −1 | 10 | 46 |
| L aI/fO | −35 | 14 | 5 |
| R aI/fO | 36 | 16 | 4 |
dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; aI/fO, anterior insula/frontal operculum.
The start-cue regions in right TPJ, left and right IPS, right frontal cortex, and the precuneus did not overlap with sustained or error-related regions. Regions of positive sustained activity in bilateral middle temporal cortex and left IPL also showed no overlap with other types of task-set signals. Figure 7B shows the start-cue, sustained, and error-related activity profiles for the regions of interest in the dACC/msFC and bilateral aI/fO defined by the conjunction of different types of task-set signals. While some may think that other effects such as “arousal” could also account for sustained signals, the convergence across three different task-set signals makes such alternative explanations unlikely.
Discussion
Posner and Petersen (1990) proposed several defining criteria for attentional control systems in the brain. According to their article, the human brain’s attentional systems should (1) be anatomically separate from the brain’s data-processing (e.g., sensory, motor) systems and (2) consist of a network of functionally diverse regions. Posner and Petersen’s article and subsequent refinements of these ideas outlined postulated attentional networks related to orienting, maintenance of vigilance, and higher-order “executive” or organizational processes. The present study is focused on a subset of the executive functions, those related to task-level organization or task-set implementation.
This study utilized a design that allowed direct visualization of signals related to the onset of a task, signals that were maintained across the trials of a task, and trial-related activity that was affected by correct versus incorrect performance. Important previous studies of task set-related processes used other means to identify task set-related signals. In one such study, extended activity was demonstrated in aPFC during the delay between cue and target (Sakai and Passingham, 2003). Using a direct method, we also found aPFC activity, but only in a limited number of tasks, so it is unlikely that this aPFC activity is related to domain-general core task-set functions.
In terms of Posner and Petersen, a task-set system should recursively maintain the attributes of attentional systems as a whole: anatomical separation from data-processing systems with multiple component regions that make different functional contributions. To relate the results described above to these concepts, we will argue five main points: (1) the dACC/msFC and aI/fO form the core of a human task-set system. They appear to carry out functions most central to the implementation of task sets. (2) Anterior prefrontal regions carry out less general task-set functions. (3) Regions showing predominantly start-cue activity may instantiate new task parameters, interrupt the current task state, or process the visual attributes of the cue. (4) Regions that show error-related activity provide or accept performance feedback information for the optimization of task sets. (5) Regions in vmPFC support the implementation of a “default” mode.
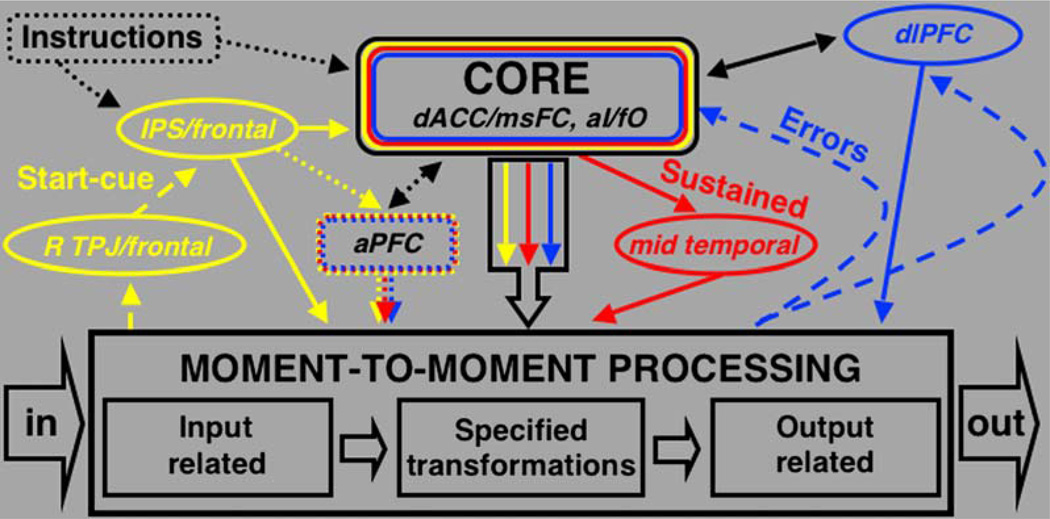
dACC/msFC and aI/fO Form “Core” of the Human Task-Set System
The dACC/msFC and bilateral aI/fO were set apart from other brain regions, in that they carried start-cue and sustained activity across most of the tasks included in our analysis. Moreover, for a subset of tasks the dACC/msFC and bilateral aI/fO carried the most reliable error-feedback activity. Much of the current literature about higher-order task-set control processes has been focused on the dACC/msFC, dlPFC, and aPFC (Botvinick et al., 2004; Braver et al., 2003; Rushworth et al., 2004; Sakai and Passingham, 2003). Based on our mixed blocked/event-related data from ten different tasks, the bilateral aI/fO should be included in future discussions of the human task-set system (Figure 8).
Figure 8. Hypothesized Framework of the Human Task-Set System.
Hypothetical task-set system consistent with the observed experimental results. Other frameworks may also be consistent. Colors are as in Figure 1B, instantiation signals in yellow, maintenance in red, and feedback in blue.
It was previously suggested that the dACC/msFC may form part of an attention or executive control system (Dehaene et al., 2003; Pardo et al., 1990; Posner and Dehaene, 1994). Our current notion that the dACC/msFC forms part of the task-set system’s core is congruent with the finding that dACC/msFC lesions can lead to difficulties initiating complex voluntary movements and actions (Barris and Schuman, 1953; Cohen et al., 1999; Rushworth et al., 2003; Williams et al., 2004). It stands to reason that impairments of task-set instantiation and maintenance might lead to paucity of complex voluntary action. Circumscribed surgical ablations of the dACC acutely cause severe impairments in task set-related processes, such as changing movement direction in response to a cue (Williams et al., 2004). Over time however, cingulotomy patients usually recover most of these functions (Dougherty et al., 2002).
It has been argued that the dACC/msFC functions as a conflict or, in the expanded version of this argument, feedback monitor (Botvinick et al., 2004; Miller and Cohen, 2001). This is consistent with our observations and prior studies, which demonstrated that the dACC/msFC responds to errors, conflict, and a variety of other types of performance feedback in a trial-related manner (Badre and Wagner, 2004; Botvinick et al., 1999; Brown and Braver, 2005; Buchel et al., 1998; Bush et al., 2002; Carter et al., 2000; Eisenberger et al., 2003; Gehring and Willoughby, 2002; Holroyd et al., 2004; Kerns et al., 2004; van Schie et al., 2004; Williams et al., 2004). Yet, it has also been claimed that the dACC/msFC plays no role in the top-down implementation of executive control (MacDonald et al., 2000).
Our observations appear to be inconsistent with such a limited role for the dACC/msFC. Our study demonstrates that the dACC/msFC commonly carries start-cue and sustained maintenance signals, making it unlikely that it functions as a pure monitoring device. Other neuroimaging studies lend support to our claim that processing in the dACC/msFC goes beyond a simple monitoring role (Bush et al., 1999; Fan et al., 2005; Pardo et al., 1991; Weissman et al., 2005). Weissmann et al. (2005), for example, recently demonstrated increased dACC/msFC activity in response to cues signaling a more difficult, control-requiring task. They interpreted their finding as evidence for the dACC/msFC’s role in directing attention toward task-relevant stimuli. Rushworth et al. (2004) have gathered evidence that the dACC/msFC may be important for maintaining associations between actions and their outcomes and the implementation of action sets.
The importance of the bilateral aI/fO for higher-order processes has often been ignored, despite the fact that it commonly is found in published tables of active regions (Crone et al., 2005; Egner and Hirsch, 2005; Fan et al., 2005; Yeung et al., 2006). Other neuroimaging studies investigating different types of higher-order processes have previously shown the dACC/msFC and aI/fO to be coactivated. For example, one event-related fMRI study showed that the dACC/msFC and aI/fO both process negative emotional feedback (Buchel et al., 1998). An fMRI study by Fan and Posner described a candidate executive attention network that included, among others, dACC/msFC and a left opercular region (−34, 20, 5) very near the region derived in our analysis (left aI/fO—−35, 14, 5; Fan et al., 2005). A study of patients with rare isolated insular infarcts showed that they often suffer from subjective anergia and underactivity, similar to patients with circumscribed dACC lesions (Manes et al., 1999).
As mentioned above, with time cingulotomy patients often recover most “executive functions” (Dougherty et al., 2002). Perhaps task-set functions recover so steeply after dACC ablation because the aI/fO functions similarly to the dACC/msFC.
aPFC May Carry Domain-Specific Task-Set Signals
Two previous neuroimaging studies of task set by Braver et al. (2003) (Tailarach—34, 48, 18) and Sakai and Passingham (2003) (36, 44, 6) identified regions in right aPFC. Consistent with these findings, we identified aPFC regions (R—27, 50, 23; L—−28, 51, 15), although our analyses identified bilateral regions. These aPFC regions showed error-related, sustained, and start-cue activity. In contrast to the dACC/msFC and aI/fO, however, start-cue and sustained activity in the aPFC were limited to a smaller subset of tasks, indicating that task-set processes in aPFC may be specific to certain types of tasks. Consistent with this idea, Braver et al. (2003) showed that right aPFC only carried significant positive sustained activity when subjects switched between two semantic classification tasks, but not when subjects performed a single task per block.
Sakai and Passingham (2003) measured activity during the delay between cue and trial for different versions of a working memory task that used letters as stimuli. They found extended pretrial activity in right aPFC. Thus, one hypothesis might be that task-set processes in aPFC are only recruited for tasks that pose high demands on working memory, such as within block task-switching. At this point, further research appears necessary to precisely identify the processing dimensions controlled by aPFC, although several important postulates have been forwarded (Duncan, 2001; Koechlin et al., 1999, 2003; Konishi et al., 2005; Ramnani and Owen, 2004).
So how does the dlPFC fit into the task-set system (Figure 8)? If dlPFC plays a central role in the top-down implementation of task-level control, one would expect it to show sustained and start-cue activity across a variety of tasks. Yet, across the ten tasks included in this analysis, dlPFC did not show consistent start-cue or sustained activity. Instead, we documented error-related signals in the dlPFC (43, 22, 34), indicating a potential role in the processing of, and top-down response to, feedback signals (Figure 6). Distinct from patients with massive bilateral midline lesions, patients with dorsolateral prefrontal lesions often show no impairments in the implementation of more basic goal-directed task sets (Luria, 1980). The imaging studies that have argued for the dlPFC as the major source of top-down control signals were directed at trial-based control effects (MacDonald et al., 2000). The data we present here do not exclude the possibility that dlPFC helps adjust task parameter settings on a trial-by-trial basis.
Start-Cue Activity Might Play Different Roles when Entering a Task State
Several regions in parietal and frontal cortex were set apart from the core regions (dACC/msFC, aI/fO), in that they showed start-cue activity but lacked sustained and error-related activity across tasks. Bilateral regions in the IPS (R—30, −61, 39; Left—−31, −59, 42) were active during the instantiation of task sets across a variety of tasks. Corbetta and Shulman (2002) have postulated that the left and right IPS are part of a dorsal frontoparietal network that controls the top-down distribution of visual attention. They contend that the left and right IPS are involved in the generation of attentional sets, or goal-directed stimulus-response mappings. Our findings support this notion. The left and right IPS might help load, transmit, or instantiate the required task-set parameters at the beginning of each task period. Corbetta and Shulman (2002) also proposed that the IPS might be important for the application of those sets during stimulus processing. Yet, the lack of sustained signals in the IPS suggests that it is recruited only transiently during the loading and instantiation of task parameters.
The right TPJ (53, −46, 17) and right lateral frontal cortex (41, 3, 36) also showed reliable start-cue activity across tasks, while lacking consistent sustained and error-related activity. This result is also in agreement with Corbetta and Shulman (2002), who have argued that the right TPJ and right lateral frontal cortex form a ventral frontoparietal network important for stimulus-driven bottom-up shifts of attention.
A straightforward explanation for the start-cue activity in fusiform cortex and extrastriate visual regions is that this activity relates to processing the visual attributes of the start-cue, since the start of a task period was always cued by a color change of the fixation cross.
Overall, our findings fit very well with the model proposed by Corbetta and Shulman (2002). An important novel observation is that the regions ascribed to both the dorsal and ventral attentional networks do not seem to play a prominent role in task-set maintenance or error-feedback processing.
Feedback
It has been argued that the processing of performance feedback should be a hallmark feature of any task-set system (Miller and Cohen, 2001). The most reliable error-feedback signals were found in dACC/msFC and aI/fO (Table 4). Right dlPFC showed error-related activity (43, 22, 34), as did bilateral cerebellar regions (L—−32, −65, −29; R—31, −61, −29). The finding of error-related activity in cerebellar regions was not surprising since previous studies had underlined the importance of the cerebellum in processing error codes (Fiez, 1996; Fiez et al., 1992).
vmPFC Supports the Brain’s Default State
Parts of vmPFC were significantly deactivated in sustained fashion in all of the tasks included in the cross-studies analysis (Figures 4A and 4B). It has been proposed that the human brain enters a default processing mode when not confronted with external task demands (Gusnard and Raichle, 2001; Raichle et al., 2001). Therefore, the task-independent negative sustained activity we observed in vmPFC could be caused by the suspension of default processes for the duration of each task period. In addition to confirming findings from previous metaanalyses of PET data (Shulman et al., 1997a, 1997b), our analysis of mixed blocked/event-related fMRI data showed that a large portion of the negative activity in vmPFC is not time locked to individual trials, but seemingly sustained for the length of a task period.
Basic Alternative Processing Modes
Across tasks, negative sustained activity in vmPFC coincided with positive sustained activity in the dACC/msFC and bilateral aI/fO (Figure 4). Hence, we hypothesize, similar to others that the human brain may exist in two alternative basic processing modes, a “default mode,” in part supported by vmPFC, and a goal-directed “task mode.” We believe that the brain’s basic goal-directed task mode is supported in part by core regions of the task-set system. This basic “task” mode still needs to be better characterized. In the end, we may discover that the brain enters the performance mode supported by the dACC/msFC and aI/fO during resource-limited tasks, but not data-limited tasks (Norman and Bobrow, 1975). Only one of the tasks included in our cross-studies analysis (#8) was data limited in the sense that it was very difficult to perceive differences between the stimuli (Gabor patches), but the operation performed on the stimuli was simple (same/different). This was also the task for which the overall profile of task set activity differed the most from all other tasks (see Figure 7B, middle panels).
The basic “task” mode may potentially be biased toward processes broadly related to linguistics, since seven of the tasks selected for the cross-studies analysis included different “linguistic” components such as orthography, semantics, syntax, and phonology. We believe this is unlikely because two tasks (#5 and #9) that were devoid of apparent linguistic processes showed reliable start-cue and sustained activity in the dACC/msFC and bilateral aI/fO.
Consistent with our ideas, a recent functional connectivity study by Fox et al. (2005b) suggests the human brain to be organized into widespread anticorrelated functional networks. The study by Fox et al. (2005b) showed spontaneous BOLD signal fluctuations in the dACC/msFC and bilateral aI/fO to be anticorrelated with those in vmPFC, while spontaneous fluctuations in the dACC/msFC and bilateral aI/fO were positively correlated with each other.
Conclusion
It is clear that across a wide range of conditions the mixed blocked/event-related design can be used to extract separate transient signals related to the start of a task and the individual trials of tasks, as well as sustained activity that endures across task trials. These different signals have overlapping but separate anatomical distributions that imply diverse functional roles. Finally, the dACC/msFC and bilateral aI/fO make up a limited set of regions with strong overlap between signal types across tasks, indicating an important general role for these regions in the implementation of goal-directed task sets.
Experimental Procedures
Task Conditions Included in the Cross-Studies Analysis
Data from a total of eight different mixed blocked/event-related experiments, using ten different tasks conducted on 183 human subjects at Washington University, were included in this analysis (Table 6). All of the experimental subjects were normal adults between 18 and 35 years of age. All of the studies included data from at least 17 subjects. All data were collected on the same Siemens MAGNETOM Vision 1.5 Tesla scanner (Erlangen, Germany). For all ten task conditions, a color change of the fixation cross from white to red or green signaled the beginning and end of task blocks. Important prior task-set experiments used visually presented words and letters as stimuli while subjects indicated their responses using a button-press (Braver et al., 2003; MacDonald et al., 2000; Sakai and Passingham, 2003; Weissman et al., 2005). We tried to broaden the task demands by including auditory stimuli, as well as images, nonwords, and abstract letter-like symbols. To control for output-specific responses, we used four tasks in which subjects indicated their responses by speaking aloud instead of pressing a button. To control for input-specific responses, two of the tasks used only auditory stimuli. Subjects were asked to perform a variety of intermediate operations, such as different semantic classifications, visual classifications, timing, naming, visual search, and reading.
Table 6.
Experimental Details for Tasks Included in Analysis
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Task condition | Letter | Verb generate/ | Object naming | Reading | Matching | Living/nonliving | Upper/lowercase and | All the same/ | Timing finger taps | Abstract/concrete |
| identification (ID) | reading | judgment | abstract/concrete | one different judgment | judgment | |||||
| Stimuli | Letters | Nouns (4–7 letters) | Images | Nonword/words (3–8 letters) | Symbols/letters | Images | Nouns | 4 Gabor patches | Tone patterns | Nouns |
| Input modality | Visual | Visual | Visual | Visual | Visual | Visual | Visual | Visual | Auditory | Auditory |
| Output modality | Speech | Speech | Speech | Speech | Button | Button | Button | Button | Button | Button |
| # Subjects | 18* | 17 | 18 | 24 | 18* | 34 | 17 | 24** | 32 | 24** |
| TR (s) | 2.5 | 2.5 | 3.18 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.625 | 2.5 |
| Task period length (TR) | 45 | 34 | 31 | 53 | 45 | 40 | 41 | 35 | 120 | 35 |
| # Task periods | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 1 | 3 |
| Baseline length (TR) | 16 | 21 | 16 | 18 | 16 | 10, 17, 13 | 25 | 15 | 24 | 15 |
| # Baseline periods | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 2 | 4 |
| Stimulus duration (ms) | 500 | 300 | 200 | 250 | 500 | 1300 | 500 | 300 | 2625 | varied |
The same subjects performed tasks #1 and #5;
the same subjects performed tasks #8 and #10.
Image Acquisitions
All images were acquired in adherence to the same standard protocol. To help stabilize head position, each subject was fitted with a thermoplastic mask fastened to holders on the head coil. All images were obtained with a Siemens MAGNETOM Vision 1.5 Tesla scanner (Erlangen, Germany). A T1-weighted sagittal MPRAGE structural image was obtained (TE = 4 ms; MR frame = 9.7 ms; TI = 300 ms; flip angle = 12°; 128 slices with 1.25 × 1 × 1 mm voxels; Mugler and Brookeman, 1990). Functional imaging was performed using a BOLD contrast-sensitive asymmetric spin-echo echo-planar sequence (T2* evolution time = 50 ms; α = 90°, in-plane resolution 3.75 × 3.75 mm). Whole-brain EPI acquisitions (MR frames) of 16 contiguous, 8 mm thick axial slices were obtained parallel to the anterior commissure-posterior commissure plane.
Preprocessing of Raw MR Data
The same preprocessing stream was used for all the studies included in the analysis. Initial data processing to remove noise and artifacts was carried out using a series of automated steps. First, a slice-by-slice normalization corrected for changes in signal intensity introduced by the acquisition of interleaved slices. Next, rigid-body translation and rotation (Snyder, 1996) was applied to every MR frame in order to realign images within and across scans. A three-dimensional cubic spline interpolation (Friston et al., 1994) was used to minimize artifacts. Finally, to allow across scan comparisons, the mode voxel value was normalized to 1000.
Analysis Using the General Linear Model
BOLD activity related to the trials, cues, and task periods were modeled using the general linear model (GLM). Additionally, baseline and trend-effect terms for each BOLD run were included in the GLM. Effects were coded according to the same principles for all task conditions. Sustained set maintenance-related activity during trial performance was modeled with a square wave, starting seven scans after the beginning of each task epoch and terminating at the end. In addition, three sorts of event-related activity were modeled using a stick function at the beginning of each event and for the subsequent six scans. This is equivalent to estimating the finite impulse response, evoked by each event and eschews assumptions about the form of the hemodynamic response function (Friston et al., 1994; Josephs et al., 1997; Miezin et al., 2000; Ollinger et al., 2001; Worsley et al., 1995; Zarahn et al., 1997a, 1997b). The three events we modeled were the start-cue, the stop-cue, and each trial. In the two conditions (#8, #10) that showed a substantial number of error trials, we modeled correct and error trials separately. As a result, we could summarize the maintenance-related activity with a single parameter and the onset transient with seven parameters. These parameters were taken to a second level for between-subject or random-effect analysis using t tests and f tests, respectively. Individual subject data were transformed into the stereotactic space of Talairach and Tournoux (1988).
Analysis of Signals
For all task conditions, we performed one-sample t tests comparing sustained activity during the task blocks to activity during the fixation-only control periods. ANOVAs were performed separately on the transient trial-related and start-cue activity. A significant main effect of time (ANOVA) indicated that the hemodynamic response was different from flat across seven TRs.
Conjunction Analyses of Sustained Activity and Start-Cue Activity
Start-cue and sustained activity are different types of neural signals, with great differences in statistical reliability and signal magnitude (Fox et al., 2005a; Konishi et al., 2001; Visscher et al., 2003). To form a conjunction map of sustained activity, we identified voxels that were significant at a p < 0.05 (uncorrected) in four or more tasks (t statistic). The probability that four or more conditions survived this criterion is (0.05)4 which is equal to 6.25 × 10−6, providing more than adequate family-wise error control over the searched brain volume. To create a conjunction map of start-cue activity, voxels significantly active at a p < 0.000001 (uncorrected) in four or more tasks (f-statistic) were identified. The stringent threshold, chosen in relation to the greater overall reliability of start-cue signals was crucial to the generation of a meaningful conjunction map. Over a long series of experiments, we have repeatedly noted that start cue-related activations are incredibly statistically reliable across subjects. Therefore, the most robust start-cue activations, which we were interested in, easily passed the chosen threshold.
Fixed-Effects Analyses of Sustained and Start-Cue Activity
Fixed-effects analyses were performed on the start-cue and sustained activations. Both the sustained T-maps (one-sample t test) and start-cue f maps (ANOVA) were transformed to z score maps using in-house fMRI analysis software (FIDL). To generate fixed-effects maps, the z score maps for all ten conditions were summed and divided by the square root of n (number of conditions = 10). This created fixed-effects statistical maps in which the z score at any given voxel represents the reliability of activation during the ten task conditions entered into the analysis.
Analysis of Error-Related Activity
Only two of the ten task conditions contained enough errors to perform an error analysis (see Table 1, Visual search #8, Abstract/concrete #10; performed by the same subjects). A correctness × time ANOVA across both conditions (coded into the same GLM) yielded a statistical map highlighting brain regions in which the time courses for error trials was significantly different from the time course for correct trials, over seven time points. The resultant f map was transformed to a z score map (Figure 6A) and thresholded at z = 4.09 (Figure 6B).
Region of Interest Definition
In order to meaningfully combine three fundamentally different types of neural signals with different statistical reliability, in a conjunction map (Figure 7), the region definition thresholds had to be set relative to the overall statistical reliability of each signal type. ROI were defined by thresholding the relevant statistical maps and clustering all surface connected voxels that passed threshold into an ROI. The clustering thresholds (start-cue z = 17; sustained z = 4.75; error-related z = 4.09) were set specifically to select the top 15 most reliably activated brain regions, in essence normalizing the thresholds. The error-related statistical map only yielded a maximum of 13 ROI at any threshold. Clusters with fewer than 66 surface connected voxels were excluded.
Acknowledgments
We thank Heather Lugar and Rebecca Coalson for their help with data collection. We thank Mark McAvoy for his help with data analysis. We thank Elizabeth Murray for pointing us toward the literature on insular infarcts. This work was supported by NIH grants NS41255 and NS46424 to SEP and the John Merck Scholars Fund, the Burroughs- Wellcome Fund, and the Dana Foundation (B.L.S.).
References
- Baddeley A. Exploring the central executive. Q J. Exp. Psychol. 1996;49A:5–28. [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Barris RW, Schuman HR. Bilateral anterior cingulate gyrus lesions. Neurology. 1953;3:44–52. doi: 10.1212/wnl.3.1.44. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Broadbent D. Task combination and selective intake of information. Acta Psychol. (Amst.) 1982;50:253–290. doi: 10.1016/0001-6918(82)90043-9. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol. Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat. Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H. Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J. Neuropsychiatry ClinNeurosci. 1999;11:444–453. doi: 10.1176/jnp.11.4.444. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb. Cortex. 2005;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Artiges E, Naccache L, Martelli C, Viard A, Schurhoff F, Recasens C, Martinot ML, Leboyer M, Martinot JL. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: the role of the anterior cingulate. Proc. Natl. Acad. Sci. USA. 2003;100:13722–13727. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. In: Cowan M, editor. Annual Review of Neuroscience. Palo Alto, CA: Annual Reviews, Inc.; 1995. pp. 193–222. [DOI] [PubMed] [Google Scholar]
- Donaldson DI. Parsing brain activity with fMRI and mixed designs: what kind of a state is neuroimaging in? Trends Neurosci. 2004;27:442–444. doi: 10.1016/j.tins.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Jenike MA, Rauch SL. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am. J. Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired nonmotor learning and error detection associated with cerebellar damage: a single-case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005a;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005b;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994;1:153–171. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K. Event-related fMRI. Hum. Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- Konishi S, Chikazoe J, Jimura K, Asari T, Miyashita Y. Neural mechanism in anterior prefrontal cortex for inhibition of prolonged set interference. Proc. Natl. Acad. Sci. USA. 2005;102:12584–12588. doi: 10.1073/pnas.0500585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol. Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. Second Edition. New York: Basic Books, Inc; 1980. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manes F, Paradiso S, Robinson RG. Neuropsychiatric effects of insular stroke. J. Nerv. Ment. Dis. 1999;187:707–712. doi: 10.1097/00005053-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J. Exp. Psychol. [Hum Learn] 1996;22:1423–1442. [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: part 1. Basic mechanisms. Psychol. Rev. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Miezin F, Maccotta L, Ollinger J, Petersen S, Buckner R. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Three-dimensional magnetization- prepared rapid gradient-echo imaging (3D MP RAGE) Magn. Reson. Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cognit. Psychol. 1975;7:44–64. [Google Scholar]
- Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. In Consciousness and Self-Regulation. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI II. Analysis. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol. Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR. Attention and cognitive control. In: Solso RL, editor. In Information Processing and Cognition: The Loyola Symposium. Hillsdale, NJ: Lawrence Erlbaum; 1975. pp. 55–85. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. Inaugural article: a default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. J. Cogn. Neurosci. 2003;15:338–353. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Saito N, Mushiake H, Sakamoto K, Itoyama Y, Tanji J. Representation of immediate and final behavioral goals in the monkey prefrontal cortex during an instructed delay period. Cereb. Cortex. 2005;15:1535–1546. doi: 10.1093/cercor/bhi032. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat. Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: Idetection, search, and attention. Psychol. Rev. 1977;84:1–53. [Google Scholar]
- Shiffrin R, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol. Rev. 1977;84:127–190. [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Fiez JA, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: Iincreases in subcortical structures and cerebellum, but not in non-visual cortex. J. Cogn. Neurosci. 1997a;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. decreases in cerebral cortex. J. Cogn. Neurosci. 1997b;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Snyder AZ. Difference image vs. ratio image error function forms in PET-PET realignment. In: Myer R, Cunningham VJ, Bailey DL, Jones T, editors. In Quantification of Brain Function Using PET. San Diego, CA: Academic Press; 1996. pp. 131–137. [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc. Natl. Acad. Sci. USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 2001;41:1359–1378. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie HT, Mars RB, Coles MG, Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nat. Neurosci. 2004;7:549–554. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb. Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Visscher KM, Miezin FM, Petersen SE, Schlaggar BL. Comparison of sustained and transient activity in children and adults using a mixed blocked/event-related fMRI design. Neuroimage. 2004;22:975–985. doi: 10.1016/j.neuroimage.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for detemining significant signals in images of cerebral activation. Hum. Brain Mapp. 1995;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-task competition and cognitive control in task switching. J. Neurosci. 2006;26:1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997a;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics: Ispatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997b;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]