Abstract
Toward the increasing demands of portable energy storage and electric vehicle applications, the widely used graphite anodes with significant drawbacks become more and more unsuitable. Herein, we report a novel scaffold of hierarchical silicon nanowires-carbon textiles anodes fabricated via a facile method. Further, complete lithium-ion batteries based on Si and commercial LiCoO2 materials were assembled to investigate their corresponding across-the-aboard performances, demonstrating their enhanced specific capacity (2950 mAh g−1 at 0.2 C), good repeatability/rate capability (even >900 mAh g−1 at high rate of 5 C), long cycling life, and excellent stability in various external conditions (curvature, temperature, and humidity). Above results light the way to principally replacing graphite anodes with silicon-based electrodes which was confirmed to have better comprehensive performances.
Rechargeable lithium-ion batteries are fast-growing technology that is attractive for potential applications in portable electronics and electric vehicles due to their relatively high energy and power densities1,2,3,4,5,6. The currently commercial graphite anodes with the low theoretical capacity (372 mAh g−1) and poor rate capability can not satisfy the requirements of high-efficient next-generation energy storage systems7. Herein, the basic strategy has been performed in order to search for various lithium insertion metals as alternative anodes8,9. In particular, silicon has been proposed as one of the most promising anode materials due to its corresponding high theoretical lithium storage capacity (4200 mAh g−1 for Li22Si5, 11.3 times that of commercial graphite), high volumetric capacity (9786 mAh cm−3), and low cost10,11,12,13,14,15,16,17,18,19,20. The major drawbacks are the volume expansion upon Li insertion of silicon itself due to the formation of LixSi alloys that causes cracking and pulverization of Si particles as well as the fracture behavior and cracking patterns in amorphous Si film-electrode, thereby leading to the loss of electrical contact and short cycle life21,22,23,24. Commonly, coating graphite and decreasing the dimensions to one-dimension, and patterning the electrode/reducing the coated-film thickness for silicon active materials are many effective techniques to solve the above-mentioned problems25. However, the complicated fabrication processes of these above Si-based electrodes may hinder their large-scale production. It is still a severe challenge to achieve high-performance silicon electrodes using a low-cost/facile route.
Up to now, great attentions have been paid to explore the potential applications of promising Si-based electrodes for lithium-ion battery. Yu et al. synthesized 3D porous Si anodes by a magnesiothermic reduction approach, showing their good cycling stability (reversible capacity of 2600 mAh g−1 over 100 cycles)26. Nam et al. produced carved Si electrodes using the laser interference lithography (LIL) technique under controlled exposure conditions. These electrodes exhibited a superior Li storage capacity and a high rate capability27. Wu et al. reported a SiNP@CT via electrospinning methods, revealing these electrodes presented long cycle life (200 cycles with 90% capacity retention) and good rate capability28. Magasinski et al. described a Si-C nano-composite granule using chemical vapor deposition (CVD) process. These materials delivered high capacity (1500 mAh g−1) at 1 C and excellent rate capability (approximately 1000 mAh g−1 at a high rate of 8 C)29. Even though the inspiring results in terms of cycling life and rate capability of these electrodes have been obtained, no information about across-the-aboard performances (large energy density, high power density, enhanced battery stability, and satisfying safety) in single complete Si-based storage unit was disclosed. Only in this unit can we principally replace existing graphite anodes with better comprehensive performance silicon-based electrodes.
Herein, we first report a novel matrix of hierarchical Si nanowires (NWs)-carbon textiles matrix as a binder-free electrode via a facile route. Full Si NWs anodes/commercial LiCoO2 cathodes batteries were successfully assembled in order to investigate their corresponding comprehensive performances. These unique Si/carbon textiles scaffold electrodes could enhance the electric conductivity and stabilize the whole structure, leading to their enhanced specific capacity (2950 mAh g−1 at 0.2 C), good repeatability/rate capability (even >950 mAh g−1 at high rate of 5 C), long cycling life, and excellent stability in various external conditions including curvature, temperature, and humidity. We provide a possibility to replace commercial graphite material with superior performance Si-based matrix.
Results
Fabrication and characterization of as-prepared silicon-carbon composites
Figure 1 shows the fabrication processes of Si NWs-carbon textiles composites. Briefly, the Si NWs were dissolved in ethanol solution to achieve a homogeneous suspension, as shown in Figure 1 inset. Then, carbon textiles were gradually coated with as-synthesized Si NWs during the fabricating process, where we can see a layer of our samples attached tightly to the carbon textiles. Finally, hierarchical Si NWs-carbon textiles anodes were achieved after thermal treatment for them. More details have been explained in Methods section.
Figure 1. Schematic illustration of the formation of hierarchical silicon-carbon textiles matrix.
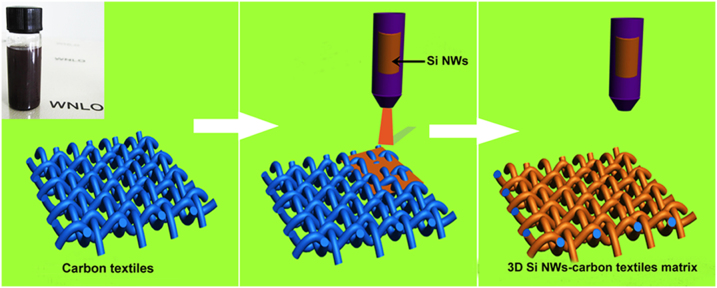
A complete route is composed of Si-based-ink preparation, spray-coating operation, and thermal treatment.
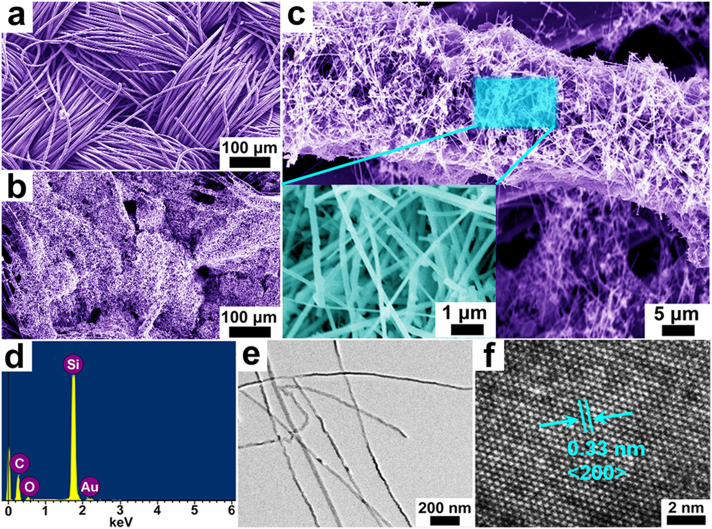
The SEM image of pure carbon textiles with smooth surfaces before coating is shown in Figure 2a and Supplementary Figure S1, which clearly illustrates their well-established texture structures and each carbon microfiber with uniform diameter of approximately 10 μm. For comparison, Figure 2b shows the FESEM image of novel samples/carbon textiles matrix. It can be seen that the carbon textiles coated with resulting materials still keep its original cloth-like feature, illustrating good mechanical-strength of this substrate. Typical SEM image of the single silicon-carbon composite fiber from this above matrix demonstrates numerous uniform silicon nanowires with length of ~10 μm sticked tightly to the surface of single carbon microfiber, as shown in Figure 2c. Figure 2c inset shows a higher-magnification SEM image of the samples, illustrating the diameter of these Si NWs with about 30–50 nm.
Figure 2. Morphological and structural analysis of hierarchical Si nanowires-carbon textiles matrix.
(a, b) Typical SEM images of samples/carbon textiles composites. (c) SEM image of a single carbon microfiber uniformly coated with Si nanowires. The EDX spectrum (d), the TEM image (e), and HR-TEM image (f) of the resulting materials.
The distributions of Si and C are also shown in Supplementary Figure S2, revealing a uniform distribution of Si and C through the whole matrix (in the SEM image area marked with an orange square). The EDX spectrum collected from the surface part of Si samples on carbon textiles (Fig. 2d) is consistent well with the above mapping results. The TEM image of Si NWs is shown in Figure 2e, the diameters of these samples are approximately 30 nm. The high-resolution TEM (HRTEM) image shown in Figure 2f reveals the inter-planar spacing of ~0.33 nm, corresponding to the <200> plane of single-crystalline Si material.
Electrochemical characterizations of coin-type half-cells and full Li-ion batteries based on Si NWs-carbon textiles matrix as a binder-free anode
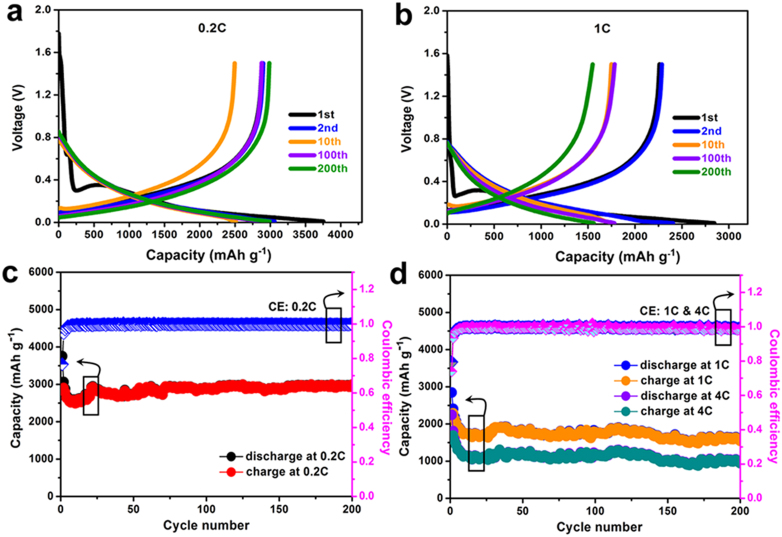
Electrochemical measurements of as-prepared Si NWs-carbon textiles electrodes were performed in coin-type half-cells at the room temperature. Figure 3a and 3b shows voltage profiles of Si NWs-carbon textiles electrodes cycled between 0.01–1.5 V at 0.2 C and 1 C for 1st, 2nd, 10th, 100th, and 200th cycles, respectively. Both first discharge curves (0.2 C and 1 C) present the flat plateaus at ~0.25 V, which corresponds to the lithiation process of crystalline Si to form amorphous LixSi phase and the formation of typical solid electrolyte interphase (SEI)30,31. Afterwards, the profiles of following charge-discharge curves almost remain the same for 200 cycles. Figure 2c demonstrates the charge/discharge curves of Si NWs-carbon textiles anodes at 0.2 C for 200 cycles. The reversible specific capacity of as high as 2950 mAh g−1, 92% of the initial value after 200 cycles is obtained, which reveals their corresponding excellent cyclability. Furthermore, the coulombic efficiency (CE) of these Si-based electrodes is approximately 99.6%, indicating highly efficient lithium insertion/extraction. In Figure 4d, we can clearly observe the specific capacities still remain 1500 and 1100 mAh g−1 after 200 cycles when the Si electrodes were cycled at the rates of 1 C and 4 C, respectively, proving their wonderful rate capabilities by introducing this novel hybrid structures. Moreover, the CE at rates of 1 C and 4 C approach 99.5%, revealing the cycling stability of this Si NWs-carbon textiles matrix during charge/discharge process. Also, galvanostatic discharge profiles were also investigated by comparing the batteries with samples/carbon textiles and carbon textiles only, as shown in Supplementary Figure S3. It can be seen that the negligible capacity of the pure carbon textiles based device, reveals that the total capacity is primarily attributed to that of the loaded silicon materials32,33,34. All these results reveal that lithium-ion batteries based on as-fabricated silicon anodes could indicate superior electrochemical activities.
Figure 3. Electrochemical characterizations of Si-based half-cells.
(a, b) Voltage profiles of as-prepared Si NWs-carbon textiles electrodes between 0.01–3.0 V at rates of 0.2 C and 1 C. (c) Cycling performance of these electrodes at 0.2 C and their corresponding coulombic efficiency. (d) The rate capability of these electrodes cycled at rates of 1 C and 4 C for as high as 200 cycles.
Figure 4. Electrochemical properties of Si-based full batteries.
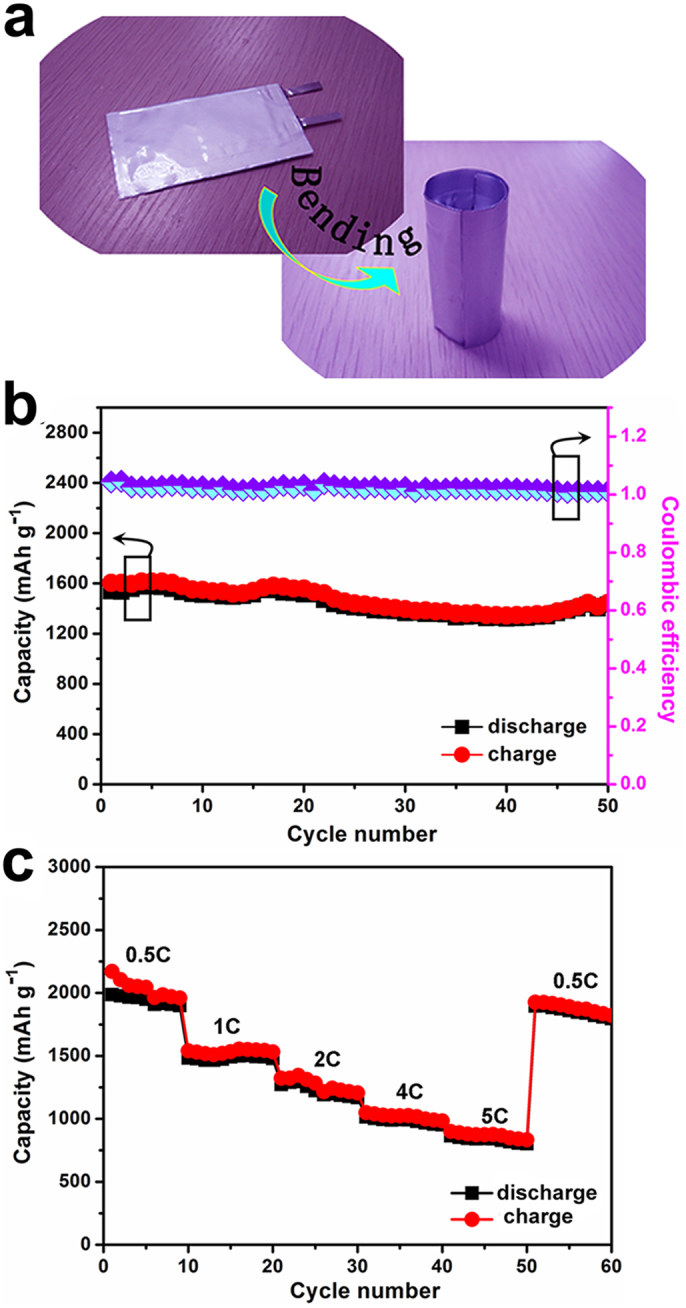
(a) Photograph of full Si-based lithium-ion battery under non-bending and bending. (b) Cycling performance of the non-bending Li-ion batteries based on silicon electrodes cycled at 1 C for 50 cycles and their CE. (c) Their corresponding rate capability cycled at various rates of 0.5 C, 1 C, 2 C, 4 C, and 5 C for total 60 cycles.
To investigate the practical applications of the Si-based electrodes, we described a novel designed full battery formed by combining a high capacity silicon-carbon anode with a high voltage of commercial LiCoO2 cathode. A full Si-based anodes/LiCoO2 cathodes battery was assembled and then evaluated by galvanostatic cycling at various C-rates. The capacities of cathode (LiCoO2) and anode (Si) are approximately 10 ~ 12 and 2.1 ~ 3.4 mAh, respectively. Therefore, it's worth mentioning that these LiCoO2 cathodes are just regarded as counter electrodes and the as-assembled full batteries are anode-limited units and 1 C rate referring to the anode weight is 4200 mAh g−1 33,35,36. Figure 4a displays a bending assembled battery rolled into a cylinder, which illustrates its outstanding flexibility. Figure 4b shows cycle performance and corresponding CE of these non-bending full Si NWs/LiCoO2 batteries at 1 C between 2.5–4.0 V over 50 cycles, from which we can see that these electrodes can deliver enhanced reversible specific capacity (as high as 1580 mAh g−1) with high CE (approximately 99.7%). Further, we investigated the corresponding rate capability with the various rates stepwise increased from 0.5 C to 5 C and then switched back, as shown in Figure 4c. Although such a high 5 C (21 A g−1) was imposed on these electrodes, their corresponding specific capacities were still as high as 950 mAh g−1. The stability to maintain a large capacity at various high rates for Si anodes can be attributed to the novel Si NWs-carbon textiles matrix, facilitating improved electric conductivity, fully accessible interior, and fast lithiation and delithiation reactions for the total Si-based architecture.
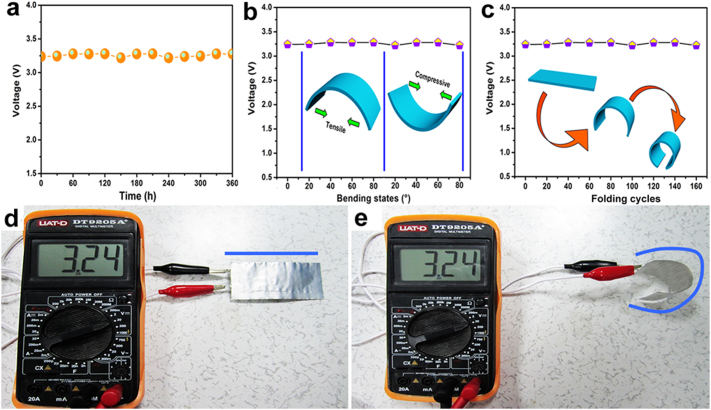
For the increasing demands of the future electronic modules with multiple functional requirements, such as portability, transparency, flexibility, and wear-ability, we designed the novel flexible full lithium-ion batteries based on Si NWs-carbon textiles. The plot of self-discharge characteristic of the flexible batteries is illustrated in Figure 5a. The voltage value remains at ~3.25 V over 360 h at the room temperature, revealing the low self-discharge performance and stable energy storage systems. Further, we investigated the impact of both tensile and compressive stress for the device along its vertical direction. Figure 5b reveals that the average voltage displays no significant change under both tensile/compressive bending states between 20° and 80°. Similarly, the voltage of the device remains stable during the 160 folded cycles. Compared with the battery at no-bending state (Fig. 5d), the voltage can still keep as high as 3.24 V, even though the battery has been bent severely (Fig. 5e). More details have been added in Supplementary Video S1. All these results vividly prove low self-discharge property, high flexibility, and excellent stability of the as-assembled lithium ion batteries.
Figure 5. Stability measurements of self-discharging and curvature situations for as-assembled batteries.
(a) Self-discharge characterization of full Si NWs-carbon textiles/LiCoO2 lithium-ion batteries. (b, c) Voltage profiles of these flexible full lithium-ion batteries for bending states. (d, e) The voltage retention test of this battery under non-bending and bending.
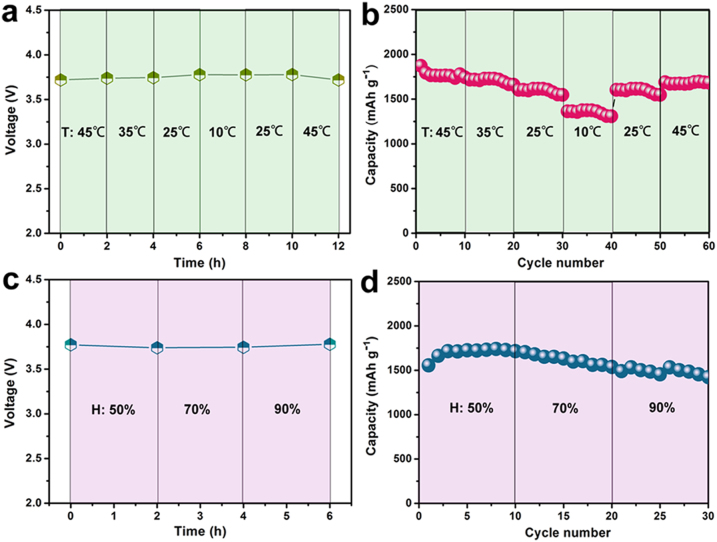
The essential performances of the as-assembled full battery were further performed with various temperature (T) and humidity (H) parameters. As shown in Figure 6a, the voltage values of the full lithium-ion batteries with the change of time are hardly affected by the change of temperature, showing as-fabricated batteries achieve the highly stable capability. Figure 6b shows the reversible capacities of Si NWs-carbon textiles anodes cycled at 1 C. The discharge capacities decline from 1700 to 1300 mAh g−1 with the ambient temperature decreased from 45°C to 10°C, which is a common phenomenon that the capacities of lithium-ion batteries can be affected by ambient temperature. When the temperature finally returns to 45°C, the initial capacity value of 1680 mAh g−1 can be recovered, revealing the stable electrochemical activity of this Li-ion battery under varied ambient temperature. Similarly, the stable feature of the cell devices at the different humidity (50%, 70%, and 90%) was also performed in this Chamber. Even though humidity is continuously raised from 50% to 90%, the voltage of a full lithium-ion battery always maintains at ~3.7 V, as shown in Figure 6c. The discharge capacities can remain a stable cyclable stage during 30 cycles at the different humidity, as illustrated in Figure 6d. These results exhibit the perfect stability, reversibility, and cyclability of our full Si/LiCoO2 batteries when applying in the special conditions.
Figure 6. Stability tests of full lithium-ion batteries under various temperature and humidity.
The as-fabricated batteries were measured at various temperature of 10, 25, 35, and 45°C and various humidity of 50, 70, and 90%, respectively.
Display of these full Si-based Li-ion batteries
Because of the advantages identified above, these full lithium-ion batteries hold the potential to supply power for promising applications. For example, two rows of nixietubes showing red visible patterns of “HUST” (Fig. 7a) and “USC” (Fig. 7b), respectively, can be successfully drived by an as-fabricated battery. More importantly, the battery can light three LED devices when the battery is used to start supplying power, as shown in Figure 7c and 7d. The brightness of devices is almost unaffected performance under greatly bending state so that the lithium-ion battery is believed to meet future portable, flexible, and ultrathin energy storage system options.
Figure 7. Display.
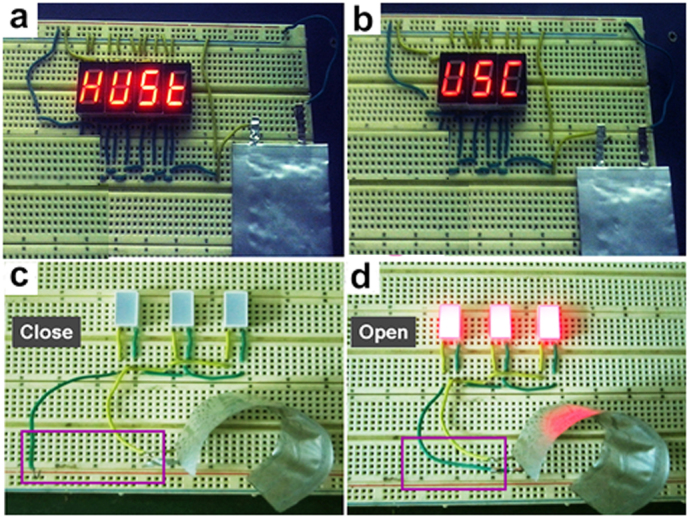
(a,b) The photographs of “HUST” and “USC” patterns lightened by an as-assembled lithium-ion battery (the initials of Huazhong University of Science and Technology & University of Southern California). (c,d) Optical images of three red LEDs driven by a full battery like a Close/Open switch.
Discussion
The above obtained superior electrochemical properties of the as-fabricated hierarchical Si NWs-carbon textiles electrodes are not simply a result of the mixture of the two units (Si & C). It is expected that these performance advancements may originate from the synergistic effect of the efficient integration of the respective advantages from both Si and C individual units in a complete cell configuration. This matrix design with the worthwhile merits achieved by combining high-capacity silicon anodes with high-conductivity carbon textiles provides several unique features: (i) the numerous 1D Si nanowires are beneficial to the insertion/extraction of Li+ among them, which also shorten the charge transfer expressway and lower the exchange resistance for lithium ions between active functional materials and electrolyte; (ii) the Si nanowires coated on carbon textiles with many spaces among them have an increased portion of exposed surfaces, which facilitates lithium-ion/electron transport inside active materials and diffusion of the electrolyte in a short time for fast energy storage; (iii) the current silicon nanowires/carbon textiles matrix can obtain outstanding electronic conductivity because of many Si nanowires tightly attached to the C substrate to form very good adhesion and electrical contact. Herein, it can be concluded that the essential performances of Si-based anodes can be indeed improved by designing the novel hierarchical network matrix.
Considering various desired persistent stability of our demonstrated electrodes during electrochemical processes, we reveal that the spray-coating method with simple, rapid, and low-cost features could keep the good adhesiveness between active materials and substrates due to electrostatic force effect among them, nano-scale raw inks, and special fabricated procedures. Therefore, this is an efficient way to fulfill large-scale industrialization of future critical Li-ion batteries based on high-performance Si anodes by introducing desired spray-coating technique.
In summary, we first report the unique matrix of hierarchical Si NWs-carbon textiles electrodes with high capacity (2950 mAh g−1 at 0.2 C), long cycle life (as long as 200 cycles), good rate capability, and excellent stability with different temperature, humidity, and curvature. Their corresponding superior across-the-aboard performances can provide the great possibility to replace the defective graphite anodes with Si-based material as a promising anode. Furthermore, this strategy to fabricate novel Si-based/3D carbon template hybrid matrix via a facile spray-coating approach can be extended to fabricating other high-performance composite electrodes for energy storage units. As-assembled Si-based lithium ion batteries hold the great potential power for the next-generation functionalities, such as optoelectronic devices, portable/curvilinear electronics, sustainable electric vehicles, etc.
Methods
Synthesis of hierarchical silicon nanowires-carbon textiles matrix
Firstly, silicon nanowires were synthesized via a CVD method. Briefly, the CVD process was carried out at 450°C for 28 min under SiH4 (111 sccm) and H2 (20 sccm) mixed gas. The chamber in tube furnace was cooled down automatically in the atmosphere and then silicon was obtained. After, these Si nanowires were mixed with 40% HF water/ethanol (1:1, v/v) solution (40 ml). The Si NWs solution was ultrasonically agitated by using a sonicator and then centrifuged at 6000 r min−1 for 2 min to separate the stable suspension of Si nanoparticles and the aggregation of Si nanoparticles, which were collected and redispersed in ethanol with sonication, respectively. These same steps were repetitively proceeded at 3 times and then as-obtained Si nanoparticles were dried under vacuum at 80°C for 4 h. Finally, 50 mg of the as-dried Si samples were also immersed in ethanol (10 mL) with sonication for 30 min, forming a homogeneous Si NWs-based solution. The cleaned carbon textiles were gradually coated with the Si NWs slurry. The Si/carbon textiles composites were heated at 300°C under high-purity N2 atmosphere for 30 min to enhance the soundness between silicon and carbon-substrate. Finally, 3D Si NWs-carbon textiles matrix was achieved via a facile spray-coating approach.
Sample characterization
The as-prepared Si NWs-carbon textiles samples were characterized with field emission scanning electron microscopy (FE-SEM, FEI Quanta 450), and transmission electron microscopy (TEM; JEOL JEM-2010 HT).
Electrochemical evaluation
For half-cells assembly, electrochemical experiments were performed using CR2032-type coin cells with Celgard 2400 as the separator and lithium foil as counter electrode. The electrolyte was 1 M LiPF6 in the mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) (1:1 in volume ratio). The working electrodes were binder-free Si NWs-carbon textiles matrix. The mass of Si NWs deposited on carbon textiles substrate was determined by weighing the substrate before and after fabricating process. The loading density of the silicon samples is calculated as 2.4–3.5 mg cm−2. Similarly, as-assembled full flexible Li-ion battery was composed of Si NWs-carbon textiles anode, commercial LiCoO2 cathode, Celgard 2400 separator, and LiPF6-based electrolyte. The coin cells and full lithium-ion batteries were assembled in an argon-filled glove box where oxygen and water concentration were strictly limited to below 1 ppm. The galvanostatic cycling measurements were performed on LAND battery testing system. The tests of the devices at various temperature and humidity were carried out in Temp & Humi Programmable Chamber.
Author Contributions
B.L., G.Z.S., D.C., Y.B.C. and C.W.Z. devised the original concept, designed the experiments, discussed the interpretation of results and co-wrote the paper. B.L. performed the electrochemical experiments. H.T.C. supported the CVD synthesis. All authors discussed the results and participated in manuscript revision.
Supplementary Material
Supplementary information
Supplementary Video
Acknowledgments
This work was supported by the National Natural Science Foundation (51002059, 21001046, 91123008), the 973 Program of China (2011CB933300), the Program for New Century Excellent Talents of the University in China (grant no. NCET-11-0179) and the Natural Science Foundation of Hubei Province (2011CDB035). Special thanks to the Analytical and Testing Center of HUST and the Center of Micro-Fabrication and Characterization (CMFC) of WNLO for using their facilities.
References
- Ellis B. L. et al.. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat. Mater. 6, 749–753 (2007). [DOI] [PubMed] [Google Scholar]
- Ji H. X. et al.. Ultrathin graphite foam: a three-dimensional conductive network for battery electrodes. Nano Lett. 12, 2446–2451 (2012). [DOI] [PubMed] [Google Scholar]
- Wang H. G., Ma D. L., Huang X. L., Huang Y. & Zhang X. B. General and controllable synthesis strategy of metal oxide/TiO2 hierarchical heterostructures with improved lithium-ion battery. Sci. Rep. 2, 701 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. T. & Cho J. Roles of nanosize in lithium reactive nanomaterials for lithium ion batteries. Nano Today 6, 28–41 (2011). [Google Scholar]
- Xia X. H. et al.. Integrated Photoelectrochemical Energy Storage: Solar Hydrogen Generation and Supercapacitor. Sci. Rep. 2, 981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. et al.. A integrated “energy wire” for both photoelectric conversion and energy storage.Angew. Chem. Tnt. Ed. 51, 11977–11980 (2012). [DOI] [PubMed] [Google Scholar]
- Jia H. P. et al.. Novel three-dimensional mesoporous silicon for high power lithium-ion battery anode material. Adv. Energy Mater. 1, 1036–1039 (2011). [Google Scholar]
- Du N. et al.. Porous Co3O4 nanotubes derived from Co4(CO)12 clusters on carbon nanotube templates: a highly efficient material for Li-battery applications. Adv. Mater. 19, 4505–4509 (2007). [Google Scholar]
- Wang H. L. et al.. Rechargeable Li-O2 batteries with a covalently coupled MnCo2O4-graphene hybrid as an oxygen cathode catalyst. Energy Environ. Sci. 5, 7931–7935 (2012). [Google Scholar]
- Chan C. K. et al.. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 3, 31–35 (2008). [DOI] [PubMed] [Google Scholar]
- Hertzberg B., Alexeev A. & Yushin G. Deformations in Si-Li anodes upon electrochemical alloying in nano-confined space. J. Am. Chem. Soc. 132, 8548–8549 (2010). [DOI] [PubMed] [Google Scholar]
- Cui Y. & Lieber C. M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 291, 851–853 (2001). [DOI] [PubMed] [Google Scholar]
- Chen P. C., Xu J., Chen H. T. & Zhou C. W. Hybrid silicon-carbon nanostructured composite as superior anodes for lithium ion batteries. Nano Res. 4, 290–296 (2011). [Google Scholar]
- Zhang G. Q., Yu L., Wu H. B., Hoster H. E. & Lou X. W. Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries. Adv. Mater. 24, 4609–4613 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou X. S., Cao A. M., Wan L. J. & Guo Y. G. Spin-coated silicon nanopaticles/graphene electrode as a binder-free anode for high-performance lithium-ion batteries. Nano Res. 5, 845–853 (2012). [Google Scholar]
- Kim H., Seo M., Park M. H. & Cho J. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 49, 2146–2149 (2010). [DOI] [PubMed] [Google Scholar]
- Yao Y. et al.. Highly conductive, mechanically robust, and electrochemical inactive TiC/C nanofiber scaffold for high-performance silicon anode batteries. ACS NANO 5, 8346–8351 (2011). [DOI] [PubMed] [Google Scholar]
- Ge M. Y., Rong J. P., Fang X. & Zhou C. W. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 12, 2318–2323 (2012). [DOI] [PubMed] [Google Scholar]
- Chen H. T. et al.. Bulk synthesis of crystalline and crystalline core/amorphous shell silicon nanowires and their application for energy storage. ACS NANO 5, 8383–8390 (2011). [DOI] [PubMed] [Google Scholar]
- McDowell M. T. & Cui Y. Single nanostructure electrochemical devices for studying electronic properties and structural changes in lithiated Si nanowires. Adv. Energy Mater. 1, 894–900 (2011). [Google Scholar]
- Li H., Wang Z. X., Chen L. Q. & Huang X. J. Research on advanced materials for Li-ion batteries. Adv. Mater. 21, 4593–4607 (2009). [Google Scholar]
- Golmon S., Maute K., Lee S. H. & Dunn M. L. Stress generation in silicon particles during lithium insertion. Appl. Phys. Lett. 97, 033111 (2010). [Google Scholar]
- Obrovac M. N. & Krause L. J. Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 154, A103–A108 (2007). [Google Scholar]
- Li J. C., Dozier A. K., Li Y. C., Yang F. Q. & Cheng Y. T. Crack pattern formation in thin film lithium-ion battery electrodes. J. Electrochem. Soc. 158, A689–A694 (2011). [Google Scholar]
- He Y., Yu X. Q., Wang Y. H., Li H. & Huang X. J. Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 23, 4938–4941 (2011). [DOI] [PubMed] [Google Scholar]
- Yu Y. et al.. Reversible storage of lithium in silver-coated three-dimensional macroporous silicon. Adv. Mater. 22, 2247–2250 (2010). [DOI] [PubMed] [Google Scholar]
- Nam S. H. et al.. Probing the lithium ion storage properties of positively and negatively carved silicon. Nano Lett. 11, 3656–3662 (2011). [DOI] [PubMed] [Google Scholar]
- Wu H. et al.. Engineering empty space between Si nanoparticles for lithium-ion battery anodes. Nano Lett. 12, 904–909 (2012). [DOI] [PubMed] [Google Scholar]
- Magasinski A. et al.. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 9, 353–358 (2010). [DOI] [PubMed] [Google Scholar]
- McDowell M. T. et al.. Studying the kinetics of crystalline silicon nanoparticle lithiation with in situ transmission electron microscopy. Adv. Mater. 24, 6034–6041 (2012). [DOI] [PubMed] [Google Scholar]
- Kim H. & Cho J. Superior lithium electroactive mesoporous Si@carbon core-shell nanowires for lithium battery anode material. Nano Lett. 8, 3688–3691 (2008). [DOI] [PubMed] [Google Scholar]
- Luo Y. S. et al.. Seed-assisted synthesis of highly ordered TiO2@α-Fe2O3 core/shell arrays on carbon textiles for lithium-ion battery applications. Energy Environ. Sci. 5, 6559–6566 (2012). [Google Scholar]
- Wang X. H. et al.. Nanostructured NiO electrode for high rate Li-ion batteries. J. Mater. Chem. 21, 3571–3573 (2011). [Google Scholar]
- Liu B. et al.. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 12, 3005–3011 (2012). [DOI] [PubMed] [Google Scholar]
- Armstrong G., Armstrong A. R., Bruce P. G., Reale P. & Scrosati B. TiO2(B) nanowires as an improved anode material for lithium-ion batteries containing LiFePO4 or LiNi0.5Mn1.5O4 cathodes and polymer electrolyte. Adv. Mater. 18, 2597–2600 (2006). [Google Scholar]
- Hassoun J., Lee K. S., Sun Y. K. & Scrosati B. An advanced lithium ion battery based on high performance electrode materials. J. Am. Chem. Soc. 133, 3139–3143 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary Video