Abstract
Endogenous cannabinoid signaling, mediated predominately by CB1 receptor activation, is involved in food intake control and body weight regulation. Despite advances in determining the role of the CB1 receptor in obesity, its involvement in the driven nature of eating pathologies has received little attention. The present study examined CB1 receptor alterations as a consequence of dietary-induced binge eating in female Sprague Dawley rats. Four control groups were used to control for calorie restriction and highly palatable food variables characterizing this behavioral model. All groups were kept on their respective feeding schedules for 6-weeks and were given a uniform 33% calorie restriction (~22 h food deprivation) prior to sacrifice. Our findings indicate regional CB1 mRNA and density were influenced by dietary conditions, but were not specific to the dietary-induced binge eating paradigm used. An increase of approximately 50% (compared with naive controls) in CB1 receptor mRNA levels in the nucleus of the solitary tract as measured by in situ hybridization was found in animals receiving continuous access to a highly palatable food (i.e., vegetable shortening with 10% sucrose). This group also had a significant increase in body weight and adiposity. An approximate 20% reduction in CB1 mRNA was observed in the cingulate cortex (area 1 and 2) in animals that were exposed to intermittent schedule of feeding, compared with groups that had ad libitum feeding schedules (i.e., continuous access and naive controls). Receptor density as measured by [3H] CP55,940 autoradiography, was reduced by approximately 30% in the nucleus accumbens shell region in groups receiving repeated access to the highly palatable food. Taken together, these findings indicate dietary conditions can differentially influence CB1 receptors in forebrain and hindbrain regions.
Keywords: Bulimia, Binge Eating Disorder, Cannabis, cnr1, food restriction, endocannabinoid
Introduction
The psychotropic and appetite stimulating effects of Cannabis are mediated by actions on the endogenous cannabinoid system [1,2]. Cannabinoid signaling is mediated through two types of membrane-bound G-protein receptors, CB1 and CB2, which share only 44% amino acid homology with one another [2-4]. Involved predominately in immune function, CB2 receptors are located on the spleen, thymus, and immune cells. In the central nervous system, CB2 receptors are principally found on microglia and increase expression under pathological conditions [5] . One exception is the caudal brainstem where CB2 receptors are located on a population of neurons in the dorsal motor nucleus of vagus and have a functional role in emesis [6]. CB1 receptors, on the other hand, are predominately distributed throughout the central, peripheral, and enteric nervous systems [7-10]. Several brain areas involved in the control of feeding and body weight have moderate to high densities of CB1 receptors and mRNA content [4,7-9,11,12]. Diet-induced obesity has been associated with alterations in both CB1 receptor expression and endogenous cannabinoid signaling [1,13,14]. Findings from several studies support the contention that endogenous cannabinoid overactivation results in obesity and associated metabolic impairment [1,14,15]. For instance, diet-induced obesity produced a down-regulation of the CB1 receptor in several brain regions [13,14,16,17], whereas prolonged treatment with a CB1 antagonist or disruptions in the CB1 receptor gene reduce food intake and adiposity, and prevents the development of diet-induced obesity [18-20]. As a result, normalization of the endogenous cannabinoid system by reducing CB1 signaling has generated considerable therapeutic interest for the treatment of obesity [21,22].
Understanding the physiological consequences of dietary conditions will help elucidate the pathophysiology of eating disorders and related pathologies [23-27]. Prolonged periods of calorie restriction, repeated failed dieting attempts, and body weight suppression are often antecedent to and/or involved in the maintenance of clinical eating pathologies [28-32]. In bulimia nervosa and binge eating disorder, bouts of overconsuming “risk” foods (typically high in sugars and/or fats) are considered to contribute to the self-sustaining nature of the pathophysiology [24,33,34]. Advances have been made in understanding the consequences of dietary influences on the neural and behavioral components of eating by utilizing rodent models of dietary-induced binge eating [33,35-39]. Indeed, a neurochemical mechanism for the motivated and driven nature of binge eating behavior has been supported by the observation of a persistence in accumbens dopamine signaling, opiate-like dependency, and differential feeding response to dopamine antagonists and GABA agonists in dietary-induced binge eating rodent models [33,39,40].
The dietary-induced binge eating model used in the present study incorporates several elements that parallel binge eating behaviors reported in clinical populations. The protocol includes “binge access” period causes an overeating pattern of intake that is produced by imposing intermittent food restriction with limited access to a highly palatable food (i.e., sweetened fat; vegetable shortening blended with 10% sucrose) [41,42]. The purpose of the present investigation was to determine whether such patterns of overeating result in alterations in the CB1 receptor mRNA and binding density in neural structures involved in body weight regulation and food intake [8,9]. Analyses were performed on frozen tissue collected from animals used in a previously published study measured mu-opioid receptor mRNA in the hindbrain and nodose ganglion [42].
Material and Methods
Animals
A total of thirty-nine adult female Sprague Dawley rats (Harlan Laboratories, Frederick, MD), with an initial weight range of 200-225g were individually housed in stainless steel wire mesh hanging cages and placed on a 12/12 h light dark schedule (lights off at 1230 h). All rats received ad libitum standard laboratory chow (Global Diet-2018, Harlan Teklad; 3.3 Kcal/g; fixed formula diet of 18% protein, 3.4% polyunsaturated fat, 1.3% monounsaturated fat, 0.9% saturated fat) unless otherwise noted. Water was available at all times during the experiments, but water intake was not measured. All the procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Feeding schedules and experimental groups
The five feeding protocols used in this experiment are described in Table 1. The highly palatable food used was “sweetened fat”, consisting of vegetable shortening, (Crisco®, a generous gift from J.M. Smucker, Co.; 33.3% monounsaturated fat, 25% saturated fat; 25% polyunsaturated fat, 12.5% trans fat) blended with 10% sucrose (8.6 Kcal/g). All animals received a 24 h pre-exposure to the sweetened fat 3 days before being allocated into the groups. Animals were distributed into five groups to reflect no initial differences in body weight or sweetened fat preferences between groups. Groups were designated relative to two independent variables, access to sweetened fat and intermittent calorie restriction. The groups included Continuous Access, Binge Access, Chow Restricted, Scheduled Access, and Naive groups (n=8 for all groups, except Chow Restricted n=7). The Binge Access and Chow Restricted group were restricted at the beginning of the dark cycle to 33% of the previous day's chow calorie intake on Days 2 and 5 of each week. On subsequent days (days 3 and 6) 2 h into the dark cycle (1430 h) (total deprivation time ~ 22 h), the Binge Access group was given access to both standard chow and sweetened fat, whereas the Chow Restricted groups were re-fed with chow alone. The Binge Access group had access to the jars of sweetened fat only for the first 2 h of each re-feeding period. In this fashion, the Binge Access group was exposed to a repeated cycle that consisted of three no restriction days (days 1, 4, and 7), two weekly episodes of calorie restriction (days 2 and 5), and two weekly episodes of scheduled re-feeding starting with a 2 h access to an optional sweetened fat (days 3 and 6). This schedule was chosen to provide the animals with combination of intermittent days of calorie restriction, palatable food access and ad libitum standard chow access within a 7-day period. A fourth group, Scheduled Access, received the 2 h access to the sweetened fat at the same time as the Binge Access groups, but did not undergo any repeated bouts of chow restriction. The Naive group received ad libitum access to the standard chow without any repeated bouts of calorie restriction or access to the sweetened fat [42].
Table 1.
Experimental groups and 7-day dietary schedules.
| Groups | Calorie Restriction (Days 2, 5) | Sweetened Fat Access (Days 3, 6) |
|---|---|---|
| Binge Access | Intermittent Daily 33% | 2 hour |
| Continuous Access | No Restriction | Ad lib access |
| Chow Restricted | Intermittent Daily 33% | No Access |
| Scheduled Access | No Restriction | 2 hour |
| Naive | No Restriction | No Access |
Binge Access, Chow Restricted, and Scheduled Access groups had ad libitum access to standard chow (Days 1, 4, and 7) Naive group had ad libitum standard chow. All groups were maintained on these schedules for 6 weeks.
Palatable food and chow intake during the feeding schedules
Animals were maintained on these feeding schedules for 6 weeks. Food intake and spillage were recorded to the nearest 0.1 g and measured separately for the 2 h feeding period and the 20 h following the re-feeding period. The 2 h and 20 h intakes were combined to represent the total intakes on days 3 and 6 (i.e., re-feeding days or “binge days”) throughout the experiment. The 24 h caloric intake was also measured on days 1 and 4 (days before the calorie restriction for Binge Access and Chow Restricted groups). Intakes for Day 7 were not recorded. A more detailed description of the feeding conditions can be found elsewhere [42].
Nodose ganglion and brain removal
After a total of 6 weeks on the feeding schedule, all groups were food-restricted beginning at the onset of the dark cycle to 33% of the previous day's caloric intake. The uniform 33% calorie restriction on the day prior to sacrifice was employed to eliminate the potential confounding effects of recent food intake, which would have varied amongst groups. On the following day, 2 h into the dark cycle at the time of expected re-feeding for Binge Access, Chow-Restricted, and Scheduled Access groups, rats were taken into a separate room and anesthetized with 1 ml/kg of a 4:3 mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml). The nodose ganglion of the vagus (left side) was removed, immediately immersed in –40°C isopentane (2-methylbutane), and stored at –80°C. The animals were then decapitated and brains were removed and processed in an identical manner to the excised nodose tissue.
Tissue sectioning
Brains were knife cut at the midbrain (coronal plane) and sectioned at 14 μm in the coronal plane on a cryostat. For the forebrain, sectioning commenced at the forceps minor corpus callosum (12.0 mm from interaural line) in an anterior-posterior approach continuing through the striatum (9.7 mm from interaural line). Sectioning of the hypothalamus commenced at the level of the emergence of the hippocampus (CA3) continuing through the fimbria (7.3 mm from the interaural line) to the posterior nucleus of the hypothalamus (5.2 mm from the interaural line). For the hindbrain, posterior-anterior sectioning commenced at the level of the obex (-5.6 mm from the interaural line) continuing rostrally to the gelatinous nucleus and the caudal aspect of the medial vestibular nucleus on the dorsal boundary (-3.6 mm from the interaural line) [43]. Nodose ganglion were embedded in brain paste (bovine) and sectioned at 14 μm in the longitudinal plane. Three or four sections were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and matched anatomically by group for each hybridization or autoradiography assay.
Riboprobes and in situ hybridization
Plasmids (bluescript SK+) containing the rat CB1 receptor construct (112-948 bp; accession number NM_012784; generous gift of M. Herkenham, NIMH and M. Abood, Temple University) were linearized by recommended restricted enzymes. Antisense and sense riboprobes for each were labeled with [35S]UTP (Specific Activity; 1200 Ci/mmol, Amersham/GE Healthcare; Piscataway NJ) utilizing in vitro transcription systems with appropriate polymerases according to the manufacturer's protocols (Promega; Madison, WI). The labeled probe was purified with mini Quick Spin RNA Columns (Roche Applied Science, Indianapolis, IN) to yield a specific activity of 5 × 108 cpm/μg. In situ hybridization was performed as described previously [42] and dehydrated slides were exposed to BioMax XAR Film (Eastman Kodak Company, New York, NY) for 3 to 5 days. Regions examined included dorsolateral caudate putamen, cingulate cortex (area 1 and 2), ventromedial hypothalamus (VMH), NTS and nodose ganglion.
Autoradiography
Sections were pre-incubated for 30 min at room temperature in 50 mM Tris HCl (pH 7.4) containing 5% bovine serum albumin (BSA). Sections were next incubated for 2 h at room temperature in the same buffer with the addition of 5 nM [3H] CP55,940 (specific activity 100 Ci/mmol, Perkin Elmer, USA). Nonspecific binding was determined in adjacent sections by incubation with 5 nM [3H] CP55,940 in the presence of 10 μM CP55,940( Tocris, Ellisville, MO). After incubation, sections were washed for 1h at 4°C in 50 mM Tris HCl (pH 7.4.) containing 1% BSA. An additional wash was carried out for 3 h in the same buffer at 4°C. A 5 min (at 4°C) final wash was performed in 50 mM Tris HCl (pH 7.4). Sections were then dipped briefly in ice cold distilled water and dried. Tissue was then juxtaposed to [H3]-Hyperfilm (Amersham/GE Healthcare; Piscataway NJ) for 21 days. Tissue was quantitated by densitometry methodology using the values from the [H3] microscales (Amersham/GE Healthcare; Piscataway NJ) exposed with the film. Regions examined included cingulate cortex (area 1 and 2), shell region of the nucleus accumbens, dorsolateral caudate putamen, entopenduncular nucleus, CA1 region of the hippocampus, and dorsal and ventral hypothalamus.
Quantitative analysis
In situ and autoradiographic images were first scanned using an Epson Professional Scanner and then quantified with Scion Image software that utilized 14C-or 3H microscales (Amersham/GE Healthcare; Piscataway NJ) as standards. Images were quantitated using the Scion Image software (Version 4.0.3.2, NIH, Bethesda, MD). Data for each animal were means of the product of hybridization area X optical density (OD) and this value was subtracted from the film background OD. There was no measurable OD of signal greater than film background in either of the sections hybridized with the labeled sense probe or unlabeled CP55,940. Data for each animal were normalized to those of Naive controls as 100% and all of the data were expressed as means ± SE.
Statistical analyses
In situ hybridization and autoradiography measurements were analyzed separately for each brain region by one-way ANOVA. To determine if body weight had an effect on the outcome of mRNA and receptor density, brain region measurements were also analyzed by ANCOVA with body weight as a covariate. Post hoc comparisons were made when appropriate with a Newman-Keuls test, unless otherwise noted. All statistical analyses were performed with Statistica software (version 7.1, StatSoft Inc., Tulsa OK), and significance was set at α = 0.05.
Results
Food intake and body weight during the 6-week feeding schedule
Intakes for all groups were previously reported [42]. Briefly, averaged 6-week total Kcal intake during the 2h re-feeding period, on days 3 and 6, for the Binge Access (57.6 ± 4 Kcal), Continuous Access (19.4 ± 2 Kcal), Chow Restricted (29.2 ± 2 Kcal), Scheduled Access (50.8 ± 5 Kcal) and Naive (11.6 ± 2 Kcal) groups. During the 2h feeding the Binge Access group consumed the most calories and the Scheduled Access group consumed the most sweetened fat. Compensatory feeding behaviors were demonstrated over other feeding intervals with Continuous Access animals consuming the most Kcals over the 6-week period. The percent body weight gains over the 6-weeks for the Binge Access (6 ± 1%), Continuous Access (14 ± 2%), Chow Restricted (6 ± 1%), Schedule Access (8 ± 2%) and Naive (9 ± 1%) groups. In addition to the highest weight gain, the Continuous Access group had significantly more subcutaneous and retroperitoneal white adipose tissue, increased plasma leptin levels, and decreased plasma total ghrelin level compared with all other groups [42].
In situ hybridization for the CB1 receptor
Of the five regions examined, only three regions demonstrated significant differences among the groups. These were the nodose ganglion [F (4, 20) = 2.9, P < 0.05], NTS [F(4, 29) = 3.2, P < 0.05], and the cingulate cortex [F (4, 31) = 4.8, P < 0.005]. For the nodose ganglion there was a trend for Continuous Access and Schedule Access groups to have lower CB1 mRNA levels than other groups, however, post-hoc testing did not reveal any differences between individual groups. For the nucleus of the solitary tract, the Continuous Access group had higher mRNA levels compared with Naive and Binge Access groups (p < 0.05 for both), see Figure 1. When performing an ANCOVA using body weight as a covariate, however, the difference among the groups did not quite reach significance [F (4, 28) = 2.7, p = 0.06]. For the cingulate cortex, mRNA levels in the Binge Access, Chow Restricted, and Schedule Access groups were all significantly less than the Continuous Access group (p < 0.05 for all). Planned comparisons revealed that all three groups exposed to intermittent feeding schedules (Binge Access, Chow Restricted, Schedule Access groups) were significantly different (P < 0.001) from groups that had ad libitum feeding schedule (Continuous Access and Naive groups), see Figure 2.
Figure 1. In situ hybridization of CB1 receptors in the nucleus of the solitary tract (NTS) following the feeding schedules.
A: Representative pseudocolor images from in situ hybridization for CB1 receptor. B: Group differences were found in the NTS (P<0.05). The Continuous Access group differed from Binge Access and Naive (*, p<0.05 for both). Dotted line represents value for the Naive group for comparison. There were no differences between groups when body weight was used as a covariate in an ANCOVA, suggesting that body weight differences influenced CB1 receptor group differences.
Figure 2. In situ hybridization of CB1 receptors in the cingulate cortex (Cg1 and Cg2) following the feeding schedules.
A: Representative pseudocolor images from in situ hybridization for CB1 receptor. B: Group differences were found in the cingulate cortex (P<0.005). The Continuous Access group differed from Binge Access, Chow Restricted, and Scheduled Access groups (*, p<0.05 for all). Dotted line represents value for the Naive group for comparison. Planned comparisons revealed there was a significant decrease in the CB1 receptor mRNA in groups that received intermittent schedules compared with those with ad libitum schedules, regardless of food palatability.
Autoradiography for the CB receptor
Of the seven regions examined, only one region demonstrated significant differences among the groups. This region was the medial region of the nucleus accumbens [F(4, 21)=5.3, P<0.005]. Post-hoc testing revealed that Binge Access, Continuous Access, and Scheduled Access groups had lower binding density than the Chow Restricted group (p<0.05 for all), see Figure 3. Differences between the Chow-Restricted and Naïve groups only approached significance (P=0.06). Vaginal cytology was taken as previously described [42] and there were no appreciable influences on CB1 receptor mRNA levels and binding densities.
Figure 3. Autoradiography of CB 1 receptor in the nucleus accumbens medial shell region.
A: Representative images from [H3] CP55940 for CB receptor densities. B: Group differences were found in the nucleus accumbens (P<0.05). Post-hoc testing revealed the Binge Access, Continuous, Scheduled Access groups demonstrated decreased receptor densities relative to the Chow Restricted group (*, p<0.05, for all).Dotted line represents value for the Naive group for comparison.
Discussion
This study sought to examine whether dietary conditions that promoted binge-like eating in female rats result in alterations in CB1 receptor mRNA and binding density levels in feeding-related neural regions. Based on the reported distribution of CB1 receptor mRNA [7,44-48], in situ hybridization was used to measure CB1 receptor mRNA in the dorsolateral caudate putamen, cingulate cortex (area 1 and 2), ventromedial nucleus of the hypothalamus, NTS and nodose ganglion. Also, a synthetic cannabinoid radioligand, [H3] CP55940, which has a relatively equal affinity for CB1 compared with CB2 receptors [7,13]. Since CB2 receptor have negligible distribution throughout forebrain regions under non-pathological conditions [5], we interpreted the CP55,940 autoradiography findings as representing CB1 receptor binding densities. A similar interpretation with CP 55450 has been used by others [13,17,49]. However, because there exists a population of CB2 receptor located in the dorsal vagal complex, we did not examine binding of this ligand in hindbrain regions [6,50-52]. Although our results indicate no alterations unique to the Binge Access group in CB1 receptor expression or density, we did observe differences between groups in several brains regions as a consequence of highly palatable food exposure or of scheduled feeding.
In the nucleus of the solitary tract, a hindbrain neural structure critical to the integration and control of food intake, we found increased CB1 receptor gene expression in animals with continuous access to the highly palatable sweetened fat diet [53,54]. These animals also displayed an obese phenotype including higher body weights, elevated plasma leptin, reduced plasma ghrelin, and more adipose tissue [42]. Correction for this body weight difference in the continuous access group accounted for the group difference in CB1 receptor expression and suggests that weight gain explained this increase in CB1receptor expression in the NTS. While involvement of endogenous cannabinoid signaling in the NTS has been previously reported [55-57], the finding that CB1 receptor gene expression is elevated as a consequence of obesity is novel. A previously published study by our lab using the same animals demonstrated a decrease in mu-opioid receptor mRNA in the NTS of the Continuous Access and Binge Access groups [42]. Additional work is needed to determine whether mu-opioid and CB1 receptors are co-localized on gastric responsive NTS neurons and how these receptor populations interact in response to dietary challenges.
Gastrointestinal sensory input is conveyed to the NTS predominately by vagal afferent and afferent responsivity is often measured by gene expression in the soma region or nodose ganglion [58-61]. Examination of the nodose ganglia of obesity-prone (OP) Sprague Dawley rats exhibited an increase in CB1 receptor gene expression after 8-weeks on a high-fat diet (compared with week 1). The OP rats also had higher CB1 levels compared with obesity-resistant rats fed a low fat diet [62]. Acute food deprivation (≥ 12 h) has been found to increase CB1 receptor mRNA in the nodose ganglion, while re-feeding or CCK administration reduce expression [63]. Even though differences in nodose CB1 receptor expression among groups were also observed in the present study, there was only a trend supporting decreased CB1 receptor mRNA in the Continuous Access group. Taken together with the increase in CB1 mRNA levels observed in the NTS, our finding suggest that independent mechanisms are likely involved in vagal CB1 receptors regulation. Future studies are needed to delineate the influence of weight gain, dietary fat content, and obesity status on gastrointestinal integration and CB1 receptor alterations.
Another region that demonstrated a differential pattern of CB1 receptor expression induced by the dietary conditions employed in the present study was the cingulate cortex (Cg1 and Cg2). The cingulate cortex is an integral neural structure in the limbic system with connections to the nucleus accumbens and ventral tegmental area [64,65], and appears to have a role in cost-benefit effort needed to obtain rewarding stimuli [66-69]. We found that regardless of diet palatability, groups with intermittent access to food demonstrated decreased CB1 receptor expression in the cingulate cortex. While the exact role of CB1 receptor function in the cingulate cortex is not known, another study by Timofeeva and colleagues reported CB1 receptor mRNA alterations in cingulate cortex as a result of diet manipulations[14]. Rats in that study were given ad libitum standard chow with continuous access to shortcake biscuits and pork spread (Palatable High Energy Diet; PHED) for 13 weeks. Compared with rats fed ad libitum standard chow, the PHED group gained ~25% more body fat and had decreased CB1 receptor expression in the cingulate cortex and ventromedial nucleus of the hypothalamus [14]. In the present study, CB1 receptor mRNA levels in the cingulate cortex did not differ between the Continuous Access and Naive controls. We also did not observe any group differences in the ventromedial nucleus of the hypothalamus. Several differences in experimental design may account for the discrepancy in findings between the present study and those of Timofeeva and colleagues. One difference was study length and maintenance on the diet following a significant increase in body weight. In the present study there was a significant difference in body weight at week 5 and animals were sacrificed a week later (total study length 6 weeks), whereas in Timofeeva and colleagues design a significant body weight difference was found at 3 weeks and animals were sacrificed 10 weeks later (total study length 13 weeks). Along these lines, regional brain differences in CB receptor density were also apparent following 3-week compared with 20-week exposure to a high fat diet in mice [17]. A second difference between our study and Timofeeva and colleagues was the percentage of unsaturated fatty acid content of the highly palatable food, since diets high in polyunsaturated fatty acid increase brain levels of endogenous cannabinoids [70,71]. Timofeeva and colleagues also noted further decreases in CB1 receptor mRNA in the cingulate cortex in the PHED group following a period of acute food restriction (12 h) compare with the PHED group ad libitum fed prior to sacrifice. No differential CB1 expression in response to food restriction was noted, however, in non-obese, chow-fed animals [14]. These data suggest that obesity or exposure to a highly palatable diet can alter the sensitivity of CB1 receptor expression in cingulate cortex. Hence, differences in dietary fat composition, time course of weight gain, and the longer period of acute deprivation (~22 h) prior to sacrifice, could have influenced CB1 receptor expression in the present study.
Another finding of the present study was the reduction in CB 1 receptor density in the nucleus accumbens medial shell region in animals with access to the highly palatable food. Reductions in CB receptor density were only significant between groups with highly palatable food access (Binge Access, Continuous Access, and Scheduled Access) and the Chow-Restricted group. Downregulation of CB 1 receptors in the nucleus accumbens has been previously observed in rats fed a highly palatable chow (33% ground chow, 33% Nestle condensed milk, 7% sucrose, and 27% water) for 10 weeks [13]. Similar to our findings, the study noted no differences CB 1 receptor binding differences in hypothalamic regions. Our findings also suggest differential cannabinoid regulation in different limbic structures as function of the feeding schedules. That is, the Continuous Access group had lower accumbens CB1 receptor binding and higher cingulate cortex CB1 receptor mRNA levels compared with the Chow-Restricted group. Interestingly, the Binge Access and Scheduled Access groups had lower CB1 receptor binding and mRNA levels in the accumbens and cingulate cortex, respectively. Considering the role the cingulate cortex and medial shell accumbens have in motivation and salience [67,72], this differences in cannabinoid regulation between the two regions is likely a consequence of the dietary schedules and availability highly palatable food rather than body weight gain or associated metabolic alterations.
Long-term treatment with a CB1 receptor antagonist/ inverse agonist (SR141617A; rimonabant) led to effective sustained weight loss in rodent studies and human clinical trials, but a large number (26%) of the treated clinical population reported a profile of psychiatric side effects (including depression, anxiety, and agitation) [2,21]. Rodent models offer the advantage of assessing various aspects of eating behavior and diet without the constraints of the complex cognitive attributes that often accompany multifactorial human eating pathologies [33,73]. Despite the absence of findings uniquely specific to our rodent model of dietary-induced binge eating, we uncovered several changes in feeding related brain regions resulting from repeated exposure to a highly palatable food or scheduled feeding. These alterations underscore the potential role of CB1 receptor signaling in sustaining motivated feeding behavior in the context of highly palatable food access and self-imposed dietary constraints. Understanding which brain regions are susceptible to dietary manipulations offers the possibility of developing more targeted cannabinoid pharmacotherapy for the treatment of obesity and eating disorders.
Research Highlights.
 Endogenous cannabinoids are involved in feeding and highly palatable food intake.
Endogenous cannabinoids are involved in feeding and highly palatable food intake.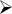 Increases in CB1 receptor mRNA levels in the nucleus of the solitary tract were demonstrated in rats with continuous access to a highly palatable sweet-fat food for 6 weeks. This increase was related to weight gain.
Increases in CB1 receptor mRNA levels in the nucleus of the solitary tract were demonstrated in rats with continuous access to a highly palatable sweet-fat food for 6 weeks. This increase was related to weight gain.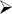 Reductions in CB1 receptor mRNA levels in the cingulate cortex were demonstrated in rats with intermittent feeding schedules.
Reductions in CB1 receptor mRNA levels in the cingulate cortex were demonstrated in rats with intermittent feeding schedules. CB receptor binding densities were reduced in nucleus accumbens shell in rats with access to a highly palatable sweet-fat food.
CB receptor binding densities were reduced in nucleus accumbens shell in rats with access to a highly palatable sweet-fat food.
Acknowledgements
This work was supported by Klarman Family Foundation Grant (A.S.G) and National Institutes of Health Grants DK-078484(N.T.B.) and DK-19302 (T.H.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carr TP, Jesch ED, Brown AW. Endocannabinoids, metabolic regulation, and the role of diet. Nutr Res. 2008;28:641–50. doi: 10.1016/j.nutres.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Viveros MP, de Fonseca FR, Bermudez-Silva FJ, McPartland JM. Critical role of the endocannabinoid system in the regulation of food intake and energy metabolism, with phylogenetic, developmental, and pathophysiological implications. Endocr Metab Immune Disord Drug Targets. 2008;8:220–30. doi: 10.2174/187153008785700082. [DOI] [PubMed] [Google Scholar]
- 3.Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Abood ME, Martin BR. Molecular neurobiology of the cannabinoid receptor. Int Rev Neurobiol. 1996;39:197–221. doi: 10.1016/s0074-7742(08)60667-4. [DOI] [PubMed] [Google Scholar]
- 5.Roche M, Finn DP. Brain CB2 receptors: Implications for Neuropsychiatric Disorders. Pharmaceuticals. 2010;3:2517–53. doi: 10.3390/ph3082517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 7.Abood ME. Molecular biology of cannabinoid receptors. Handb Exp Pharmacol. 2005:81–115. doi: 10.1007/3-540-26573-2_3. [DOI] [PubMed] [Google Scholar]
- 8.Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–74. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 9.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuece B, Sibaev A, Broedl UC, Marsicano G, Goke B, Lutz B, et al. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007;19:744–53. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin CR, Abood ME. Developmental expression of cannabinoid receptor mRNA. Brain Res Dev Brain Res. 1993;76:75–8. doi: 10.1016/0165-3806(93)90124-s. [DOI] [PubMed] [Google Scholar]
- 12.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–8. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- 14.Timofeeva E, Baraboi ED, Poulin AM, Richard D. Palatable high-energy diet decreases the expression of cannabinoid type 1 receptor messenger RNA in specific brain regions in the rat. J Neuroendocrinol. 2009;21:982–92. doi: 10.1111/j.1365-2826.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 15.Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 95:375–82. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Harrold JA, Williams G. The cannabinoid system: a role in both the homeostatic and hedonic control of eating? Br J Nutr. 2003;90:729–34. doi: 10.1079/bjn2003942. [DOI] [PubMed] [Google Scholar]
- 17.South T, Huang XF. Temporal and site-specific brain alterations in CB1 receptor binding in high fat diet-induced obesity in C57Bl/6 mice. J Neuroendocrinol. 2008;20:1288–94. doi: 10.1111/j.1365-2826.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 18.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–31. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–8. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–32. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- 21.Arias Horcajadas F. Cannabinoids in eating disorders and obesity. Mol Neurobiol. 2007;36:113–28. doi: 10.1007/s12035-007-0018-x. [DOI] [PubMed] [Google Scholar]
- 22.Smith RA, Fathi Z. Recent advances in the research and development of CB1 antagonists. IDrugs. 2005;8:53–66. [PubMed] [Google Scholar]
- 23.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–91. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 24.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–64. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 25.Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. 2008;29:1588–95. doi: 10.1016/j.peptides.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsen GC, Berman Y, Carr KD. Curve-shift analysis of self-stimulation in food-restricted rats: relationship between daily meal, plasma corticosterone and reward sensitization. Brain Res. 1995;695:186–94. doi: 10.1016/0006-8993(95)00764-h. [DOI] [PubMed] [Google Scholar]
- 27.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–20. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. Task Force on DSM-IV. [Google Scholar]
- 29.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe MR, Thomas JG, Safer DL, Butryn ML. The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. Int J Eat Disord. 2007;40:640–4. doi: 10.1002/eat.20405. [DOI] [PubMed] [Google Scholar]
- 31.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: a study of former prisoners of war. J Abnorm Psychol. 1994;103:409–11. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- 32.Elmore DK, de Castro JM. Meal patterns of normal, untreated bulimia nervosa and recovered bulimic women. Physiol Behav. 1991;49:99–105. doi: 10.1016/0031-9384(91)90238-j. [DOI] [PubMed] [Google Scholar]
- 33.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broft AI, Berner LA, Martinez D, Walsh BT. Bulimia nervosa and evidence for striatal dopamine dysregulation: A conceptual review. Physiol Behav. doi: 10.1016/j.physbeh.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–13. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 37.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 38.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 39.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 40.Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–48. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- 41.Bello NT, Guarda AS, Terrillion CE, Redgrave GW, Coughlin JW, Moran TH. Repeated binge access to a palatable food alters feeding behavior, hormone profile, and hindbrain c-Fos responses to a test meal in adult male rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R622–31. doi: 10.1152/ajpregu.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bello NT, Patinkin ZW, Moran TH. Opioidergic consequences of dietary-induced binge eating. Physiol Behav. doi: 10.1016/j.physbeh.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- 44.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–31. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 45.Jelsing J, Larsen PJ, Vrang N. Identification of cannabinoid type 1 receptor expressing cocaine amphetamine-regulated transcript neurons in the rat hypothalamus and brainstem using in situ hybridization and immunohistochemistry. Neuroscience. 2008;154:641–52. doi: 10.1016/j.neuroscience.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 47.Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res Mol Brain Res. 1997;46:100–8. doi: 10.1016/s0169-328x(96)00277-x. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–50. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 49.Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience. 1994;63:637–52. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 50.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–68. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–30. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–46. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci. 2006;361:1275–80. doi: 10.1098/rstb.2006.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33(Suppl 1):S11–5. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 55.Himmi T, Perrin J, El Ouazzani T, Orsini JC. Neuronal responses to cannabinoid receptor ligands in the solitary tract nucleus. Eur J Pharmacol. 1998;359:49–54. doi: 10.1016/s0014-2999(98)00630-x. [DOI] [PubMed] [Google Scholar]
- 56.Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–74. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- 57.Accorsi-Mendonca D, Almado CE, Dagostin AL, Machado BH, Leao RM. Inhibition of spontaneous neurotransmission in the nucleus of solitary tract of the rat by the cannabinoid agonist WIN 55212-2 is not via CB1 or CB2 receptors. Brain Res. 2008;1200:1–9. doi: 10.1016/j.brainres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 59.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 60.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–9. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav. 2001;72:123–8. doi: 10.1016/s0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 62.Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–15. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 65.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–78. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 66.Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–41. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–9. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schweimer J, Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 2005;12:334–42. doi: 10.1101/lm.90605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci. 2005;119:1687–92. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- 70.Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A. 2001;98:6402–6. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crozier Willi G, Berger A, Di Marzo V, Bisogno T, De Petrocellis L, Fride E, et al. Lipids in neural function: modulation of behavior by oral administration of endocannabinoids found in foods. Nestle Nutr Workshop Ser Clin Perform Programme. 2001;5:169–84. doi: 10.1159/000061850. discussion 85-7. [DOI] [PubMed] [Google Scholar]
- 72.de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 73.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]





