Abstract
This report describes the development of elastomeric capture microparticles (ECμPs) and their use with acoustophoretic separation to perform microparticle assays via flow cytometry. We have developed simple methods to form ECμPsby crosslinking droplets of common commercially available silicone precursors in suspension followed by surface functionalization with biomolecular recognition reagents. The ECμPs are compressible particles that exhibit negative acoustic contrast in ultrasound when suspended in aqueous media, blood serum or diluted blood. In this study, these particles have been functionalized with antibodies to bind prostate specific antigen and immunoglobulin (IgG). Specific separation of the ECμPs from blood cells is achieved by flowing them through a microfluidic acoustophoretic device that uses an ultrasonic standing wave to align the blood cells, which exhibit positive acoustic contrast, at a node in the acoustic pressure distribution while aligning the negative acoustic contrast ECμPs at the antinodes. Laminar flow of the separated particles to downstream collection ports allows for collection of the separated negative contrast (ECμPs) and positive contrast particles (cells). Separated ECμPs were analyzed via flow cytometry to demonstrate nanomolar detection for prostate specific antigen in aqueous buffer and picomolar detection for IgG in plasma and diluted blood samples. This approach has potential applications in the development of rapid assays that detect the presence of low concentrations of biomarkers in a number of biological sample types.
INTRODUCTION
Acoustic microfluidic systems are of significant utility in the analysis and separation of a number of biological samples, including blood.1–4 Whole blood comprises 45–50% hematocrit (blood cell volume) and a plethora of serum proteins. Many serum proteins are biomarkers for a number of pathologies, including cancers, inflammatory responses, and infectious diseases.5–9 Sensitive and rapid quantification of such biomarkers is very useful for early diagnoses and for monitoring the therapeutic success of many clinical treatments.10 To detect and quantify low concentrations of serum biomarkers, it is often necessary to remove blood cells prior to analysis.11 This is most commonly performed via centrifugation,12,13 which is time consuming, labor intensive, and requires milliliters of blood. Once the serum protein containing supernatant is obtained, immunoassays can be performed to detect and quantify low concentrations of the desired biomarker present in the serum,14,15. Other more specific protein purification and enrichment methods such as: 2-D gel electrophoresis, chromatography, precipitation or filtration can also be performed prior to analysis, for example using mass spectrometry.16–19
Acoustic standing waves offer an alternative to centrifugation for blood cell separation. Initial work on this approach concentrated blood cells, which generally exhibit positive acoustic contrast, to nodes in a standing wave where they aggregated and sedimented out of solution to leave clarified plasma.20 Based on similar acoustic principles, many acoustophoretic flow through systems have been developed by Laurell et al. to separate blood cells from serum, lipids, and platelets.21–26 In general this approach uses flow channels in a rigid substrate to which a acoustic driver (e.g., a PZT crystal) is attached to generate a standing acoustic wave across the channel. Drive frequencies are chosen to match the dimensions of the channel such that the wavelength of the standing wave has fractional harmonics across the channel (e.g., 1/2λ, 3/2λ, etc.), which creates acoustic nodes and antinodes across the channel. Cells are concentrated to the nodes and separated from the plasma by collecting the focused cells downstream in microfluidic channels. As described below there is a significant dependence of particle size to the magnitude of the force imparted on a particle by the standing wave, which allows for size dependent acoustophoretic fractionation of components such as platelets.25 Of interest here is the observation that particles exhibiting negative contrast, such as lipids, can be separated by collecting them as they are driven to the antinode.21,22,23
The primary acoustic force on particles in an acoustic standing wave field can be calculated from the following equations.22,23,27
| (1) |
| (2) |
The magnitude of the primary acoustic force is directly proportional to the volume of the particles(Vp), the pressure amplitude of the field(P2) the applied frequency (1/λ), and the acoustic contrast factor ϕ(β, ρ) The value of the acoustic contrast factor depends on the density of the particles (ρp) and the suspension media (ρo) and the compressibility of the particles (βp) and the suspension media (βo). If ϕ(β, ρ) has a positive value then the acoustic field will exert a time-averaged force moving particles to the acoustic pressure node of a standing wave (positive contrast particles). However, if ϕ(β, ρ) has a negative value then the acoustic field will exert a time-averaged force moving particles to acoustic pressure antinodes in a standing wave (negative contrast particles). A more detailed description of the acoustic forces exerted on particles under an acoustic standing wave field can be found in Bruus et al.28
In this report we demonstrate facile synthesis of elastomeric particles from a common water insoluble elastomer, crosslinked polydimethylsiloxane (Dow Corning Sylgard 184), which we hypothesized would (i) exhibit negative acoustic contrast, and thus (ii) allow their continuous separation from blood cells in an acoustic microfluidic chip. We introduce the concept of negative contrast elastomeric capture microparticles (ECμPs) and demonstrate the use of ECμPs in model antigen and antibody capture assays conducted in buffer, plasma and diluted whole blood in an acoustic sample preparation chip. As ECμPs are separated continuously from blood cells they are collected into fractions, which are then analyzed using flow cytometry.29 This continuous separation of ECμPs via acoustophoresis has the potential to greatly simplify immunoassays by obviating the need for centrifugation and lysis steps that are normally used to remove the background generating blood cells that are present at 5×109 cells per mL. Finally, simple and rapid separations, such as acoustophoretic ECμPs displaying high affinity antibodies, have the potential to improve the sensitivity of detection for biomarkers by reducing the number of sample preparation steps that can lead to non-specific loss of biomarkers of interest. 30 This analysis could be performed directly on ECμPs (e.g., through particle based immunoassays and flow cytometric detections) or through other subsequent analysis methods (e.g., mass spectrometry) on separated ECμPs.
METHODS AND MATERIALS
Acoustic Focusing and Separation
Samples were flowed (45 μL/min) through the acoustic sample preparation chip (see supplementary information (SI) for details of fabrication of the chip) using a microsyringe pump (Nexus 3000, Chemyx Inc. Stafford, TX). To focus and separate particles and blood cells, the PZT was actuated using a waveform generator (33250A, Agilent, Santa Clara, CA) at a frequency of 2.91 MHz at 10 volts peak-to-peak. To monitor and image the focusing and separation of particles and blood cells, commercially available Nile Red (NR)-polystyrene particles (PS) (Spherotech Inc., Lake Forest, IL) were used, and Nile Red dye (Sigma-Aldrich, St. Louis, MO) was used to label elastomeric particles and blood cells; this was accomplished by incubation in an aqueous NileRed solution (100 μM) in 1 mL of phosphate buffered saline (1X PBS with 7.4 pH: 10 mM Na2HPO4; 1 mM KH2PO4; 138 mM NaCl; 3 mM KCl) for 30 minutes followed by centrifugal washing (PBS) to remove unbound dye. Focusing and separation of particles (and blood cells) was monitored and imaged using an epifluorescence microscope (Zeiss Axio Imager) and a Luca S EMCCD camera (Andor Technology, Belfast N., Ireland) with Andor imaging software (Andor-Solis). This software was used to perform intensity analysis of line scans across the width of the microchannel. The average of 17 scans from bottom to top was used to generate fluorescence histograms using PRISM® software (version 5.0b, GraphPad).
Synthesis of Elastomeric Particles
Sylgard 184 (1 g, 10:2 ratio of PDMS prepolymer to crosslinking agent) (Dow Corning Corp., Midland, MI) was emulsified in 10 mL of ultrapure water (18.2 MΩ •cm @ 25 °C; Synergy®, EMD Millipore), using a homogenizer (Power Gen 125, Fisher Scientific) set at 6K RPM for ~ 60 seconds. The droplets were cured at 100 °C for ~ 1 hour to form crosslinked elastomeric particles. Elastomeric particles were imaged using bright field microscopy (BH-2, Olympus) and a 4300 cool pix camera (Nikon). The scale bar was calibrated based on standardized monodisperse particles (mean = 30.1 μm; standard deviation = ± 2.1 μm; and a coefficient of variation = 6.6%) (Thermo Scientific). A Coulter counter (Z2 Coulter Particle Count and Size Analyzer, Becton Dickinson) was used to determine the concentration and the size distribution of the polydisperse elastomeric particles.
Biofunctionalization of Polydisperse Elastomeric Particles
Elastomeric particles (2.5 × 107) where incubated in 1 μM avidin (Molecular Probes, Eugene, Oregon) in PBS for 30 minutes with continuous rocking at room temperature. They were then centrifugally (2900 g for 5 minutes) washed and resuspended in washing/blocking buffer (PBS with 0.1 % BSA). A biotinylated mouse anti-human prostate specific antigen (PSA) monoclonal antibody (capture antibody; HyTest Ltd., Turku, Finland; catalog # 4P33B Mab8A6; lot # 10/11-P33B-8A6) was added to make a 6 nM solution; the solution was incubated for 30 minutes with rocking at room temperature. The resulting ECμPs were centrifugally washed and resuspended in the washing/blocking buffer.
Separation Efficiency Measurements
1.5 × 105 ECμPs were fluorescently labeled with a goat anti-mouse antibody (PE) and mixed with 0.1% porcine whole blood in 200 μL of total volume (diluted in PBS with 0.1% BSA). The initial fraction of ligand-bound ECμPs to porcine blood cells was determined based on flow cytometry gating ((fluorescence (585±20 nm) vs. forward side scatter). The mixture was then flowed (45 μL/min) through the acoustic sample preparation chip with the acoustic field on (2.91 MHz at 10 Volts peak-to-peak) and the 3 separated fractions at the trifurcation were collected through the outlet silicone tubing. Once collected, the fractions were analyzed in an Accuri C6 flow cytometer where gating was performed, as mentioned-above, to determine the percentages of ligand-bound ECμPs and porcine blood cells in each of the collected fractions.
Preparation of Plasma
1 mL samples of whole porcine blood containing sodium heparin (Bioreclamation) were centrifuged in 1.7 mL polypropylene microcentrifuge tubes using a Galaxy 14D microcentrifuge (VWR, Radnor, PA) at 2000 g for 10 minutes. Supernatant (plasma) was carefully pipetted into microcentrifuge tubes and stored in 0.5 mL aliquots at −20 °C.
PSA Titration in Physiological buffer
5 × 105 ECμPs –elastomeric particles functionalized with mouse anti-human PSA monoclonal antibodies (HyTest Inc., Turku, Finland)– were incubated with different concentrations (0,1,5,10,20,30 nM) of prostate specific antigen (PSA) (Meridian Life Sciences Inc., Memphis, TN) in 200 μL of washing/blocking buffer for 30 minutes with continuous rocking at room temperature. Without washing, enough mouse anti-human PSA monoclonal antibody-FITC (detection antibody) (HyTest Inc., Turku, Finland) was added to make a 1 nM solution that was incubated for 30 minutes with rocking at room temperature. The ECμPs were then analyzed with an Accuri C6 flow cytometer without washing. All bioassay data was fitted to a one-site binding curve with the following equation, using PRISM (Graph Pad) version 5.0b. The dependent variable (Y) is the median fluorescence intensity (MFI, y-axis), the independent variable (x) is the ligand analyte concentration (x-axis), Kd is the dissociation constant and Fmax is the maximum MFI.
IgG-PE Titration in 10% Plasma
5 × 105 ECμPs –elastomeric particles functionalized with mouse anti-human PSA monoclonal antibodies (Abcam, Cambridge MA)– were incubated with different concentrations, (0, 21, 42, 84, 168, 336, 672 pM) of goat anti-mouse IgG-phycoerythrin (PE) (Abcam, Cambridge MA) in 200 μL of 10% volume porcine plasma (diluted in the washing/blocking buffer) for 30 minutes with continuous rocking at room temperature. ECμPs were then analyzed in an Accuri C6 flow cytometer without prior washing.
Titration in 0.1% Blood, Acoustic Separation, and Flow Cytometry
ECμPs –again, elastomeric particles (5 × 105) functionalized with mouse anti-human PSA monoclonal antibodies– were incubated with different concentrations (0, 21, 42, 84, 168, 336, 672 pM) of goat anti mouse IgG-(PE) (Abcam, Cambridge MA) in 200 μL of 0.1 % volume whole porcine blood (porcine blood was diluted in washing/blocking buffer) for 30 minutes with continuous rocking at room temperature. Samples were then flowed (45 μL/min) through the acoustic sample preparation chip with the acoustic field on (2.91 MHz; 10 V peak-to-peak supplied to the PZT) and collected through the outlet silicone tubings. Once collected, ligand-bound ECμPs were analyzed in an Accuri C6 flow cytometer without prior washing.
Flow Cytometry Gating in Bioassays
Flow cytometry (Accuri C6) data on ECμPs was acquired by gating on forward and side scatter parameters to exclude debris and doublets. The median fluorescence intensity of gated ECμPs was used to formulate the binding curves shown within the manuscript.
RESULTS
Particle Separation Approach
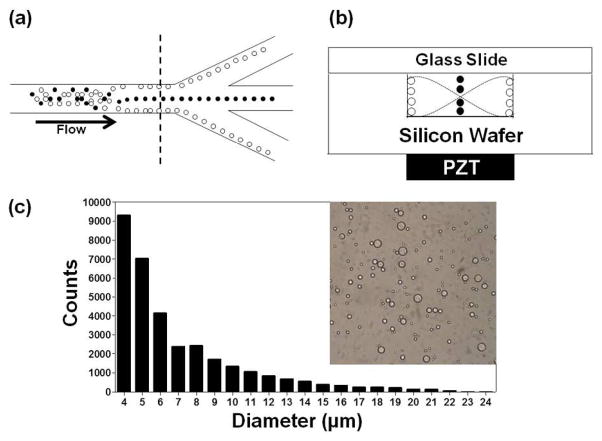
Particles (or cells) with different acoustic contrast properties can be focused (i.e., acoustically positioned to nodal or antinodal planes) and then separated using an acoustic sample preparation chip with a downstream trifurcation (Figure 1a) (see SI Figure S1 for an image of an actual acoustic sample preparation chip).22 After acoustic focusing, laminar flow carries particles continuously into outlet channels at the trifurcation for collection (Figure 1a). The attached acoustic transducer(PZT) has an appropriate size to allow resonance at the frequency (2.91 MHz) that corresponds to a wavelength that is twice the width (252 μm) of the acoustic focusing channel (Figure 1b). Thus a resonant acoustic standing wave is established in the fluid-filled cavity of the chip and the field exerts a time-averaged force that focuses positive contrast particles (e.g., blood cells) to the center pressure node and negative contrast particles (e.g., elastomeric particles) to the two pressure antinodes at the sides of the channel (Figure 1b).21,22
Figure 1.
(a) Schematic diagram depicting the separation approach for elastomeric negative acoustic contrast particles (white) from positive acoustic contrast particles (e.g., blood cells) (black) at the trifurcation in a silicon acoustic sample preparation chip. (b) Cross-section, at dashed line in (a), of the chip, with the positive contrast particles focused at the pressure node and the negative contrast particles focused at the pressure antinodes under an acoustic standing wave field. Note: Images are not drawn to scale. (c) Bright field image and size histogram of PDMS-based elastomeric particles prepared by bulk emulsification.
Particle Synthesis
Polydisperse elastomeric particles were synthesized using an oil-in-water bulk emulsion process without the use of detergent. The synthesis method is straightforward and allows polydisperse elastomeric particles to be rapidly synthesized (~1 hour) with a bulk concentration of 1.3 × 108 particles/mL. The diameters of particles produced by this method varied from submicron to approximately 21 μm in diameter (Figure 1c). As prepared, these particles were unstable in regards to particle aggregation; adsorption of avidin allowed the elastomeric particles to maintain stability during centrifugal washes (2900 g for 5 minutes) performed in a washing/blocking buffer.
Acoustic Focusing and Separation
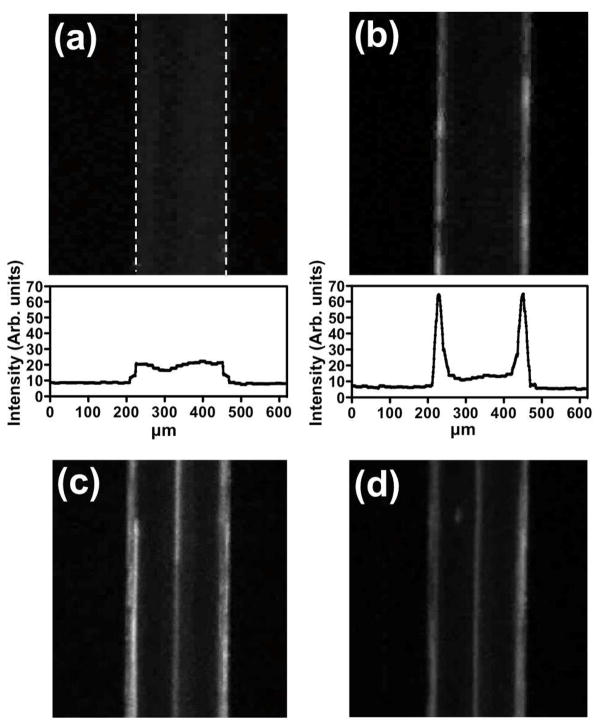
Acoustic focusing experiments were performed on Nile Red labeled ECμPs (NR-ECμPs) to examine their acoustic contrast properties and the optimal operating conditions of the acoustic sample preparation chip (e.g., particle concentrations, flow rates, resonance frequency, and applied voltage on the actuating PZT). The field of view of the epifluorescence microscopic objective (2.5x, NA of 0.3) was large enough to capture the entire width(252 μm) of the central micro-channel in the acoustic sample preparation chip and was positioned to capture fluorescent images in the central micro-channel upstream of the trifurcation. NR-ECμPs (2.5 × 107 particle/mL) were flowed (45 μL/min) continuously through the acoustic sample preparation chip with the acoustic field off (Figure 2a) and then on (Figure 2b); the concomitant increase in Nile Red fluorescence at the sides of the micro-channel, indicated an increase in the concentration of NR-ECμPs at the pressure antinodes. Histograms of the average fluorescence intensity measured across the channel width confirm the relatively uniform distribution of fluorescence particles across the channel width in the absence of the acoustic field (Figure 2a), and the focusing of the NR-ECμPs at the side-walls of the channels upon imposition of the acoustic field (Figure 2b). The NR-ECμPs thus exhibit negative acoustic contrast and are focused to the pressure antinodes along the sides of the micro-channel walls upon imposition of a resonant frequency (2.91 MHz)to the acoustic sample preparation chip.
Figure 2.
ECμPs function as negative contrast particles and can be separated from positive contrast particles using an acoustic sample preparation chip. These images were captured via an epifluorescence microscope with a 2.5x objective. Each sample was flowing at 45 μL/min. Fluorescence microscopy images along with histograms of average intensity profiles for Nile Red (NR) stained ECμPs with the field (a) off and then (b) on. (c) NR-ECμPs separated from NR-PS particles with the field on. (d) NR-ECμPs separated from NR-blood cells with the field on. Note: white dashed lines in (a) indicate micro-channel borders.
To evaluate the ability of ECμPs to be acoustically separated from positive contrast particles and the ability of the acoustic sample preparation chip to support effective separations, NR-ECμPs (2.5 × 107 particles/mL) were separated from positive contrast, NileRed labeled polystyrene particles (NR-PS: 2.5 × 106 particles/mL) (Figure 2c). Further, to demonstrate that ECμPs could be continuously separated from blood cells NR-ECμPs (2.5 × 107 particles/mL) were separated from Nile Red stained blood cells (NR-blood cells: 0.1% volume whole porcine blood) (Figure 2d) using the same acoustic set-up described above.
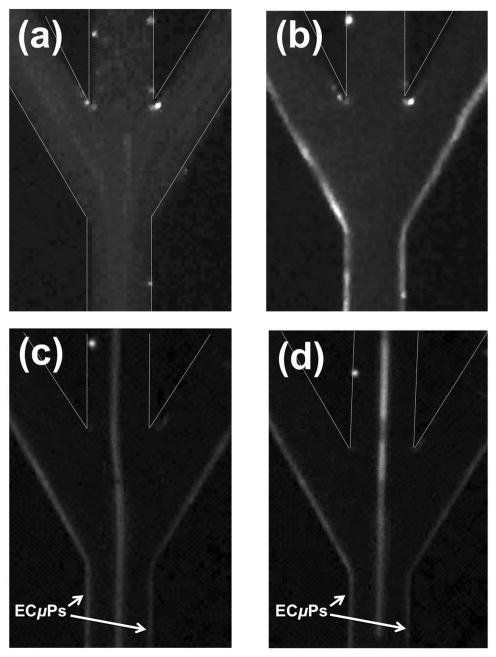
Separation at Trifurcation
As a result of laminar flow within the micro-channel (Re = 7.4), acoustically focused ECμPs flow directly into the peripheral outlet channels at the trifurcation for collection. NR-ECμPs (2.5 × 107 particles/mL) were continuously flowed (45 μL/min) through the acoustic sample preparation chip. Figure 3 shows images at the trifurcation, with the field off (Figure 3a) and then on (Figure 3b); the concomitant increase in Nile Red fluorescence at the sidewalls of the side outlet channels of the trifurcation demonstrated that ECμPs were acoustically manipulated into side outlet channels for continuous collection. To evaluate whether ECμPs can be separated, concentrated, and collected apart from positive contrast particles, polydisperse NR-ECμPs (2.5 × 107 particles/mL) were continuously separated from NR-PS particles (2.5 × 106 particle/mL) at the trifurcation (Figure 3c). Laminar flow also allowed acoustically focused and separated NR-ECμPs and NR-blood cells (from 0.1% porcine whole blood) to maintain focusing and allow shunting into designated outlet channels at the trifurcation for collection. Focused NR-ECμPs flowed into side outlet channels and NR-blood cells flowed directly into the center outlet channel at the trifurcation (Figure 3d).
Figure 3.
(a) Fluorescence microscopy images of NR-ECμPs with the field (a) off and then (b) on. (c) NR-ECμPs separated from NR-PS particles with the field on. (d) NR-ECμPs separated from NR-blood cells (0.1% volume whole porcine blood) with the field on. Each sample was flowing at 45 μL/min. Note: white dashed lines indicate micro-channel borders that are not otherwise visible due to presence of NR-ECμPs.
ECμPs used in Bioassays
Biospecific Functionalization of Negative Contrast Particles
Biofunctionalization of elastomeric particles was accomplished by non-specifically adsorbing avidin to the hydrophobic surface of the elastomeric particles. To demonstrate their specific biotin-binding ability, avidinylated elastomeric particles were titrated with biotin-4-fluorescein; controls were performed where avidinylated elastomeric particles were pre-incubated with free biotin (biotin-block) followed by incubation with biotin-4-fluorescein (see SI Figure S2). Our results indicate that (i) avidin protein can be non-specifically adsorbed to bare elastomeric particles, and (ii) adsorbed avidin protein maintains biotin-binding functionality and binds biotin with minimal non-specific adsorption. Once elastomeric particles have been functionalized with avidin they can be further functionalized with a biotinylated capture antibody (see SI Figure S3). These results demonstrate that negative contrast elastomeric particles can be biofunctionalized using simple avidin/biotin conjugation reagents.
PSA Sandwich Assays Performed in Physiological Buffer
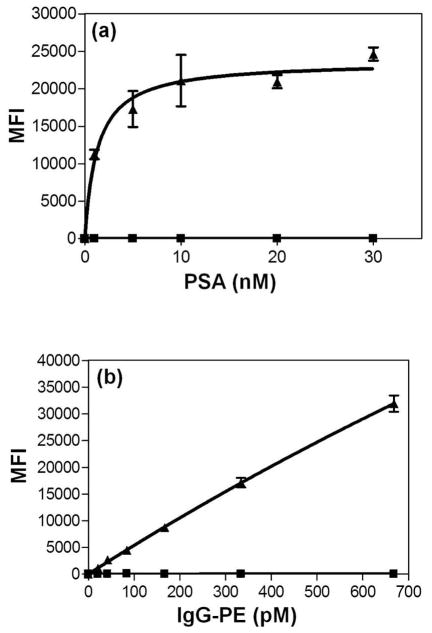
ECμPs were used in a titration assay for prostate specific antigen (PSA) in PBS (0.1 % BSA) in which anti-human PSA monoclonal antibody-FITC was used as the secondary (detection) antibody in a sandwich assay configuration where the lowest concentration of human PSA used was 1 nM. The assay data exhibited high signal-to-noise (Figure 4a) and very low background indicative of biospecific analysis and were fit to a one-site binding curve (R2=0.89) Though a specific limit of detection for this assay was not determined, the signal above noise at 1 nM suggests that detection levels below 1 nM may be achievable for PSA in simple buffer solutions.
Figure 4.
ECμPs as platforms for protein capture assays in flow cytometry. Note: (▲)denotes ECμPs with capture antibody and (■) denotes particles without capture antibody. (a) ECμPs used in a sandwich assay for prostate specific antigen (PSA) in physiological buffer (PBS). (b) ECμPs used in a binding assay for goat anti-mouse IgG-phycoerythrin performed in 10% volume porcine plasma diluted with PBS. Note: all data points were obtained from triplicate experiments analyzed by gated flow cytometric analysis. Error bars represent the standard deviation of the mean for 3 separate determinations of median fluorescence intensity.
IgG-PE Binding Assays Performed in 10% Porcine Plasma
To examine biospecific binding of ECμPs in a complex solution of proteins, an IgG capture assay was performed in 10% whole porcine plasma (diluted with 0.1% BSA in PBS). ECμPs were used in a titration binding assay fora polyclonal goat anti-mouse IgG-phycoerythrin (PE), where the lowest concentration used was 21 pM. The standard assay data exhibited high signal-to-noise (Figure 4b) and very low background indicative of biospecific adsorption and were fitted to a one-site binding curve. This fit well(R2=0.99)but provided a linear response implying that we did not achieve saturation over our titration range. Nonetheless, this assay demonstrated a high signal-to-noise ratio and indicates that detection of pM concentrations of non-species IgG in plasma is readily possible (Figure 4b).
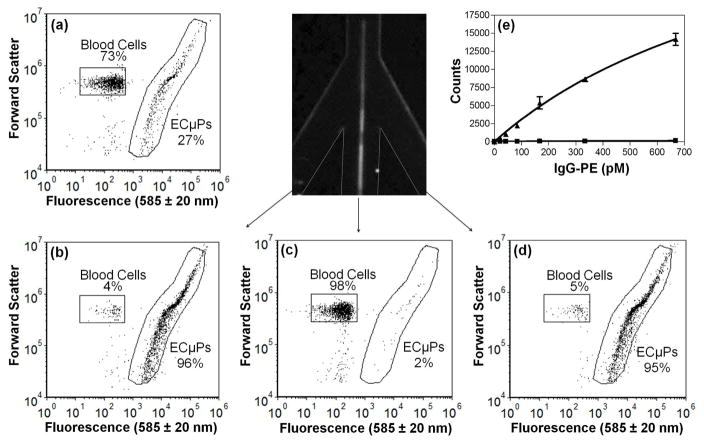
ECμPs and Blood Cells Collected from Trifurcation
Ligand-bound ECμPs (Ligand = goat anti mouse IgG antibody-PE) were separated from porcine blood cells using an acoustic sample preparation chip. To determine the effectiveness of separating ECμPs from blood cells, separation efficiency measurements were performed by analysis via flow cytometry of fractions collected from each leg of a trifurcation. Control experiments (see SI Figure S4) were performed to determine gating regions in flow cytometry scatter plots (fluorescence (585 ± 20 nm) versus forward scatter) that allow identification and quantitation of ligand-bound ECμPs and blood cells. A mixture (73% blood cells; 27% ligand-bound ECμPs, Figure 5a) was prepared and then flowed through the acoustic sample preparation chip with the acoustic field on. The outputs from the two peripheral outlet channels were collected and consisted mostly of ligand-bound ECμPs (95–96% ligand-bound ECμPs, 4–5% blood cells; see Figures 5b, d); whereas the output from the central outlet channel consisted mostly of blood cells (98% blood cells, 2% ligand-bound ECμPs; see Figure 5c). Our results thus indicate that ligand-bound ECμPs can be efficiently separated away from blood cells using an acoustic sample preparation chip with a downstream trifurcation.
Figure 5.
ECμPs used in an assay in diluted blood where acoustic separation and collection was achieved using the acoustic sample preparation chip prior to flow cytometry analysis. (a)Flow cytometry scatter plot (forward scatter versus fluorescence (585 ± 20 nm)) showing the initial mixture (inlet) of ligand-bound ECμPs and blood cells. (b) Scatter plot of the collected fraction of the left peripheral outlet channel. (c) Scatter plot of the collected fraction from the central outlet channel. (d) Scatter plot of the collected fraction of the right peripheral outlet channel. (e) IgG-PE binding assay in 0.1 % porcine blood from ECμPs separated and collected using an acoustic sample preparation chip, prior to flow cytometry analysis. (▲) denotes ECμPs with capture antibody and (■) denotes particles without capture antibody. Note: all data points were obtained in triplicate. Error bars represent the standard deviation of the mean for 3 separate determinations of median fluorescence intensity.
IgG-PE Binding Performed in 0.1% Blood with Acoustic Separation Prior to Assay
ECμPs were used in an IgG-PE assay in which IgG capture was performed in 0.1 % porcine blood prior to separation from blood cells, collection of the ECμPs using an acoustic sample preparation chip, and their subsequent analysis via flow cytometry. The blood was diluted to 0.1% (or 5 × 106 cells/ml) to eliminate acoustic scattering from cells that is known to occur in whole or high concentration blood.31 High concentrations of red cells under an acoustic standing wave field can generate acoustic scattering that results in secondary acoustic forces (i.e., interparticle forces on red cells).31 These secondary acoustic forces can cause red cells to aggregate or experience repulsive forces,31 and thus result in decreased focusing and lower separation efficiencies. Future work will examine the maximum concentration of blood that can be used in conjunction with ECμPs. Nonetheless, the blood concentrations used are consistent with those used in ELISA assays.32 The assay data exhibited high signal-to-noise and very low background indicative of biospecific binding and were fit to a one-site binding curve (R2=0.99) where we had a near linear response over our titration range (Figure 5e). The lowest detected concentration was 21 pM (mean - background (control)/std. dev. of mean = 60). The high signal-to-noise ratio suggests that pM detection levels can be readily achieved using this approach.
DISCUSSION
We have synthesized PDMS-based ECμPs that possess negative acoustic contrast and specific biorecognition properties. We have demonstrated that ECμPs enable binding and acoustophoretic separation of ligand analytes from complex biological samples (e.g., blood) containing large concentrations of cells. Furthermore, we have demonstrated that when the separated ECHPs are analyzed via flow cytometry, highly sensitivity and selective detection of serum proteins is possible.
Sylgard 184™ and PDMS-based elastomers, in general, are water insoluble elastomers whose compressibility (inverse of bulk modulus) can be adjusted based on the amount of crosslinking agent added.33 To date, we have only used Sylgard 184™ based elastomeric microparticles that were synthesized using standard PDMS prepolymer to crosslinking agent ratios of 10:1 and 10:2;34 both samples exhibited negative acoustic contrast. Incorporating other amounts, i.e., increasing or decreasing the amount of crosslinking agent, may allow adjustments to be made in how elastomeric microparticles respond to acoustic radiation fields. Other compressible water-insoluble elastomers such as other silicones, natural rubbers, polyurethanes, butyl rubbers, polybutadienes, styrene butadienes, fluoroelastomers, polyether block amides, ethylene-vinyl acetates, and polyacrylic rubber35, have the potential to be synthesized into negative contrast particles using emulsion-based methodologies such as those employed herein.
The acoustic separation of ligand-bound ECμPss from blood cells in the acoustic sample preparation chip occurs rapidly, within seconds or less, and occurs with continuous flow. The acoustic sample preparation chip allows for ligand-bound ECμPs to be rapidly collected without (i) performing time-consuming centrifugal washes to remove blood cells, and (ii) without other time-consuming protein isolation steps such as 2-D gel electrophoresis or other protein separation steps (e.g., precipitation, chromatography, filtering) that are performed prior to mass spectrometry analysis. The use of flow cytometry, as compared to other detection methods of analysis (e.g., ELISA, spectrofluorimetry), is advantageous in that it allows ECμPs to be used in homogeneous (no wash) blood-based assays where unbound fluorescently labeled ligands provide minimal background.36
The PSA assay was conducted in PBS buffer, and was primarily used to demonstrate that ECμPs can be used as platforms for binding of medically-relevant antigens. We note that the PSA concentration range tested was not in the diagnostically-relevant range (4 – 10 ng/mL).37 However, our lowest PSA concentration (1 nM) was detected with a high signal-to-noise ratio and thus shows potential to detect PSA at even lower concentrations. Future work will emphasize the use of optimal antibodies and fluorophores for such assays to further improve limits of detection. Furthermore, the use of monodisperse elastomeric microparticles may provide greater potential to optimize the functionalization of ECμPs; thus enabling detection of PSA at lower concentration levels.
The ability to bind ligands specifically in the presence of abundant serum proteins is a necessary component of accurate and sensitive immunoassay platforms for the detection of low concentration levels of biomarkers in blood. Our detection of 21 pM IgG-PE (~3 ng/mL) in blood-based samples (Figures 4b and 5e) is commensurate with the clinically relevant detection limits for other biomarkers commonly detected in diagnostics using blood-based samples.37 Furthermore, the continuous flow format of acoustophoresis will make it possible to couple this approach directly to a conventional flow cytometer or to perform cytometry directly on the focused streams as has been done in other microfluidic systems.4,38
We present the first demonstration of engineered particles with negative contrast in acoustic based microfluidic bioanalytical systems. The negative contrast property of ECμPs, along with the use of acoustic sample preparation microfluidic systems have the potential to enable the development of bioassay systems where the acoustic sample preparation chip is coupled directly to a flow cytometer. The continuous feed of separated ligand-bound ECμPs directly into a flow cytometer may allow for a significantly decreased time-to-analysis along with increased analysis rates. Further improvements could use ECμPs in portable systems where a disposable acoustic flow cell is used to separate ECμPs from blood cells before analysis by a low cost flow cytometer.4,38 In pursuit of making ligand-bound ECμP measurements more accurate and sensitive using flow cytometry, we are developing methods (such as those based on microfluidics, see SI Figure S5) to synthesize monodisperse elastomeric particles. Such monodisperse particles would be simpler to identify in flow cytometry as discreet populations with consistent optical properties. Furthermore, their singular size would make their time dependent movement in an acoustic standing waves more predictable and simplify efficient separations using ECμPs.
Supplementary Material
Acknowledgments
We thank Travis Woods and Robert Applegate for technical assistance. We also appreciate helpful discussions with Andrew Goumas, Carl Brown, Greg Kaduchak, and Michael Ward. We are grateful for the funding that supported this work from the National Science Foundation (Research Triangle MRSEC: DMR 1121107, DMR 0611616) and the National Institutes of Health (NIH RR020064, NIH RR001315).
Footnotes
Supporting Information: Five figures as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Laurell T, Petersson F, Nilsson A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chemical Society Reviews. 2007;36(3):492–506. doi: 10.1039/b601326k. [DOI] [PubMed] [Google Scholar]
- 2.Nam J, Lim H, Kim D, Shin S. Separation of platelets from whole blood using standing surface acoustic waves in a microchannel. Lab on a Chip. 2011;11(19):3361–3364. doi: 10.1039/c1lc20346k. [DOI] [PubMed] [Google Scholar]
- 3.Gossett DR, Weaver WM, Mach AJ, Hur SC, Tse HTK, Lee W, Amini H, DiCarlo D. Label-free cell separation and sorting in microfluidic systems. Analytical and bioanalytical chemistry. 2010;397(8):3249–3267. doi: 10.1007/s00216-010-3721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piyasena ME, Suthanthiraraj PPA, Applegate RW, Jr, Goumas AM, Woods TA, Lopez GP, Graves SW. Multinode Acoustic Focusing for Parallel Flow Cytometry. Analytical Chemistry. 2012;84(4):1831–1839. doi: 10.1021/ac200963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng Z, Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. Journal of Proteomics. 2011;74(12):2650–2659. doi: 10.1016/j.jprot.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald J, Burstein G, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Current Infectious Disease Reports. 2006;8 (2):125–131. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 7.Stevens EV, Liotta LA, Kohn EC. Proteomic analysis for early detection of ovarian cancer: A realistic approach? International Journal of Gynecological Cancer. 2003;13:133–139. doi: 10.1111/j.1525-1438.2003.13358.x. [DOI] [PubMed] [Google Scholar]
- 8.Toner M, Irimia D. Blood-on-a-chip. Annual Review of Biomedical Engineering. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP (TM) assay. Journal of Immunological Methods. 2002;260(1–2):207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nature Reviews Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 11.Stern E, Vacic A, Rajan NK, Criscione JM, Park J, Ilic BR, Mooney DJ, Reed MA, Fahmy TM. Label-free biomarker detection from whole blood. Nature Nanotechnology. 2010;5(2):138–142. doi: 10.1038/nnano.2009.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou HW, Bhagat AAS, Lee WC, Huang S, Han J, Lim CT. Microfluidic Devices for Blood Fractionation. Micromachines. 2011;2(3):319–343. [Google Scholar]
- 13.Gervais L, de Rooij N, Delamarche E. Microfluidic Chips for Point-of-Care Immunodiagnostics. Advanced Materials. 2011;23(24):H151–H176. doi: 10.1002/adma.201100464. [DOI] [PubMed] [Google Scholar]
- 14.Wang RE, Tian L, Chang YH. A homogeneous fluorescent sensor for humanserum albumin. Journal of Pharmaceutical and Biomedical Analysis. 2012;63:165–169. doi: 10.1016/j.jpba.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jani IV, Janossy G, Brown DWG, Mandy F. Multiplexed immunoassays by flow cytometry for diagnosis and surveillance of infectious diseases in resource-poor settings. The Lancet infectious diseases. 2002;2(4):243–250. doi: 10.1016/s1473-3099(02)00242-6. [DOI] [PubMed] [Google Scholar]
- 16.Williams D, Ackloo S, Zhu P, Bowden P, Evans KR, Addison CL, Lock C, Marshall JG. Precipitation and selective extraction of human serum endogenous peptides with analysis by quadrupole time-of-flight mass spectrometry reveals posttranslational modifications and low-abundance peptides. Analytical and Bioanalytical Chemistry. 2010;396(3):1223–1247. doi: 10.1007/s00216-009-3345-0. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff R, Luider TM. Methodological advances in the discovery of protein and peptide disease markers. Journal of Chromatography B. 2004;803(1):27–40. doi: 10.1016/j.jchromb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Prazeres S, Santos MA, Ferreira HG, Sobrinho LG. A practical method for the detection of macroprolactinaemia using ultrafiltration. Clinical endocrinology. 2003;58(6):686–690. doi: 10.1046/j.1365-2265.2003.01721.x. [DOI] [PubMed] [Google Scholar]
- 19.Harper RG, Workman SR, Schuetzner S, Timperman AT, Sutton JN. Low-molecular-weight human serum proteome using ultrafiltration, isoelectric focusing, and mass spectrometry. Electrophoresis. 2004;25(9):1299–1306. doi: 10.1002/elps.200405864. [DOI] [PubMed] [Google Scholar]
- 20.Cousins C, Holownia P, Hawkes J, Price C, Keay P, Coakley W. Clarification of plasma from whole human blood using ultrasound. Ultrasonics. 2000;38(1):654–656. doi: 10.1016/s0041-624x(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 21.Petersson F, Nilsson A, Holm C, Jönsson H, Laurell T. Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces. Lab on a Chip. 2005;5(1):20–22. doi: 10.1039/b405748c. [DOI] [PubMed] [Google Scholar]
- 22.Petersson F, Nilsson A, Holm C, Jönsson H, Laurell T. Separation of lipids from blood utilizing ultrasonic standing waves in microfluidic channels. Analyst. 2004;129(10):938–943. doi: 10.1039/b409139f. [DOI] [PubMed] [Google Scholar]
- 23.Grenvall C, Augustsson P, Folkenberg JR, Laurell T. Harmonic microchip acoustophoresis: a route to online raw milk sample precondition in protein and lipid content quality control. Analytical chemistry. 2009;81(15):6195–6200. doi: 10.1021/ac900723q. [DOI] [PubMed] [Google Scholar]
- 24.Lenshof A, Ahmad-Tajudin A, Järås K, Swärd-Nilsson AM, Åberg L, Marko- Varga G, Malm J, Lilja H, Laurell T. Acoustic whole blood plasmapheresis chip for prostate specific antigen microarray diagnostics. Analytical chemistry. 2009;81(15):6030–6037. doi: 10.1021/ac9013572. [DOI] [PubMed] [Google Scholar]
- 25.Lenshof A, Laurell T. Continuous separation of cells and particles in microfluidic systems. Chemical Society Reviews. 2010;39(3):1203–1217. doi: 10.1039/b915999c. [DOI] [PubMed] [Google Scholar]
- 26.Dykes J, Lenshof A, Åstrand-Grundström B, Laurell T, Scheding S. Efficient Removal of Platelets from Peripheral Blood Progenitor Cell Products Using a Novel Micro-Chip Based Acoustophoretic Platform. PLoS One. 2011;6(8):e23074. doi: 10.1371/journal.pone.0023074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo J, Svendsen WE, Dimaki M. Micro and nano techniques for the handling of biological samples. CRC Press; 2011. [DOI] [PubMed] [Google Scholar]
- 28.Bruus H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab on a Chip. 2012;12(6):1014–1021. doi: 10.1039/c2lc21068a. [DOI] [PubMed] [Google Scholar]
- 29.Galbraith DW. Analysis of higher plants by flow cytometry and cell sorting. Int Rev Cytol. 1989;116:165–228. [Google Scholar]
- 30.Nash MA, Yager P, Hoffman AS, Stayton PS. A Mixed Stimuli-Responsive Magnetic and Gold Nanoparticle System for Rapid Purification, Enrichment, and Detection of Biomarkers. Bioconjugate chemistry. 2010;21(12):2197. doi: 10.1021/bc100180q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiser M, Apfel R, Neppiras E. Interparticle forces on red cells in a standing wave field. Acta Acustica united with Acustica. 1984;56 (2):114–119. [Google Scholar]
- 32.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLoS Pathogens. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilder EA, Guo S, Lin-Gibson S, Fasolka MJ, Stafford CM. Measuring the modulus of soft polymer networks via a buckling-based metrology. Macromolecules. 2006;39(12):4138–4143. [Google Scholar]
- 34.Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomedical microdevices. 2005;7(4):281–293. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 35.Lopez GP, Carroll N, Cushing K, Petsev DN. US Patent Application 20, 120, 065, 329. Synthesis of Stable Elastomeric Negative Acoustic Contrast Particles. 2012
- 36.Nolan JP, Lauer S, Prossnitz ER, Sklar LA. Flow cytometry: a versatile tool for all phases of drug discovery. Drug discovery today. 1999;4(4):173–180. doi: 10.1016/S1359-6446(99)01320-3. [DOI] [PubMed] [Google Scholar]
- 37.Paul B, Dhir R, Landsittel D, Hitchens MR, Getzenberg RH. Detection of prostate cancer with a blood-based assay for early prostate cancer antigen. Cancer research. 2005;65 (10):4097. doi: 10.1158/0008-5472.CAN-04-4523. [DOI] [PubMed] [Google Scholar]
- 38.Austin Suthanthiraraj PP, Piyasena ME, Woods TA, Naivar MA, Lopez GP, Graves SW. One-dimensional acoustic standing waves in rectangular channels for flow cytometry. Methods. 2012 doi: 10.1016/j.ymeth.2012.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.