Abstract
Background: Concerns for arsenic exposure are not limited to toxic waste sites and massive poisoning events. Chronic exposure continues to be a major public health problem worldwide, affecting hundreds of millions of persons.
Objectives: We reviewed recent information on worldwide concerns for arsenic exposures and public health to heighten awareness of the current scope of arsenic exposure and health outcomes and the importance of reducing exposure, particularly during pregnancy and early life.
Methods: We synthesized the large body of current research pertaining to arsenic exposure and health outcomes with an emphasis on recent publications.
Discussion: Locations of high arsenic exposure via drinking water span from Bangladesh, Chile, and Taiwan to the United States. The U.S. Environmental Protection Agency maximum contaminant level (MCL) in drinking water is 10 µg/L; however, concentrations of > 3,000 µg/L have been found in wells in the United States. In addition, exposure through diet is of growing concern. Knowledge of the scope of arsenic-associated health effects has broadened; arsenic leaves essentially no bodily system untouched. Arsenic is a known carcinogen associated with skin, lung, bladder, kidney, and liver cancer. Dermatological, developmental, neurological, respiratory, cardiovascular, immunological, and endocrine effects are also evident. Most remarkably, early-life exposure may be related to increased risks for several types of cancer and other diseases during adulthood.
Conclusions: These data call for heightened awareness of arsenic-related pathologies in broader contexts than previously perceived. Testing foods and drinking water for arsenic, including individual private wells, should be a top priority to reduce exposure, particularly for pregnant women and children, given the potential for life-long effects of developmental exposure.
Keywords: arsenic, arsenic health effects, cancer, chronic arsenic exposure, development, drinking water, skin lesions
Ongoing exposures to toxic chemicals such as arsenic continue to pose a significant threat to public health. The World Health Organization (WHO) estimates that > 200 million persons worldwide might be chronically exposed to arsenic in drinking water at concentrations above the WHO safety standard of 10 µg/L (WHO 2008) (Table 1). Arsenic is a metalloid element that is encountered primarily as arsenical compounds. Within these compounds, arsenic occurs in different valence states, the most common of which are AsIII (arsenites) and AsV (arsenates). Arsenic in drinking water is typically found in the inorganic form, either as AsIII or AsV, whereas arsenic in food is found in the organic and inorganic forms, depending on the specific food [Agency for Toxic Substances and Disease Registry (ATSDR) 2007; European Food Safety Authority (EFSA) 2009] Sources of arsenic contamination include natural deposits as well as anthropogenic sources such as mining and electronics manufacturing processes and metal smelting (ATSDR 2007).
Table 1.
Arsenic exposure concerns worldwide.
| Country | Estimated exposed population (millions)a | Arsenic concentration in drinking water (µg/L) | References |
|---|---|---|---|
| Argentina | 2.0 | < 1 to 7,550 | Bates et al. 2004; Moore et al. 2004; Steinmaus et al. 2010 |
| Bangladesh | 35–77 | < 10 to > 2,500 | Kinniburgh and Smedley 2001 |
| Chileb | 0.4 | 600 to 800 | Ferreccio et al. 2000; Smith et al. 1998, 2000a |
| China | 0.5–2.0 | < 50 to 4,400 | Yu et al. 2007 |
| Ghana | < 0.1 | < 2 to 175 | Asante et al. 2007; Smedley 1996 |
| India | > 1.0 | < 10 to > 800 | Acharyya et al. 1999 |
| Mexico | 0.4 | 5 to 43 | Calderón et al. 2001; Camacho et al. 2011; Meza et al. 2004, 2005 |
| Taiwan | NA | < 1 to > 3,000 | Chen et al. 2010a, 2010b |
| United States | > 3.0 | < 1 to > 3,100 | Anning et al. 2012; Ayotte et al. 2003; Burgess et al. 2007; Nielsen et al. 2010; NRDC 2000; Peters 2008; Sanders et al. 2012; Thundiyil et al. 2007; Xue et al. 2010 |
| Vietnam | > 3.0 | < 0.1 to 810 | Winkel et al. 2011 |
| Abbreviations: NA, not available; NRDC, National Resources Defense Council. aEstimated number of persons exposed to > 10 µg/L arsenic in drinking water. Estimates were obtained from cited references and usually refer to a specific city or region within each country. The actual number of exposed persons in each country could be higher. bThe population in one region of Chile was exposed to high levels of arsenic from 1958 to 1971, and studies of long-term and latent effects are ongoing. | |||
Arsenic holds the highest ranking on the current U.S. ATSDR 2011 substance priority list (ATSDR 2011b) (Table 2). ATSDR ranks chemicals using an algorithm that translates potential public health hazards into a points-scaled system based on the frequency of occurrence at National Priority List (NPL) Superfund sites as well as toxicity and potential for human exposure. Arsenic tops the list in spite of the fact that this ranking does not include full consideration of exposure from drinking water, diet, copper-chromated arsenic-treated wood, coal- and wood-burning stoves, arsenical pesticides, and homeopathic remedies (ATSDR 2007, 2011b; Akter et al. 2005; EFSA 2009; Rose et al. 2007). Therefore, the threat to human health posed by arsenic is even greater than its top ATSDR ranking would suggest. In regard to toxicity, the International Agency for Research on Cancer (IARC) defines arsenic as a Group I known human carcinogen that also induces a wide array of other noncancer effects, leaving essentially no bodily system free from potential harm (ATSDR 2007; IARC 2012; National Research Council 2001; WHO 2008).
Table 2.
The ATSDR 2011 substance priority list.
| Rank | Substance name | Points | CAS number |
|---|---|---|---|
| 1 | Arsenic | 1665.5 | 007440-38-2 |
| 2 | Lead | 1529.1 | 007439-92-1 |
| 3 | Mercury | 1460.9 | 007439-97-6 |
| 4 | Vinyl chloride | 1361.1 | 000075-01-4 |
| 5 | Polychlorinated biphenyls (PCBs) | 1344.1 | 001336-36-3 |
| 6 | Benzene | 1332.0 | 000071-43-2 |
| 7 | Cadmium | 1318.7 | 007440-43-9 |
| 8 | Polycyclic aromatic hydrocarbons | 1282.3 | 130498-29-2 |
| 9 | Benzo[a]pyrene | 1305.7 | 000050-32-8 |
| 10 | Benzo[b]fluoranthene | 1252.4 | 000205-99-2 |
| This list was generated by the ATSDR (2011) using an algorithm that translates potential public health hazards into a points-scaled system based on the frequency of occurrence at NPL Superfund sites and on toxicity and potential for human exposure. | |||
Here we synthesize the large body of current research pertaining to arsenic exposure and health effects and emphasize the broadening scope of predicted and observed impacts of arsenic on public health. Understanding the wide range of these impacts drives home the importance of testing drinking-water sources and monitoring foods for arsenic. Whereas municipalities test public drinking-water sources, private wells can go untested. Recent data also raise concerns for arsenic exposure via foods including rice and organic brown rice syrup [Davis et al. 2012; EFSA 2009; Food and Drug Administration (FDA) 2012; Gilbert-Diamond et al. 2011; Jackson et al. 2012] as well as chicken feather meal products that are used in the human food system (Nachman et al. 2012).
Even as assessments of dietary exposure continue to unfold, drinking water remains a major concern for arsenic exposure. There are known, large-scale drinking-water contamination problems in countries such as Bangladesh (Ahsan et al. 2006; Argos et al. 2010, 2012; Smith et al. 2000b). However, chronic arsenic exposure is a concern in many parts of the world (Table 1). For example, arsenic concentrations in drinking water from some private wells in the United States are as high as 3,100 µg/L, which is in the range of the highest concentrations reported in Bangladesh (Nielsen et al. 2010; Yang et al. 2009). Yet detection of arsenic contamination even at these high levels remains problematic because it is tasteless, colorless, and odorless.
Given the large number of studies that address the broad range of information provided here, it is impractical to include all pertinent studies. Rather, we present a synthesis of information and cite recent studies that help illustrate the breadth and scope of the problems. When available, we cite current reviews that can serve as a resource for a more complete listing of relevant resources, most often focusing on single health issues such as cardiovascular disease (States et al. 2009). Detailed discussions of arsenic exposure and health effects can be found elsewhere (ATSDR 2007; EFSA 2009; Gibb et al. 2011; States et al. 2011).
As awareness of arsenic exposure increases, so should knowledge of its health effects because the impact of chronic arsenic exposure on public health is substantial. In addition to skin lesions and skin cancer (ATSDR 2011a; Sengupta et al. 2008; Smith et al. 2000a), neurological, respiratory, cardiovascular, and developmental effects and more are linked to chronic arsenic exposure (Table 3) (Argos et al. 2012; Smith and Steinmaus 2009; States et al. 2011). Acute poisonings still occur but are uncommon (Bronstein et al. 2011). Arsenic renders its toxicity via numerous mechanisms: Arsenic is genotoxic and has multiple effects on cellular signaling, cellular proliferation, DNA structure, epigenetic regulation, and apoptosis (Flora 2011; Ren et al. 2011; States et al. 2011).
Table 3.
Arsenic affects a broad range of organs and systems.
| Targets | Health effects | References |
|---|---|---|
| Skin | Skin lesions | Argos et al. 2011; Haque et al. 2003; Smith et al. 2000a |
| Skin cancer | Tseng 1977, 2007; Yu et al. 2006 | |
| Developmental processes | Increased infant mortality | Milton et al. 2005; Rahman et al. 2010a |
| Reduced birth weight | Rahman et al. 2009 | |
| Altered DNA methylation of tumor promoter regions in cord blood and maternal leukocytes | Intarasunanont et al. 2012; Kile et al. 2012 | |
| Neurological impairments in children | Dong and Su 2009; Hamadani et al. 2011; Wasserman et al. 2004, 2007 | |
| Early-life exposure associated with increased cancer risk as adults | Bates et al. 2004; Chen CL et al. 2010b; Liaw et al. 2008; Marshall et al. 2007; Su et al. 2011; Yuan et al. 2010 | |
| Nervous system | Impaired intellectual function in children and adults | Hamadani et al. 2011; Wasserman et al. 2004, 2007; Dong and Su 2009 |
| Impaired motor function | Gong et al. 2011; Parvez et al. 2011 | |
| Neuropathy | Vahidnia et al. 2007 | |
| Respiratory system | Increased mortality from | |
| Pulmonary tuberculosis | Smith et al. 2011 | |
| Bronchiectasis | Smith et al. 2006 | |
| Lung cancer | Heck et al. 2009; Marshall et al. 2007; Smith et al. 2009 | |
| Cardiovascular system | Coronary and ischemic heart disease | Chen Y et al. 2011; Gong and O’Bryant 2012 |
| Acute myocardial infarction | Yuan et al. 2007 | |
| Hypertension | Abhyankar et al. 2012; Abir et al. 2012 | |
| Liver, kidney, and bladder | Liver cancer | Chen and Ahsan 2004; Chiu et al. 2004; Liaw et al. 2008; Liu and Waalkes 2008 |
| Kidney cancer | Bates et al. 2004; Yuan et al. 2010 | |
| Bladder and other urinary cancers | Chen et al. 2010b; Chiou et al. 2001; Gibb et al. 2011; Marshall et al. 2007 | |
| Immune system | Altered immune-related gene expression and cytokine expression | Ahmed et al 2011; Andrew et al. 2008; Kile et al. 2012 |
| Inflammation | Ahmed et al. 2011 | |
| Increased infant morbidity from infectious diseases | Rahman et al. 2010b; Spivey 2011 | |
| Endocrine system | Diabetes | Chen et al. 2007; Del Razo et al. 2011; Islam et al. 2012; Jovanovic et al. 2012 |
| Impaired glucose tolerance in pregnant women | Ettinger et al. 2009 | |
| Disrupted thyroid hormone, retinoic acid, and glucocorticoid receptor pathways in mice and amphibians | Barr et al. 2009; Davey et al. 2007, 2008 | |
| The list of references is not intended to be comprehensive but rather to provide examples of health effects across multiple bodily systems. | ||
A wealth of data comes from ongoing epidemiological studies of large populations exposed to a wide range of arsenic levels in drinking water in regions such as Taiwan, Bangladesh, Chile, India, and Argentina (Ahsan et al. 2006; Argos et al. 2012; Chen CL et al. 2010a; Smith et al. 2011; Yuan et al. 2010). In Taiwan, a stable population in an arsenic-endemic region had been exposed to arsenic via drinking water since the 1900s [Chen CJ et al. 1988b, 1992; Gibb et al. 2011; Tseng 1977; U.S. Environmental Protection Agency (EPA) 2001; Wu et al. 1989]. In Bangladesh, tube wells were dug in the 1970s as a source of drinking water to avoid microbial contamination, only to later learn that the tube wells are contaminated with naturally occurring arsenic (Smith et al. 2000b). Researchers established a cohort in Bangladesh with over 10,000 persons enrolled as part of the Health Effects of Arsenic Longitudinal Study (HEALS) (Ahsan et al. 2006; Argos et al. 2012). Researchers are also studying a population in Chile where some cities were exposed to high concentrations of arsenic for a defined, limited period of time (1958–1971), at which point, systems were installed to remove arsenic from drinking water (Biggs et al. 1998). This population is particularly well suited for studies related to latency periods for chronic diseases and susceptibility during development (Dauphine et al. 2011; Liaw et al. 2008; Marshall et al. 2007; Yuan et al. 2010). Major findings from these cohorts and other studies are described in the following sections.
In light of accumulated research, there is increasing awareness that arsenic exposure might be affecting more persons and contributing to more chronic disease than previously thought. In the HEALS cohort, approximately 21.4% of all deaths and 23.5% of deaths associated with chronic disease could be attributed to arsenic at > 10 µg/L in drinking water (Argos et al. 2010). Here we present an overview and synthesis of recent information on worldwide concerns for arsenic exposures and public health. The enormity of potential public health impacts is striking. Given this potential, testing and remediating arsenic in drinking water at the level of single private wells and reducing dietary exposure are critical to protecting public health.
Worldwide Concerns for Arsenic Exposure
Arsenic exposure is a major environmental public health concern worldwide and a primary concern for exposure is via drinking water (Table 1). The WHO and Australia set or confirmed a guideline level of 10 μg/L for arsenic in drinking water in 2008 and 2011, respectively (National Health and Medical Research Council 2011; WHO 2008). The U.S. EPA promulgated that it lowered the maximum contaminant level (MCL) from 50 μg/L to 10 μg/L, effective in 2002 (U.S. EPA 2001). In many developing countries, including Bangladesh, 50 μg/L is still the commonly adopted guideline, primarily because of difficulties in remediating arsenic below that level (WHO 2008). The excess cancer risk associated with lifetime arsenic exposure at water concentrations of > 10 µg/L is approximately 1 in 300, which is 30–300 times higher than the cancer risks estimated for exposure to other known carcinogens in drinking water at concentrations equal to current U.S. drinking-water standards (Smith et al. 2002).
What is the extent of chronic exposure via drinking water? The answer varies greatly depending on regional and local sources of arsenic (Table 1). For example, in Maine, the U.S. Geological Survey reported that 18.4% of wells tested had > 10 µg/L arsenic and estimated that 24,000–44,000 households might be affected (Nielsen et al. 2010). A recent study predicted that 42.7% of the area of aquifers in the southwestern United States has arsenic concentrations of ≥ 10 µg/L, although portions of these areas are in remote regions (Anning et al. 2012). Of 63,000 wells tested in North Carolina, 1,436 (2.3%) had arsenic concentrations of > 10 µg/L with a maximum of 806 µg/L (Sanders et al. 2012). In comparison, in Bangladesh in 1998, shortly after discovery of arsenic contamination, it was estimated that up to 94% of tube wells in certain regions and 35% of all wells in the country contained > 50 µg/L arsenic (Smith et al. 2000b). In Chile, San Pedro de Atacama drew most of its public drinking water from the Vilama River, which contained approximately 600–680 µg/L arsenic, and some homes with no public supply drew water from the San Pedro River, which contained 170 µg/L; in contrast, a town 40 km away had an average drinking-water arsenic concentration of 15 µg/L (Hopenhayn-Rich et al. 1996).
Testing is required to determine whether a given source of drinking water has high levels of arsenic. Even if the local municipality does not test private wells for arsenic, test kits are available worldwide through local municipalities, public health offices, and commercial sources accessible via the Internet (Water Quality Association 2012; Massachusetts Department of Environmental Protection 2011). Hot spots of arsenic contamination of drinking-water sources can occur because of proximity to naturally occurring arsenic found in certain types of bedrock and sediments as well as proximity to hazardous waste sites. Therefore, drinking-water sources with high arsenic concentrations can exist in very close proximity to sources with low arsenic concentrations, with differences noted even in neighboring individual wells.
Another source of growing concern for arsenic exposure is through diet. For persons with limited exposure to arsenic via drinking water, diet is the major source of exposure (EFSA 2009). Rice, organic rice syrup, fruits, juices, and other grains can contain significant amounts of arsenic (FDA 2012; Jackson et al. 2012; Norton et al. 2012). Furthermore, rice consumption has been shown to be associated with urinary arsenic levels in pregnant women and children (Davis et al. 2012; Gilbert-Diamond et al. 2011). Because of their level of consumption of rice products, children < 3 years of age are estimated to have the greatest exposures to arsenic via diet (EFSA 2009).
Health Outcomes of Arsenic Exposure
Dermatological effects. Cutaneous lesions are one of the best-known clinical manifestations of chronic arsenic exposure and can occur within months or after several years of exposure (Das and Sengupta 2008; WHO 2005). Clinical photos of different types of arsenic-associated lesions are shown in Figure 1. Melanosis (hyperpigmentation) is considered an early and more common manifestation (Figure 1A), whereas keratosis (Figure 1B) is considered a sensitive marker of more advanced stages of arsenicosis (Das and Sengupta 2008; Sengupta et al. 2008). Leucomelanosis (hypopigmentation) also occurs but less frequently than melanosis or keratosis. Arsenic-related melanosis can be diffuse or patchy, or exhibit a distinctive “rain drop” pattern, and these lesions often appear on the trunk of the body. Keratotic lesions tend to appear mainly on the palms and soles. Sudden increases in the size of keratotic lesions, or cracks or bleeding of lesions, suggest malignant transformation—often to squamous cell carcinoma (Figure 1C,D). Analyses of numerous epidemiological studies of skin lesions suggest that most persons with skin lesions had consumed water with arsenic concentrations of > 100 µg/L, although lesions have been reported at arsenic concentrations of < 50 µg/L (Argos et al. 2011; Smith and Steinmaus 2009). Nutritional, economic, and smoking status are contributing factors for susceptibility to skin lesions as are sex and age, with a greater prevalence of skin lesions in older men (Pierce et al. 2010). A recent report from the HEALS prospective study found that the risk of skin lesions did not decrease after reducing exposure for up to several years (Argos et al. 2011). Therefore, lesions can appear several years after exposure diminishes. The vast majority of exposed individuals (even with high levels of chronic exposure) will not develop skin lesions but are still at risk of arsenic-related skin and internal cancers and other noncancer diseases (Argos et al. 2010; Chen Y et al. 2011; Parvez et al. 2010).
Figure 1.
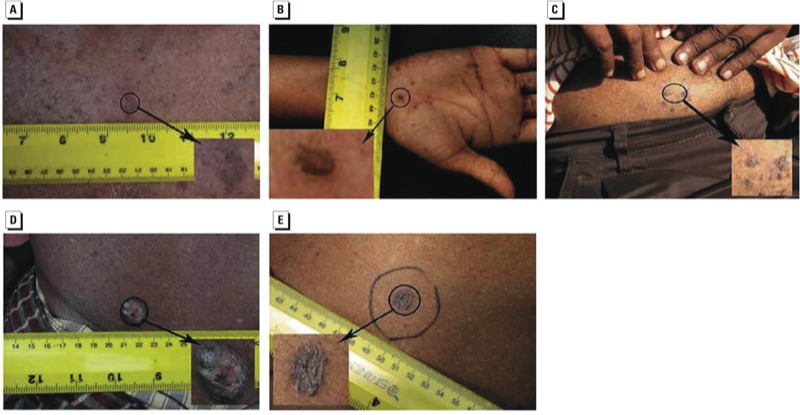
Skin manifestations of chronic arsenic exposure. (A) Hyperpigmentation (melanosis); (B) hyperkeratosis (keratosis); (C) squamous cell carcinoma in situ (Bowen’s disease); (D) invasive squamous cell carcinoma; and (E) basal cell cancer.
Arsenic exposure and cancer. Arsenic is a known carcinogen in skin, lung, bladder, liver, and kidney, with evidence suggesting lung cancer is the most common cause of arsenic-related mortality (IARC 2012; National Toxicology Program 2011). Skin cancer has long been associated with chronic arsenic exposure (ATSDR 2007; Yu et al. 2006). Squamous cell carcinoma in situ (Bowen’s disease; Figure 1C), invasive squamous cell carcinoma (Figure 1D), and basal cell carcinoma (Figure 1E) are the most common types of skin cancer associated with chronic arsenic exposure. Studies from arsenic-endemic regions of Taiwan revealed that the overall prevalence of skin cancer was 10.6 per 1,000 persons and was associated with increased arsenic drinking-water concentrations (Tseng 1977) and increased urinary concentrations of certain arsenic metabolites (Tseng 2007). In the United States, where arsenic exposure is generally lower, significantly increased risks for squamous cell and basal cell carcinomas occurred in individuals in the top 97th percentile of toenail arsenic concentrations (Karagas et al. 2001), particularly among individuals carrying susceptible genotypes for the nucleotide excision repair genes (Applebaum et al. 2007).
Chronic arsenic exposure is also associated with an increased risk of lung cancer (IARC 2012). In the Chilean cohort that was exposed to high arsenic concentrations in drinking water (> 850 µg/L) for a limited period of time (1958–1971), the peak mortality rate ratio (MRR) for lung cancer was highest at 3.61 (95% CI: 3.13, 4.16) for men in 1992–1994 (Table 4), suggesting a 34- to 36-year latency period (Marshall et al. 2007). Arsenic is carcinogenic in the lung regardless of oral or inhalation pathways of exposure, and it is well established that lung cancer is associated with exposure to > 100 µg/L arsenic in drinking water. However, it is unclear whether such an association exists for exposure to < 100 µg/L arsenic (Chen CL et al. 2010a; Heck et al. 2009; Putila and Guo 2011; Smith et al. 2009; Steinmaus et al. 2010).
Table 4.
Peak mortality ratios for internal cancers and bronchiectasis in Chilean cohort studies.
| Disease | Reference | Peak mortality ratio (95% CI) | Type of mortality ratio | Subpopulation with peak ratio |
|---|---|---|---|---|
| Lung cancer | Marshall et al. 2007 | 3.61 (3.13, 4.16) | MRR | Men, 22–24 years after exposure reduction |
| Bladder cancer | Marshall et al. 2007 | 13.8 (7.74, 24.5) | MRR | Women, 22–24 years after exposure reduction |
| Childhood liver cancer | Liaw et al. 2008 | 14.1 (1.6, 126.2) | MRR | Girls born 1950–1957 (exposed during childhood), 0–19 years of age |
| Kidney cancer | Yuan et al. 2010 | 4.37 (2.98, 6.41) | MRR | Women, 21–25 years after exposure reduction |
| 9.52 (2.56, 24.4) | MRR | Women born 1950–1970 (exposed in utero and during childhood), 21–25 years after exposure reduction | ||
| Bronchiectasis | Smith et al. 2006 | 50.1 (20.0, 103) | SMR | Women born 1958–1970 (exposed in utero and during childhood), 18–29 years after exposure reduction |
| Abbreviations: MRR, mortality rate ratio; SMR, standardized mortality ratio. For the exposed group, arsenic concentrations in drinking water were high (about 870 µg/L) between 1958 and 1970, at which point, filtration systems were installed thereby lowering the arsenic exposure. At the time of exposure reduction, the exposed population ages ranged from prenatal through adulthood. | ||||
Increasing evidence supports the hypothesis that arsenic exposure can increase cancer risks in other organs. Increased risk of bladder cancer is significantly associated with increasing arsenic exposure, particularly with longer exposure periods (> 40 years) and higher drinking-water concentrations (> 600 µg/L) (Chen CJ et al. 1992; Chen CL et al. 2010b; Chiou et al. 2001; Gibb et al. 2011; Marshall et al. 2007). For kidney cancer, mortality rates increased in a dose-dependent manner for drinking-water concentrations ranging from 170 to 800 µg/L in Taiwan (Chen CJ et al. 1988a); the MRRs at 800 µg/L were 196 for men and 37.0 for women. Results from other studies in Taiwan support this finding (Smith et al. 1992). More studies with larger sample sizes are warranted to evaluate associations at drinking-water concentrations of < 100 µg/L.
A causal association between arsenic exposure and liver cancer, particularly liver angiosarcoma, was suspected as early as 1957, and several studies have substantiated that suspicion (e.g., Liaw et al. 2008; Smith et al. 1992). A number of studies from the Taiwan cohort have demonstrated increases in liver cancer deaths with increasing concentrations of arsenic in drinking water. For example, a significant dose-dependent linear trend in MRRs for liver cancer was reported with increasing arsenic concentrations in drinking water ranging from 170 to 800 µg/L (Chen CJ et al. 1988a; Wu et al. 1989). Links between arsenic exposure and liver cancer have also been supported by other reports (Chen CL et al. 2010b; Chen and Ahsan 2004; Chiu et al. 2004; Liaw et al. 2008; Morales et al. 2000). Taking epidemiological, rodent, and in vitro studies together, the evidence shows that the liver is a target organ of arsenic carcinogenicity (Liu and Waalkes 2008).
Other effects on multiple bodily systems. A multitude of other health effects are linked to chronic arsenic exposure. These arsenic-associated health problems affect nearly every major organ and organ system in the body (Table 3). A comprehensive review of the literature for these effects is beyond the scope of this review; therefore, this section addresses the broad range of harmful effects of arsenic in the human body and makes apparent the impact of arsenic-contaminated drinking water on public health. Taken together, the body of data drives home the critical importance of monitoring for arsenic in food sources and drinking-water sources, including private wells.
Significant neurological impairments are evident in children and adults who exhibit impaired cognitive abilities and motor functions after arsenic exposure (Chen Y et al. 2009; Dong and Su 2009; Gong et al. 2011; Hamadani et al. 2011; Parvez et al. 2011; Vahidnia et al. 2007; Wasserman et al. 2004, 2007). Cognitive impairments were observed in children at 6 and 10 years of age (Wasserman et al. 2004, 2007). One recent study reported impairments in verbal and full-scale IQ in girls but not boys (Hamadani et al. 2011). In adults, arsenic exposure in drinking water is linked to significantly lower scores on tests of cognitive ability as well as lower education levels (Gong et al. 2011). Peripheral neuropathy and painful muscle spasms are also known to occur with arsenic exposure (Sengupta et al. 2008; Vahidnia et al. 2007).
In addition to lung cancer, chronic arsenic exposure is associated with other respiratory system effects. Mortality from pulmonary tuberculosis was increased in arsenic-exposed individuals in the Chilean cohort (Smith et al. 2011). In the same cohort, increased mortality from bronchiectasis was significant for those exposed to arsenic during early life with a standardized mortality ratio (SMR) of 50.1 (Table 4) (Smith et al. 2006). Reduced forced expiratory volume and forced vital capacity is associated with early-life exposure to arsenic, with a magnitude of reduction similar to smoking throughout adulthood (Dauphine et al. 2011). Other respiratory symptoms include chronic cough, blood in the sputum, and other breathing problems (Parvez et al. 2010).
The cardiovascular system is affected in several ways by arsenic (Abhyankar et al. 2012; Chen Y et al. 2009, 2011; States et al. 2009; Yuan et al. 2007). Cardiovascular effects include carotid atherosclerosis (Huang et al. 2009) and ischemic heart disease (Abhyankar et al. 2012; Chen Y et al. 2011; States et al. 2009). Furthermore, an association between hypertension and arsenic exposure is evident in some studies, and additional larger studies are needed to substantiate the link (Abhyankar et al. 2012; Abir et al. 2012).
Immune system effects of arsenic exposure are evident in several contexts. Effects include altered immune-related gene expression and cytokine production in lymphocytes (Andrew et al. 2008; Morzadec et al. 2012) and in lung (Lantz et al. 2007). Arsenic is significantly associated with increased infant morbidity from infectious diseases (Rahman et al. 2010b). Furthermore, maternal urinary arsenic during pregnancy is significantly associated with increased inflammation and reduced numbers of T cells as well as altered cytokine profiles in cord blood (Ahmed et al. 2011) and reduced thymic function in infants (Ahmed et al. 2012).
Multiple endocrine effects of arsenic exposure are suggested from studies in human and animal studies. These include affecting hormone regulation via the retinoic acid, thyroid hormone, and estrogen receptors (Barr et al. 2009; Davey et al. 2007, 2008; Ettinger et al. 2009; Smith and Steinmaus 2009; Watson and Yager 2007). Increased occurrence of diabetes is also linked to arsenic exposure, particularly at higher doses and with exposure periods of > 10 years (Chen CJ et al. 2007; Del Razo et al. 2011; Islam et al. 2012; Jovanovic et al. 2012).
Varied Susceptibilities
Genetic and nutritional factors in susceptibility. The variety of biological systems often simultaneously affected by arsenic is further complicated by varied individual susceptibilities to its toxic effects. For example, interindividual variation in the ability to methylate arsenic is associated with differential susceptibility to the effects of arsenic exposure (Hall and Gamble 2012; Steinmaus et al. 2010). Genetic polymorphisms have also been shown to be a contributing factor (Agusa et al. 2012; Ahsan et al. 2007; Applebaum et al. 2007; Argos et al. 2012; Pierce et al. 2012; Porter et al. 2010; Reichard and Puga 2010). A recent large, comprehensive genome-wide association study identified specific genetic variations associated with risk for skin lesions as well as differences in arsenic metabolism (Pierce et al. 2012). Evidence is also building that nutritional factors, notably folate, appear to play an important role in arsenic methylation and elimination (Basu et al. 2011; Chen Y et al. 2009; Gamble et al. 2007; Hall and Gamble 2012; Pilsner et al. 2009). For example, low folate and hyperhomocysteinemia are associated with increased risk of skin lesions (Pilsner et al. 2009). Together, current information about arsenic metabolism across individuals sheds light on possibilities for new strategies for the prevention and amelioration of the toxicity of arsenic.
Susceptibility during development and long-term latency. Adverse pregnancy and developmental outcomes are associated with early-life exposure to arsenic (Vahter 2008). Arsenic exposure is significantly associated with increased infant mortality and, in some studies, increased spontaneous abortion and stillbirth (Milton et al. 2005; Rahman et al. 2010a; von Ehrenstein et al. 2006) as well as reduced birth weight (Rahman et al. 2009). Early-life arsenic exposure is also associated with neurological impairments in children (Hamadani et al. 2011; Parvez et al. 2011; Wasserman et al. 2004, 2007). For example, motor function in children, as well as verbal and full-scale IQ in girls, are both inversely associated with arsenic exposure (Hamadani et al. 2011; Parvez et al. 2011). Prenatal exposure also affects the developing immune system. Maternal urinary arsenic concentrations are associated with increased inflammation as well as altered cytokine profiles in cord blood and reduced thymus size and function in newborns (Ahmed et al. 2011, 2012). Altered immune responses are consistent with the observation of increased risk for lower respiratory infections and diarrhea in infants with increasing arsenic exposure (Rahman et al. 2010b).
The impacts of early-life arsenic exposure can continue into adulthood (Vahter 2008). Exposure during pregnancy and childhood is associated with an increased occurrence and/or severity of lung disease, cardiovascular disease, and cancer in childhood and later in life, with evidence of decades-long latency periods for these health conditions (Table 4) (Dauphine et al. 2011; Liaw et al. 2008; Marshall et al. 2007; Smith et al. 2011; Yuan et al. 2010). Childhood liver cancer MRRs were 9–14 times higher for those exposed as young children as compared with controls (Liaw et al. 2008). Other reports of latency periods extending over 50 years include skin cancer (Haque et al. 2003), urinary cancers (Bates et al. 2004; Chen CL et al. 2010b; Marshall et al. 2007; Su et al. 2011), and lung cancer (Marshall et al. 2007; Su et al. 2011). For example, peak SMRs for childhood liver cancer and bronchiectasis were 14.1 and 50.1 times higher, respectively, for individuals exposed to arsenic in utero and during childhood as compared with individuals exposed during other periods of their lives (Table 4) (Smith et al. 2006). Bladder cancer mortality peaked 25–36 years from the initiation of exposure (Marshall et al. 2007), and kidney cancer MRR peaked 21–25 years from initiation of exposure and was highest for women (Yuan et al. 2010). Regarding noncancer health effects, early-life arsenic exposure is associated with increased adult mortality from pulmonary tuberculosis (Smith et al. 2011), bronchiectasis (Smith et al. 2006), and myocardial infarction (Yuan et al. 2007).
Together the data indicate a sensitivity during development to health effects that can be long lasting and latent for > 50 years. The implications are profound and make it clear that every effort should be made to prevent exposure of pregnant women, women of childbearing age, infants, and children to arsenic in order to prevent a multitude of health effects, particularly cancer, later in life.
Conclusions
Environmental health issues are not limited to toxic waste sites and poisoning events: some deleterious exposures come from naturally occurring substances, such as arsenic often found in drinking water. Arsenic affects multiple biological systems, sometimes years or decades after exposure reductions. Studies that reveal the complex nature of the origins and toxicity of arsenic highlight the importance of heightened awareness of arsenic-related health effects in broader contexts than previously perceived. In spite of current efforts, over 200 million persons globally are at risk of arsenic exposure at levels of concern for human health. Although specific regulatory levels might be debatable, all would agree that minimizing arsenic exposure is the best solution, especially prenatal and early-life exposure. Therefore, testing drinking water for arsenic is particularly important for pregnant women and women of childbearing age, given the potential for neurological and other lifelong effects of early-life exposure. The return on the investment can be substantial when measured in the reduced incidence of chronic disease and reduced rates of cancer worldwide.
Footnotes
M.F.N. is supported through a contract with the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) (contract GS-OOF-0001S, Health and Human Services order CR700013). H.A. is supported by National Institutes of Health and NIEHS SRP grants P42ES10349, RO1CA107431, and RO1CA102484. J.G. is supported by NIEHS SRP grant P42ES10349.
The authors declare they have no actual or potential competing financial interests.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abir T, Rahman B, D’Este C, Farooq A, Milton AH.2012The association between chronic arsenic exposure and hypertension: a meta-analysis. J Toxicol 2012:198793; doi: 10.1155/2012/198793[Online 8 March 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya SK, Chakraborty P, Lahiri S, Raymahashay BC, Guha S, Bhowmik A. Arsenic poisoning in the Ganges delta. Nature. 1999;401(6753):545. doi: 10.1038/44052. [Brief Discussion] [DOI] [PubMed] [Google Scholar]
- Agusa T, Kunito T, Tue NM, Lan VT, Fujihara J, Takeshita H, et al. Individual variations in arsenic metabolism in Vietnamese: the association with arsenic exposure and GSTP1 genetic polymorphism. Metallomics. 2012;4(1):91–100. doi: 10.1039/c1mt00133g. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Ahsan KB, Kippler M, Mily A, Wagatsuma Y, Hoque AM, et al. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicol Sci. 2012;129(2):305–314. doi: 10.1093/toxsci/kfs202. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Khoda SM, Rekha RS, Gardner RM, Ameer SS, Moore S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119:258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, Jasmine F, et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16(2):191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- Akter KF, Owens G, Davey DE, Naidu R. Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol. 2005;184:97–149. doi: 10.1007/0-387-27565-7_3. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116:524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anning DW, Paul AP, McKinney TS, Huntington JM, Bexfield LM, Thiros SA. Predicted Nitrate and Arsenic Concentrations in Basin-Fill Aquifers of the Southwestern United States. 2012. Available: http://pubs.usgs.gov/sir/2012/5065/ [accessed 1 October 2012]
- Applebaum KM, Karagas MR, Hunter DJ, Catalano PJ, Byler SH, Morris S, et al. Polymorphisms in nucleotide excision repair genes, arsenic exposure, and non-melanoma skin cancer in New Hampshire. Environ Health Perspect. 2007;115:1231–1236. doi: 10.1289/ehp.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Ahsan H, Graziano JH.2012Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health; doi: 10.1515/reveh-2012-0021[Online 10 September 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Kalra T, Pierce BL, Chen Y, Parvez F, Islam T, et al. A prospective study of arsenic exposure from drinking water and incidence of skin lesions in Bangladesh. Am J Epidemiol. 2011;174(2):185–194. doi: 10.1093/aje/kwr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376(9737):252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante KA, Agusa T, Subramanian A, Ansa-Asare OD, Biney CA, Tanabe S. Contamination status of arsenic and other trace elements in drinking water and residents from Tarkwa, a historic mining township in Ghana. Chemosphere. 2007;66(8):1513–1522. doi: 10.1016/j.chemosphere.2006.08.022. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Arsenic Toxicological Profile. Atlanta, GA:ATSDR. 2007. Available: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=22&tid=3 [accessed 2 October 2012] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry) Case Studies in Environmental Medicine: Arsenic Toxicity. Atlanta, GA:ATSDR. 2011a. Available: http://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0 [accessed 2 October 2012] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry) The ATSDR 2011 Substance Priority List. Atlanta, GA:ATSDR. 2011b. Available: http://www.atsdr.cdc.gov/SPL/index.html [accessed 2 October 2012]
- Ayotte JD, Montgomery DL, Flanagan SM, Robinson KW. Arsenic in groundwater in eastern New England: occurrence, controls, and human health implications. Environ Sci Technol. 2003;37(10):2075–2083. doi: 10.1021/es026211g. [DOI] [PubMed] [Google Scholar]
- Barr FD, Krohmer LJ, Hamilton JW, Sheldon LA.2009Disruption of histone modification and CARM1 recruitment by arsenic represses transcription at glucocorticoid receptor-regulated promoters. PLoS One. 48e6766; doi: 10.1371/journal.pone.0006766[Online 27 August 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Mitra S, Chung J, Guha Mazumder DN, Ghose N, Kalman DA, et al. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect. 2011;119:1308–1313. doi: 10.1289/ehp.1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, et al. Case-control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol. 2004;159(4):381–389. doi: 10.1093/aje/kwh054. [DOI] [PubMed] [Google Scholar]
- Biggs ML, Haque R, Moore L, Smith A, Ferreccio C, Hopenhayn-Rich C. Arsenic-laced water in Chile. Science. 1998;281(5378):785. doi: 10.1126/science.281.5378.783g. [Letter] [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Dart RC. 2010 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol. 2011;49(10):910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Meza MM, Josyula AB, Poplin GS, Kopplin MJ, McClellen HE, et al. Environmental arsenic exposure and urinary 8-OHdG in Arizona and Sonora. Clin Toxicol. 2007;45(5):490–498. doi: 10.1080/15563650701354119. [DOI] [PubMed] [Google Scholar]
- Calderón J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, et al. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res. 2001;85(2):69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Camacho LM, Gutiérrez M, Alarcón-Herrera MT, Villalba Mde L, Deng S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere. 2011;83(3):211–225. doi: 10.1016/j.chemosphere.2010.12.067. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Brit J Cancer. 1992;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. 1988a;1(8582):414–415. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wang SL, Chiou JM, Tseng CH, Chiou HY, Hsueh YM, et al. Arsenic and diabetes and hypertension in human populations: a review. Toxicol Applied Pharmacol. 2007;222(3):298–304. doi: 10.1016/j.taap.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wu MM, Lee SS, Wang JD, Cheng SH, Wu HY. Atherogenicity and carcinogenicity of high-arsenic artesian well water: multiple risk factors and related malignant neoplasms of blackfoot disease. Arteriosclerosis. 1988b;8(5):452–460. doi: 10.1161/01.atv.8.5.452. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Chen CJ. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ Res. 2010a;110(5):455–462. doi: 10.1016/j.envres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Wang YH, et al. Arsenic in drinking water and risk of urinary tract cancer: a follow-up study from northeastern Taiwan. Cancer Epidemiol Biomarkers Prev. 2010b;19(1):101–110. doi: 10.1158/1055-9965.EPI-09-0333. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ahsan H. Cancer burden from arsenic in drinking water in Bangladesh. Am J Public Health. 2004;94(5):741–744. doi: 10.2105/ajph.94.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. 2011Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ 342d2431; doi: 10.1136/bmj.d2431[Online 5 May 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009;239(2):184–192. doi: 10.1016/j.taap.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, et al. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol. 2001;153(5):411–418. doi: 10.1093/aje/153.5.411. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Ho SC, Wang LY, Wu TN, Yang CY. Does arsenic exposure increase the risk for liver cancer? J Toxicol Environ Health A. 2004;67(19):1491–1500. doi: 10.1080/15287390490486806. [DOI] [PubMed] [Google Scholar]
- Das NK, Sengupta SR. Arsenicosis: diagnosis and treatment. Indian J Dermatol Venereol Leprol. 2008;74(6):571–581. doi: 10.4103/0378-6323.45098. [DOI] [PubMed] [Google Scholar]
- Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84(6):591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci. 2007;98(1):75–86. doi: 10.1093/toxsci/kfm013. [DOI] [PubMed] [Google Scholar]
- Davey JC, Nomikos AP, Wungjiranirun M, Sherman JR, Ingram L, Batki C, et al. Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis. Environ Health Perspect. 2008;116:165–172. doi: 10.1289/ehp.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo L, García-Vargas G, Valenzuela O, Castellanos E, Sánchez-Peña L, Currier J, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health. 2011;10(1):73–84. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Su SY. The association between arsenic and children’s intelligence: a meta-analysis. Biol Trace Elem Res. 2009;129(103):88–93. doi: 10.1007/s12011-008-8298-1. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Scientific Opinion on Arsenic in Food. EFSA J. 2009;7(10):60–71. [Google Scholar]
- Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H, et al. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect. 2009;117:1059–1064. doi: 10.1289/ehp0800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) Arsenic in Rice. 2012. Available: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Metals/ucm319870.htm [accessed 1 October 2012]
- Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11(6):673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51(2):257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86(4):1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb H, Haver C, Gaylor D, Ramasamy S, Lee JS, Lobdell D, et al. Utility of recent studies to assess the National Research Council 2001 estimates of cancer risk from ingested arsenic. Environ Health Perspect. 2011;119:284–290. doi: 10.1289/ehp.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in U.S. women. Proc Natl Acad Sci USA. 2011;108(51):20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Hargrave KA, Hobson V, Spallholz J, Boylan M, Lefforge D, et al. Low-level groundwater arsenic exposure impacts cognition: a project FRONTIER study. J Environ Health. 2011;74(2):16–22. [PubMed] [Google Scholar]
- Gong G, O’Bryant SE. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environ Res. 2012;113:52–57. doi: 10.1016/j.envres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Hall MN, Gamble MV.2012Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol 2012:595307, doi: [Online 14 March 2012] 10.1155/2012/595307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40(6):1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Haque R, Mazumder DN, Samanta S, Ghosh N, Kalman D, Smith MM, et al. Arsenic in drinking water and skin lesions: dose-response data from West Bengal, India. Epidemiology. 2003;14(2):174–182. doi: 10.1097/01.EDE.0000040361.55051.54. [DOI] [PubMed] [Google Scholar]
- Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117:1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407(8):2608–2614. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts. Lyon:World Health Organization Press. 2012. Available: http://monographs.iarc.fr/ENG/Monographs/vol100C/ [accessed 2 October 2012]
- Intarasunanont P, Navasumrit P, Woraprasit S, Chaisatra K, Suk WA, Mahidol C, et al. 2012Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ Health 11131; doi: 10.1186/1476-069X-11-31[Online 2 May 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MRD, Khan IP, Hassan SMD, McEvoy MM, D’Este CP, Attia JP, et al. 2012Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 11138; doi: 10.1186/1476-069X-11-38[Online 7 June 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. Arsenic, organic foods, and brown rice syrup. Environ Health Perspect. 2012;120:623–626. doi: 10.1289/ehp.1104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic D, Rasic-Milutinovic Z, Paunovic K, Jakovljevic B, Plavsic S, Milosevic J.2012Low levels of arsenic in drinking water and type 2 diabetes in Middle Banat region, Serbia. Int J Hyg Environ Health; doi: 10.1016/j.ijheh.2012.01.001[Online 10 February 2012] [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, et al. Skin cancer risk in relation to toenail arsenic concentrations in a U.S. population-based case-control study. Am J Epidemiol. 2001;153(6):559–565. doi: 10.1093/aje/153.6.559. [DOI] [PubMed] [Google Scholar]
- Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Perspect. 2012;120:1061–1066. doi: 10.1289/ehp.1104173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburgh D, Smedley P. Arsenic Contamination of Groundwater in Bangladesh. Vol. 2: Final Report. Keyworth: British Geological Survey and Department of Public Health Engineering (Bangladesh). 2001. Available: http://www.bgs.ac.uk/downloads/directDownload.cfm?id=2223&noexcl=true&t=Phase%202%20Volume%202%3A%20Final%20Report%20cover%2C%20preliminary%20 [accessed 23 January 2013]
- Lantz RC, Lynch BJ, Boitano S, Poplin GS, Littau S, Tsaprailis G, et al. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic. Environ Health Perspect. 2007;115:586–591. doi: 10.1289/ehp.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1982–1987. doi: 10.1158/1055-9965.EPI-07-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008;105(1):24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G, Ferreccio C, Yuan Y, Bates MN, Steinmaus C, Selvin S, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;99(12):920–928. doi: 10.1093/jnci/djm004. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Environmental Protection. Certified Laboratories for Testing Arsenic & Uranium. 2011. Available: http://www.mass.gov/dep/water/drinking/au/aulabs.htm [accessed 16 December 2012]
- Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ Res. 2004;96(2):119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Meza MM, Yu L, Rodriguez YY, Guild M, Thompson D, Gandolfi AJ, et al. Developmentally restricted genetic determinants of human arsenic metabolism: association between urinary methylated arsenic and CYT19 polymorphisms in children. Environ Health Perspect. 2005;113:775–781. doi: 10.1289/ehp.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, et al. Chronic arsenic exposure and adverse pregnancy outcomes in Bangladesh. Epidemiology. 2005;16(1):82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- Moore LE, Wiencke JK, Bates MN, Zheng S, Rey OA, Smith AH. Investigation of genetic polymorphisms and smoking in a bladder cancer case–control study in Argentina. Cancer Lett. 2004;211(2):199–207. doi: 10.1016/j.canlet.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–661. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzadec C, Bouezzedine F, Macoch M, Fardel O, Vernhet L. Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology. 2012;300(1–2):46–56. doi: 10.1016/j.tox.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Raber G, Francesconi KA, Navas-Acien A, Love DC. Arsenic species in poultry feather meal. Sci Total Environ. 2012;417–418:183–188. doi: 10.1016/j.scitotenv.2011.12.022. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council. National Water Quality Management Strategy: Australian Drinking Water Guidelines 6. Canberra:National Health and Medical Research Council. 2011. Available: http://www.nhmrc.gov.au/guidelines/publications/eh52 [accessed 2 October 2012]
- National Research Council. Washington, DC: National Academy Press; 2001. Arsenic in Drinking Water: 2001 Update. [Google Scholar]
- National Toxicology Program. Report on Carcinogens, 12th ed. Research Triangle Park, NC:NTP. 2011. Available: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/Arsenic.pdf [accessed 2 October 2012]
- Nielsen MG, Lombard PJ, Schalk LK. Assessment of arsenic concentrations in domestic well water, by town, in Maine, 2005–09: U.S. Geological Survey Scientific Investigations Report 2010–5199. 2010. Available: http://pubs.usgs.gov/sir/2010/5199/ [accessed 2 October 2012]
- Norton GJ, Pinson SR, Alexander J, McKay S, Hansen H, Duan GL, et al. Variation in grain arsenic assessed in a diverse panel of rice (Oryza sativa) grown in multiple sites. New Phytol. 2012;193(3):650–664. doi: 10.1111/j.1469-8137.2011.03983.x. [DOI] [PubMed] [Google Scholar]
- NRDC (National Resources Defense Council) Arsenic and old laws: a scientific and public health analysis of arsenic occurrence in drinking water, its health effects, and EPA’s outdated arsenic tap water standard. 2000. Available: http://www.nrdc.org/water/drinking/arsenic/aolinx.asp [accessed 2 October 2012]
- Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 2010;65(6):528–533. doi: 10.1136/thx.2009.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SC. Arsenic in groundwaters in the Northern Appalachian Mountain belt: a review of patterns and processes. J Contam Hydrol. 2008;99(1–4):8–21. doi: 10.1016/j.jconhyd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Argos M, Chen Y, Melkonian S, Parvez F, Islam T, et al. Arsenic exposure, dietary patterns, and skin lesion risk in Bangladesh: a prospective study. Am J Epidemiol. 2010;173(3):345–354. doi: 10.1093/aje/kwq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Kibriya MG, Tong L, Jasmine F, Argos M, Roy S, et al. 2012Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet 82e1002522; doi: 10.1371/journal.pgen.1002522[Online 23 February 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect. 2009;117:254–260. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KE, Basu A, Hubbard AE, Bates MN, Kalman D, Rey O, et al. Association of genetic variation in cystathionine-β-synthase and arsenic metabolism. Environ Res. 2010;110:580–587. doi: 10.1016/j.envres.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putila JJ, Guo NL.2011Association of arsenic exposure with lung cancer incidence rates in the United States. PloS One. 610e25886; doi: 10.1371/journal.pone.0025886[Online 18 October 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010a;21(6):797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2010b;119:719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2(1):87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;19:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45(7):1263–1267. doi: 10.1016/j.fct.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Messier KP, Shehee M, Rudo K, Serre ML, Fry RC. Arsenic in North Carolina: public health implications. Environ Int. 2012;38(1):10–16. doi: 10.1016/j.envint.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta SR, Das NK, Datta PK. Pathogenesis, clinical features and pathology of chronic arsenicosis. Indian J Dermatol Venereol Leprol. 2008;74(6):559–570. [PubMed] [Google Scholar]
- Smedley P. Arsenic in rural groundwater in Ghana. J African Earth Sci. 1996;22:459–470. [Google Scholar]
- Smith AH, Arroyo AP, Mazumder DN, Kosnett MJ, Hernandez AL, Beeris M, et al. Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect. 2000a;108:617–620. doi: 10.1289/ehp.00108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol. 2009;19(4):343–348. doi: 10.1038/jes.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147(7):660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, et al. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000b;78(9):1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Arsenic epidemiology and drinking water standards. Science. 2002;296(5576):2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Liaw J, Ferreccio C, Steinmaus C. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2011;173(4):414–420. doi: 10.1093/aje/kwq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey A. Arsenic and infectious disease: a potential factor in morbidity among Bangladeshi children. Environ Health Perspect. 2011;119:A218. doi: 10.1289/ehp.119-a218a. [Science Selection] [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC. Arsenic toxicology: translating between experimental models and human pathology. Environ Health Perspect. 2011;119:1356–1363. doi: 10.1289/ehp.1103441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247(2):138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CC, Lu JL, Tsai KY, Lian Ie B. Reduction in arsenic intake from water has different impacts on lung cancer and bladder cancer in an arseniasis endemic area in Taiwan. Cancer Causes Control. 2011;22(1):101–108. doi: 10.1007/s10552-010-9679-2. [DOI] [PubMed] [Google Scholar]
- Thundiyil JG, Yuan Y, Smith AH, Steinmaus C. Seasonal variation of arsenic concentration in wells in Nevada. Environ Res. 2007;104(3):367–373. doi: 10.1016/j.envres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(1):1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- Tseng WP. Effects and dose–response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency) Arsenic Rule. Washington, DC:U.S. EPA. 2001. Available: http://water.epa.gov/lawsregs/rulesregs/sdwa/arsenic/regulations.cfm [accessed 2 October 2012]
- Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity—a review. Hum Exp Toxicol. 2007;26(10):823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- Vahter M. Health effects of early life exposure to arsenic. Basic Clin Pharmacol Toxicol. 2008;102(2):204–211. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, Ghosh N, Yuan Y, Windham G, et al. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2006;163(7):662–669. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect. 2007;115:285–289. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, et al. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112:1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Water Quality Association. 2012. Arsenic. http://www.wqa.org/sitelogic.cfm?ID=2346 [accessed 16 December 2012]
- Watson WH, Yager JD. Arsenic: extension of its endocrine disruption potential to interference with estrogen receptor-mediated signaling. Toxicol Sci. 2007;98(1):1–4. doi: 10.1093/toxsci/kfm111. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) A field guide for detection, management and surveillance of arsenicosis cases. Geneva:WHO Press. 2005. Available: http://www.searo.who.int/LinkFiles/Publications_seaTP30.pdf [accessed 2 October 2012]
- WHO (World Health Organization) Guidelines for Drinking-water Quality: Incorporating First and Second Addenda to Third Edition. Vol. 1—Recommendations. Geneva:WHO Press. 2008. Available: http://www.who.int/water_sanitation_health/dwq/gdwq3/en/index.html [accessed 1 October 2012]
- Winkel LH, Pham TK, Vi ML, Stengel C, Amini M, Nguyen TH, et al. Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. Proc Natl Acad Sci USA. 2011;108(4):1246–1251. doi: 10.1073/pnas.1011915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Jung HB, Culbertson CW, Marvinney RG, Loiselle MC, Locke DB, et al. Spatial pattern of groundwater arsenic occurrence and association with bedrock geology in greater Augusta, Maine. Environ Sci Technol. 2009;43(8):2714–2719. doi: 10.1021/es803141m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Sun D, Zheng Y. Health effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrence. Environ Health Perspect. 2007;115(4):636–642. doi: 10.1289/ehp.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J Biomed Sci. 2006;13(5):657–666. doi: 10.1007/s11373-006-9092-8. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Liaw J, Bates M, et al. Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;21(1):103–108. doi: 10.1097/EDE.0b013e3181c21e46. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166(12):1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]


