Abstract
A randomized study was planned to compare the effects of whey and egg albumin protein supplements in dialysis patients. Fifty adult patients were randomized to receive either whey protein or egg albumin as per their deficit calculated from K/DOQI recommendations. Actual intake was calculated from three-day dietary diary. Assessment of nutritional status was done by serum albumin and bioelectric impedance analysis (BIA). Repeat evaluation was done after 6 months. The mean initial intake of protein in whey and egg albumin group was 0.74 ± 0.3 vs. 0.69 ± 0.2 g/kg/day, (P = 0.5) and calorie intake was 20 ± 5.6 vs. 20.5 ± 5.1 kcal/kg/day, (P = 0.8), respectively. Out of 50 patients, two died within 2 months and were excluded from the study and 14 (28%) dropped out within one month because of side effects. The most common side effect in drop-outs was nausea and vomiting (43%). Out of remaining 34 patients who completed the study, 80% could not consume >50% of the recommended supplement because of side effects. The protein and calorie intake remained similar at baseline and 6 months in both the groups. The main side effects in whey group were bloating and nausea with vomiting, and in egg protein group were nausea with vomiting, bloating and anorexia. Oral protein supplements were not tolerated in dialysis patients and side effects resulted in high degree of non-compliance.
Keywords: Dialysis, malnutrition, protein supplement
Introduction
Malnutrition is common in dialysis patients.[1–3] It predicts morbidity and mortality in hemodialysis[1,4,5] as well as peritoneal dialysis.[6] Hence, nutritional supplements are routinely suggested in such patients to keep their nutritional status appropriate.[7,8] However, non-compliance is reported to be common, and long-term studies with oral protein supplements are not available. Different protein formulae with different protein concentrations are available in the market. The two common ingredients to supplement protein in such patients are whey protein and egg albumin. A comparative study was carried out to look for the effect of the two oral formulae supplements on nutritional parameters in dialysis population.
Materials and Methods
All adult patients on hemodialysis and peritoneal dialysis were eligible for the study. The total number of patients to be studied was 50, randomly divided into 25 patients to get whey protein and 25 to get egg albumin supplement. Both these proteins were commercially available with 100 gm of whey powder containing 46 g protein and 230 Kcal energy, and egg albumin powder containing 70 g protein and 316 Kcal energy per 100 gram. Patients, who were on thrice a week hemodialysis or more than 3 exchanges per day of peritoneal dialysis were included. Adequacy of hemodialysis was observed by urea reduction ratio (URR) every month, and for peritoneal dialysis by KT/V urea every 6 months. Vegetarians were excluded because randomization was between whey protein and egg protein. Patients with active infection in last four weeks, hepatitis B, hepatitis C, or HIV infection were also excluded. Patient enrolment was done on first come first basis after fulfilling the inclusion and exclusion criteria. After consent, at the enrolment, daily dietary intake of calories and proteins was calculated from 3-day dietary diary. Deficiency of calorie and protein intake was calculated from standard requirement and actual intake was calculated from the dietary diary. Standard requirement was fixed at 35 kcal/kg of calories and 1.2 g/kg of protein in hemodialysis patients and 1.3 g/kg of protein in peritoneal dialysis patients.[8] Patients were divided into two groups according to random number generated by computer. Group A was given whey protein supplement and group B was given egg protein powder as supplement.
According to randomization and protein deficit, each patient was given the protein supplement to match the requirement and counseled to improve their calorie intake. Weekly feedback was taken in dialysis unit (hemodialysis patients) or on telephone (peritoneal dialysis patients) and compliance emphasized. Monthly supply of the protein supplements were given on ‘as required’ or on monthly visit to the clinic. Side effects or any other incident was noted accordingly. The amount of supplement consumed was calculated from the refills taken, divided by number of days.
Bioelectrical impedance analysis (BIA) was carried out for ‘body fat mass’ (BFM) and ‘fat-free mass’ (FFM). BIA was carried out by direct segmental multi-frequency method using ‘In-Body-230 body composition analyzer’ (Biospace 1996-2009, Biospace Co. Ltd). Baseline laboratory investigations were also carried out. At the end of 6 months, BIA and investigations were repeated. Their actual calorie and protein intake at 6 months was also calculated from three-day dietary diary. The two groups were then compared.
Continuous variables were recorded as mean and standard deviation and compared between the two groups using independent sample T-test. Comparison of variables between baseline and at 6 months was carried out by paired t-test. Categorical variables were compared using Chi-square test. Data were analyzed using SPSS 17 (SPSS Inc. Chicago, IL, USA).
Results
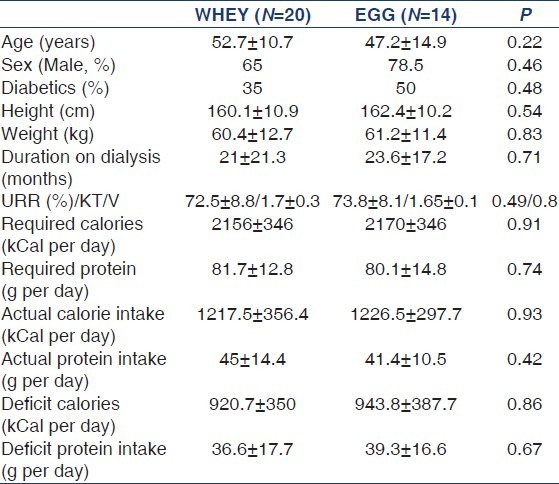
Out of 50 patients randomized, 14 (28%) patients were excluded because of side effects leading to discontinuation of the supplements within the first month. Two patients died within 2 months of enrolment and were also excluded, cause of death being sudden cardiac death and myocardial infarction respectively. Finally, only 34 patients could complete the study [Figure 1]. Sixteen out of 34 patients (47%) were on peritoneal dialysis. Table 1 shows the demographic profile and initial dietary intake between the two groups, which was similar. The mean initial daily protein intake was 0.74 ± 0.3 vs. 0.69 ± 0.2 g/kg, P = 0.5 (45 ± 14.4 vs. 41.4 ± 10.5, P = 0.4) and calorie intake was 20 ± 5.6 vs. 20.5 ± 5.1 kcal/kg, P = 0.8 (1217.5 ± 356.4 vs. 1226.5 ± 297.7 kCal/d, P = 0.9) in whey and egg albumin group respectively. The deficit calculated was almost half the required intake (protein deficit 45% in whey and 49% in egg albumin; calories deficit 56% in both the groups). The deficit was intended to be supplemented by respective protein formulae divided in two to three servings.
Figure 1.
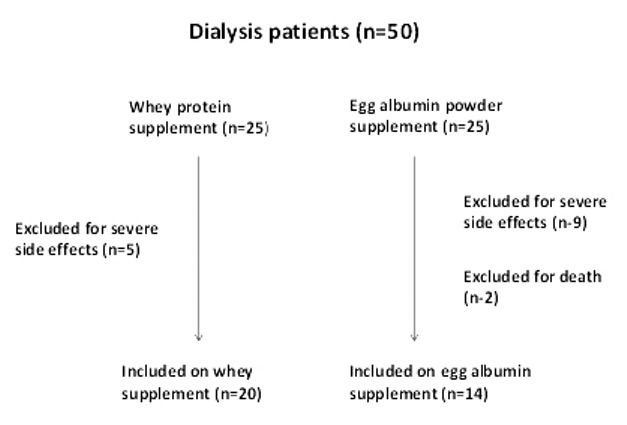
Flow of patients into the study
Table 1.
Demographic profile of patients included in the study
Many patients became irregular in their ‘refills’ after initial few weeks. Despite repeated counseling, significant number withdrew complaining of intolerance (as mentioned above and excluded from the study). Remaining patients wanted to continue with the supplements, but at decreased quantity, as higher quantity was intolerant.
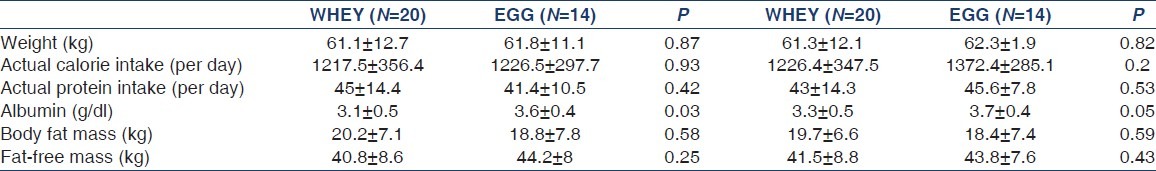
Comparison of dietary intake of proteins and calories, serum albumin, body fat mass and fat free mass at baseline and at 6 months are shown in Table 2. There was no difference in any of these parameters between or within the groups. The actual total intake of proteins and calories remained the same despite suggested protein formulae supplementation. The body fat mass and fat-free mass were also same for pre- and post- intervention. Other biochemical laboratory tests were also comparable between the groups (data not shown).
Table 2.
Dietary intake, serum albumin, and bioelectric impedance analysis comparison between the two groups pre- and post-intervention
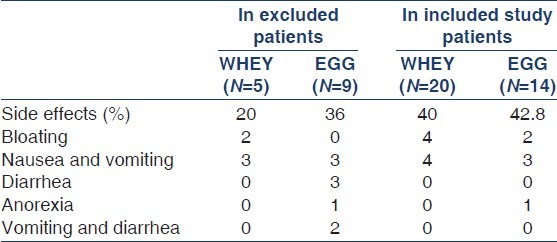
During the follow-up, despite regular counseling and emphasizing the need for protein supplementation, only 20% (7/34) patients were estimated to consume >50% of suggested protein formula. Rest 80% patients were infrequent and taking <50% of the suggested quantity because of intolerance and side effects. The side effects are shown in Table 3. Predominant side effects were bloating, nausea, and vomiting. The side effects were equal in both the groups. Patients also reported intolerance whenever they tried to increase the quantity of the supplement.
Table 3.
Side effects noted in patients in both the groups leading to drop out as well as in patients completing the study
Discussion
Malnutrition is a strong adverse predictive marker in outcome of dialysis patients.[1,4–6] Our study showed significant reduction in protein and calorie intake in our dialysis patients. On an average they took 50% of the recommended calories and proteins per day. Low intake was also shown in the HEMO study cohort, where 76% patients had calorie intake of <28 kcal/kg/day and 61% patients had protein intake of <1 gm/kg/day.[3]
Protein supplements are recommended to correct the deficit and improve outcomes.[7,8] Kopple also mentions lack of randomized studies after observing the effects of nutritional supplements.[8] Cockram et al.[9] studied the effects of medical nutritional products in hemodialysis patients and found good tolerability but in short-term study of 10 days. Phillips[10] studied with oral essential amino acid supplements in only 16 patients. Caglar et al.[11] used oral supplements during dialysis, to improve the compliance. The supplements had only 16.6 g of protein and reported 31% drop outs. We tried a larger, long-term study on the effects of protein supplement in dialysis patients. In our study, when supplemented with protein formulae, 28% patients discontinued the supplements because of poor tolerance and severe side effects. The predominant side effect was gastrointestinal with bloating and vomiting being most frequent. Even in those who continued, the intake was below 50% of the suggested quantity, again because of side effects. Patients limited their intake of supplements to avoid side effects and complained fear of increasing the quantity recommended. Hence, at the end of the study, no increase in total protein or calories intake was recorded in either group. It was not surprising that no difference was observed in any of the nutritional parameters like serum albumin or fat-free mass (BIA).
Protein assimilation (digestion and absorption) is impaired in small intestine in chronic kidney disease[12] as well as end-stage renal disease patients on dialysis (both hemodialysis and peritoneal dialysis), as shown by Bammens et al.[13] Undigested protein passes into the colon and is then fermented by the microbial flora of the colon forming metabolites like thiols, phenols, ammonia, indoles, and amines, which are potentially toxic.[14] Gastric acid suppression by drugs, which many of these patients are on, also leads to decreased protein assimilation and increased fermentation in the colon.[15] Supplementary load of dietary protein can further enhance fermentation process[16] Many of these fermented metabolites have been shown to be associated with gastro-intestinal diseases and malabsorption.[14] So, it is likely that poor protein assimilation, compounded by gastric acid suppressive drugs and excess protein load leads to increased fermentation of undigested proteins resulting in toxic metabolites, which may be the actual pathogenesis of adverse effects seen in these patients.
Despite several limitations, this study gives an important insight in the management of malnutrition in dialysis patients. This study shows that oral protein supplements in high dose and for longer period of time are not well tolerated in dialysis patients. Another drawback is the heterogeneous population of hemodialysis and peritoneal dialysis patients studied together. However, similar studies have been carried out earlier to compare the effect of protein supplements in physiologically similar set of patients.[17,18] It can be concluded from our study that both protein and calorie intakes are poor in our dialysis patients and oral supplements are poorly tolerated amounting to significant non-compliance. It is possible that instead of oral protein supplements, parenteral protein supplements may be a better alternative in managing malnutrition in dialysis patients. Further studies may be required comparing parenteral versus oral supplements to prove the hypothesis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis. 1994;24:1002–9. doi: 10.1016/s0272-6386(12)81075-4. [DOI] [PubMed] [Google Scholar]
- 2.Sharma RK, Sahu KM. Nutrition in dialysis patients. J Indian Med Assoc. 2001;99:210–1. [PubMed] [Google Scholar]
- 3.Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, et al. Nutritional status in the HEMO study cohort at baseline. Hemodialysis. Am J Kidney Dis. 2002;39:245–56. doi: 10.1053/ajkd.2002.30543. [DOI] [PubMed] [Google Scholar]
- 4.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–45. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR. Relationship between serum protein and mortality in adults on long-term hemodialysis: Exhaustive review and meta-analysis. Nutrition. 2010;26:10–32. doi: 10.1016/j.nut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in chronic peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 7.Kopple JD. Therapeutic approaches to malnutrition in chronic dialysis patients: The different modalities of nutritional support. Am J Kidney Dis. 1999;33:180–5. doi: 10.1016/s0272-6386(99)70280-5. [DOI] [PubMed] [Google Scholar]
- 8.Kopple JD National Kidney Foundation K/DOQI Work Group. The national kidney foundation K/DOQI clinical practice guidelines for dietary protein intake for chronic dialysis patients. Am J Kidney Dis. 2001;38:S68–73. doi: 10.1053/ajkd.2001.27578. [DOI] [PubMed] [Google Scholar]
- 9.Cockram DB, Hensley MK, Rodriguez M, Agarwal G, Wennberg A, Ruey P, et al. Safety and tolerance of medical nutritional products as sole sources of nutrition in people on hemodialysis. J Ren Nutr. 1998;8:25–33. doi: 10.1016/s1051-2276(98)90034-6. [DOI] [PubMed] [Google Scholar]
- 10.Phillips ME, Havard J, Howard JP. Oral essential amino acid supplementation in patients on maintenance hemodialysis. Clin Nephrol. 1978;9:241–8. [PubMed] [Google Scholar]
- 11.Caglar K, Fedje L, Dimmitt R, Hakim RM, Shyr Y, Ikizler TA. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002;62:1054–9. doi: 10.1046/j.1523-1755.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- 12.Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003;64:2196–203. doi: 10.1046/j.1523-1755.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- 13.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Impairment of small intestinal protein assimilation in patients with end-stage renal disease: Extending the malnutrition-inflammation-atherosclerosis concept. Am J Clin Nutr. 2004;80:1536–43. doi: 10.1093/ajcn/80.6.1536. [DOI] [PubMed] [Google Scholar]
- 14.McFarlane GT, Cummings JH. The colonic flora, fermentation, and large bowel digestive function. In: Phillips SF, Pemberton H, Shorter RG, editors. The large intestine: Physiology, Pathophysiology and disease. New York: Raven press; 1991. pp. 51–92. [Google Scholar]
- 15.Evenepoel P, Claus D, Geypens B, Maes B, Hiele M, Rutgeerts P, et al. Evidence for impaired assimilation and increased colonic fermentation of protein, related to gastric acid suppression therapy. Aliment Pharmacol Ther. 1998;12:1011–9. doi: 10.1046/j.1365-2036.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 16.Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, Peeters M, et al. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut. 1997;41:70–6. doi: 10.1136/gut.41.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eustace JA, Coresh J, Kutchey C, Te PL, Gimenez LF, Scheel PJ, et al. Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int. 2000;57:2527–38. doi: 10.1046/j.1523-1755.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 18.Moretti HD, Johnson AM, Keeling-Hathaway TJ. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. J Ren Nutr. 2009;19:298–303. doi: 10.1053/j.jrn.2009.01.029. [DOI] [PubMed] [Google Scholar]


