Abstract
BACKGROUND
The global obesity epidemic has paralleled a decrease in semen quality. Yet, the association between obesity and sperm parameters remains controversial. The purpose of this report was to update the evidence on the association between BMI and sperm count through a systematic review with meta-analysis.
METHODS
A systematic review of available literature (with no language restriction) was performed to investigate the impact of BMI on sperm count. Relevant studies published until June 2012 were identified from a Pubmed and EMBASE search. We also included unpublished data (n = 717 men) obtained from the Infertility Center of Bondy, France. Abstracts of relevant articles were examined and studies that could be included in this review were retrieved. Authors of relevant studies for the meta-analysis were contacted by email and asked to provide standardized data.
RESULTS
A total of 21 studies were included in the meta-analysis, resulting in a sample of 13 077 men from the general population and attending fertility clinics. Data were stratified according to the total sperm count as normozoospermia, oligozoospermia and azoospermia. Standardized weighted mean differences in sperm concentration did not differ significantly across BMI categories. There was a J-shaped relationship between BMI categories and risk of oligozoospermia or azoospermia. Compared with men of normal weight, the odds ratio (95% confidence interval) for oligozoospermia or azoospermia was 1.15 (0.93–1.43) for underweight, 1.11 (1.01–1.21) for overweight, 1.28 (1.06–1.55) for obese and 2.04 (1.59–2.62) for morbidly obese men.
CONCLUSIONS
Overweight and obesity were associated with an increased prevalence of azoospermia or oligozoospermia. The main limitation of this report is that studied populations varied, with men recruited from both the general population and infertile couples. Whether weight normalization could improve sperm parameters should be evaluated further.
Keywords: obesity, BMI, sperm concentration, total sperm count, meta-analysis
Introduction
Subfertility affects ∼15% of couples who seek to obtain a pregnancy and a male contribution is identified in 20–50% of the cases (Thonneau et al., 1991). A gradual decrease in sperm quality since the 1970s, particularly of sperm count, has been suggested by two meta-analyses (Carlsen et al., 1992; Swan and Elkin, 1999). This reported secular trend has traditionally been attributed to various methodological (standardization of the techniques, abstinence delay) or environmental (geography, season, genetic, ethnic group, tobacco, toxins) factors (Jouannet et al., 2001) but has also coincided with a worldwide increase in the prevalence of overweight and obesity (Finucane et al., 2011).
The association between high adiposity and subfertility has not been clearly demonstrated in men. Data from three large-scale epidemiological studies suggest an elevated risk for infertility among couples when the male partner is overweight or obese (Sallmen et al., 2006; Nguyen et al., 2007; Ramlau-Hansen et al., 2007). Results of studies investigating the links between BMI and sperm parameters, the gold standard for evaluation of male fertility potential, remain controversial. Several reports have shown an inverse correlation between BMI and sperm concentration or total sperm count (TSC) (Jensen et al., 2004; Paasch et al., 2010) but others have failed to document this association (Aggerholm et al., 2008; Duits et al., 2010). A previous meta-analysis published in 2010 concluded that there was no evidence of an association between BMI and sperm concentration or TSC (MacDonald et al., 2010). However, data from most studies could not be aggregated for the meta-analysis and the conclusion was based on five publications only (Jensen et al., 2004; Koloszar et al., 2005; Fejes et al., 2006; Qin et al., 2007; Aggerholm et al., 2008). Moreover, ∼30 original studies have been published since then. In a preliminary report, we showed that overweight and obesity were associated with an increased risk of presenting with oligozoospermia or azoospermia, compared with normal weight (Sermondade et al., 2012a).
The purpose of the current study is to update the systematic review on the relationship between BMI and sperm count and to perform a meta-analysis.
Methods
Literature search
A systematic review of available literature was performed to investigate the impact of BMI on sperm parameters in human males according to the PRISMA statement (Liberati et al., 2009). Relevant studies published until June 2012 were identified from PubMed and EMBASE using a combined free text and the following MeSH search strategy: (‘overweight’ OR ‘weight’ OR ‘obesity’ OR ‘BMI’ OR ‘body fat’ OR ‘body weight’ OR ‘body mass index’ OR ‘adiposity’) AND (‘sperm’ OR ‘semen’ OR ‘spermatozoa’ OR ‘sperm count’ OR ‘sperm concentration’ OR ‘semen quality’ OR ‘semen parameters’ OR ‘sperm quantity’ OR ‘total sperm count’ OR ‘oligozoospermia’ OR ‘azoospermia’). References from these studies were also scrutinized to identify other relevant studies. No language restriction was applied.
Study selection and data extraction
Titles of all articles retrieved from the database searches were screened. We excluded studies without results on the relationship between BMI and sperm parameters, case reports, reviews, experimental or interventional studies, studies restricted to men with a particular pathology (such as a varicocele) and studies comparing exposed/non-exposed men. The abstracts of relevant articles investigating the relationship between BMI and sperm parameters were examined and all studies that could potentially be included in this review were retrieved, regardless of population size, origin or age. References from these studies and previous reviews were also scanned for any other relevant articles. Two reviewers independently extracted data (N.S. and C.F.) and there was no disagreement over eligibility of studies.
Owing to the wide variety of statistical methods and outcomes used in published studies (different BMI categories, mean or median, sperm concentration or TSC), authors of studies selected to be included in the present meta-analysis were contacted by email and asked to complete a standardized data extraction form indicating TSCs according to BMI categories, as specified by the World Health Organization (WHO; World Health Organization, 2000). We also included previously unpublished data obtained from all patients seen at the Infertility Center of Jean Verdier Hospital, Bondy, France, between January 2007 and December 2010, assigned as ‘Levy et al. (unpublished)’ study in the following text, table and figures.
Data synthesis and analysis
Analyses were performed using the following BMI categories: <18.5 (underweight), 18.5–24.9 (normal weight), 25.0–29.9 (overweight), 30.0–39.9 (obesity) and ≥40.0 (morbid obesity) kg/m² (World Health Organization, 2000). Participants with a BMI between 18.5 and 24.9 kg/m2 were considered as the reference group. Random effects models were used to obtain summary estimates in order to account for inter-study variation. Studies were weighted according to an estimate of statistical size defined as the inverse of the variance of the log odds ratio (OR; Woodward, 2005). Prevalence ORs and their 95% confidence intervals (95% CIs) were obtained by comparing the prevalence of abnormal sperm count in each BMI category with the BMI reference category (see above). Statistical significances were obtained using the χ2 test. The percentage of variability across studies attributable to heterogeneity was estimated using the I2 statistic (Higgins and Thompson, 2002; Higgins et al., 2003).
First, mean sperm concentrations and TSC were compared using standardized weighted mean differences (SMD) across BMI categories. Secondly, data were stratified according to TSC as normozoospermia (≥40 × 106 spermatozoa per ejaculate), oligozoospermia (<40 × 106 but >0 spermatozoa per ejaculate) and azoospermia (absence of spermatozoa) according to WHO guidelines (World Health Organization, 1999). We tested whether the association between BMI and abnormal sperm count was the same for men with oligozoospermia or azoospermia by performing separate analyses on each of these outcomes. As there was no significant heterogeneity, further analyses were performed by combining oligozoospermia and azoospermia as a single outcome in order to increase the statistical power of the analyses. The prevalence of subjects having abnormal sperm count was compared across BMI categories as described above, as well as the prevalence of men having decreased sperm concentration according to WHO guidelines (<15 M/ml; Cooper et al., 2010; World Health Organization, 2010). The Egger regression test was performed to assess publication bias (Egger et al., 1997). All analyses were performed using STATA software (Release 10; STATA Corporation, College Station, TX, USA).
Results
Study characteristics
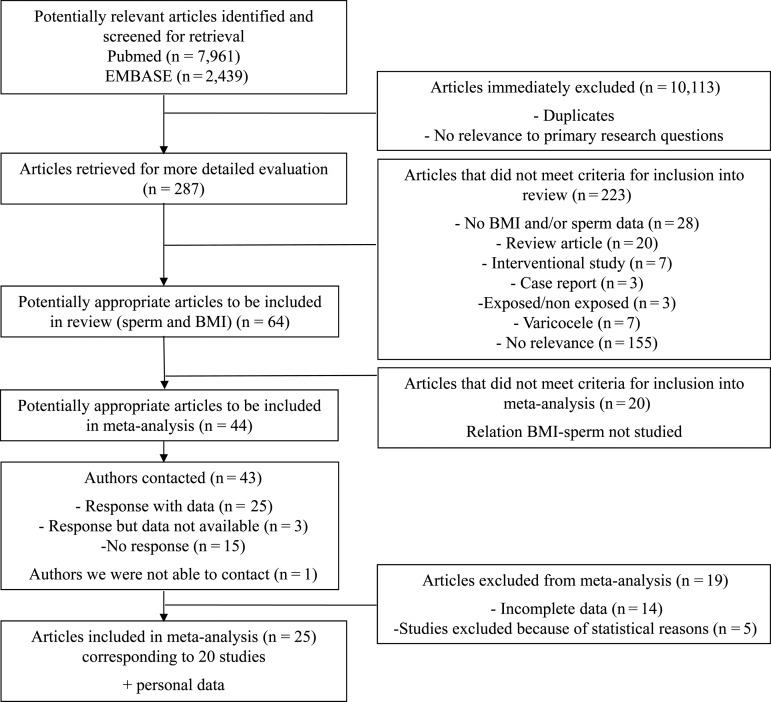
The search strategy identified a total of 10 400 articles, including duplicates and articles that had no relevance to the primary research questions. After review of 287 abstracts, 64 articles providing BMI and sperm data were selected. Among them, 44 articles investigating the relationship between BMI and sperm parameters seemed potentially appropriate to be included in the meta-analysis (Fig. 1). We were able to contact 43 of the 44 authors by email (one email address was not available), allowing us to obtain original and complete data for 20 studies corresponding to 25 published articles (Eskenazi et al., 2003; Jensen et al., 2004; Fejes et al., 2005, 2006; Koloszar et al., 2005; Magnusdottir et al., 2005; Zorn et al., 2007, 2012; Aggerholm et al., 2008; Li et al., 2009; Vujkovic et al., 2009; Chavarro et al., 2010; Duits et al., 2010; Keltz et al., 2010; Martini et al., 2010; Ramlau-Hansen et al., 2010; Hammiche et al., 2011, 2012; Lotti et al., 2011; Relwani et al., 2011; Shayeb et al., 2011; Tunc et al., 2011; Braga et al., 2012; Eskandar et al., 2012; La Vignera et al., 2012). Three authors could not contribute to the meta-analysis because of incomplete data (Strain et al., 1982; Nicopoulou et al., 2009; Paasch et al., 2010). We included previously unpublished data obtained from Jean Verdier Infertility Center, Bondy, France. Data from 19 articles, totaling 8359 men, which addressed the association between BMI and sperm parameters could not be analyzed (Strain et al., 1982; Parazzini et al., 1993; Kort et al., 2006; Gao et al., 2007; Qin et al., 2007; Hammoud et al., 2008, 2010; Pauli et al., 2008; Robeva et al., 2008; Nicopoulou et al., 2009; Stewart et al., 2009; Bak et al., 2010; Hofny et al., 2010; Paasch et al., 2010; Sekhavat and Moein, 2010; Wegner et al., 2010; Egwurugwu et al., 2011; Rybar et al., 2011; Fariello et al., 2012).
Figure 1.
Flow chart of screening for relevant articles in systematic review and meta-analysis of data on BMI and sperm count.
The present meta-analysis included a total of 21 eligible studies. All were cross-sectional studies, except two prospective cohort studies (Vujkovic et al., 2009; Hammiche et al., 2011, 2012). The study sample sizes ranged from 72 (Magnusdottir et al., 2005) to 1966 (Shayeb et al., 2011) and totaled 13 077 individuals, including men from Jean Verdier Hospital Infertility Center (n = 717) (Table I). Study participants were from diverse countries, including Australia (Tunc et al., 2011), China (Li et al., 2009), Saudi Arabia (Eskandar et al., 2012), Argentina (Martini et al., 2010), Brazil (Braga et al., 2012), USA (Eskenazi et al., 2003; Chavarro et al., 2010; Keltz et al., 2010; Relwani et al., 2011), Denmark (Jensen et al., 2004; Aggerholm et al., 2008; Ramlau-Hansen et al., 2010), Hungary (Fejes et al., 2005, 2006; Koloszar et al., 2005), Iceland (Magnusdottir et al., 2005), Italy (Lotti et al., 2011; La Vignera et al., 2012), the Netherlands (Vujkovic et al., 2009; Duits et al., 2010; Hammiche et al., 2011, 2012), Slovenia (Zorn et al., 2007, 2012), UK (Shayeb et al., 2011) and France (Levy et al., unpublished data). They were recruited from the general population, including volunteers during military conscription, or fertility clinics (Table I). Sperm analysis was performed according to WHO 1999 guidelines (World Health Organization, 1999) for all studies, except one (Hammiche et al., 2012) which followed WHO 2010 guidelines (World Health Organization, 2010).
Table I.
Characteristics of studies included in the meta-analysis.
| Study | Populationa | Ascertainment of BMI | Repeated semen collection | Age (years, mean ± SD) | Percentage by BMI category (kg/m2) |
Percentage by TSC category |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 (%) | 25–29.9 (%) | 30–39.9 (%) | ≥40 (%) | Azoospermia | Oligozoospermia (%) | Normozoospermia (%) | |||||
| Aggerholm et al. (2008), Denmark | 1669 male volunteers from general population | Self-reported | Once | 33.9 ± 8.8 | 0.5% | 52.0 | 39.4 | 8.1 | 0 | 1.2% | 11.1 | 87.7 |
| Jensen et al. (2004), Denmark | 1558 young male military recruits | Measured on site | Once | 19.5 ± 1.3 | 3.5% | 77.3 | 15.4 | 3.7 | 0.1 | 0.3% | 45.2 | 54.5 |
| Li et al. (2009), China | 1338 healthy male volunteers | Measured on site | Once | 32.4 ± 5.5 | 6.9% | 74.1 | 17.8 | 1.2 | 0 | 0% | 8.4 | 91.6 |
| Ramlau-Hansen et al. (2010), Denmark | 259 sons of mothers recruited during their pregnancy in 1984–1987 | Self-reported | Once | 20.1 ± 0.8 | 3.9% | 72.2 | 17.8 | 6.1 | 0 | 0.8% | 20.5 | 78.7 |
| La Vignera et al. (2012), Italy | 150 healthy non-smoking male volunteers | Self-reported | Twice | 31.4 ± 2.3 | 0% | 33.3 | 33.3 | 26.7 | 6.7 | 2.7% | 41.3 | 56.0 |
| Eskenazi et al. (2003), USA | 97 non-smoking male volunteers without known fertility problems | Self-reported | Once | 46.4 ± 15.9 | 0% | 50.5 | 42.3 | 7.2 | 0 | 4.1% | 12.4 | 83.5 |
| Shayeb et al. (2011), UK | 1966 male partners from subfertile couples presenting in fertility center | Measured on site | Once | 33.1 ± 6.0 | 0.9% | 40.8 | 44.9 | 12.5 | 0.9 | EXC | 18.2 | 81.8 |
| Duits et al. (2010), The Netherlands | 1401 male partners from subfertile couples presenting in fertility center | Self-reported | Twice | 36.4 ± 6.5 | 0.4% | 47.3 | 41.9 | 9.7 | 0.7 | 6.3% | 17.5 | 76.2 |
| Martini et al. (2010), Argentina | 793 male partners from subfertile couples presenting in fertility center | Measured on site | Once | 34.9 ± 6.2 | EXC | 31.0 | 49.4 | 18.5 | 1.1 | 1.9% | 52.7 | 45.4 |
| Lévy et al. (unpublished data), France | 717 male partners from subfertile couples presenting in fertility center | Self-reported | Once | 37.4 ± 7.5 | 0.4% | 45.5 | 38.9 | 13.5 | 1.7 | 8.2% | 27.6 | 64.2 |
| Eskandar et al. (2012), Saudi Arabia | 500 male partners from subfertile couples presenting in fertility center | Measured on site | Twice | 34.8 ± 7.7 | 11.0% | 13.4 | 24.0 | 26.4 | 25.2 | 1.4% | 29.0 | 69.6 |
| Chavarro et al. (2010), USA | 483 male partners from subfertile couples presenting in fertility center | Measured on site | Once | 36.3 ± 5.4 | EXC | 25.5 | 48.2 | 23.8 | 2.5 | EXC | 10.8 | 89.2 |
| Koloszar et al. (2005) and Fejes et al. (2005, 2006), Hungary | 473 male partners from subfertile couples presenting in fertility center | Measured on site | Twice | 29.5 ± 3.6 | 6.3% | 33.6 | 32.4 | 22.0 | 5.7 | 4.4% | 30.0 | 65.6 |
| Hammiche et al. (2012), The Netherland | 449 male partners from subfertile couples presenting in fertility center | Measured on site | Once | 35.4 ± 6.5 | 1.1% | 34.1 | 49.2 | 15.2 | 0.4 | 5.8% | 35.2 | 59.0 |
| Braga et al. (2012), Brazil | 250 male partners from subfertile couples during IVF/ICSI cycles | Measured on site | Once | 38.4 ± 9.3 | 2.0% | 50.0 | 40.0 | 4.0 | 4.0 | EXC | 34.4 | 65.6 |
| Vujkovic et al. (2009) and Hammiche et al. (2011), The Netherland | 225 male partners from subfertile couples during IVF/ICSI cycles | Self-reported | Once | 37.4 ± 5.3 | 0.9% | 45.3 | 45.3 | 8.5 | 0 | EXC | 40.9 | 59.1 |
| Lotti et al. (2011), Italy | 222 male partners from subfertile couples presenting in fertility center | Measured on site | Once | 35.3 ± 7.0 | 0% | 59.0 | 32.0 | 9.0 | 0 | 20.3% | 37.8 | 41.9 |
| Zorn et al. (2007), Slovenia | 189 male partners from subfertile couples presenting in fertility center | Self-reported | Once | 34.4 ± 5.8 | 0% | 43.9 | 41.8 | 14.3 | 0 | 22.2% | 11.7 | 66.1 |
| Keltz et al. (2010) and Relwani et al. (2011), USA | 185 male partners from subfertile couples during IVF/ICSI cycles | Self-reported | Once | 37.5 ± 8.0 | 0.5% | 22.2 | 47.0 | 29.2 | 1.1 | EXC | 44.9 | 55.1 |
| Tunc et al. (2011), Australia | 81 male partners from subfertile couples presenting in fertility center | Self-reported | Once | 36.8 ± 5.2 | 0% | 25.9 | 45.7 | 28.4 | 0 | EXC | 28.4 | 71.6 |
| Magnusdottir et al. (2005), Iceland | 72 male partners from subfertile couples presenting in fertility center | Self-reported | Once | 37.0 ± 5.4 | 0% | 36.1 | 44.4 | 15.3 | 4.2 | 2.8% | 27.8 | 69.4 |
EXC, excluded; TSC, total sperm count.
aSize of the population corresponds to the size used for the main studied outcome.
Association between BMI and sperm count abnormality
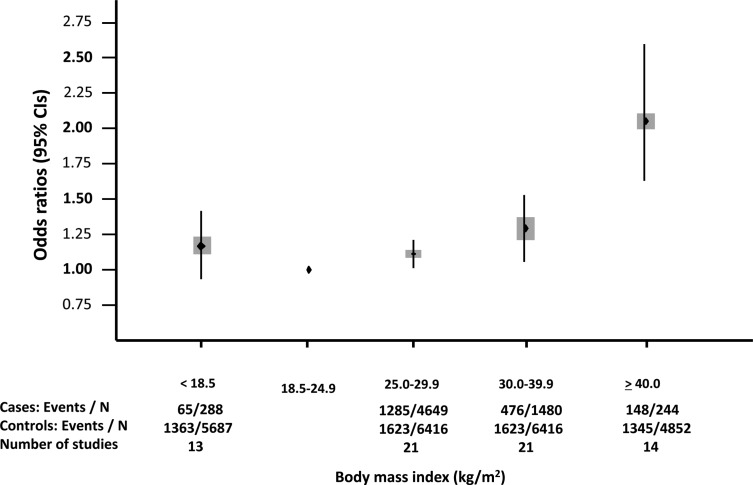
With azoospermia and oligozoospermia considered as a single outcome, a J-shaped association was found between BMI and abnormal sperm count (<40 M/ejaculate) (Fig. 2; n = 13 077 men analyzed). Compared with normal weight men, the ORs (95% CI) for oligozoospermia or azoospermia were 1.15 (0.93–1.43) for underweight men, 1.11 (1.01–1.21) for overweight men, 1.28 (1.06–1.55) for obese men and 2.04 (1.59–2.62) for morbidly obese men (see also Supplementary data, Figs S1–IV).
Figure 2.
Association between BMI and abnormal TCS (oligozoospermia or azoospermia) according to categories of BMI.
A similar J-shaped association was observed between BMI and abnormal sperm concentration (<15 M/ml; n = 13 453 men analyzed). Compared with normal weight men, the ORs (95% CI) for oligozoospermia or azoospermia were 1.46 (1.14–1.88) for underweight men, 1.06 (0.95–1.18) for overweight men, 1.31 (1.07–1.61) for obese men and 1.97 (1.27–3.07) for morbidly obese men.
Sensitivity analyses
Using fixed effects models did not substantially modify the results (underweight: 1.03, 0.83–1.28; overweight: 1.12, 1.05–1.19; obese: 1.26, 1.15–1.38; morbidly obese: 2.36, 1.93–2.89). Also, excluding data from Levy et al. (unpublished) did not influence the results: when this study was excluded, the ORs (95% CI) for abnormal sperm count were 1.10 (0.89–1.37) for underweight, 1.10 (1.00–1.22) for overweight, 1.31 (1.08–1.60) for obesity and 2.11 (1.59–2.80) for morbid obesity.
Possible sources of heterogeneity were investigated by stratifying the studies according to study population type (general population or clinical population, see Supplementary data, Fig. SV).
Assessment of publication bias
The Egger test provided no evidence of publication bias when analyses were performed for underweight (P = 0.92), overweight (P = 0.66) or obesity (P = 0.79) using ‘oligozoospermia or azoospermia’ as abnormal sperm count. Similar results were obtained for oligozoospermia or azoospermia analyzed separately.
Discussion
This meta-analysis based on 13 077 men showed a J-shaped association between BMI and abnormal sperm count: underweight was associated with an increased but non-significant risk of abnormal sperm count, whereas overweight and obese men had a significantly elevated risk of abnormal sperm count compared with normal weight men.
The relationship between obesity and alteration of sperm parameters or male subfertility is likely to be multifactorial, and different pathophysiological hypotheses have been raised. First, alterations of the hypothalamic–pituitary–gonadal axis have been suggested to be involved in this process. Indeed, aromatization of steroids to estrogens in peripheral tissues leads to the hypogonadotropic hyperestrogenic hypogonadism previously described in obese men (Schneider et al., 1979), with a significant decrease in total and free testosterone levels and increase in estradiol (E2), both leading to deleterious effects on spermatogenesis. Moreover, studies showed a decrease of sex hormone-binding globulin among obese men, notably mediated by hyperinsulinemia, emphasizing the negative feedback effect of elevated total E2 levels (Stellato et al., 2000). Obesity is also associated with an increase of endorphins leading to a both lower LH pulse amplitude and GnRH production (Blank et al., 1994). Some authors have also suggested that obesity may directly alter spermatogenesis and Sertoli cell function (Winters et al., 2006), as indicated by the more severe decrease of inhibin B levels compared with the decrease of FSH. Another hypothesis is the increase of scrotal temperature caused by hip and abdominal fat tissue accumulation, or even scrotal fat deposition (Shafik and Olfat, 1981), which would involve spermatogenesis disturbances. Preferential accumulation in fatty tissue of toxic substances and liposoluble endocrine disruptors would amplify those alterations, as indicated by serum organochlorine levels being correlated with BMI (Magnusdottir et al., 2005).
When mean sperm concentrations were compared using SMD across BMI categories, no significant difference was observed (data not shown) in agreement with a previous meta-analysis (MacDonald et al., 2010). Our analysis based on dichotomized sperm count or concentration, however, is in sharp contrast with the previous meta-analysis. We believe the current meta-analysis overcomes many of the limitations of previous attempts to summarize the association between BMI and semen quality. First, because sperm count has a highly skewed distribution, it is not unexpected that our analyses comparing means across BMI categories or previous analyses based on correlation statistics suggested no association between BMI and sperm count. We believe our alternative approach of dichotomizing sperm count at a prespecified and clinically relevant cutoff is more informative clinically and more adequate analytically. Secondly, ∼30 articles assessing the association of BMI with sperm parameters have been published since the previous meta-analysis by MacDonald et al. (2010). Lastly, given the wide variety of statistical methods, BMI categories and outcomes used in published studies, we obtained individual patient data rather than relying on published information only. Thanks to this strategy, we achieve a more homogeneous meta-analysis.
Our study has several limitations. First, despite our efforts, incomplete data or absence of response from contacted authors led to the exclusion of 19 studies (Strain et al., 1982; Parazzini et al., 1993; Kort et al., 2006; Gao et al., 2007; Qin et al., 2007; Hammoud et al., 2008, 2010; Pauli et al., 2008; Robeva et al., 2008; Nicopoulou et al., 2009; Stewart et al., 2009; Bak et al., 2010; Hofny et al., 2010; Paasch et al., 2010; Sekhavat and Moein, 2010; Wegner et al., 2010; Egwurugwu et al., 2011; Rybar et al., 2011; Fariello et al., 2012). Among them, 10 studies corresponding to 4809 men (Kort et al., 2006; Hammoud et al., 2008, 2010; Robeva et al., 2008; Stewart et al., 2009; Bak et al., 2010; Hofny et al., 2010; Paasch et al., 2010; Sekhavat and Moein, 2010; Egwurugwu et al., 2011) argued for a relationship between and BMI and sperm parameters, whereas 9 studies investigating 3550 men (Strain et al., 1982; Parazzini et al., 1993; Gao et al., 2007; Qin et al., 2007; Pauli et al., 2008; Nicopoulou et al., 2009; Wegner et al., 2010; Rybar et al., 2011; Fariello et al., 2012) did not. A selective outcome reporting can then probably be rejected and, owing to the high number of excluded studies showing an inverse association between BMI and sperm parameters, it is likely that this exclusion led to an underestimation of the computed ORs. Secondly, study populations varied, with men recruited from the general population or infertile couples. However, this variability also suggests that both the clinical population and the general population would benefit from our findings. Thirdly, BMI and conventional semen parameters were considered relevant enough to estimate body fat content and assess male fertility. BMI may not be the best indicator, as suggested by the questions about thresholds (Prentice and Jebb, 2001) and its inability to distinguish body fat composition or distribution, such as with waist circumference or waist-to-hip ratio (Fejes et al., 2005; Akpinar et al., 2007; Hammiche et al., 2012). Nevertheless, our findings will prove easy to apply, as BMI is a marker widely used in clinical and research settings. Similarly, conventional semen parameters suffer from high uncertainty of measurement and only provide partial information about sperm functions. For example, functional tests, such as the hemizona assay or zona-binding test, have been suggested to be more relevant to predict fertilization outcome (Sifer et al., 2005). Cutoff values for sperm parameters have also been blamed to be of insufficient clinical relevance because of variations in semen analysis results, related to both physiological variations and limitations of the techniques used (Björndahl, 2011). However, conventional semen parameters remain the gold standard for primary clinical evaluation of male fertility. Notably sperm count is a relatively consensual and objective semen parameter (Auger et al., 2000; Eustache and Auger, 2003) and TSC is a readily available parameter that most laboratories would assess fairly consistently with a WHO cutoff that can be used. We believe that, beyond controversies about reference limits, our meta-analysis offers several strengths, including the largest sample size ever published and the original use of standardized aggregated data.
In conclusion, a J-shaped association was found between BMI and the risk of abnormal sperm count, defined as oligozoospermia or azoospermia. Our systematic review with meta-analysis is in contradiction with a previous one that did not find associations of overweight and obesity with sperm concentration and TSC. Several methodological issues and updates in the literature have helped in understanding such a discrepancy. Although the risk may remain moderate at an individual level, our data indicate that high BMI affects sperm production. It is currently unclear whether weight loss can reverse this effect. Whereas weight loss was associated with an increase in TSC in a recent pilot cohort study (Hakonsen et al., 2011), others reported a severe worsening of semen parameters during the months after bariatric surgery (Sermondade et al., 2012b). Longitudinal studies and randomized controlled trials will then be required to evaluate whether weight normalization through diet modification and physical activity or bariatric surgery could improve sperm parameters and therefore male fertility.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors' roles
N.S., C.F. and S.C. have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. R.L., S.C., N.S., L.F. studied the concept and designed the same. N.S., C.F., A.G.S., J.P.B., T.K.J., M.V.W., J.C., A.C.M., M.E., J.E.C., S.K., J.M.T., C.H.R.-H., E.B., F.L., R.P.M.S.-T., B.Z., A.J.P., S.L.V., B.E., K.T., E.V.M., I.F. were involved in acquisition of data. R.L., S.C., N.S., C.F., L.F., S.H. performed the analysis and interpretation of data. N.S., S.C., L.F. were helpful in the drafting of the manuscript. N.S., C.F., L.F., A.G.S., J.P.B., T.K.J., M.V.W., J.C., A.C.M., M.E., J.E.C., S.K., J.M.T., C.H.R.-H., E.B., F.L., R.P.M.S.-T., B.Z., A.J.P., S.L.V., B.E., K.T., E.V.M., I.F., S.H., R.L., S.C. contributed to the critical revision of the manuscript for important intellectual content. L.F. made the statistical analyses. S.C. supervised the study.
Funding
J.E.C. was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant 5P30DK046200-19 and B.E. was supported in part by National Institutes of Health grant P42ES04705.
Conflicts of interest
None declared.
Supplementary Material
Acknowledgements
Niels Jorgensen, Rigshospitalet, Copenhagen, Denmark; Yafei Li, Third Military Medical University, Chongqing, China; Zhihong Cui, Third Military Medical University, Chongqing, China; Rosa Molina, Laboratorio de Andrologia y Reproduccion, Cordoba, Argentina; Ruben Daniel Ruiz, Facultad de Ciencias Medicas, Universidad Nacional de Cordoba, Argentina; Thomas L. Toth, Harvard Medical School, Boston MA, USA; Russ Hauser, Harvard School of Public Health, Boston MA, USA; Janos Szollosi, University of Szeged, Hungary; Ane Marie Thulstrup, Aarhus University Hospital, Aarhus, Denmark; Daniela Braga, Fertility-Assisted Fertilization Centre, Sao Paulo, Brazil; Gabriela Halpern, Fertility-Assisted Fertilization Centre, Sao Paulo, Brazil; Mario Maggi, University of Florence, Italy; Joop Laven, Erasmus University Medical Center, Rotterdam, The Netherlands; Marijana Vujkovic, Erasmus University Medical Center, Rotterdam, The Netherlands; Fatima Hammiche, Erasmus University Medical Center, Rotterdam, The Netherlands; Gregor Majdic, Center for Animal Genomics, Veterinary Faculty, University of Ljubljana, Slovenia; Sangita Jindal, Albert Einstein College of Medicine And Montefiore Medical Center, Bronx, NY, USA; Rosita A. Condorelli, University of Catania, Catania, Italy; Rosana Hernandez Weldon, UC Berkeley School of Public Health, Berkeley CA, USA; Andrew J. Wyrobek, Lawrence Berkeley National Laboratory, Berkeley CA, USA; Ozlem Tunc, Repromed, Adelaide, South Australia; Tanja Thorsteinsson, Department of Assisted Reproduction, Landspitali University Hospital (current address: Art Medica), Iceland; Zoltan Zavaczki, Landstinget Gavleborg, Hudiksvall, Sweden.
References
- Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90:619–626. doi: 10.1016/j.fertnstert.2007.07.1292. doi:10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- Akpinar E, Bashan I, Bozdemir N, Saatci E. Which is the best anthropometric technique to identify obesity: body mass index, waist circumference or waist–hip ratio? Coll Antropol. 2007;31:387–393. [PubMed] [Google Scholar]
- Auger J, Eustache F, Ducot B, Blandin T, Daudin M, Diaz I, Matribi SE, Gony B, Keskes L, Kolbezen M, et al. Intra- and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum Reprod. 2000;15:2360–2368. doi: 10.1093/humrep/15.11.2360. doi:10.1093/humrep/15.11.2360. [DOI] [PubMed] [Google Scholar]
- Bak CW, Song SH, Yoon TK, Lim JJ, Shin TE, Sung S. Natural course of idiopathic oligozoospermia: comparison of mild, moderate and severe forms. Int J Urol. 2010;17:937–943. doi: 10.1111/j.1442-2042.2010.02628.x. doi:10.1111/j.1442-2042.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- Björndahl L. What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen analysis results. Hum Fertil (Camb) 2011;14:179–186. doi: 10.3109/14647273.2011.580823. doi:10.3109/14647273.2011.580823. [DOI] [PubMed] [Google Scholar]
- Blank DM, Clark RV, Heymsfield SB, Rudman DR, Blank MS. Endogenous opioids and hypogonadism in human obesity. Brain Res Bull. 1994;34:571–574. doi: 10.1016/0361-9230(94)90142-2. doi:10.1016/0361-9230(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Braga DP, Halpern G, Figueira Rde C, Setti AS, Iaconelli A, Jr, Borges E., Jr Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97:53–59. doi: 10.1016/j.fertnstert.2011.10.011. doi:10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br Med J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. doi:10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. doi:10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. doi:10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- Duits FH, van Wely M, van der Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. 2010;94:1356–1359. doi: 10.1016/j.fertnstert.2009.05.075. doi:10.1016/j.fertnstert.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. doi:10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwurugwu JN, Nwafor A, Chike CP, Ufearo CS, Uchefuna RC, Iwuji SC, Okwara JE, Alawu EA. The relationship between body mass index, semen and sex hormones in adult male. Niger J Physiol Sci. 2011;26:29–34. [PubMed] [Google Scholar]
- Eskandar M, Al-Asmari M, Babu Chaduvula S, Al-Shahrani M, Al-Sunaidi M, Almushait M, Donia O, Al-Fifi S. Impact of male obesity on semen quality and serum sex hormones. Adv Urol. 2012;2012:407601. doi: 10.1155/2012/407601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–454. doi: 10.1093/humrep/deg107. doi:10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- Eustache F, Auger J. Inter-individual variability in the morphological assessment of human sperm: effect of the level of experience and the use of standard methods. Hum Reprod. 2003;18:1018–1022. doi: 10.1093/humrep/deg197. doi:10.1093/humrep/deg197. [DOI] [PubMed] [Google Scholar]
- Fariello RM, Pariz JR, Spaine DM, Cedenho AP, Bertolla RP, Fraietta R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. 2012;110:863–867. doi: 10.1111/j.1464-410X.2011.10813.x. [DOI] [PubMed] [Google Scholar]
- Fejes I, Koloszar S, Szollosi J, Zavaczki Z, Pal A. Is semen quality affected by male body fat distribution? Andrologia. 2005;37:155–159. doi: 10.1111/j.1439-0272.2005.00671.x. doi:10.1111/j.1439-0272.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- Fejes I, Koloszar S, Zavaczki Z, Daru J, Szollosi J, Pal A. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch Androl. 2006;52:97–102. doi: 10.1080/01485010500315479. doi:10.1080/01485010500315479. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. doi:10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Gao ES, Yang Q, Walker M, Wu JQ, Zhou WJ, Wen SW. Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod. 2007;22:477–484. doi: 10.1093/humrep/del383. doi:10.1093/humrep/del383. [DOI] [PubMed] [Google Scholar]
- Hakonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, Bungum M, Ernst EH, Hansen ML, Ramlau-Hansen CH. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health. 2011;8:24. doi: 10.1186/1742-4755-8-24. doi:10.1186/1742-4755-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammiche F, Laven JS, Boxmeer JC, Dohle GR, Steegers EA, Steegers-Theunissen RP. Sperm quality decline among men below 60 years of age undergoing IVF or ICSI treatment. J Androl. 2011;32:70–76. doi: 10.2164/jandrol.109.009647. doi:10.2164/jandrol.109.009647. [DOI] [PubMed] [Google Scholar]
- Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27:2365–2372. doi: 10.1093/humrep/des177. doi:10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. doi:10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Griffin J, Meikle AW, Gibson M, Peterson CM, Carrell DT. Association of aromatase (TTTAn) repeat polymorphism length and the relationship between obesity and decreased sperm concentration. Hum Reprod. 2010;25:3146–3151. doi: 10.1093/humrep/deq255. doi:10.1093/humrep/deq255. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. doi:10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofny ER, Ali ME, Abdel-Hafez HZ, Kamal Eel D, Mohamed EE, Abd El-Azeem HG, Mostafa T. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril. 2010;94:581–584. doi: 10.1016/j.fertnstert.2009.03.085. doi:10.1016/j.fertnstert.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. doi:10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Jouannet P, Wang C, Eustache F, Kold-Jensen T, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS. 2001;109:333–344. doi: 10.1034/j.1600-0463.2001.090502.x. doi:10.1034/j.1600-0463.2001.090502.x. [DOI] [PubMed] [Google Scholar]
- Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27:539–544. doi: 10.1007/s10815-010-9439-y. doi:10.1007/s10815-010-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloszar S, Fejes I, Zavaczki Z, Daru J, Szollosi J, Pal A. Effect of body weight on sperm concentration in normozoospermic males. Arch Androl. 2005;51:299–304. doi: 10.1080/01485010590919701. doi:10.1080/01485010590919701. [DOI] [PubMed] [Google Scholar]
- Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. doi:10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Condorelli RA, Vicari E, Calogero AE. Negative effect of increased body weight on sperm conventional and nonconventional flow cytometric sperm parameters. J Androl. 2012;33:53–58. doi: 10.2164/jandrol.110.012120. doi:10.2164/jandrol.110.012120. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin H, Ma M, Li L, Cai M, Zhou N, Han X, Bao H, Huang L, Zhu C, et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod. 2009;24:459–469. doi: 10.1093/humrep/den399. doi:10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. doi:10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti F, Corona G, Colpi GM, Filimberti E, Degli Innocenti S, Mancini M, Baldi E, Noci I, Forti G, Adorini L, et al. Elevated body mass index correlates with higher seminal plasma interleukin 8 levels and ultrasonographic abnormalities of the prostate in men attending an andrology clinic for infertility. J Endocrinol Invest. 2011;34:e336–e342. doi: 10.3275/7855. [DOI] [PubMed] [Google Scholar]
- MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. doi:10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005;20:208–215. doi: 10.1093/humrep/deh569. doi:10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- Martini AC, Tissera A, Estofan D, Molina RI, Mangeaud A, de Cuneo MF, Ruiz RD. Overweight and seminal quality: a study of 794 patients. Fertil Steril. 2010;94:1739–1743. doi: 10.1016/j.fertnstert.2009.11.017. doi:10.1016/j.fertnstert.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. doi:10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- Nicopoulou SC, Alexiou M, Michalakis K, Ilias I, Venaki E, Koukkou E, Mitios G, Billa E, Adamopoulos DA. Body mass index vis-a-vis total sperm count in attendees of a single andrology clinic. Fertil Steril. 2009;92:1016–1017. doi: 10.1016/j.fertnstert.2008.12.093. doi:10.1016/j.fertnstert.2008.12.093. [DOI] [PubMed] [Google Scholar]
- Paasch U, Grunewald S, Kratzsch J, Glander HJ. Obesity and age affect male fertility potential. Fertil Steril. 2010;94:2898–2901. doi: 10.1016/j.fertnstert.2010.06.047. doi:10.1016/j.fertnstert.2010.06.047. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Marchini M, Tozzi L, Mezzopane R, Fedele L. Risk factors for unexplained dyspermia in infertile men: a case–control study. Arch Androl. 1993;31:105–113. doi: 10.3109/01485019308988387. doi:10.3109/01485019308988387. [DOI] [PubMed] [Google Scholar]
- Pauli EM, Legro RS, Demers LM, Kunselman AR, Dodson WC, Lee PA. Diminished paternity and gonadal function with increasing obesity in men. Fertil Steril. 2008;90:346–351. doi: 10.1016/j.fertnstert.2007.06.046. doi:10.1016/j.fertnstert.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. doi:10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- Qin DD, Yuan W, Zhou WJ, Cui YQ, Wu JQ, Gao ES. Do reproductive hormones explain the association between body mass index and semen quality? Asian J Androl. 2007;9:827–834. doi: 10.1111/j.1745-7262.2007.00268.x. doi:10.1111/j.1745-7262.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. doi:10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Hansen M, Jensen CR, Olsen J, Bonde JP, Thulstrup AM. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: two decades of follow-up. Fertil Steril. 2010;94:610–618. doi: 10.1016/j.fertnstert.2009.01.142. doi:10.1016/j.fertnstert.2009.01.142. [DOI] [PubMed] [Google Scholar]
- Relwani R, Berger D, Santoro N, Hickmon C, Nihsen M, Zapantis A, Werner M, Polotsky AJ, Jindal S. Semen parameters are unrelated to BMI but vary with SSRI use and prior urological surgery. Reprod Sci. 2011;18:391–397. doi: 10.1177/1933719110385708. doi:10.1177/1933719110385708. [DOI] [PubMed] [Google Scholar]
- Robeva R, Sestrimska N, Atanasova I, Mekhandzhiev T, Tomova A, Kumanov F. [Sperm disorder in males with obesity and metabolic syndrome—pilot study] Akush Ginekol (Sofiia) 2008;47:11–14. [PubMed] [Google Scholar]
- Rybar R, Kopecka V, Prinosilova P, Markova P, Rubes J. Male obesity and age in relationship to semen parameters and sperm chromatin integrity. Andrologia. 2011;43:286–291. doi: 10.1111/j.1439-0272.2010.01057.x. doi:10.1111/j.1439-0272.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. doi:10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. doi:10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- Sekhavat L, Moein MR. The effect of male body mass index on sperm parameters. Aging Male. 2010;13:155–158. doi: 10.3109/13685530903536643. doi:10.3109/13685530903536643. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S Obesity-Fertility Collaborative Group. Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med. 2012a;172:440–442. doi: 10.1001/archinternmed.2011.1382. doi:10.1001/archinternmed.2011.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermondade N, Massin N, Boitrelle F, Pfeffer J, Eustache F, Sifer C, Czernichow S, Levy R. Sperm parameters and male fertility after bariatric surgery: three case series. Reprod Biomed Online. 2012b;24:206–210. doi: 10.1016/j.rbmo.2011.10.014. doi:10.1016/j.rbmo.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Shafik A, Olfat S. Scrotal lipomatosis. Br J Urol. 1981;53:50–54. doi: 10.1111/j.1464-410x.1981.tb03128.x. doi:10.1111/j.1464-410X.1981.tb03128.x. [DOI] [PubMed] [Google Scholar]
- Shayeb AG, Harrild K, Mathers E, Bhattacharya S. An exploration of the association between male body mass index and semen quality. Reprod Biomed Online. 2011;23:717–723. doi: 10.1016/j.rbmo.2011.07.018. doi:10.1016/j.rbmo.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Sifer C, Sasportes T, Barraud V, Poncelet C, Rudant J, Porcher R, Cedrin-Durnerin I, Martin-Pont B, Hugues JN, Wolf JP. World Health Organization grade ‘a’ motility and zona-binding test accurately predict IVF outcome for mild male factor and unexplained infertilities. Hum Reprod. 2005;20:2769–2775. doi: 10.1093/humrep/dei118. doi:10.1093/humrep/dei118. [DOI] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. doi:10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Stewart TM, Liu DY, Garrett C, Jorgensen N, Brown EH, Baker HW. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod. 2009;24:1561–1568. doi: 10.1093/humrep/dep075. doi:10.1093/humrep/dep075. [DOI] [PubMed] [Google Scholar]
- Strain GW, Zumoff B, Kream J, Strain JJ, Deucher R, Rosenfeld RS, Levin J, Fukushima DK. Mild hypogonadotropic hypogonadism in obese men. Metabolism. 1982;31:871–875. doi: 10.1016/0026-0495(82)90175-5. doi:10.1016/0026-0495(82)90175-5. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP. Declining semen quality: can the past inform the present? Bioessays. 1999;21:614–621. doi: 10.1002/(SICI)1521-1878(199907)21:7<614::AID-BIES10>3.0.CO;2-B. doi:10.1002/(SICI)1521-1878(199907)21:7<614::AID-BIES10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. doi:10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24:1304–1312. doi: 10.1093/humrep/dep024. doi:10.1093/humrep/dep024. [DOI] [PubMed] [Google Scholar]
- Wegner CC, Clifford AL, Jilbert PM, Henry MA, Gentry WL. Abnormally high body mass index and tobacco use are associated with poor sperm quality as revealed by reduced sperm binding to hyaluronan-coated slides. Fertil Steril. 2010;93:332–334. doi: 10.1016/j.fertnstert.2009.07.970. doi:10.1016/j.fertnstert.2009.07.970. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Wang C, Abdelrahaman E, Hadeed V, Dyky MA, Brufsky A. Inhibin-B levels in healthy young adult men and prepubertal boys: is obesity the cause for the contemporary decline in sperm count because of fewer Sertoli cells? J Androl. 2006;27:560–564. doi: 10.2164/jandrol.05193. doi:10.2164/jandrol.05193. [DOI] [PubMed] [Google Scholar]
- Woodward M. Epidemiology: Study Design and Data Analysis. Boca Raton, FL, USA: Chapman & Hall/CRC; 2005. [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 4th edn. New York: Cambridge University Press; 1999. [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 5th edn. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- Zorn B, Osredkar J, Meden-Vrtovec H, Majdic G. Leptin levels in infertile male patients are correlated with inhibin B, testosterone and SHBG but not with sperm characteristics. Int J Androl. 2007;30:439–444. doi: 10.1111/j.1365-2605.2006.00728.x. doi:10.1111/j.1365-2605.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- Zorn B, Golob B, Ihan A, Kopitar A, Kolbezen M. Apoptotic sperm biomarkers and their correlation with conventional sperm parameters and male fertility potential. J Assist Reprod Genet. 2012;29:357–364. doi: 10.1007/s10815-012-9718-x. doi:10.1007/s10815-012-9718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.