Abstract
Background
We have shown previously that male and female adolescents differ in their responses to caffeine, but to date, the mechanisms underlying these gender differences are unknown.
Objective
The purpose of this study was to test the hypothesis that differences in circulating steroid hormones mediate gender differences in response to caffeine.
Methods
Subjective and physiological responses to caffeine were tested in adolescents using a double-blind, placebo controlled, crossover design. Participants were tested every 2 weeks for 8 weeks and received placebo and caffeine (2 mg/kg) twice each. Females were tested with placebo and caffeine in each phase of their menstrual cycle. Salivary concentrations of testosterone, estradiol, and progesterone were also measured.
Results
Males showed greater positive subjective effects than females. In females, higher levels of estradiol were associated with little or no subjective responses to caffeine, but lower levels of estradiol were associated with negative subjective responses to caffeine relative to placebo. There were gender differences in cardiovascular responses to caffeine, with males showing greater decreases in heart rate after caffeine administration than females, but females showing greater increases in diastolic blood pressure than males after caffeine administration. These gender differences may be related to steroid hormone concentrations. Blood pressure responses to caffeine were lower in males when estradiol was high, but higher in females when estradiol was high.
Conclusions
When taken together, these findings suggest that males and females differ in their responses to caffeine and that these differences may be mediated by changes in circulating steroid hormones.
Introduction
Caffeine is the most widely used psychoactive substance in the world.1 Caffeine has physiological, psychological, and behavioral effects in humans that vary widely among individuals.2–4 Although generally thought of as safe, consumption recommendations are primarily based on observations from adults. Despite the fact that children and adolescents are the fastest growing segment of the caffeine consumption market and are being specifically targeted for sales of caffeine containing beverages, little research exists on the effects of caffeine within this population.4 Although we would expect responses to caffeine in adults and in postpubertal adolescents to be similar, there are reasons why differences may exist. For example, adolescents use less caffeine on average and use caffeine more sporadically than do adults.5 Therefore, adolescents may be less likely to have developed tolerance to the effects of caffeine and may be less likely to experience withdrawal.6,7 As withdrawal reversal is proposed to be a major motivator for caffeine use and to be a mechanism for some of the effects of caffeine,8 differences between adults and adolescents are noteworthy.
Childhood and adolescence represent critical time periods for physical,9 neurobiological,10 and social development.11 Adolescence is also the time in which pubertal development occurs. During this period, circulating steroid hormones increase, which can affect the metabolism of and responsivity to drugs.12,13 It has been well established that males and females differ in their response to drugs of abuse.14 Thus, there is a strong interest in determining the mechanisms that underlie these gender differences to understand risk factors for drug use and to improve treatment of drug abusers. Because illicit drugs cannot be readily administered to children and adolescents, using a licit drug, such as caffeine, that can be administered to children and that activates common neurobiological circuits as some illicit drugs15–17 may allow us to gain an understanding of factors that affect drug use in general. Our previous work has demonstrated gender differences in the reinforcing and physiological responses to caffeine,18,19 but a mechanism for these gender differences had not been established. The purpose of the study presented here was to determine if gender differences in the subjective and physiological effects of caffeine are related to levels of circulating steroid hormones. We hypothesized that elevated levels of estradiol, such as during the luteal phase, would reduce responses to caffeine in females.
Methods
Participants and recruitment
Participants were 24, 15–16-year-old boys and girls, recruited through direct mailings, flyers distributed at local middle and high schools, as well as flyers posted around the University at Buffalo and the surrounding community. Eligibility criteria included the following: previous ingestion of caffeine without adverse reactions, having signs of pubertal maturation, not using hormone based contraceptives, not smoking, not taking medication known to have interactions with caffeine, and willingness to visit the laboratory on four occasions for 90 minutes each. We had a total of 28 participants complete informed consent. We lost two females due to scheduling conflicts and two additional females who never completed follow-up phone calls to report menstrual cycle phase and complete scheduling. Data from these four females were not included in this article.
General experimental procedures
Qualifying males were scheduled for four visits, 2 weeks apart. Females were asked to schedule their first and third visits to our laboratory within 3–8 days from day 1 of menstruation (follicular phase). Their second and fourth visits were scheduled 2 weeks later (luteal phase). Participants were provided a list of caffeine-containing products from which to abstain for 24 hours before their scheduled appointment and were instructed not to not eat or drink anything other than water for 2 hours before the session. Participants were also asked to refrain from engaging in physical activity on the day of each laboratory visit.
Upon arrival to the laboratory for the first session, parents and participants read and signed consent and assent forms. To remove subject expectations about the effects of caffeine, participants were told that the beverage they would be consuming “may have levels of one or more of the following substances manipulated: sugar, aspartame, Splenda, caffeine, or artificial coloring.” This deception was considered acceptable because it involved no greater than minimal risk, and was necessary to prevent potential preconceptions of caffeine's effects from altering experimental results.
Participants then completed a same-day and previous-day dietary recall to confirm caffeine abstinence,20 whereas the parent completed a demographic questionnaire. Parents were then escorted from the room and the participant had height and weight measured (first visit only). Participants then provided a 3-mL saliva sample into a sterile tube that was analyzed for steroid hormones. Parent and child (while separated) were asked to assess the child's pubertal development via the “tanner stage evaluation” (first visit only). Next, the participant completed the “caffeine consumption questionnaire” to determine caffeine use habits and the “profile of mood states” (POMS) questionnaire (see descriptions below).
The participant then had baseline blood pressure and heart rate readings taken. Then, the participant consumed a 350 mL portion of Sprite™, lemonade, or orange juice, containing either placebo or caffeine (2 mg/kg). Participants received two doses each of placebo and caffeine over the course of four separate visits. Before the first visit, participants were randomized to receive caffeine or placebo first and subsequent drug administration was counterbalanced. Immediately after beverage consumption, blood pressure and heart rate measurements were taken every 10 minutes for a total of 60 minutes while participants watched a video. After each 10-minute interval, the video was paused to obtain vitals and the “drug effects questionnaire (DEQ)” was administered. “The addiction research center inventory (ARCI)” was implemented at 0, 30, and 60 minutes21 to determine the time point where maximum caffeine effects would occur After 1 hour, participants completed the POMS a second time. After the final session, participants had their height and weight measured again. Both the participant and their parent were debriefed and compensated for participation. Participants were compensated $20.00 for each visit they completed, for a total of $80.00 in the form of a gift card to a store of their choice. Parents were also compensated $20.00 in the form of a gift card to help defray the cost of transportation and any potential child care costs that may have been incurred. All study procedures were conducted in accordance with National Institutes of Health (NIH) guidelines for the use of humans in research and with the approval of the University at Buffalo Social and Behavioral Sciences Institutional Review Board.
Caffeine and beverage preparation
Caffeine and placebo treatments were prepared by an experimenter who was not involved in the data collection for this study. Caffeine was added to Sprite to mask its bitter taste and heated to 140°F and stirred for 25 minutes. Flattened Sprite without added caffeine was used as the placebo. The caffeine or placebo solutions were then aliquoted into 14-mL vials, labeled A or B, and frozen. On the day of the visit, the appropriate vial was thawed for >1 hour at room temperature. Participants were able to choose to drink orange juice, lemonade, or Sprite, which are all caffeine free. We verified that this dose of caffeine could not reliably be detected by taste in a previous study.18 While the participant was providing a saliva sample and completing questionnaires, the researcher prepared a mixture of 350 mL of the chosen beverage and volume of A or B equivalent to 2 mg caffeine/kg body weight.
Measurements
Cardiovascular responses
An automated heart rate and blood pressure monitor (Tango; SunTech Medical, Inc., Morrisville, NC) was used to collect cardiovascular measurements. The participants were seated in reclined position and instructed to relax. The blood pressure cuff was placed on the nondominant arm, with the microphone positioned over the brachial artery. Electrodes were placed on each forearm and on the chest above the heart. A baseline reading was conducted before consumption of the test beverage. Immediately after caffeine or placebo consumption, blood pressure and heart rate readings were taken every 10 minutes for 60 minutes.
Salivary steroid hormone measurement
Saliva collection was conducted at the beginning of each laboratory visit. Participants were instructed to expectorate into a tube with a funnel attached. They provided 3 mL of saliva, without air bubbles. This line was indicated for them on the outside of the sterile vile with a sticker labeled with participant number and visit code. Participants were offered a piece of wax which they could chew to facilitate saliva production. Samples were stored at −20°C until analyzed. Analyses of estradiol, testosterone, and progesterone hormone content were conducted by Salimetrics (State College, PA) using standard radioimmunilogical techniques.
Caffeine consumption
Average daily caffeine consumption was calculated based on the participant's self-report of daily or weekly intake of caffeine on a “caffeine use questionnaire” adapted from Miller22 that was designed to assess sources, amounts, and frequency of caffeinated food and beverage intake as well as reasons why adolescents use and/or do not use caffeine. Amounts of caffeine contained within each major source were calculated based on information from the U.S. Department of Nutritional Services and include the following: tea (40 mg/5 oz), soda (40 mg/12 oz), coffee (100 mg/5 oz), energy drinks (∼150 mg/12 oz), chocolate (10 mg/oz), and caffeine-containing pills (Excedrin or No-Doze–130 mg–200 mg/pill).
Weight, height, and body mass index measurements
Participant weight was assessed by use of a digital scale (SECA, Hanover, MD). Height was assessed using a SECA stadiometer. On the basis of the height and weight data, body mass index (BMI) was calculated according to the following formula: weight in kg/height in m2.
Questionnaires
Tanner stage evaluation
Participants were given line drawings of the five stages of pubertal development and asked to circle the one that looked most like them. Girls were given drawings of breast and pubic hair development and boys were given drawings of genital and public hair development. Parents were given the same drawings and asked to circle the one that they felt best resembled their child. If there were discrepancies between self-report and parental report, the higher of the two numbers was used. These drawings were given to the children in an envelope along with verbal instructions to circle the picture that looked most like their body and return the questionnaire to the envelope. The experimenter left the room and returned 2 minutes later. Self-assessment of pubertal stage has been shown to be accurate and an acceptable substitute when physical examinations are not feasible.23,24
Demographic questionnaire
A general demographics questionnaire was used to assess parental education, annual household income, parental employment status, and parent and participants' race and ethnicity.
Addiction research center inventory
Participants completed the short form of the ARCI,25 which is a 49-item questionnaire consisting of five subscales: amphetamine, benzedrine group, lysergic acid diethylamide, morphine-benzedrine group, and pentobarbital-chlorpromazine alcohol group. Subjects completed the ARCI at baseline and 30 and 60 minutes after drug administration.
Profile of mood states
This version of the POMS measures eight dimensions of affect or mood, including anxiety, depression, anger, vigor, fatigue, confusion, friendliness, and elation. Each was assessed on a 5-point Likert Scale, with 1 being “not at all” and 5, “extremely.” Within these eight areas are two mood super-scales: arousal and positive mood.26
Drug effects questionnaire
Participant was asked to indicate whether they “felt any drug effects,” “liked the drug effects,” “felt high from the drug,” and “wanted more of the drug” on a 100 mm visual analog scale anchored by “not at all” and “very much.”27 They completed this questionnaire at baseline and then every 10 minutes for 60 minutes.
Analytic plan
Gender differences in participant characteristics were analyzed using either a one-way analysis of variance (BMI, age, caffeine consumption, and tanner stage) or chi-squared analyses for categorical variables (race, household income, and parental education). The pattern of diastolic blood pressure (DBP) and systolic blood pressure (SBP), heart rate, and answers on the DEQ, the ARCI, and the POMS were analyzed using mixed effects regression models with gender, and caffeine use (mg/day) as time invariant predictors and time (from 10 to 60 minutes after drug administration), drug treatment (placebo vs. caffeine), and steroid hormone levels as time variant predictors and baseline blood pressure and heart rate as covariates.28 To correct for multiple comparisons on the POMS, a Bonferroni correction was applied here as well and data were only considered significant if p<0.006. Finally, the ARCI had five subscales, so with the Bonferroni correction, data were considered significant if p<0.01. Cardiovascular response data and data from the DEQ were considered significantly different if p<0.05 and data analyses were conducted using SYSTAT 11.0 (Chicago, IL).
Results
Participant characteristics
Participants were 15 males and 9 females with an average age of 15.6±0.12 years. Before recruitment, we conducted a power analysis on data collected in a previous study and determined that with a power of 0.84 and an effect size of 0.8 we could achieve statistically significant gender differences in subjective responses with an n of 8 per cell. The sample was predominantly Caucasian (79%) from middle class households. The average self-reported tanner stage was 4.25±0.14 and tanner stage reported by parents was 4.2±0.15. Females had higher levels of estradiol [F (1, 94)=17.84, p<0.0001] and progesterone [F (1, 94)=12.3, p=0.001] and males had higher levels of testosterone [F (1, 94)=39.6, p<0.0001]. There were no differences as a function of gender in BMI, age, tanner stage, caffeine use, parental education, household income, or race (all p>0.10; Table 1).
Table 1.
Participant Characteristics Shown by Gender
| |
Males |
Females |
|---|---|---|
| Mean±SEM | Mean±SEM | |
| Age | 15.7±0.2 | 15.6±0.2 |
| Body mass index | 24.1±1.3 | 24.3±1.2 |
| Caffeine use (mg/day) | 60.4±10.1 | 60.9±18.1 |
| Tanner stage—parental report | 4.1±0.2 | 4.3±.3 |
| Tanner stage—self-report | 4.4±0.2 | 4.0±0.2 |
| Estradiol | 1.8±0.1 | 2.6±0.2a |
| Progesterone | 44.2±3.7 | 74.1±9.2a |
| Testosterone | 80.9±3.6a | 47.1±3.5 |
| n | n | |
|---|---|---|
| Race | ||
| Caucasian | 13 | 6 |
| African American | 0 | 1 |
| Asian | 0 | 1 |
| Other | 0 | 0 |
| Parental education | ||
| High school | 0 | 2 |
| College | 15 | 4 |
| Graduate school | 0 | 3 |
| Household income | ||
| <$30,000 | 1 | 3 |
| $30,000–$50,000 | 7 | 2 |
| $50,000–$70,000 | 2 | 2 |
| >$70,000 | 0 | 1 |
Significant gender difference (all p<0.01).
Cardiovascular responses to caffeine
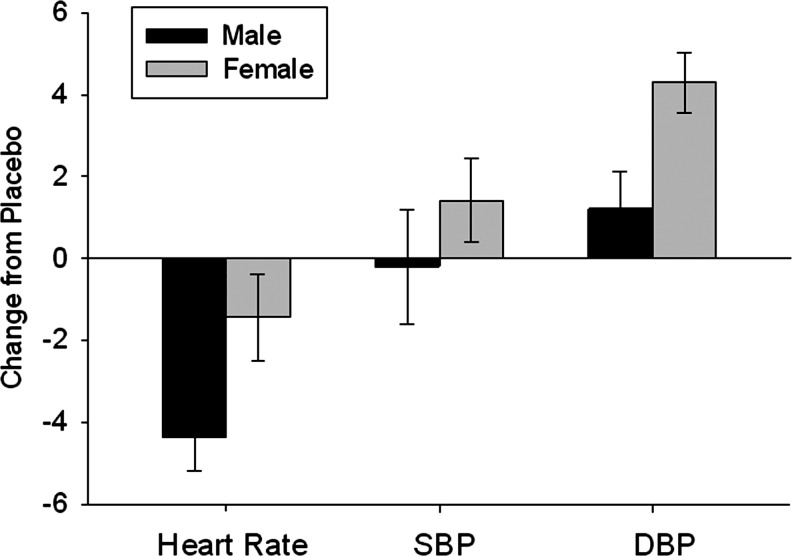
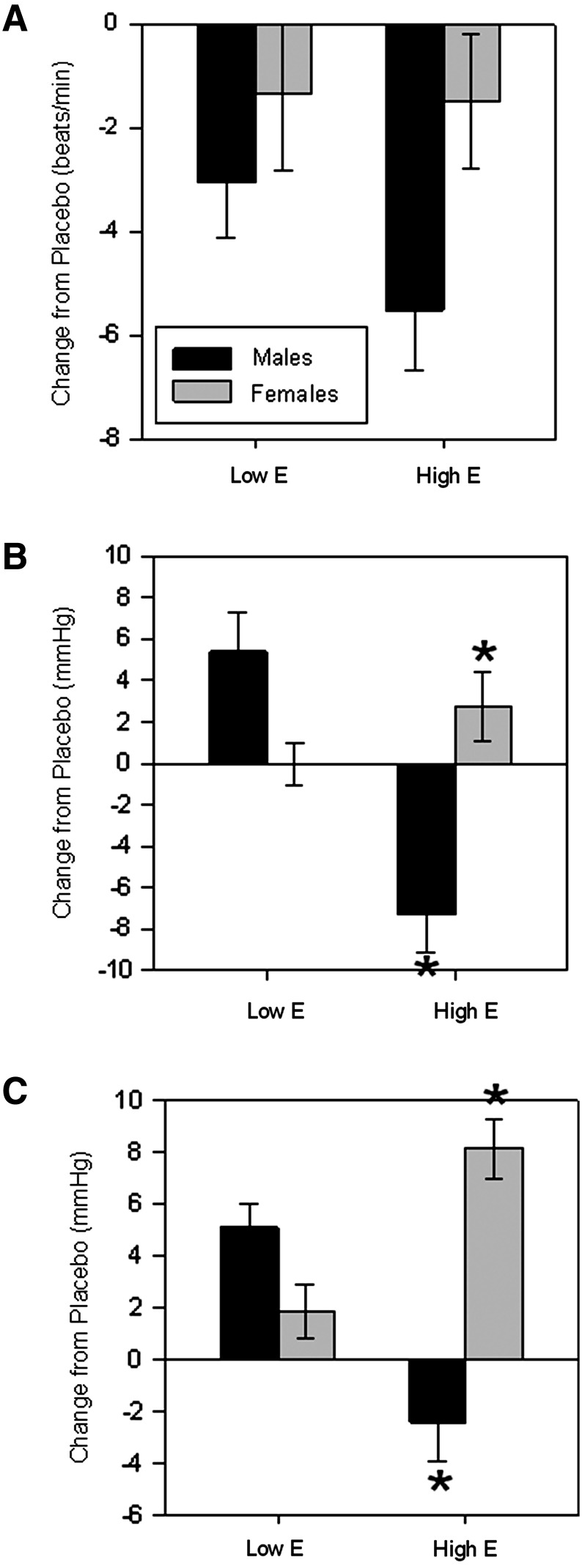
Caffeine administration significantly decreased heart rate (p=0.034) and increased DBP (p=0.001). There were no main effects of gender on cardiovascular responses, but there were significant interactions between drug treatment and gender on heart rate (p<0.0001) and DBP (p=0.005). Males showed a greater decrease in heart rate after caffeine administration than females (Fig. 1). However, females showed a greater increase in DBP after caffeine administration than did males (Fig. 1). There were no main effects or interactions with gender on SBP. When steroid hormones were included in the model, there was a main effect of estradiol level on DBP (p=0.008) and SBP (p=0.001). There were also two way interactions between estradiol level and gender (p=0.002 and p<0.0001, respectively) and estradiol level and caffeine administration on DBP (p=0.002 and p=0.004, respectively) and SBP (p<0.0001 for both). Finally, there was a three-way interaction among gender, estradiol levels, and caffeine administration on DBP (p=0.001) and SBP (p<0.0001; Fig. 2). For both DBP and SBP, males had a significant decrease after caffeine administration relative to placebo when estradiol was high and significant increases relative to placebo when estradiol was low. Females had greater blood pressure responses to caffeine when estradiol was high compared to when estradiol was low.
FIG. 1.
Mean±SEM change from placebo in heart rate (beats/min) and diastolic blood pressure (DBP) and systolic blood pressure (SBP) (mmHg) after administration of 2 mg/kg of caffeine in males (black bars) and females (gray bars). There was a significant interaction between gender and caffeine administration on heart rate (p=0.005) and DBP (p=0.001), with males showing a greater response to caffeine than females for heart rate, but females showing a greater change in blood pressure after caffeine administration than males.
FIG. 2.
Mean±SEM change from placebo in (A) heart rate (beats/min) and (B) DBP and (C) SBP (mmHg) after administration of 2 mg/kg of caffeine in males (black bars) and females (gray bars) who were either low or high in estradiol. There was a significant interaction between gender, estradiol levels, and caffeine administration on DBP (p=0.008) and SBP (p = 0.001), with males showing a greater response to caffeine than females for heart rate, but females showing a greater change in blood pressure after caffeine administration than males. In the statistical analyses, estradiol was included as a continuous variable, but for the purposes of making graphs, a median split was performed for estradiol levels within each gender to determine who was “low” and who was “high.” *Significant difference within each gender as a function of estradiol level (p<0.01).
Drug effects
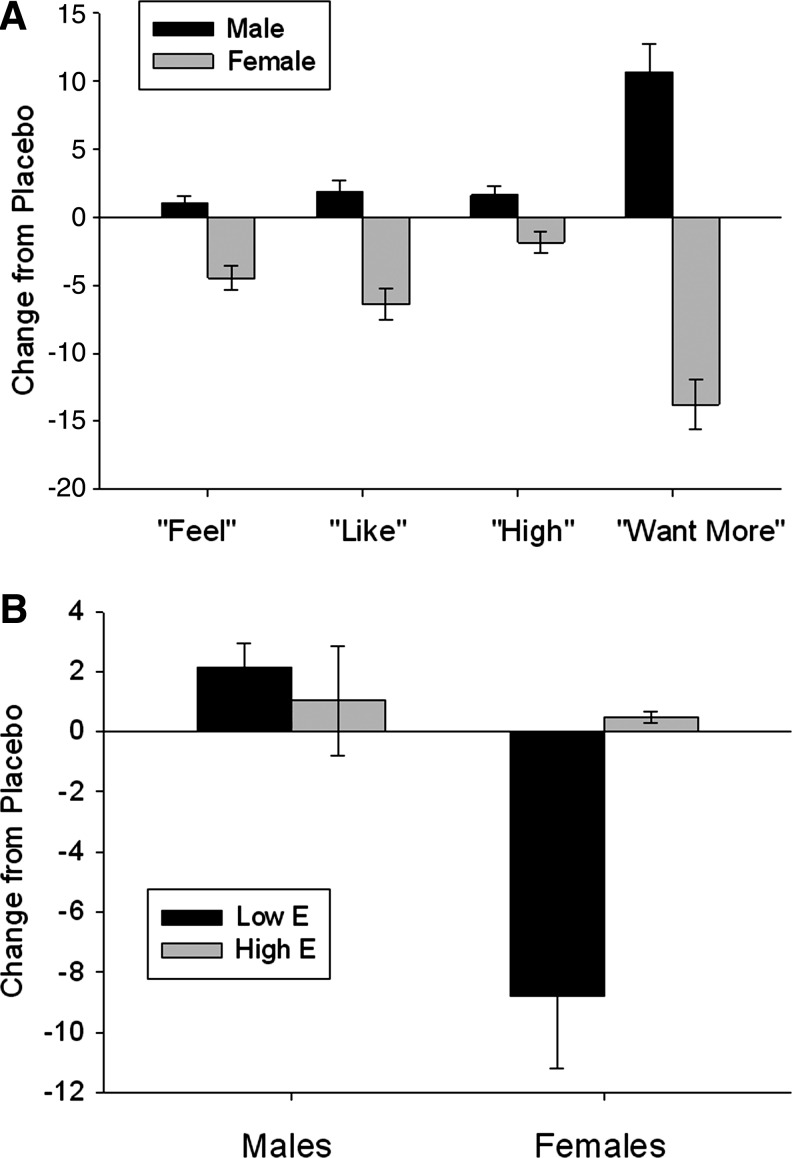
For responses on the DEQ (Table 2), we found main effects of caffeine administration on reports of “feeling drug effects” (p=0.01), “liking drug effects” (p=0.036), and “wanting more of the drug” (p=0.007), with caffeine increasing the “feeling” and “liking” of the drug effects, but significantly decreasing the “wanting more of the drug.” There was also a main effect of gender on “feeling drug effects” (p=0.03), with males reporting greater “feeling drug effects” than females. There were interactions between gender and caffeine administration on “feeling drug effects” (p=0.02), “liking drug effects” (p=0.04), and “wanting more of the drug” (p<0.0001). When these interactions were explored, males reported significantly great “feeling of drug effect” and “liking of the drug effect” than did females after caffeine administration compared to placebo (Fig. 3A).
Table 2.
Table of Z Scores from the Regression Analyses of Cardiovascular and Subjective Responses to Caffeine as a Function of Gender and Salivary Steroid Hormone Levels
| |
Cardiovascular |
Drug effects |
|||||
|---|---|---|---|---|---|---|---|
| HR | DBP | SBP | Feel | Like | High | Want | |
| Caffeine | NS | −3.1 | 3.1 | 4.7 | 5.3 | 2.7 | 6.5 |
| Gender | NS | 1.9 | 4.9 | 3.5 | 2.9 | 2.1 | 5.6 |
| Estradiol levels | NS | 2.7 | 3.7 | 2.1 | NS | NS | NS |
| Progesterone levels | NS | NS | 2.6 | NS | NS | NS | NS |
| Testosterone levels | NS | NS | NS | NS | NS | NS | NS |
| Caffeine*Gender | 2.4 | −2.1 | −4.1 | −4.4 | −4.4 | −2.9 | −7.6 |
| Estradiol*Caffeine | NS | −3.1 | −4.3 | −3.0 | −2.6 | NS | NS |
| Estradiol*Gender | NS | −2.9 | −4.6 | −2.6 | NS | NS | NS |
| Progesterone*Caffeine | NS | NS | −3.9 | NS | NS | NS | −2.1 |
| Progesterone*Gender | NS | NS | −3.4 | NS | NS | NS | NS |
| Testosterone*Caffeine | NS | 2.2 | NS | NS | NS | NS | −2.4 |
| Testosterone*Gender | NS | NS | NS | NS | NS | NS | −2.6 |
| Estradiol*Gender*Caffeine | NS | 3.2 | 5.3 | NS | NS | NS | NS |
| Progesterone*Gender*Caffeine | −2.9 | NS | 4.9 | NS | NS | NS | 2.8 |
| Testosterone*Gender*Caffeine | NS | NS | 2.2 | NS | NS | NS | 3.0 |
Numbers in the cells indicate the Z scores for main effects or interactions with p-values of <0.05.
HR, heart rate; DBP, diastolic blood pressure; SBP, systolic blood pressure; NS, not significant.
Represents interactions among different predictor variables.
FIG. 3.
Mean±SEM change from placebo in responses on the drug effects questionnaire after administration of 2 mg/kg of caffeine in (A) (black bars, males; gray bars, females). There was a significant interaction between gender and caffeine administration on “feeling drug effects” (p=0.02), “liking drug effects” (p=0.04), and “wanting more of the drug” (p<0.0001). When these interactions were explored, males reported significantly great feeling of drug effect and liking of the drug effect than did females. (B) Mean±SEM change from placebo in responses on self-reported feelings of drug effects after administration of 2 mg/kg of caffeine in males (left) and females (right) with low levels of estradiol (black bars) or high levels of estradiol (gray bars). There was a significant interaction between gender, salivary estradiol levels, and caffeine administration on “feeling drug effects” (p<0.0001). When this interaction was explored, males showed no differences in subjective effects related to estradiol levels, but females had strong negative responses to the caffeine relative to placebo when estradiol was low.
When we examined the relationship between drug effects and steroid hormone levels, there were significant two-way interactions between estradiol levels and caffeine administration on “feeling drug effects” (p=0.006), “liking drug effects” (p<0.001), and “feeling high from the drug” (p=0.004). When we examined these interactions, we found that reports of “feeling drug effects” and “liking” were higher when estradiol levels were high, but “feeling high” was lower when estradiol levels were high. There was also a three-way interaction among gender, caffeine administration and estradiol levels for “feeling drug effects” (p<0.0001). In males, there were no differences as a function of estradiol levels, but in females, lower estradiol levels were associated with a greater negative change from placebo than higher estradiol levels (Fig. 3B).
Profile of mood states
There were trends for effects of caffeine administration and gender on the arousal (p=0.006 and p=0.008, respectively) and fatigue (p=0.017 and p=0.03, respectively) subscales of the POMS as well as trends for interactions between gender and caffeine administration on scores on the vigorous (p=0.04), fatigue (p=0.04), and arousal (p=0.01) subscales of the POMS. However, after the Bonferroni correction was applied, none of these remained significantly different. There were no significant interactions when steroid hormone levels were included in the model (all p>0.08).
Addiction research center inventory
There were no main effects or interactions of caffeine administration, gender, or steroid hormone levels on responses on the ARCI (all p>0.08).
Discussion
The purpose of this study was to test the hypothesis that gender differences in responses to caffeine administration are due to differences in circulating steroid hormones. We administered caffeine or placebo once every 2 weeks for 8 weeks and measured cardiovascular, psychological, and subjective responses in adolescent males and females. We found gender differences in cardiovascular and subjective responses to caffeine. Females showed greater blood pressure changes than males, but weaker changes in heart rate. Effects of caffeine on blood pressure also differed as a function of salivary estradiol levels. Specifically, males had reductions in both SBP and DBP when estradiol was higher, but increases when estradiol was lower. Females showed positive changes in both SBP and DBP after caffeine administration, but these responses were greater when esrtadiol levels were higher. Females also showed weaker subjective responses compared to males. When steroid hormone levels were included in the model, we found that females reported “feeling” the effects of the caffeine when estradiol was high, but reported lower feeling of drug effects for caffeine than for placebo when estradiol was low. In males, steroid hormone concentrations had no impact on subjective responses. When taken together, these data suggest that males and females respond differently to caffeine administration and that these gender differences may be mediated by changes in circulating steroid hormones.
We have previously shown gender differences in physiological responses to caffeine.18,19 Specifically, males differed in cardiovascular responses to caffeine based on their level of typical caffeine use (high users responded less than low users), but females did not differ in their responses as a function of typical caffeine use.19 In the current study, we found that females had greater changes in blood pressure after caffeine administration than did males. Males, conversely, showed greater decreases in heart rate in response to caffeine than did females. One explanation for these effects is that males have a more sensitive baroreflex than females, which has been demonstrated by a number of investigators.29–31 These gender differences may be related to changes in circulating steroid hormones, as studies in animals32 and humans33 have shown that baroreflex sensitivity varies as a function of estrus and menstrual cycles. When we examined the relationship between salivary hormone levels and cardiovascular responses to caffeine, we found that males showed an increase in blood pressure in response to caffeine administration only when estradiol levels were low. When estradiol was high in males, they had reductions in blood pressure. In females, blood pressure increases were greater in the presence of higher levels of estradiol, which is consistent with previously reported changes in baroreflex sensitivity.33 This suggests that males and females have different cardiovascular responses to caffeine and that differences in estradiol concentrations have opposite effects on this response in males and females.
In addition to cardiovascular responses, we measured subjective responses to caffeine. In our previous study, we found that boys reported using caffeine to get “high” or to get a “rush” more than did girls.19 This study sought to expand upon the previous study by including questionnaires specifically designed to test subjective effects of drugs. We found that caffeine increased reports of feeling and liking drug effects in males, but decreased these responses in females. Other investigators have reported gender differences in subjective responses to caffeine. For example, a study by Adan et al. showed that males had subjective state responses to caffeine at lower doses than females.34 Another study examined gender differences in interactions between licit substance use and impulsivity and found that higher caffeine use was associated with increased impulsivity in males, but not in females.14 When taken together, these studies suggest that males are more responsive to caffeine than are females. Our data on subjective responses are similar in that males are showing greater positive responses to caffeine than are females, but they differ in that females are showing large negative subjective responses to caffeine relative to placebo.
Gender differences may be due to differences in circulating steroid hormones. There were differences in responses to caffeine on the DEQ as a function of circulating steroid hormones, with increases or no change in “feeling” and “liking” of the drug effects of caffeine when estradiol was high, but decreases in “feeling” and “liking” of caffeine when estradiol was high relative to responses for placebo. These differences were most pronounced in reports of “feeling drug effects.” Females had large differences as a function of estradiol levels, but males showed no change in response to steroid hormones. One explanation for this finding is that when estradiol is low, the girls were experiencing fewer drug effects. In addition, although we did not have the statistical power to examine interaction between regular caffeine use, gender, and steroid hormones in subjective responses, many of the participants were chronic caffeine users and, perhaps, tolerant to the drug effects of caffeine at the dose administered here. Finally, it is possible that instead of the caffeine effects being weak that the placebo response was particularly strong, resulting in a greater difference between placebo and caffeine. The study by Adan et al. found that women had stronger subjective responses to administration of decaffeinated coffee than did males.34 Perhaps women are more prone to placebo effect during certain phases of the menstrual cycle.
This study had several strengths. First, it was a double-blind, placebo-controlled design. Second, we verified pubertal status using parental and self-report of tanner stage characteristics and examined females across different menstrual cycle phases. Third, we measured salivary steroid hormones instead of relying on self-report of menstrual cycle phase. Finally, we collected physiological and subjective state data at the same time within individuals. This study was not without weaknesses. First, we had a small number of participants and, although we collected repeated measures on these participants, we did not have enough power to examine differences as a function of typical caffeine use. Our previous studies have found that responses to caffeine often depend on the level of typical caffeine use. Second, our population was very homogenous in terms of race, age, and socioeconomic status. While this likely reduced variability, it makes our findings difficult to generalize to other populations and ages. Third, although we attempted to minimize expectancies about caffeine, because participants were told to abstain from caffeine, they may have been aware that caffeine was being manipulated. Fourth, we did not adequately control for potential effects of withdrawal reversal. There is a growing consensus among caffeine researchers that many of the positive effects of caffeine may be related to reversal of negative symptoms associated with caffeine withdrawal. Our participants were withdrawn from caffeine for 24 hours, but the relative effects of this withdrawal may differ as a function of gender and/or level of regular caffeine use. It would be interesting to know if males experience stronger withdrawal effects than females and, therefore, report greater positive effects after caffeine administration. Future studies will address this more effectively. Despite these weaknesses, these data provide preliminary evidence of mechanisms that underlie gender difference in responses to caffeine and suggests that the regulation of physiological and subjective responses to caffeine is dissociated or responsive to different factors. Although these data are preliminary, they provide a foundation on which future studies can build to improve our understanding of gender differences in responses to caffeine and, perhaps, to other licit and illicit drugs.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (KO1DA021759) to J.L.T. We thank Amber Dewey for help with data collection.
Author Disclosure Statement
Neither author of this article has any conflicts of interest to report.
References
- 1.Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23:563–576. doi: 10.1016/s0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths RR. Mumford CK. Psychopharmacology: The Fourth Generation in Progress. In: Bloom F.E., editor; Kupfer D.J., editor. Caffeine: a drug of abuse? New York: Raven Press; 1995. pp. 1699–1713. [Google Scholar]
- 3.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 4.Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33:793–806. doi: 10.1016/j.neubiorev.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frary CD. Johnson RK. Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein GA. Carroll ME. Dean NW. Crosby RD. Perwien AR. Benowitz NL. Caffeine withdrawal in normal school-age children. J Am Acad Child Adolesc Psychiatry. 1998;37:858–865. doi: 10.1097/00004583-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Oberstar JV. Bernstein GA. Thuras PD. Caffeine use and dependence in adolescents: one-year follow-up. J Child Adolesc Psychopharmacol. Summer. 2002;12:127–135. doi: 10.1089/104454602760219162. [DOI] [PubMed] [Google Scholar]
- 8.James JE. Rogers PJ. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacology (Berl) 2005;182:1–8. doi: 10.1007/s00213-005-0084-6. [DOI] [PubMed] [Google Scholar]
- 9.Hills AP. Byrne NM. An overview of physical growth and maturation. Med Sport Sci. 2010;55:1–13. doi: 10.1159/000321968. [DOI] [PubMed] [Google Scholar]
- 10.Ernst M. Korelitz KE. Cerebral maturation in adolescence: behavioral vulnerability. Encephale. 2009;35(Suppl 6):S182–S189. doi: 10.1016/S0013-7006(09)73469-4. [DOI] [PubMed] [Google Scholar]
- 11.Smetana JG. Campione-Barr N. Metzger A. Adolescent development in interpersonal and societal contexts. Annu Rev Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- 12.Pollak CP. Bright D. Caffeine consumption and weekly sleep patterns in US seventh-, eighth-, and ninth-graders. Pediatrics. 2003;111:42–46. doi: 10.1542/peds.111.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Terner JM. de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Waldeck TL. Miller LS. Gender and impulsivity differences in licit substance use. J Subst Abuse. 1997;9:269–275. doi: 10.1016/s0899-3289(97)90021-3. [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K. Agnati LF. Jacobsen K, et al. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson's disease. Neurology. 2003;61(11 Suppl 6):S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- 16.Kudlacek O. Just H. Korkhov VM, et al. The human D2 dopamine receptor synergizes with the A2A adenosine receptor to stimulate adenylyl cyclase in PC12 cells. Neuropsychopharmacology. 2003;28:1317–1327. doi: 10.1038/sj.npp.1300181. [DOI] [PubMed] [Google Scholar]
- 17.Salim H. Ferre S. Dalal A, et al. Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J Neurochem. 2000;74:432–439. doi: 10.1046/j.1471-4159.2000.0740432.x. [DOI] [PubMed] [Google Scholar]
- 18.Temple JL. Bulkley AM. Briatico L. Dewey AM. Sex differences in reinforcing value of caffeinated beverages in adolescents. Behav Pharmacol. 2009;20:731–741. doi: 10.1097/FBP.0b013e328333b27c. [DOI] [PubMed] [Google Scholar]
- 19.Temple JL. Dewey AM. Briatico LN. Effects of acute caffeine administration on adolescents. Exp Clin Psychopharmacol. 2010;18:510–520. doi: 10.1037/a0021651. [DOI] [PubMed] [Google Scholar]
- 20.Tran KM. Johnson RK. Soultanakis RP. Matthews DE. In-person vs telephone-administered multiple-pass 24-hour recalls in women: validation with doubly labeled water. J Am Diet Assoc. 2000;100:777–783. doi: 10.1016/S0002-8223(00)00227-3. [DOI] [PubMed] [Google Scholar]
- 21.Oliveto AH. Bickel WK. Hughes JR. Terry SY. Higgins ST. Badger GJ. Pharmacological specificity of the caffeine discriminative stimulus in humans: effects of theophylline, methylphenidate and buspirone. Behav Pharmacol. 1993;4:237–246. [PubMed] [Google Scholar]
- 22.Miller KE. Wired: energy drinks, jock identity, masculine norms, and risk taking. J Am Coll Health. 2008;56:481–489. doi: 10.3200/JACH.56.5.481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonat S. Pathomvanich A. Keil MF. Field AE. Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110:743–747. doi: 10.1542/peds.110.4.743. [DOI] [PubMed] [Google Scholar]
- 24.Schlossberger NM. Turner RA. Irwin CE., Jr Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13:109–113. doi: 10.1016/1054-139x(92)90075-m. [DOI] [PubMed] [Google Scholar]
- 25.Martin WR. Sloan JW. Sapira JD. Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 26.Cella DF. Tross S. Psychological adjustment to survival from Hodgkin's disease. J Consult Clin Psychol. 1986;54:616–622. doi: 10.1037//0022-006x.54.5.616. [DOI] [PubMed] [Google Scholar]
- 27.Leddy JJ. Epstein LH. Jaroni JL, et al. Influence of methylphenidate on eating in obese men. Obes Res. 2004;12:224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell E. de Leon CF. Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870–878. doi: 10.1097/01.psy.0000239144.91689.ca. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Rahman AR. Merrill RH. Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J Appl Physiol. 1994;77:606–613. doi: 10.1152/jappl.1994.77.2.606. [DOI] [PubMed] [Google Scholar]
- 30.Beske SD. Alvarez GE. Ballard TP. Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol. 2001;91:2088–2092. doi: 10.1152/jappl.2001.91.5.2088. [DOI] [PubMed] [Google Scholar]
- 31.Laitinen T. Hartikainen J. Vanninen E. Niskanen L. Geelen G. Lansimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 32.Goldman RK. Azar AS. Mulvaney JM. Hinojosa-Laborde C. Haywood JR. Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1419–R1426. doi: 10.1152/ajpregu.91030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minson CT. Kaplan P. Meendering JR. Torgrimson BN. Miller NP. Comments on Women, hormones, and clinical trials: a beginning, not an end. J Appl Physiol. 2006;100:373. doi: 10.1152/japplphysiol.01183.2005. [DOI] [PubMed] [Google Scholar]
- 34.Adan A. Prat G. Fabbri M. Sanchez-Turet M. Early effects of caffeinated and decaffeinated coffee on subjective state and gender differences. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1698–1703. doi: 10.1016/j.pnpbp.2008.07.005. [DOI] [PubMed] [Google Scholar]